Abstract
Purpose
Intensive voice therapy (LSVT®LOUD) can effectively manage voice and speech symptoms associated with idiopathic Parkinson disease (PD). This small-group study evaluated voice and speech in individuals with and without deep brain stimulation of the subthalamic nucleus (STN-DBS) before and after LSVT LOUD, to determine whether outcomes for surgical subjects were comparable to non-surgical cohorts.
Methods
Eight subjects with PD (four with STN-DBS and four without) received LSVT LOUD four times a week for four weeks. Four additional subjects with PD remained untreated. Voice intensity (SPL), Vowel Articulation Index (VAI), the Voice Handicap Index (VHI), and a structured interview were evaluated before and after treatment and again six months later.
Results
Both treated groups showed significant increases in SPL from pre to post and six-month follow up. VAI was significantly higher for the treated groups compared to the untreated subjects at follow up. Several treated individuals had significant clinical improvement in VHI scores, particularly within the LSVT-DBS group. Treated individuals reported improvements in voice and speech in structured interviews; however, answers suggest more variable long-term maintenance within the LSVT-DBS group. The untreated group exhibited no significant changes in any measure throughout the study.
Conclusions
Results support LSVT LOUD for treating voice and speech in individuals with PD following STN-DBS surgery. However, modifications may be required to maintain functional improvements.
Keywords: Parkinson’s disease, dysarthria, LSVT®, deep brain stimulation, behavioral treatment, subthalamic nucleus
1. Introduction
Up to 90% of individuals with idiopathic Parkinson disease (PD) experience voice and speech problems during the course of their disease (Hartelius & Svensson, 1994; Ho, Iansek, Marigliani, Bradshaw, & Gates, 1998; Logemann, Fisher, Boshes, & Blonsky, 1978). Collectively termed hypokinetic dysarthria (Darley, Aronson, & Brown, 1975), voice and speech deficits may appear in any or all subsystems of speech production, caused by abnormalities in respiration, vocal loudness, voice quality, pitch and loudness variability, articulation, and fluency (Forrest, Weismer, & Turner, 1989; Fox & Ramig, 1997; Holmes, Oates, Phyland, & Hughes, 2000; Solomon & Hixon, 1993). These changes in speech production can have a significant, negative influence on social interaction and quality of life (Miller et al., 2007; Miller, Noble, Jones, & Burn, 2006).
The history of treating voice and speech disorders in PD is notable for the failure of surgical and pharmacological treatments to have consistent, positive effects on communication commensurate with documented improvements in limb function (Jones, Kendall, Sudhyadhom, & Rosenbek, 2007; Pinto et al., 2004a; Schulz, 2002). Early speech treatment approaches also failed to achieve functional and lasting improvements in PD-related speech deficits (Sarno, 1968). However, research over the last 20 years, including a series of randomized controlled trials (RCTs), has established the Lee Silverman Voice Treatment (LSVT® LOUD) as an efficacious behavioral treatment for voice and speech problems associated with PD (Pinto et al., 2004a; Ramig et al., 2001a; Ramig, Countryman, Thompson, & Horii, 1995; Ramig, Sapir, Fox, & Countryman, 2001b; Schulz, 2002; Yorkston, Spencer, & Duffy, 2003). Using a cognitively simple and intensive approach, LSVT LOUD promotes a healthy increase in vocal loudness which has been found to generalize to improvements not only in voice production but also speech articulation and intelligibility (Halpern, Spielman, Ramig, Sharpley, & Panzer, 2008; Sapir, Spielman, Ramig, Story, & Fox, 2007). Preliminary data suggest LSVT LOUD may also positively affect swallowing (El-Sharkawi A. et al., 2002), facial expression (Spielman, Borod, & Ramig, 2003), and neural function as revealed by PET (Liotti et al., 2003; Narayana et al., 2010) in people with PD, and it has been used successfully on individuals with Parkinson plus syndromes, multiple sclerosis, ataxia, traumatic brain injury, Down syndrome, and cerebral palsy, with no modifications to the basic treatment protocol (Countryman, Ramig, & Pawlas, 1994; Fox et al., 2006; Sapir et al., 2003; Wenke, Theodoros, & Cornwell, 2008).
