Abstract
Pollen tubes extend rapidly in an oscillatory manner by the extreme form of polarized growth, tip growth, and provide an exciting system for studying the spatiotemporal control of polarized cell growth. The Rho-family ROP GTPase is a key signaling molecule in this growth control and is periodically activated at the apical plasma membrane to spatially define the apical growth region and temporally precede the burst of growth. The spatiotemporal dynamics of ROP GTPase is interconnected with actin dynamics and polar exocytosis that is required for tip-targeted membrane and wall expansion. Recent advances in the study of the mechanistic interlinks between ROP-centered signaling and spatiotemporal dynamics of cell membrane and wall remodeling will be discussed.
Keywords: ROP signaling, vesicular trafficking, cell wall mechanics, actin dynamics, oscillation
1. Introduction
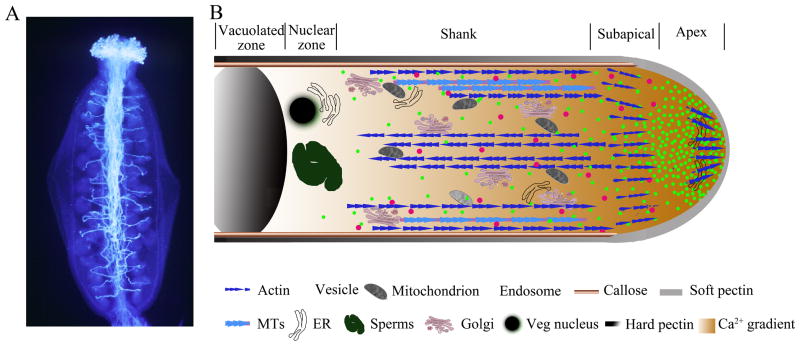
As a male gametophyte, pollen germinates on the flower stigma into a single pollen tube, which navigates through several female tissues to reach the ovule (Figure. 1A). This guided tip growth, which is remarkably similar to neuronal guidance, involves signal-mediated attraction to female tissues as well as repulsion and competition among pollen tubes [1–3]. In addition to the fascinating biology, the pollen tube provides an excellent experimental system. Unlike most plant cells, which dedifferentiate and lose polarity upon in vitro culture, cultured pollen maintains its polarity and developmental identity. In vitro pollen tubes grow synchronously and uniformly, and exhibit highly polarized cytoplasmic organization with the apical region packed with exocytic vesicles (Figure. 1B). Although each pollen tube contains two sperm cells embedded in the vegetative cytoplasm, the sperm genome does not seem to contribute to the genetic control of pollen tube growth. Instead, it is controlled by the haploid genome carried by the vegetative nucleus, and thus lethal mutations affecting tube growth can be maintained in heterozygous plants, which facilitates genetic analysis of essential genes involved in polarity and growth control. These advantages, combined with the ease with which live imaging is performed with pollen tubes, make it one of the most exciting systems for the studies of polarity and tip growth.
Figure 1. The pollen tube system: Directional and polarized cell growth.
(A) Pollen tubes in the pistil, aniline blue staining of pollen tubes in the Arabidopsis pistil. (B) Schematic diagram of the internal zonation and structural elements of a growing pollen tube. The growing tube displays a tip-focused cytoplamic Ca2+ gradient and contains a single soft pectin apical wall and two layers shank wall, the inner sheath of callose and outer coating of hard pectin, which are non-plastic and able to resist turgor pressure. The apical clear zone is characterized by a V shaped accumulation of secretory vesicles that facilitate massive tip-targeted exocytosis. The subapical organelle-rich zone is followed by a nuclear and a vacuole zone. Microtubules (MTs) and long actin cables axially aligned in the shank and are excluded from the apical zone. A collar-like actin microfilaments (F-actin) structure is present in the subapical region and a population of fine and short F-actin is detected in the extreme apex.
To efficiently reach their target in the ovary, pollen tubes elongate at an astonishing rate (up to 1 cm/hr) to an extraordinary length (e.g., the length of corn silk) by polarized tip growth, which is strictly dependent on polar exocytosis that delivers cell membrane and wall materials to the growing tip as in other tip growing systems [4–8]. An intriguing question is how pollen tubes design their structural and molecular machineries to achieve such rapid polar growth. At the sub-cellular level, pollen tube growth requires a highly polarized cytoplasmic organization [5, 6]. As the pollen tube grows, periodic cross-wall callose deposition isolates the pollen protoplast, which contains the biosynthetic machinery and the male germ unit, in the tip region of the elongating tube. This process is analogous to septum formation in fungal hyphae. The tip region displays four distinct zonings: an apical zone essentially packed with exocytic vesicles accumulated as a typical V shape to facilitate massive tip-targeted exocytosis, a subapical organelle-rich zone, a nuclear zone, and a vacuolated zone that may extend toward the grain [4]. Cytoplasmic streaming drives organelles moving rapidly back and forth along the main axis of the pollen tube in a reverse fountain pattern, which maintains the distribution of membranous structures and releases exocytic vesicles to the apical zone [9]. A complete picture of the cytoskeletal elements and dynamics that regulate the polar organization of the tube cytoplasm and targeted exocytosis is emerging [7, 9–15]. Due to a high global turgor pressure, the exocytosis-based membrane and wall extension needs to be coupled with the spatiotemporal regulation of cell wall mechanics. Both experimental and computational approaches have recently provided important insights into the cell wall mechanics during pollen tip growth.
At the molecular level, recent studies have uncovered a Rho GTPase-based self-organizing signaling network that controls tip growth in pollen tubes via its inter-connection with the cytoskeletal elements and the polarized exocytosis [13, 16–20]. Several excellent recent reviews provide important insights into the molecular mechanisms under pollen tube tip growth [6–8, 21–24]. Our current review will focus on the latest advances in the structural basis of this process and its interface with the Rho GTPase-based signaling network. Emphasis will be given to the comparison and contrast of the mechanisms for tip growth of pollen tubes with those of other systems.
2. The structural system: Roles in structure and regulation
2.1. The cytoskeleton
Pollen tubes contain two major cytoskeletal elements, microtubules (MTs) and actin microfilaments (F-actin), which are highly organized and dynamic through their interaction with various actin-binding proteins and microtubule-associated proteins [22, 25–27]. MTs are involved in the organization of spitzenköper (the exocytosis organizing center) and organelle movement and regulate the efficiency of tip growth but not essential for this growth [28–30]. In contrast, F-actin structures are quintessential for tip growth in pollen tubes. Multiple forms of F-actin in different regions of pollen tube support its pivotal role for tip growth. Abundant long actin cables axially aligned in the shank provide the main tracks for movement of organelles and vesicles and regulate cytoplasmic streaming [9, 10]. The formin 3 (FH3) actin nucleation factor is responsible for the formation of these actin cables [15]. RNAi-mediated FH3 down-regulation abolished the actin cables and altered cytoplasmic streaming pattern [15]. FH3RNAi tubes were much shorter and wider than wild type tubes [15], suggesting that the actin cables play an important role in tip growth. Although the mechanism by which the axial actin cables regulate tip growth is unclear, cables-dependent cytoplasmic streaming may rapidly bring exocytic vesicles to the subapical zone. In this zone, a collection of shorter and thinner actin cables constitutes a collar, ring- or funnel-like F-actin structure. This highly dynamic subapical F-actin structure is assembled by the tip-located, cell membrane-anchored formin 5 (FH5) in pollen tubes [14]. FH5RNAi pollen tubes were devoid of the subapical F-actin and exhibited abnormal twists and turns [11, 14]. Thus the subapical F-actin apparently maintains the direction of tip growth. It was proposed that the subapical F-actin participates in vesicular trafficking in the apical region [11, 14]. However, the vesicular trafficking mediated by the subapical F-actin alone cannot satisfactorily explain how exocytic vesicles accumulate in the apical zone in a V-shape with the highest density of vesicles in the cortex of the extreme apex (Figure. 1B). The subapical F-actin could capture vesicles released from the axial actin cables and then transport them to the apical zone. In the apical zone, a population of fine and less abundant microfilaments is present in the extreme apex [11, 31]. Evidence suggests these fine F-actin filaments promote the accumulation of exocytic vesicles to the extreme apex in a V-shape pattern [13]. The dynamic apical F-actin is regulated by Rho-related GTPase of plants (ROP) (see below) [11–13, 31], but the nature of the nucleation factor responsible for the assembly of this F-actin remains unknown.