The current need for a behavioral treatment to manage speech and voice disorders in PD has not diminished hope that a single pharmacological or surgical approach might eventually improve both speech and non-speech symptoms. Although pharmacological and surgical treatments available for managing PD-related motor symptoms typically demonstrate little or no benefit to voice and speech, recent literature examining deep brain stimulation of the subthalamic nucleus (STN-DBS) for management of PD symptoms has reported positive effects of this surgery on select aspects of oromotor, laryngeal and velopharyngeal function (Gentil, Garcia-Ruiz, Pollak, & Benabid, 2000; Hammer, Barlow, Lyons, & Pahwa, 2010; Hammer, Barlow, Lyons, & Pahwa, 2011), acoustic voice variables (Dromey, Kumar, Lang, & Lozano, 2000; Pinto et al., 2004b), stuttering (Walker et al., 2009), and glottic tremor (D’Alatri et al., 2008; Klostermann et al., 2008). Unfortunately, these changes are considered by some researchers to be too small to reach clinical significance (Dromey et al., 2000; Jones et al., 2007). In addition, many of these studies have relied on the UPDRS speech item (item 18) as a means of measuring functional speech improvement, which may be insufficiently sensitive for measuring changes in voice and speech (Rousseaux et al., 2004).
In contrast to studies reporting positive effects of STN-DBS on voice and speech, a growing body of literature is documenting degradation of speech function and intelligibility following surgery (Iulianella, Adams, & Gow, 2008; Tripoliti et al., 2011). Rousseaux et al. (2004) found that bilateral STN stimulation reduced intelligibility during reading and spontaneous speech, and that two of seven subjects were observed to have post-operatively worsened dysarthria. Similarly, Tripoliti et al. (2006) reported a large decrease in intelligibility on the Assessment of Intelligibility of Dysarthric Speech (AIDS) (Yorkston & Beukelman, 1981) for a subset of patients in the stimulation-on vs. stimulation-off condition. Narayana and colleagues reported deterioration in both acoustic and perceptual measures for an individual during stimulation-on vs. stimulation-off conditions (Narayana et al., 2009). Some participants in these studies have also reported negative effects of STN-DBS surgery on their own speech. Santens et al. (2003) found that despite no measurable change in speech for bilateral stimulation in the on vs. off condition, “all [seven] patients mentioned a subjective decrease in their intelligibility following their bilateral STN stimulation” (p. 255). In addition, these authors observed a worsening of speech symptoms with left-sided as compared to right-sided stimulation, a finding also reported by Tripoliti et al. (2011), Narayana et al. (2009), and Wang and colleagues (Wang, Verhagen, Bakay, Arzbaecher, & Bernard, 2003; Wang et al., 2006).
While there is not uniform agreement that STN-DBS stimulation exacerbates speech problems in PD, at least two studies have reported reductions in speech intelligibility directly related to increases in stimulator amplitude and/or frequency settings (Tornqvist, Schalen, & Rehncrona, 2005; Tripoliti et al., 2008). Of further concern is that these deficits in articulation and intelligibility are unlike those typically observed in PD, and may or may not respond to current therapy approaches. Given the possibility of increased articulatory deficits following STN-DBS, there is a need to evaluate whether LSVT LOUD, with its focus on voice, remains efficacious in this population. In 2 pilot studies (Mahler, Spielman, Sapir, Ramig, & Halpern, 2008; Spielman, Petska, Halpern, & Ramig, 2006), we reported on three-to-four subjects with idiopathic PD and bilateral STN-DBS who successfully completed LSVT LOUD, made improvements in vocal intensity and vowel articulation, and reduced voice handicap. The present study compares in detail four idiopathic PD subjects with bilateral STN-DBS receiving LSVT LOUD to two non-surgical groups of subjects with PD, one also treated with LSVT LOUD and one untreated, to begin to evaluate whether individuals with STN-DBS benefit from behavioral voice therapy to the same extent as their non-surgical cohorts.
2. Methods
2.1 Participants
Twelve individuals with idiopathic PD (four with bilateral STN-DBS, eight without) participated in this study. Subject characteristics, medications (reported as Levodopa Equivalents [LE] plus additional PD medications) and stimulator settings appear in Table 1. The Levodopa Equivalent formula calculates the effects of each individual’s anti-parkinsonian drug in terms of 100 mg of immediate release levodopa, and then combines them into a single LE value reflecting total daily medication dosage (Tomlinson et al., 2010). This makes for an easier comparison of medication dosages across subjects. Subjects were recruited from the Denver, Colorado area and signed consent forms approved by the Colorado Multiple Institutional Review Board (IRB) and the University of Colorado, Boulder IRB.
Table 1.
Biographical and medical information for all subjects (N=12). Stimulator settings for the LSVT-DBS group.
| Subject and Gender | Age | Years Since Diagnosis | Years Since Surgery | Stage | Levodopa Equivalents (LE); other PD meds | Voice and Speech Severity | Stim Settings | Stim Mode | Active Contacts | Amp (V) | Freq (Hz) | Pulse Width (μsec) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBS-1 (F) | 71 | 9 | 3.75 | N/A | LE = 0; Amantadine | Severe | Left | Mono | 2- | 3.0 | 185 | 60 |
| Right | Bi | 2- | 3.6 | 185 | 120 | |||||||
| 3+ | ||||||||||||
| DBS-2 (M) | 73 | 24 | 1 | N/A | LE = 450 | Severe | Left | Mono | 2- | 3.0 | 185 | 60 |
| Right | Mono | 0- | 3.6 | 185 | 60 | |||||||
| DBS-3 (F) | 50 | 9 | 1.25 | N/A | LE = 747 | Moderate | Left | Mono | 1- | 3.0 | 185 | 60 |
| Right | Mono | 2- | 2.0 | 160 | 60 | |||||||
| 3- | ||||||||||||
| DBS-4 (M) | 84 | 21 | 4.5 | N/A | LE = 400; Amantadine | Severe | Left | Bi | 0- | 3.0 | 185 | 60 |
| 1- | ||||||||||||
| 2+ | ||||||||||||
| Right | Mono | 1- | 3.6 | 185 | 90 | |||||||
| 2- | ||||||||||||
|
| ||||||||||||
| LSVT-5 (F) | 72 | 8 | 3 | LE = 720; Selegiline | Severe | |||||||
| LSVT-6 (F) | 63 | 31 | 2.5 | LE = 1296; Selegiline | Severe | |||||||
| LSVT-54 (M) | 82 | 8 | 3 | LE = 701; Rasagiline | Moderate | |||||||
| LSVT-65 (M) | 60 | 6 | 2 | LE = 900; Selegiline | Moderate | |||||||
|
| ||||||||||||
| NT-8 (F) | 48 | 2 | 2 | LE = 20 | Severe | |||||||
| NT-18 (M) | 61 | 11 | 2.5 | LE = 1160; Amantadine | Moderate | |||||||
| NT-48 (M) | 81 | 14 | 2 | LE = 1000; Amantadine | Moderate | |||||||
| NT-70 (M) | 67 | 12 | 2 | LE = 600; Amantadine, Selegiline | Mild | |||||||
Notes: Stage based on Hoehn & Yahr 1-5, higher stages indicate greater severity; N/A = not available; Levodopa Equivalent is based on the following formula: LE=[(levodopa+levodopa CR×0.7)×1.1 if on COMT inhibitor] + pramipexole×100 + pergolide×100 + ropinirole×33; Mono = monopolar; Bi = Bipolar; Amp = Amplitude; V = volts; Freq = Frequency; Hz = Hertz; μsec = microseconds
All subjects were recruited as part of a larger study evaluating the effects of different types of speech treatment on communication in PD. All four participants with STN-DBS were assigned directly to a group receiving intensive voice treatment (henceforth LSVT-DBS) and are reported here. The non-surgical subjects selected for this analysis were assigned to either an intensive voice treatment group (henceforth LSVT) or an untreated control group (henceforth NT) upon entry into the study, on the basis of age, gender, time since diagnosis, stage of PD (Hoehn & Yahr, 1967), perceptual severity of voice and speech symptoms (as judged by two experienced speech-language pathologists), severity of swallowing disorder, cognitive status, and depression (Beck, Steer, & Brown, 1996). Because people who receive STN-DBS may generally have more severe and advanced symptoms than non-surgical participants, the eight non-surgical subjects in the present analysis were selected from the larger LSVT and NT subject pools on the basis of time since diagnosis, PD stage, age, perceptual severity of speech and voice symptoms, gender, and depression, in order to minimize performance differences that may be attributed to these factors. Subjects in all three groups reported here entered the study during the same period of time. Specific voice and speech characteristics for subjects can be found in Table 2.