2.2. Exocytosis and endocytosis
The rapid and continuous tip growth has to rely on efficient and ample supply of cell wall components, membrane materials, enzymes, and signaling molecules, which is dependent on polarized exocytosis. The quintessential role of exocytosis in pollen tube tip growth has been inferred from the massive apical accumulation of vesicles and the cessation of tip growth by treatments with brefeldin A and chemicals that disrupt F-actin [32, 33]. Tobacco and Arabidopsis homologs of Rab11 are associated with vesicles accumulated in the extreme apex [34, 35]. Subsequently, the octameric exocyst complex will facilitate the exocytic vesicles targeting and tethering to the PM [36]. In yeast, it is proposed that six of the eight exocyst subunits (Sec5p, Sec6p, Sec8p, Sec10p, Sec15p and Exo84p) ride the exocytic vesicle along actin cables to exocytic sites [37], while the other two components Sec3p and Exo70p bind to plasma membrane (PM) localized Rho1-GTP and Rho3-GTP respectively and define the location of exocytic sites [38, 39]. Thus, the assembly of the eight subunits of exocyst determines where and when vesicles are tethered in preparation for fusion [40]. Mutations of the homologs of exocyst subunits, such as SEC3, SEC8 and Exo70, in Arabidopsis result in defective root hairs or pollen tube growth [41–43]. Moreover, Sec3 is shown to interact with ROP effector, ICR1 [44], suggesting that exocyst plays crucial roles in polar tip growth through integration with ROP regulation of polar exocytosis.
Two different views of vesicular trafficking in pollen tube tips have been proposed. Recently it was proposed that exocytosis occurs at the shoulder of the tip while the apical vesicles are endocytic vesicles [45, 46]. This contrasts with the long-standing prevalent model, which proposes that exocytosis occurs at the extreme apex, whereas endocytosis occurs preferentially in the subapical area [47–49]. The localization of exocyst to the tip of pollen tubes supports the prevalent model [42]. To definitely distinguish these two models, a method for direct measurements of exocytic activity in the tip of pollen tubes is needed. Recently a novel strategy involving fluorescence recovery after photobleaching (FRAP) of receptor-like kinase (RLK)-GFP, whose targeting to the PM completely depends on exocytosis, was developed to visualize exocytosis in pollen tube tips and led to the conclusion that exocytosis of the RLK-GFP tracer protein was indeed restricted to the PM apex with a tip-high gradient, which corresponds to the gradient of active ROP1 that has been shown to promote the tip-targeted exocytosis [13]. The polarized exocytosis is dependent upon the dynamics of the apical F-actin that is regulated by the ROP1 Rho-family GTPase via two counteracting RIC4 and RIC3 downstream pathways that promote actin assembly and disassembly, respectively [11, 13, 31].
By monitoring the changes in the amount of exocytic wall material and the surface appearance of a direct exocytosis marker, pectin methylesterase (PME), it was shown that exocytosis in pollen tubes oscillates temporally and serves as key factor in the initiation and regulation of oscillatory pollen tube growth [50]. Evidence suggests that exocytosis delivers to the apical PM PRK2 receptor kinase that recruits and activates ROP1 upstream activators, RopGEFs, via phosphorylation regulation to release the C-terminal inhibition of RopGEFs [51–53]. Therefore, exocytosis participates in the positive feedback activation of ROP1 and might create an autocrine signaling mechanism underlying the speedy establishment and regeneration of the active ROP1 cap. Moreover, exocytosis also participates in the negative feedback loop to restrict the positive feedback-based lateral amplification of ROP1 activation to the tip growth domain through targeting ROP1 negative regulators, such as REN1 RhoGAP, to the tube apical PM [17–19]. In addition to the spatiotemporal regulation of the dynamic apical cap of ROP1, exocytosis might also regulate cell wall mechanics (see below).
Exocytosis in growing pollen tubes is balanced by the retrieval of excess PM and wall materials and associated signaling molecules by endocytosis [54]. Clathrin-dependent internalization is the predominant endocytic system in plants and is required for the generation of cell polarity [55]. Clathrin-dependent endocytosis occurs preferentially in the shoulder (or subapical) area of the pollen tube tip [47, 56], which also coincides with the localization of the subapical F-actin (see above). Thus it would be reasonable to speculate that rapid exocytosis that occurs at the extreme apex is coordinated with clathrin-dependent endocytosis in the subapical area to maintain the identity of the tube apex, and that endocytic vesicles are transported via the subapical F-actin to the apex for recycling. This notion is supported by several observations. First, FM dyes that stain endocytic vesicles accumulate as the canonical V-shaped pattern in the tip as exocytic vesicles [48, 56]. Second, inhibition of clathrin-dependent endocytosis disrupted canonical V-shaped fluorescence in the tip region and uptake of negatively charged nanogold [57]. Third, the depletion of the subapical F-actin by FH5RNAi also leads to the collapse of the V-shaped vesicle accumulation [14]. Interestingly, clathrin-dependent endocytosis also occurs in the subapical area of fungal hyphae [58, 59] implying that the complementary apical exocytosis and subapical endocytosis may be a conserved design principle underlying rapid tip growth.
The machinery for clathrin-dependent endocytosis appears to be conserved [56]. For example, phosphatidylinositol 4,5-bisphosphate (PIP2) localized in the inner lea et of the PM is known to promote the early stages of clathrin-dependent endocytosis in yeast and animal cells [60], and has also been shown to do so in pollen tubes [56]. Earlier studies in yeast suggest that the hydrolysis of PIP2 to phosphatidylinositol 4-phosphate (PI4P) is important for the late stage of endocytosis [60]. Our work in pollen tubes suggests that it is actually the hydrolytic product PI4P that is required for the completion of clathrin-dependent endocytosis [56].
How does the pollen tube control the site of clathrin-dependent endocytosis in the subapical area? Given PIP2’s role in initiating the recruitment of clathrin coat onto the inner leaflet of the PM site for endocytosis, it would be important to know the mechanism for the polar PIP2 distribution. Consistent with the site of endocytosis, in growing pollen tube PIP2 is preferentially compartmentalized in the subapical area of the PM through the subapical distribution of PI4P-5 kinases that generate PIP2 from PI4P, which is distributed throughout the whole apical region [56, 61, 62]. In non-growing tubes, PIP2 appears to be compartmentalized in the apical PM [63], implying a potential role for the subapical endocytosis in the regulation of tip growth. Thus the mechanism underlying PI4P5K localization and activation is anticipated to be the signaling events governing the spatial control of clathrin-dependent endocytosis. Interestingly, a ROP/Rac GTPase was reported to be physically associated with a PIP5K activity in tobacco pollen tubes, although direct evidence for ROP/Rac regulation of clathrin-dependent endocytosis is missing [63]. In the budding yeast, clathrin-dependent endocytosis is regulated by the Cdc42 member of the Rho GTPase family via its effect on actin dynamics [64]. Rho-family GTPase regulation of PIP signaling has also been reported in other systems [65, 66]. Thus, Rho GTPase signaling may provide a conserved mechanism for the modulation of endocytosis during polarized cell growth.
2.3. Cell wall composition and mechanics
Because of high turgor pressure in pollen tubes as in other plant cells, biosynthesis-based growth via exocytic delivery of cell wall materials has to be coordinated with the mechanics of the cell wall that facilitate localized cell expansion at the tip. It was reported that the growing region of pollen tubes spatially coincides with a region of lower stiffness and that the growth rate oscillations in pollen tubes are correlated with spatially confined dynamic changes in the mechanical properties of the apical cell wall [67–69]. Mathematical simulations of cell wall mechanics combined with experimental manipulation of cell wall composition provide an important approach for understanding the complex tip growth process not only in pollen tube but also other tip-growing walled cells, such as root hairs and fungal hyphae [70, 71]. Given that globally acting turgor pressure drives a walled cell to expand in the wall region that is “soft” or extensible [72], the spatiotemporal regulation of cell wall mechanics learned from pollen tubes may provide insights into the mechanisms of shape formation and polar growth for all walled cells. The apical exocytosis could regulate the wall mechanics through its targeting of methylated soft pectin and PME inhibitors to the tip. Likewise the subapical endocytosis might also contribute to the wall mechanics by retrieving specific wall components or their modifiers, e.g., demethylated pectin [73, 74]. Therefore we speculate that intracellular signaling pathways leading the localized exocytosis and endocytosis may coordinate the spatiotemporal control of cell wall growth with that of cell wall mechanics.
3. The regulatory system: Self-organization and structural regulation
Pollen grains generate pollen tubes in vitro in the absence of external signals, suggesting that their tip growth is controlled by a self-organizing system. Studies over the last decade have demonstrated the existence of a self organizing signaling network, which is centered on a tip-localized Rho GTPase (ROP1) and tip-focused calcium gradients and their interactions with the actin cytoskeleton and vesicular trafficking [11, 13, 16–18, 31, 56, 75–79].
3.1. ROPs
As in all other polar growth systems, Rho-family GTPases play an essential role in the control of pollen tube growth. All plant Rho-like GTPases fall into a single subfamily named ROP, which was first identified from pea [80]. Arabidopsis has 11 ROPs, six (ROP1 to ROP6) of which are known to regulate cell polarity formation and polar cell growth [6, 81–84]. ROP1 protein exhibits polar localization to the apical domain of the PM of pollen tubes [76]. Active ROP1 is distributed as an apical cap, which corresponds to the site of exocytosis [11, 12, 77, 85]. Blocking ROP1 signaling by expressing DN-ROP1 or by microinjecting anti-ROP1 antibody inhibited pollen-tube tip growth, whereas expression of a CA-ROP1 and overexpression of WT-ROP1 induced a dramatically enlarged active ROP1 cap and growth depolarization [63, 77, 86–88]. These observations led to the proposal that the apical cap of active ROP1 defines the tip growth region, termed tip growth domain, and activates tip growth, and that efficient tube elongation requires an optimum level of apical ROP1 activity that is dynamically self-organized to maintain an optimal cap size [18, 63, 77, 86–88].
Apical ROP1 signaling must be regulated by a self-organizing mechanism because spontaneous polar growth occurs in vitro in the absence of external spatial cues. To understand the spatial regulation of ROP1, a GFP-based reporter, ΔRIC4-GFP, in which the CRIB (Cdc42/Rac-interactive binding) domain of the RIC4 ROP1 effector is fused with GFP, was developed to monitor the distribution of active ROP1 [16]. ΔRIC4-GFP was localized to the PM as an apical cap in a ROP1 activation-dependent manner, and ΔRIC4-GFP imaging reveals an oscillation of ROP1 activity along with the growth oscillation of the pollen tubes [16, 18]. The maximal cap of active ROP1 defines the tip growth domain [16, 18]. The apical ROP1 cap is generated by lateral propagation of a localized ROP activity and is regulated by downstream events including tip F-actin and Ca2+ [16–18, 31]. The stabilization of tip F-actin causes dramatic enlargement of the apical cap, generating a ballooned tip, as does knocking out the REN1 RhoGAP [16, 17, 19, 31]. Elevation of Ca2+ or actin depolymerization reduces the apical ROP1 activity [16, 18, 19, 31]. Thus tip F-actin and Ca2+ feedback activate and inhibit ROP1, respectively [19].
Exocytosis in pollen tubes is tightly regulated in time and space. In oscillating pollen tube, polar exocytosis oscillates in a phase just slightly behind ROP1 activity, but well ahead of tip growth [13]. Tip F-actin oscillates in the same phase with ROP1 activity and is required for exocytosis [13, 16]. The effect of tip F-actin on the positive ROP1 feedback regulation may be due to actin-mediated exocytosis by targeting of ROP1 activators such as PRK2 receptor kinase that could activate ROP1 upstream activators RopGEFs, to the tip [51–53]. In addition, ROP1 interacts with its effecter RIP1/ICR1, which subsequently targets to exocyst subunit SEC3, to regulate polarized exocytosis [20, 44, 89]. This exocytosis-dependent positive feedback loop coupled with diffusion may be responsible for the formation and regeneration of the apical ROP1 cap as well as the lateral propagation of active ROP1 cap (Figure. 2) [18].
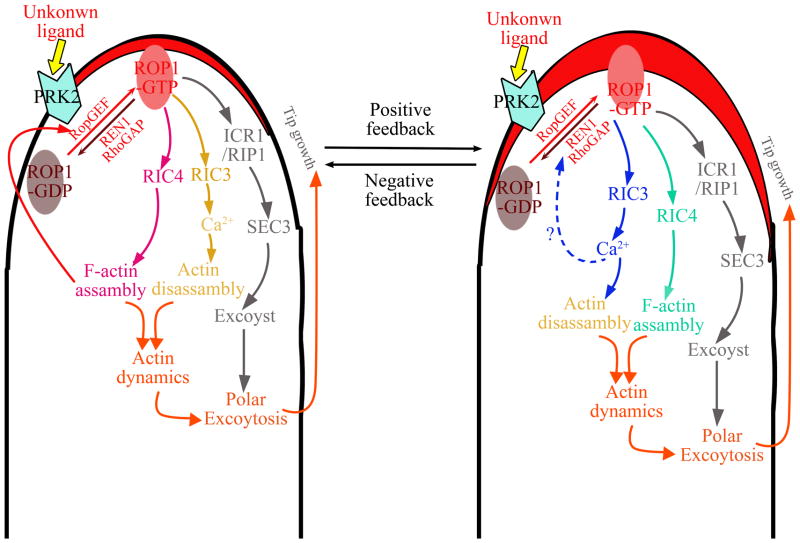
Figure 2. The ROP1 signaling network that control pollen tube tip growth.
The network is composed of several pathways coordinately promoting tip-targeted exocytosis and positive and negative feedback loops, which may balance each other to maintain a certain size of the apical ROP1 cap that defines tip growth domain or may allow the oscillation of the ROP1 activity. ROP1 is locally activated in the PM to determine the site of exocytosis and activates multiple pathways leading to polar exocytosis. The RIC4 pathway promotes F-actin assembly and induces the accumulation of exocytic vesicles to the tip, and promotes positive feedback loops to increase the area of active ROP1 probably by targeting ROP1 upstream components such as RopGEFs and PRK2. Positive feedbacks coupled with diffusion rapidly generate the apical cap of active ROP1 that defines the tip growth domain. Meanwhile, ROP1 also activates the RIC3–calcium pathway. RIC3-dependent Ca2+ promotes tip F-actin disassembly and facilitates exocytosis. Polar exocytosis is also promoted by another likely ROP1 effector, RIP1/ICR1, which subsequently target recruits the SEC3 exocyst subunit that mediates the tethering of exocytic vesicles on the PM. Polarized exocytosis brings the REN1 RhoGAP to the apical PM, which deactivate PM-localized active ROP1. Thus the REN1-based negative-feedback globally inhibits ROP1, prevents excess ROP1 activation in the apical PM, and restricts the enlargement of the apical cap to the tip growth domain.