Table 2.
Voice and speech characteristics, rated by two expert speech-language pathologists. Ratings were scaled from 0 (no disorder) to 5 (severe disorder), and based on samples of reading, connected speech, and sustained phonation.
| Subject | Overall Voice and Speech Severity | Voice severity rating | Articulation severity rating | Voice and speech characteristics |
|---|---|---|---|---|
| DBS-1 | Severe | 3 | 3 | Reduced loudness, imprecise consonants, slurring, hoarse voice, monopitch |
| DBS-2 | Severe | 4 | 2.5 | Reduced loudness, breathy voice, monopitch and loudness, imprecise consonants, slow rate. |
| DBS-3 | Moderate | 2 | 2 | Hoarse/pressed voice, imprecise consonants. |
| DBS-4 | Severe | 5 | 1 | Reduced loudness, breathy voice, high pitch, imprecise consonants. |
|
| ||||
| LSVT-5 | Severe | 4.5 | 1.5 | Reduced loudness, hoarse/pressed voice, mono pitch, imprecise consonants. |
| LSVT-6 | Severe | 3.5 | 4.5 | Reduced loudness, monoloud, breathy voice, imprecise consonants, slurring. |
| LSVT-54 | Moderate | 3 | 1 | Hoarse voice, breathy voice, imprecise consonants, reduced loudness, |
| LSVT-65 | Moderate | 3 | 0.25 | Reduced loudness, hoarse voice, pressed voice, breathy voice |
|
| ||||
| NT-8 | Severe | 4 | 0 | Reduced loudness, breathy/pressed voice, monoloud and monopitch. |
| NT-18 | Moderate | 3 | 1 | Reduced loudness, hoarse voice, breathy voice, imprecise consonants. |
| NT-48 | Moderate | 3 | 0 | Reduced loudness, breathy voice, hoarse voice. |
| NT-70 | Moderate | 2 | 0.5 | Reduced loudness, breathy voice, slurring |
No subject had received speech or voice therapy prior to entering the study except for one participant (DBS-1), who failed to disclose that she had received voice treatment twice a week for eight weeks two years prior to this study; this was discovered only later through medical records. All treated subjects were required to refrain from participating in any additional private or group therapy until completing their 6-month follow-up recordings. Subjects in the NT group were also required to refrain from participating in any voice or speech therapy throughout the entire time period, Individuals who failed a hearing screening or presented with moderate or severe dementia, severe untreated depression, or laryngeal pathology on exam (as determined by a laryngologist) were excluded from the study. All subjects were right-handed. Any subject with neurological illness or complication as a result of the STN-DBS surgery was excluded from the surgical group.
Subjects were stable on their anti-parkinson medications for at least one month prior to beginning the study and asked not to make changes during the study. However, one subject (LSVT-5) began self-regulating Carbidopa/Levodopa during treatment (reducing her LE by about 200 mg). By follow up DBS-3 had been taken off Carbidopa/Levodopa entirely (LE = 0), and LSVT-6 reported a number of medication changes, including an “as needed” supplement of Carbidopa/Levodopa, making it difficult to calculate an accurate LE. However, it is believed that pharmacological treatments for PD generally have little or no systematic effect on voice and speech disorders (Ho, Bradshaw, & Iansek, 2008; Pinto et al., 2004a; Schulz, 2002), and therefore these changes are unlikely to have substantially influenced speech treatment outcomes reported here.