To maintain an optimal apical ROP1 cap and generate the apical ROP1 activity oscillation required for efficient pollen-tube elongation, a negative feedback mechanism is required to limit the positive feedback-based lateral propagation of apical ROP1 activity [18]. A global inhibition mechanism governed by ROP1 negative regulators, RopGAPs (Rop GTPase activating proteins) and RhoGDIs (Rho guanine nucleotide dissociation inhibitors), is shown to restrict the lateral amplification once the apical ROP1 cap reaches a certain size, thus preventing excess lateral propagation and finally terminating one cycle of ROP1 activity increase (Figure. 4) [18]. A screen for mutations that enhance ROP1-overexpression-induced depolarization of pollen tube growth identified a novel RhoGAP, termed REN1, which was demonstrated to play a primary role in restricting active ROP1 to the pollen tube tip, by globally inhibiting ROP1 GTPase at the tube apex [17]. REN1 localizes to the apical cap and exocytic vesicles in the pollen tube tip, implying that exocytosis is also involved in the negative feedback inhibition of ROP1 activity [17]. In addition to exocytosis, Ca2+ signal is also involved in the negative feedback inhibition of ROP1 to balance the positive feedback of ROP1 activity and to generate the oscillation of the apical ROP1 activity [19] (see below).
3.2. Calcium gradients
Calcium has long been recognized as a critical intracellular signal regulating tip growth in pollen tubes. Growing pollen tubes display a tip-focused Ca2+ gradient [79, 90], which oscillates with the same periodicity as pollen tube growth, but the calcium pulses slightly lag behind those of growth rates [91]. The dissipation of the gradients using Ca2+ channel blockers or applying extra Ca2+ into growth media leads to growth arrest [79]. Manipulations that alter the focal point of the gradients reorient the direction of pollen tube growth [92]. These observations suggest that calcium gradients are essential for pollen tube growth and regulate tip growth polarity, a role that parallels that of ROP1. Indeed several recent studies suggest that ROP1 and calcium are interwoven in the control of tip growth (Figures 2 and 3). It was shown that apical ROP1 activity oscillates ahead of growth rate and the apical calcium [16]. Tip-localized ROP1 GTPase activates downstream effector RIC3, which mediates the formation of the calcium gradients [31]. By promoting F-actin depolymerization, calcium regulates the F-actin dynamics at the tip, which in turn promotes exocytosis (Figure. 3) [13, 31]. This role for calcium is also supported by the observation that low Ca2+ concentrations caused an excessive quantity of vesicles to accumulate at the tip, while high Ca2+ concentrations accelerates vesicle fusion at the tip [93]. Calcium might also play a direct role in the regulation of exocytosis as in neuronal cells, although evidence for this is lacking.
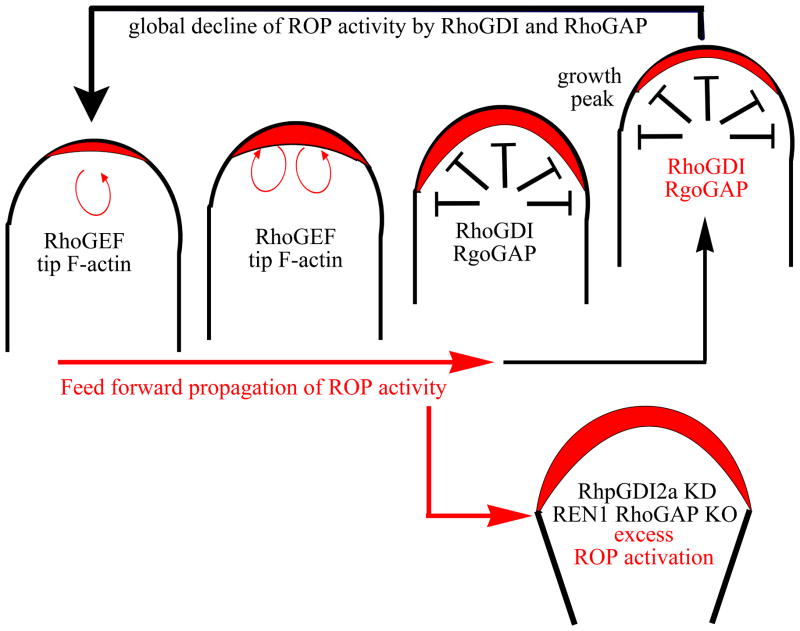
Figure 3. A model for the generation and maintenance of the apical cap of active ROP1 in growing pollen tubes.
The localized ROP1 activity in the center of tube apical PM is amplified through a positive-feedback loop of ROP1 activation, such as recruitment of RhoGEF or other upstream ROP activator, which induces a rapid increase of local ROP1 activity and then its lateral propagation through the apex, generating the active ROP1 cap. The ROP1-mediated tip F-actin dynamic might also contribute to the rapid lateral propagation of ROP1 activity by facilitating the diffusion of ROP1 and its regulators in the PM through polarized exocytosis. RhoGAP and RhoGDI globally inhibit ROP1 in the apex, preventing excess lateral propagation and finally terminating one cycle of ROP1 activity increase. ROP1 activity starts to increase again, probably via positive feedback from the remnant of the previous active ROP1 cap. A tightly balanced interaction of ROP1 activation and inactivation might continuously generate the dynamic apical ROP1 activity for the continuous tip growth. When the balance is broken by loss of critical RhoGDI or RhoGAP activity (RhoGDI2a and REN1 RhoGAP in Arabidopsis pollen tube), ROP1 becomes activated, resulting in the depolarization of apical ROP1 cap and pollen-tube tip growth.
Adding to the complexity of calcium-ROP1 interaction is the finding that increased rate of calcium accumulation actually suppresses ROP1 activity and balances the RIC4- and F-actin-dependent apical ROP1 activation [19]. Thus calcium functions in the negative feedback regulation of the apical ROP1 activity necessary for growth oscillation [19]. The complex roles of calcium and its interaction with ROP1 are consistent with the large number of calcium sensors expressed in pollen, including actin-binding proteins, calcium-dependent protein kinases, and calcineurin-interacting proteins [27, 75, 94, 95]. It will be important to elucidate the functions of these calcium sensors in the regulation of tip growth in pollen tubes and their relationship with ROP1 signaling. Calcium also regulates tip growth in root hairs of plants and likely in other rapid tip growth systems such as fungal hyphae [96, 97]. Thus, the Rho-calcium interplay might provide a universal mechanism underpinning rapid tip growth.
The mechanisms regulating the generation and the oscillation of the Ca2+ gradients are complex as well, probably involving multiple pathways and transport systems. The ROP1 effector RIC3 is proposed to promote calcium influxes through the plasma membrane [31], but the underlying mechanism is unknown. The cyclic nucleotide-gated channel (CNGC) 18 localized to the PM at the growing tip of pollen tube is shown to function as a Ca2+-permeable channel and regulate pollen tube growth, but it seems not be the target of RIC3 signaling, even though its tip PM expression pattern is affected by ROP1 signaling [98, 99]. An important breakthrough is the newly uncovered function of glutamate receptor-like (GLR) family in the generation of Ca2+ gradients and oscillations through facilitating Ca2+ influx across the apical PM [100]. Regulation of intracellular calcium stores and PM-localized calcium pumps is expected to participate in Ca2+ gradient formation and oscillations, but their connection to ROP1 signaling has yet to be explored.
3.3. ROS
NADPH oxidase (NOX)-dependent reactive oxygen species (ROS), which is localized in the pollen tube tip, is also implicated in pollen tube growth [101], and is likely linked to ROP1 signaling. NOX directly interacts with and appears to be a ROP/Rac effector in cultured rice cells and Arabidopsis during stress responses [102, 103]. ROS’ function and linkage with ROP signaling has been well studied with regards to tip growth in root hair, a process that share many mechanistic similarities with pollen tubes. In root hair, its regulation of tip growth is tightly connected with calcium signal. ROS activates Ca2+ permeable channels required for generation of tip-focused Ca2+ gradient in root hairs [104]. Establishment and maintenance of tip growing site in root hair is regulated by ROS produced by RHD2, a PM localized NOX, and Ca2+ mediated positive feedback loop [105]. SCN1, a RhoGDI isoform spatially regulates RHD2-catalyzed production of ROS to hair tips, implying that ROP may play a pivotal role in the spatial regulation of ROS production [106]. In addition to spatial regulation, ROP controls ROS production through regulation of the enzyme activity of NOX in root hair. CA-rop2 expression promotes ROS production in root hairs in a RHD2-dependent manner, whereas DN-rop2 decreases ROS formation [102, 107]. ROPs regulate ROS production most likely through both direct activation of NOX and ROP-dependent Ca2+, which in turn increases the enzyme activity of RHD2 [105]. ROP regulation of ROS may also occurs in pollen tubes [101]. Similarly Cdc42 has been shown to modulate tip-localized ROS production in fungal hyphae, which is required for hyphal morphgenesis and growth [108, 109]. Thus the Rho GTPase-ROS signaling module appears to provide a new universal mechanism regulating tip growth in various systems.
4. A working model for the interface between intracellular signaling and cellular structures
Based on the recent findings discussed above, we propose a working model for the mechanisms behind pollen tube tip growth that overarch the intracellular signaling pathways, cellular structures, and vesicular trafficking (Figures 2 and 3). ROP1 is locally activated in the PM to determine the site of exocytosis and activates multiple pathways leading to polar exocytosis [11, 12, 63, 76, 77, 85, 87]. The RIC4 pathway promotes F-actin assembly and induces the accumulation of exocytic vesicles to the tip, and promotes positive feedback loops to increase the area of active ROP1 probably by targeting ROP1 upstream components such as RopGEFs and PRK2 [13, 31, 51–53]. Positive feedbacks coupled with diffusion rapidly generate the apical cap of active ROP1 that defines the tip growth domain. Meanwhile, ROP1 also activates the RIC3–calcium pathway, which promotes the depolymerization of tip F-actin, allowing exocytic vesicles to tether on and fuse with the PM [13, 31]. The tethering is promoted by another potential ROP1 effector, RIP1/ICR1, which may regulate tip targeting of the SEC3 exocyst subunit [20, 44, 89]. Polarized exocytosis brings the REN1 RhoGAP to the apical PM [17]. The PM-localized REN1 may not be active until Ca2+ is further increased to a threshold level, which could then trigger REN1 activation to deactivate PM-localized active ROP1 [17, 19]. Thus the REN1-dependent global inhibition of ROP1 prevents excess ROP1 activation in the apical PM, and restricts the enlargement of the apical cap to the tip growth domain [18]. Following ROP1 inactivation, REN1 returns to the cytosol, thus allowing ROP1 activity to increase again. Consequently, tip F-actin and polar exocytosis oscillates in a phase just slightly behind ROP1 activity, but well ahead of tip growth, whereas the apical Ca2+ oscillation lags behind the growth rate [16, 18]. This interlinked positive and negative feedback loops explain the self-organization of the active ROP1 cap as well as the oscillation of the apical ROP1 activity. As more information becomes available, other signaling loops and pathways need to be integrated into this ROP1- and Ca2+-based self-organizing framework. For example, ROP1 may also regulate a NOX-ROS-calcium signaling loop to boost calcium accumulation [105]. Other signaling factors such as actin-binding proteins (e.g., ADFs) [110], RopGAPs [111], PLC [112, 113] should also be incorporated. ROP signaling may also coordinate endocytosis and cell wall mechanics to drive rapid tip growth in pollen tubes.
5. Extracellular signals for directional and polarized tip growth
5.1. Potential signals for tip growth: An implication for autocrine signaling
Evidence suggests that the interaction between PRK2 and RopGEFs recruits RopGEFs to the PM, leading to the activation of ROP signaling [53]. PRK2 is required for pollen tube growth in vitro in the absence of exogenous signals, implying that PRK-based ROP activation is critical for pollen tube growth. As a trans-membrane cell surface receptor, PRK2 is expected to detect and transduce an extracellular signal(s) that is produced by pollen, constituting an autocrine signaling system. Potential PRK2 ligands include LTP5 and LAT52 [114–116]. LTP5 is a member of the lipid-transfer protein family and is presumably secreted from pollen [116]. Interestingly, dominant-active mutation of LTP5 causes depolarized swollen tip [116], resembling the abnormal morphology of pollen tubes overexpressing ROP1 GTPase or its upstream activator RopGEFs or PRK2 [11, 51, 53, 77]. The physical interaction between pollen secreted extracellular protein LAT52 and pollen receptor kinase LePRK2, suggests that ligand-receptor kinase system regulates pollen tube growth [114, 115].
5.2. Signals that direct tip growth
In vivo pollen tubes are precisely targeted to the ovule for sperm delivery, which is expected to require multiple guidance signals from the female tissues. To date, only two molecules, a cyanin-like small protein from lily stigma called chemocyanin [117] and a cysteine-rich small protein from Torenia synergids called LURE [118], have been implicated as guidance signals, and no receptors for any guidance signals have been identified. The RLK superfamily is the predominant receptors in plants, and is involved in sensing a wide range of signals including small molecules, peptides, and cell wall components. More than 100 RLKs are expressed in pollen, implying that they could perceive various guidance signals [119]. The pollen-expressed RLK, PRK2, interacts directly with RopGEFs in the control of polar tip growth in pollen tubes [53], and the localization of active ROP1 in the apical PM region predicts the future direction of pollen tube turning [16, 18]. Therefore it is likely that possible guidance signal-sensing RLKs also interact with RopGEFs to activate ROP1 signaling; consequently modulating the spatial distribution of ROP1 signaling and directing pollen tube growth in response to the guidance signals. Identification of various guidance signals and their receptors and determination of their intracellular signaling pathways in pollen tubes are in the forefront of exploration of guided tip growth in pollen tubes, a phenomenon that is shared by other tip growing systems such as fungal hyphae and neuronal axon.
6. Conclusion
Knowledge of regulatory and structural mechanisms underlying polarized tip growth in pollen tubes have expanded and evolved over the past several decades in virtue of extensive studies. The advent of innovative tools (e.g., FRAP-based visualization of exocytosis in growing pollen tube [13]) and new approaches (e.g., screen for the ren mutations that enhance ROP1 overexpression-induced depolarization [17]) combined with mathematical simulations significantly advances the investigation of tip growth processes. Systems-based and quantitative approaches are needed to produce a comprehensive view of the pollen tube tip growth system with regards to what all major structural and regulatory components and circuitries are and how they are integrated to achieve rapid and directional tip growth. Pollen tubes use conserved mechanisms such as conserved Rho family GTPase signaling to coordinate F-actin dynamics and polar exocytosis leading to rapid tip growth as observed in root hairs, yeasts, fungal hyphae and neuronal cells. Elucidations of the regulatory principles that govern polarized pollen tube growth may help to enlighten the mechanisms underlying tip growth across eukaryotic kingdoms.