Stimulator settings were gathered from medical records released at the time of enrollment into the study, ranging from 1-4 months prior to starting therapy. No DBS subjects reported changes in their settings between pre and post recordings. Subsequent medical records indicated that minor changes were made to various settings between post treatment and six-month follow up for DBS-1 and DBS-4. Specifically, DBS-1’s stimulation mode was adjusted on the right side to monopolar, (electrode 2-), and DBS-4 underwent a right-sided amplitude increase of 0.1 volt. It is unknown whether DBS-3 had any changes in settings between post and follow up recordings, although she did report that her stimulator required rewiring due to a fall that resulted in a fracture in the hardware system one month before follow up. DBS-2 did not complete a 6-month follow up evaluation. The potential effects of stimulator settings on voice and speech outcomes are discussed in more detail below (see Discussion).
2.2 Data Collection
All subjects participated in two pre-treatment (PRE), two post-treatment (POST) and two follow-up (FU) data collection sessions. PRE data were collected during the week immediately prior to treatment, POST data were collected during the week immediately following treatment, and FU data were collected six months after treatment, within one week. Data were collected in an IAC sound treated booth using a head-mounted AKG 420 condenser microphone positioned 8 cm from the lips. The microphone was calibrated to a Type I sound level meter (Brüel and Kjaer 2238) at a distance of 30 cm, using standard procedures (Svec, Popolo, & Titze, 2003). Subjects were asked to read the Rainbow Passage (Fairbanks, 1960), describe a picture, repeat a sentence (“the stewpot is packed with peas”) 10 times, and talk about a self-selected topic for one minute. All tasks except sentence repetition were collected on both day 1 and day 2 sessions. Data collection took place at the same time of day for each subject across all six sessions, and was scheduled for the same time in relation to their medications. No treating therapist collected data. Therapists also made themselves unavailable during data collection days in order to lessen the chance that subjects would be cued to use speech techniques learned in treatment.
2.3 Treatment
LSVT LOUD was administered by three speech-language pathologists (SLPs) with expertise in the delivery of this therapy. Treatment consisted of 16 sessions of individual therapy (4 1-hour sessions a week for 4 weeks) delivered according to the standard LSVT LOUD protocol, as published elsewhere (Ramig et al., 1995). Using intensive, high effort voice exercises, LSVT LOUD trains healthy vocal loudness as a trigger for distributed effects across the speech production system (voice, articulation, rate) using the simple cue “Think Loud,” avoiding any direct training focus on respiration or articulation. Through daily “calibration” activities that focus on establishing an internal effort level required for a speaker to be heard and understood, LSVT LOUD simultaneously retrains the sensorimotor processing and internal cueing disorders which limit generalization of treatment strategies into daily living in PD. Unlike other forms of speech treatment, LSVT LOUD uses a mode of delivery that is an intensive, high effort exercise, adhering to many of the fundamental principles of exercise and motor training that have been shown to promote neural plasticity and brain reorganization (Kleim & Jones, 2008).
One LSVT-DBS subject (DBS-2) received 19 sessions of LSVT LOUD over six weeks due to a period of illness during the study, and was unavailable at follow up. Specifically, he completed all of Week 1 and three days of Week 2, was unable to participate for two weeks, and then resumed treatment starting at the beginning of Week 2. The NT group did nothing throughout the treatment phase. Untreated individuals received LSVT LOUD free of charge at the completion of the study.
2.4 Data Analysis
Sound pressure level (SPL) – the acoustic correlate of vocal loudness – was measured for sentence repetition, reading, picture description and spontaneous monologue using the calibrated microphone signal and a customized SPL extraction program. The calibrated microphone audio file for each task was edited to remove coughs, laughs, filled pauses, loud breaths, and other non-speech sounds, and then subjected to SPL extraction using a custom-built program designed to emulate a sound level meter (Matos, 2003). Specifically, the SPL program analyzes each 1-second segment of a digitized audio file in 250-msec windows and then selects the peak SPL value from the four values measured per second. The array of peak values is then averaged for the task. The noise floor for SPL extraction was set to 60 dB, and silences lasting 300 msec or longer were considered pauses and automatically excluded from the sample.