Acknowledgments
We thank Lihua Zhao for her preparation of the artwork for this paper. This work was supported by the Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, and NIH grant (GM081451) to ZY.
Abbreviations
- CA
constitutively active
- CNGC
cyclic nucleotide-gated channel
- CRIB
Cdc42/Rac-interactive binding
- DN
dominant negative
- ER
endoplasmic reticulum
- F-actin
actin laments
- FH3
formin3
- FH5
formin 5
- FRAP
fluorescence recovery after photobleaching
- GAP
GTPase activating protein
- GDI
guanine nucleotide dissociation inhibitor
- GEF
guanine nucleotide exchange factor
- GLR
glutamate receptor-like
- GTPase
guanosine triphosphatase
- ICR
interactor of constitutive active ROPs
- IP3
1,4,5-trisphosphate
- MTs
microtubules
- NOX
NADPH oxidase
- PI
phosphatidylinositol
- PM
plasma membrane
- PME
pectin methylesterase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PI4P
phosphatidylinositol 4-phosphate
- PI4PK
phosphatidylinositol-4-phosphate kinase
- PRK
pollen receptor kinase
- RLK
receptor like kinase
- RIC
ROP-interactive CRIB motif containing protein
- RIP
ROP interactive partner
- ROP
Rho-related GTPase of plants
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson MA, Preuss D. Plotting a course: multiple signals guide pollen tubes to their targets. Dev Cell. 2002;2(3):273–81. doi: 10.1016/s1534-5807(02)00130-2. [DOI] [PubMed] [Google Scholar]
- 2.Qin Y, et al. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet. 2009;5(8):e1000621. doi: 10.1371/journal.pgen.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palanivelu R, Preuss D. Pollen tube targeting and axon guidance: parallels in tip growth mechanisms. Trends Cell Biol. 2000;10(12):517–24. doi: 10.1016/s0962-8924(00)01849-3. [DOI] [PubMed] [Google Scholar]
- 4.Hepler PK, Vidali L, Cheung AY. Polarized cell growth in higher plants. Annu Rev Cell Dev Biol. 2001;17:159–87. doi: 10.1146/annurev.cellbio.17.1.159. [DOI] [PubMed] [Google Scholar]
- 5.Yang Z. Signaling tip growth in plants. Curr Opin Plant Biol. 1998;1(6):525–30. doi: 10.1016/s1369-5266(98)80046-0. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z. Cell polarity signaling in Arabidopsis. Annu Rev Cell Dev Biol. 2008;24:551–75. doi: 10.1146/annurev.cellbio.23.090506.123233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung AY, Wu HM. Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol. 2008;59:547–72. doi: 10.1146/annurev.arplant.59.032607.092921. [DOI] [PubMed] [Google Scholar]
- 8.Lee YJ, Yang Z. Tip growth: signaling in the apical dome. Curr Opin Plant Biol. 2008;11(6):662–71. doi: 10.1016/j.pbi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai G, Cresti M. Organelle motility in the pollen tube: a tale of 20 years. J Exp Bot. 2009;60(2):495–508. doi: 10.1093/jxb/ern321. [DOI] [PubMed] [Google Scholar]
- 10.Staiger CJ, et al. Microinjected profilin affects cytoplasmic streaming in plant cells by rapidly depolymerizing actin microfilaments. Curr Biol. 1994;4(3):215–9. doi: 10.1016/s0960-9822(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y, Wu G, Yang Z. Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J Cell Biol. 2001;152(5):1019–32. doi: 10.1083/jcb.152.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Y, et al. ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J Exp Bot. 2003;54(380):93–101. doi: 10.1093/jxb/erg035. [DOI] [PubMed] [Google Scholar]
- 13.Lee YJ, et al. Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J Cell Biol. 2008;181(7):1155–68. doi: 10.1083/jcb.200801086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung AY, et al. A transmembrane formin nucleates subapical actin assembly and controls tip-focused growth in pollen tubes. Proc Natl Acad Sci U S A. 2010;107(37):16390–5. doi: 10.1073/pnas.1008527107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye J, et al. Arabidopsis formin3 directs the formation of actin cables and polarized growth in pollen tubes. Plant Cell. 2009;21(12):3868–84. doi: 10.1105/tpc.109.068700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang JU, et al. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell. 2005;16(11):5385–99. doi: 10.1091/mbc.E05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang JU, et al. A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr Biol. 2008;18(24):1907–16. doi: 10.1016/j.cub.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang JU, et al. Pollen-tube tip growth requires a balance of lateral propagation and global inhibition of Rho-family GTPase activity. J Cell Sci. 2010;123(Pt 3):340–50. doi: 10.1242/jcs.039180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan A, Xu G, Yang ZB. Calcium participates in feedback regulation of the oscillating ROP1 Rho GTPase in pollen tubes. Proc Natl Acad Sci U S A. 2009;106(51):22002–7. doi: 10.1073/pnas.0910811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, et al. RIP1 (ROP Interactive Partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen growth. Mol Plant. 2008;1(6):1021–35. doi: 10.1093/mp/ssn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geitmann A. How to shape a cylinder: pollen tube as a model system for the generation of complex cellular geometry. Sex Plant Reprod. 2010;23(1):63–71. doi: 10.1007/s00497-009-0121-4. [DOI] [PubMed] [Google Scholar]
- 22.Fu Y. The actin cytoskeleton and signaling network during pollen tube tip growth. J Integr Plant Biol. 2010;52(2):131–7. doi: 10.1111/j.1744-7909.2010.00922.x. [DOI] [PubMed] [Google Scholar]
- 23.Krichevsky A, et al. How pollen tubes grow. Dev Biol. 2007;303(2):405–20. doi: 10.1016/j.ydbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Kato N, He H, Steger AP. A systems model of vesicle trafficking in Arabidopsis pollen tubes. Plant Physiol. 2010;152(2):590–601. doi: 10.1104/pp.109.148700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrhardt DW, Shaw SL. Microtubule dynamics and organization in the plant cortical array. Annu Rev Plant Biol. 2006;57:859–75. doi: 10.1146/annurev.arplant.57.032905.105329. [DOI] [PubMed] [Google Scholar]
- 26.Staiger CJ, Blanchoin L. Actin dynamics: old friends with new stories. Curr Opin Plant Biol. 2006;9(6):554–62. doi: 10.1016/j.pbi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Staiger CJ, et al. Regulation of actin dynamics by actin-binding proteins in pollen. J Exp Bot. 2010;61(7):1969–86. doi: 10.1093/jxb/erq012. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs U, Manns I, Steinberg G. Microtubules are dispensable for the initial pathogenic development but required for long-distance hyphal growth in the corn smut fungus Ustilago maydis. Mol Biol Cell. 2005;16(6):2746–58. doi: 10.1091/mbc.E05-03-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mourino-Perez RR, Roberson RW, Bartnicki-Garcia S. Microtubule dynamics and organization during hyphal growth and branching in Neurospora crassa. Fungal Genet Biol. 2006;43(6):389–400. doi: 10.1016/j.fgb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Raudaskoski M, Mao WZ, Yli-Mattila T. Microtubule cytoskeleton in hyphal growth. Response to nocodazole in a sensitive and a tolerant strain of the homobasidiomycete Schizophyllum commune. Eur J Cell Biol. 1994;64(1):131–41. [PubMed] [Google Scholar]
- 31.Gu Y, et al. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol. 2005;169(1):127–38. doi: 10.1083/jcb.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baluska F, et al. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 2002;130(1):422–31. doi: 10.1104/pp.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibbon BC, Kovar DR, Staiger CJ. Latrunculin B has different effects on pollen germination and tube growth. Plant Cell. 1999;11(12):2349–63. doi: 10.1105/tpc.11.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szumlanski AL, Nielsen E. The Rab GTPase RabA4d regulates pollen tube tip growth in Arabidopsis thaliana. Plant Cell. 2009;21(2):526–44. doi: 10.1105/tpc.108.060277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Graaf BH, et al. Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant Cell. 2005;17(9):2564–79. doi: 10.1105/tpc.105.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grote E, Carr CM, Novick PJ. Ordering the final events in yeast exocytosis. J Cell Biol. 2000;151(2):439–52. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyd C, et al. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J Cell Biol. 2004;167(5):889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong G, et al. The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat Struct Mol Biol. 2005;12(12):1094–100. doi: 10.1038/nsmb1017. [DOI] [PubMed] [Google Scholar]
- 39.Guo W, Tamanoi F, Novick P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol. 2001;3(4):353–60. doi: 10.1038/35070029. [DOI] [PubMed] [Google Scholar]
- 40.Novick P, et al. Interactions between Rabs, tethers, SNAREs and their regulators in exocytosis. Biochem Soc Trans. 2006;34(Pt 5):683–6. doi: 10.1042/BST0340683. [DOI] [PubMed] [Google Scholar]
- 41.Cole RA, et al. SEC8, a subunit of the putative Arabidopsis exocyst complex, facilitates pollen germination and competitive pollen tube growth. Plant Physiol. 2005;138(4):2005–18. doi: 10.1104/pp.105.062273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hala M, et al. An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell. 2008;20(5):1330–45. doi: 10.1105/tpc.108.059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Synek L, et al. AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J. 2006;48 (1):54–72. doi: 10.1111/j.1365-313X.2006.02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavy M, et al. A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol. 2007;17(11):947–52. doi: 10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 45.Bove J, et al. Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching. Plant Physiol. 2008;147(4):1646–58. doi: 10.1104/pp.108.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zonia L, Munnik T. Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J Exp Bot. 2008;59(4):861–73. doi: 10.1093/jxb/ern007. [DOI] [PubMed] [Google Scholar]
- 47.Moscatelli A, et al. Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J Cell Sci. 2007;120(Pt 21):3804–19. doi: 10.1242/jcs.012138. [DOI] [PubMed] [Google Scholar]
- 48.Parton RM, et al. Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J Cell Sci. 2001;114(Pt 14):2685–95. doi: 10.1242/jcs.114.14.2685. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q, et al. Effects of brefeldin A on pollen germination and tube growth. Antagonistic effects on endocytosis and secretion. Plant Physiol. 2005;139(4):1692–703. doi: 10.1104/pp.105.069765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKenna ST, et al. Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell. 2009;21(10):3026–40. doi: 10.1105/tpc.109.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu Y, et al. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell. 2006;18(2):366–81. doi: 10.1105/tpc.105.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaothien P, et al. Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J. 2005;42(4):492–503. doi: 10.1111/j.1365-313X.2005.02388.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2007;104(47):18830–5. doi: 10.1073/pnas.0705874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picton JM, Steer MW. Membrane recycling and the control of secretory activity in pollen tubes. J Cell Sci. 1983;63:303–10. doi: 10.1242/jcs.63.1.303. [DOI] [PubMed] [Google Scholar]
- 55.Dhonukshe P, et al. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol. 2007;17(6):520–7. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Y, et al. Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant Cell. 2010;22(12):4031–44. doi: 10.1105/tpc.110.076760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moscatelli A, Idilli AI. Pollen tube growth: a delicate equilibrium between secretory and endocytic pathways. J Integr Plant Biol. 2009;51(8):727–39. doi: 10.1111/j.1744-7909.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 58.Araujo-Bazan L, Penalva MA, Espeso EA. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol Microbiol. 2008;67(4):891–905. doi: 10.1111/j.1365-2958.2007.06102.x. [DOI] [PubMed] [Google Scholar]
- 59.Higuchi Y, Arioka M, Kitamoto K. Endocytic recycling at the tip region in the filamentous fungus Aspergillus oryzae. Commun Integr Biol. 2009;2(4):327–8. doi: 10.4161/cib.2.4.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mousavi SA, et al. Clathrin-dependent endocytosis. Biochem J. 2004;377(Pt 1):1–16. doi: 10.1042/BJ20031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sousa E, Kost B, Malho R. Arabidopsis phosphatidylinositol-4-monophosphate 5-kinase 4 regulates pollen tube growth and polarity by modulating membrane recycling. Plant Cell. 2008;20(11):3050–64. doi: 10.1105/tpc.108.058826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ischebeck T, Stenzel I, Heilmann I. Type B phosphatidylinositol-4-phosphate 5-kinases mediate Arabidopsis and Nicotiana tabacum pollen tube growth by regulating apical pectin secretion. Plant Cell. 2008;20(12):3312–30. doi: 10.1105/tpc.108.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kost B, et al. Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;145(2):317–30. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris KP, Tepass U. Cdc42 and vesicle trafficking in polarized cells. Traffic. 2010;11(10):1272–9. doi: 10.1111/j.1600-0854.2010.01102.x. [DOI] [PubMed] [Google Scholar]
- 65.Oinuma I, Katoh H, Negishi M. R-Ras controls axon specification upstream of glycogen synthase kinase-3beta through integrin-linked kinase. J Biol Chem. 2007;282(1):303–18. doi: 10.1074/jbc.M607979200. [DOI] [PubMed] [Google Scholar]
- 66.Chen F, et al. Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr Biol. 2000;10(13):758–65. doi: 10.1016/s0960-9822(00)00571-6. [DOI] [PubMed] [Google Scholar]
- 67.Zerzour R, Kroeger J, Geitmann A. Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev Biol. 2009;334(2):437–46. doi: 10.1016/j.ydbio.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 68.Bolduc JE, et al. Finite-element analysis of geometrical factors in micro-indentation of pollen tubes. Biomech Model Mechanobiol. 2006;5(4):227–36. doi: 10.1007/s10237-005-0010-1. [DOI] [PubMed] [Google Scholar]
- 69.Winship LJ, et al. Under pressure, cell walls set the pace. Trends Plant Sci. 2010;15(7):363–9. doi: 10.1016/j.tplants.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fayant P, et al. Finite element model of polar growth in pollen tubes. Plant Cell. 2010;22(8):2579–93. doi: 10.1105/tpc.110.075754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Campas O, Mahadevan L. Shape and dynamics of tip-growing cells. Curr Biol. 2009;19(24):2102–7. doi: 10.1016/j.cub.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 72.Slaughter B, Li R. Toward a molecular interpretation of the surface stress theory for yeast morphogenesis. Curr Opin Cell Biol. 2006;18(1):47–53. doi: 10.1016/j.ceb.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Bosch M, Hepler PK. Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell. 2005;17(12):3219–26. doi: 10.1105/tpc.105.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rockel N, et al. Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J. 2008;53(1):133–43. doi: 10.1111/j.1365-313X.2007.03325.x. [DOI] [PubMed] [Google Scholar]
- 75.Myers C, et al. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. 2009;59(4):528–39. doi: 10.1111/j.1365-313X.2009.03894.x. [DOI] [PubMed] [Google Scholar]
- 76.Lin Y, et al. Localization of a Rho GTPase Implies a Role in Tip Growth and Movement of the Generative Cell in Pollen Tubes. Plant Cell. 1996;8(2):293–303. doi: 10.1105/tpc.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H, et al. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell. 1999;11(9):1731–42. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu P, et al. Lipid microdomain polarization is required for NADPH oxidase-dependent ROS signaling in Picea meyeri pollen tube tip growth. Plant J. 2009;60(2):303–13. doi: 10.1111/j.1365-313X.2009.03955.x. [DOI] [PubMed] [Google Scholar]