In addition to reduced vocal intensity it is not uncommon for vowel articulation to be compromised in individuals with PD, resulting in compacted vowel space (Tjaden & Wilding, 2004; Weismer, Jeng, Laures, Kent, & Kent, 2001). The present study examined vowel production through the Vowel Articulation Index (VAI), a ratio formula. (Roy, Nissen, Dromey, & Sapir, 2009; Sapir, 2007; Skodda, Visser, & Schlegel, 2010). The VAI is comprised of formant elements F1 and F2 of the corner vowels /i/, /u/, and /α/ and expressed by the formula (F2i+F1α)/(F2u+F2α+F1i+F1u), where F1i is the first formant frequency of the vowel /i/, F2i is the second formant frequency, and so on. This ratio formula is intended to overcome some of the limitations of traditional vowel measures like the triangular Vowel Space Area, considered an unreliable measure for statistical differentiation of dysarthric from normal speech likely due to large inter-speaker variability (Sapir, Ramig, Spielman, & Fox, 2010).
In acoustical terms, centralized vowel production is generally reflected in a decrease of typically high formant frequencies (found in the VAI numerator) and an increase of typically low frequencies (found in the denominator), ultimately resulting in a low VAI value. One recent application of the VAI formula to dysarthric speech demonstrated significantly lower VAI values in individuals with PD compared to those without (Skodda et al., 2010). Therefore, an increase in VAI from pre to post treatment would suggest less vowel centralization and, theoretically, improved vowel articulation.
To obtain raw data for the VAI, formant frequencies (F1 and F2) for vowels /u/, /α/, and /i/ were extracted from all 10 repetitions of “the stewpot is packed with peas” at each time period (PRE, POST, and FU). Vowel formants were extracted from either the 30-msec midpoint (/α/ and /i/) or endpoint (/u/) of the associated target word (“pot”, “peas” and “stew”) using TF32 software (Milenkovic, 2004) and following standard procedures. Further details describing these vowel formant extraction methods can be found elsewhere (Sapir et al., 2007). VAI values were obtained for each of the 10 sets of vowels per subject and recording, such that each group had 40 PRE VAI values (4 subjects × 10 sentence repetitions), 40 POST, and 40 FU (except for LSVT-DBS at FU, which had only 30 as a result of one unavailable subject).
The Voice Handicap Index (VHI) (Jacobson et al., 1997) – a 30-item self-rating questionnaire measuring the impact of voice disorders on physical, functional, and emotional well being – was administered to evaluate changes in the self-perception of voice disorder and its impact on daily life. A change in score of 18 points or more is considered clinically significant. The VHI was completed by all subjects once at PRE, POST, and FU. Subjects also answered structured interview questions about their voice and speech before and after therapy.
2.5 Statistical and Non-statistical Analyses
The Lilliefors Test, a variant of the Kolgoromov-Smirnov test, was used to determine whether acoustic data in this study were normally distributed. Analysis indicated that the null hypothesis could not be rejected for either SPL (p = 0.39) or VAI (p = 0.45) and the data were deemed appropriate for parametric statistics. Pairwise comparisons, corrected for multiple comparisons (Tukey-Kramer HSD), revealed no significant differences between days 1 and 2 within PRE, POST, or FU sessions for any of the repeated speech tasks (reading, picture description, or conversation); therefore, SPL data for each task were pooled within each time period. To examine SPL for changes over time within and between groups, data were submitted to a fully repeated measures analysis of variance (RM-ANOVA) using a general linear model to allow for missing data points, using NCSS software (Hintze, 2007). In this analysis, SPL was treated as the response variable, Group was treated as the between group factor, and Task and Time were treated as within group factors. Post hoc planned comparisons, corrected for multiple comparisons (Tukey-Kramer HSD), examined changes in SPL for each group at each of the three time periods. Because the number of subjects in each group is small, Huynh-Feldt corrections were applied to the statistical results to correct for violations of sphericity.
VAI scores for each subject were treated as the response variable in a RM-ANOVA. Group was treated as the between group factor, and Time and Token number were each treated as within group factors. In this analysis, Token number was treated as a random variable with repeated observations, and Huynh-Feldt corrections were applied to the results to correct for violations of sphericity.
Effect size (ES) measures (Cohen’s d) were calculated in order to evaluate the magnitude of differences between means from pre to post and pre to follow up time periods within each group, using a pooled variance method (Cohen, 1988). Using this approach, an ES of .20 is considered small, an ES of .50 is considered medium, and an ES of .80 or above is considered large.
The VHI was evaluated using published standards, which require a change of 18 points or more to reach clinical significance for an individual speaker (Jacobson et al., 1997).