- 79.Holdaway-Clarke TL, et al. Pollen Tube Growth and the Intracellular Cytosolic Calcium Gradient Oscillate in Phase while Extracellular Calcium Influx Is Delayed. Plant Cell. 1997;9(11):1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Z, Watson JC. Molecular cloning and characterization of rho, a ras-related small GTP-binding protein from the garden pea. Proc Natl Acad Sci U S A. 1993;90(18):8732–6. doi: 10.1073/pnas.90.18.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gu Y, Wang Z, Yang Z. ROP/RAC GTPase: an old new master regulator for plant signaling. Curr Opin Plant Biol. 2004;7(5):527–36. doi: 10.1016/j.pbi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Yang Z. Small GTPases: versatile signaling switches in plants. Plant Cell. 2002;14(Suppl):S375–88. doi: 10.1105/tpc.001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng ZL, Yang Z. The Rop GTPase: an emerging signaling switch in plants. Plant Mol Biol. 2000;44(1):1–9. doi: 10.1023/a:1006402628948. [DOI] [PubMed] [Google Scholar]
- 84.Fu Y, Yang Z. Rop GTPase: a master switch of cell polarity development in plants. Trends Plant Sci. 2001;6(12):545–7. doi: 10.1016/s1360-1385(01)02130-6. [DOI] [PubMed] [Google Scholar]
- 85.Li H, et al. Arabidopsis Rho-related GTPases: differential gene expression in pollen and polar localization in fission yeast. Plant Physiol. 1998;118(2):407–17. doi: 10.1104/pp.118.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li H, et al. The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol. 2001;126(2):670–84. doi: 10.1104/pp.126.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Y, Yang Z. Inhibition of Pollen Tube Elongation by Microinjected Anti-Rop1Ps Antibodies Suggests a Crucial Role for Rho-Type GTPases in the Control of Tip Growth. Plant Cell. 1997;9(9):1647–1659. doi: 10.1105/tpc.9.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu G, et al. A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell. 2001;13(12):2841–56. doi: 10.1105/tpc.010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hazak O, et al. A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol. 2010;8(1):e1000282. doi: 10.1371/journal.pbio.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pierson ES, et al. Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol. 1996;174(1):160–73. doi: 10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- 91.Messerli MA, et al. Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev Biol. 2000;222(1):84–98. doi: 10.1006/dbio.2000.9709. [DOI] [PubMed] [Google Scholar]
- 92.Malho R, Trewavas AJ. Localized Apical Increases of Cytosolic Free Calcium Control Pollen Tube Orientation. Plant Cell. 1996;8(11):1935–1949. doi: 10.1105/tpc.8.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roy SJ, et al. Uncoupling secretion and tip growth in lily pollen tubes: evidence for the role of calcium in exocytosis. Plant J. 1999;19(4):379–86. doi: 10.1046/j.1365-313x.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- 94.Zhou L, Fu Y, Yang Z. A genome-wide functional characterization of Arabidopsis regulatory calcium sensors in pollen tubes. J Integr Plant Biol. 2009;51(8):751–61. doi: 10.1111/j.1744-7909.2009.00847.x. [DOI] [PubMed] [Google Scholar]
- 95.Yoon GM, et al. Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell. 2006;18(4):867–78. doi: 10.1105/tpc.105.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dauphin A, et al. Fungal hypaphorine reduces growth and induces cytosolic calcium increase in root hairs of Eucalyptus globulus. Protoplasma. 2007;231(1-2):83–8. doi: 10.1007/s00709-006-0240-9. [DOI] [PubMed] [Google Scholar]
- 97.Bibikova TN, Zhigilei A, Gilroy S. Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta. 1997;203(4):495–505. doi: 10.1007/s004250050219. [DOI] [PubMed] [Google Scholar]
- 98.Frietsch S, et al. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci U S A. 2007;104(36):14531–6. doi: 10.1073/pnas.0701781104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang F, et al. A putative calcium-permeable cyclic nucleotide-gated channel, CNGC18, regulates polarized pollen tube growth. Journal of Integrative Plant Biology. 2007;49(8):1261–1270. [Google Scholar]
- 100.Michard E, et al. Glutamate Receptor-Like Genes Form Ca2+ Channels in Pollen Tubes and Are Regulated by Pistil D-Serine. Science. 2011 doi: 10.1126/science.1201101. [DOI] [PubMed] [Google Scholar]
- 101.Potocky M, et al. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007;174(4):742–51. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- 102.Wong HL, et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19(12):4022–34. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park J, et al. Phosphatidic acid induces leaf cell death in Arabidopsis by activating the Rho-related small G protein GTPase-mediated pathway of reactive oxygen species generation. Plant Physiol. 2004;134(1):129–36. doi: 10.1104/pp.103.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: lessons from root hairs. J Exp Bot. 2006;57(8):1829–34. doi: 10.1093/jxb/erj201. [DOI] [PubMed] [Google Scholar]
- 105.Takeda S, et al. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319(5867):1241–4. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 106.Carol RJ, et al. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438(7070):1013–6. doi: 10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- 107.Jones MA, et al. NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J Exp Bot. 2007;58(6):1261–70. doi: 10.1093/jxb/erl279. [DOI] [PubMed] [Google Scholar]
- 108.Takemoto D, et al. Polarity proteins Bem1 and Cdc24 are components of the filamentous fungal NADPH oxidase complex. Proc Natl Acad Sci U S A. 2011;108(7):2861–6. doi: 10.1073/pnas.1017309108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Semighini CP, Harris SD. Regulation of apical dominance in Aspergillus nidulans hyphae by reactive oxygen species. Genetics. 2008;179(4):1919–32. doi: 10.1534/genetics.108.089318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen CY, Cheung AY, Wu HM. Actin-depolymerizing factor mediates Rac/Rop GTPase-regulated pollen tube growth. Plant Cell. 2003;15(1):237–49. doi: 10.1105/tpc.007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell. 2006;18(11):3033–46. doi: 10.1105/tpc.106.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Helling D, et al. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell. 2006;18(12):3519–34. doi: 10.1105/tpc.106.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dowd PE, et al. Petunia phospholipase c1 is involved in pollen tube growth. Plant Cell. 2006;18(6):1438–53. doi: 10.1105/tpc.106.041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnson MA, Preuss D. On your mark, get set, GROW! LePRK2-LAT52 interactions regulate pollen tube growth. Trends Plant Sci. 2003;8(3):97–9. doi: 10.1016/S1360-1385(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 115.Tang W, et al. A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell. 2002;14(9):2277–87. doi: 10.1105/tpc.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chae K, et al. A gain-of-function mutation of Arabidopsis lipid transfer protein 5 disturbs pollen tube tip growth and fertilization. Plant Cell. 2009;21(12):3902–14. doi: 10.1105/tpc.109.070854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim S, et al. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci U S A. 2003;100(26):16125–30. doi: 10.1073/pnas.2533800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Okuda S, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458(7236):357–61. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- 119.Shiu SH, Bleecker AB. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci U S A. 2001;98(19):10763–8. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]