3. Results
3.1 Reliability
A second investigator (LM) independently re-analyzed 75% of SPL measures – including all file editing and calibration calculations – in order to test metric reliability. A correlation analysis of the two independent sets of observations revealed a Pearson product-moment correlation of r = 0.94, with a standard error of 1.0 dB. The same investigator also reanalyzed a random subset (18%) of vowel formant measures, resulting in correlations of r = 0.99 and standard errors of 25 Hz and 53 Hz for F1 and F2 respectively, consistent with the literature (Sapir et al., 2007). Validity and reliability information for the VHI have been reported elsewhere (Jacobson et al., 1997; Spielman, Gilley, Halpern, & Ramig, 2010).
3.2 Acoustic Measures
3.2.1 Sound pressure level
Averaged dB SPL data for each subject, as well as PRE to POST and PRE to FU differences, appear in Table 3. Figure 1 presents mean SPL with standard error of the mean (SEM) at each time period for each group.
Table 3.
Average Sound Pressure Level (SPL dB) data (at 30 cm), differences in SPL from PRE to POST and PRE to FU, and group Effect Sizes (ES).
| Group/Subject | PRE | POST | FU | POST-PRE* | FU-PRE* |
|---|---|---|---|---|---|
| DBS-1 | 69.6 | 78.3 | 73.5 | 8.7 | 3.9 |
| sd | 1.3 | 1.8 | 1.9 | ||
| DBS-2 | 69.1 | 73.1 | n/a | 4.0 | n/a |
| sd | 1.8 | 2.4 | |||
| DBS-3 | 68.3 | 76.6 | 75.8 | 8.3 | 7.4 |
| sd | 1.4 | 2.6 | 2.4 | ||
| DBS-4 | 73.5 | 79.3 | 75.7 | 5.8 | 2.2 |
| sd | 1.0 | 1.9 | 1.3 | ||
| Group mean | 70.1 | 76.8 | 75.0 | 6.7 | 4.5 |
| sd | 2.3 | 2.7 | 1.3 | 2.2 | 2.7 |
| Effect Size | 2.5 | 1.8 | |||
|
| |||||
| LSVT-5 | 70.1 | 72.8 | 72.9 | 2.7 | 2.8 |
| sd | 0.7 | 1.4 | 1.8 | ||
| LSVT-6 | 70.2 | 76.2 | 73.5 | 6.0 | 3.3 |
| sd | 1.5 | 1.5 | 1.7 | ||
| LSVT-54 | 73.5 | 75.6 | 75.4 | 2.1 | 1.9 |
| sd | 1.6 | 1.9 | 1.5 | ||
| LSVT-65 | 72.0 | 76.5 | 74.3 | 4.5 | 2.4 |
| sd | 1.4 | 0.9 | 1.3 | ||
| Group mean | 71.4 | 75.3 | 74.0 | 3.8 | 2.6 |
| sd | 1.6 | 1.7 | 1.1 | 1.7 | 0.6 |
| Effect Size | 2.0 | 1.4 | |||
|
| |||||
| NT-8 | 67.6 | 67.5 | 67.2 | -0.1 | -0.4 |
| sd | 1.6 | 1.9 | 1.4 | ||
| NT-18 | 73.3 | 73.5 | 72.1 | 0.2 | -1.2 |
| sd | 1.2 | 1.3 | 0.8 | ||
| NT-48 | 72.9 | 72.2 | 73.8 | -0.7 | 0.9 |
| sd | 2.4 | 2.0 | 2.2 | ||
| NT-70 | 66.9 | 68.7 | 68.7 | 1.7 | 1.8 |
| sd | 2.2 | 0.9 | 1.1 | ||
| Group mean | 70.2 | 70.5 | 70.5 | 0.3 | 0.3 |
| sd | 3.4 | 2.8 | 3.0 | 1.1 | 1.4 |
| Effect Size | 0.2 | 0.2 | |||
All mean PRE to POST and PRE to FU changes are significant for the LSVT-DBS and LSVT groups (p < .05) and for all speech tasks (p < .01). Changes for individual subjects were not analyzed statistically. ES measures of .20, .50, and .80 are considered small, medium and large respectively.
Figure 1.
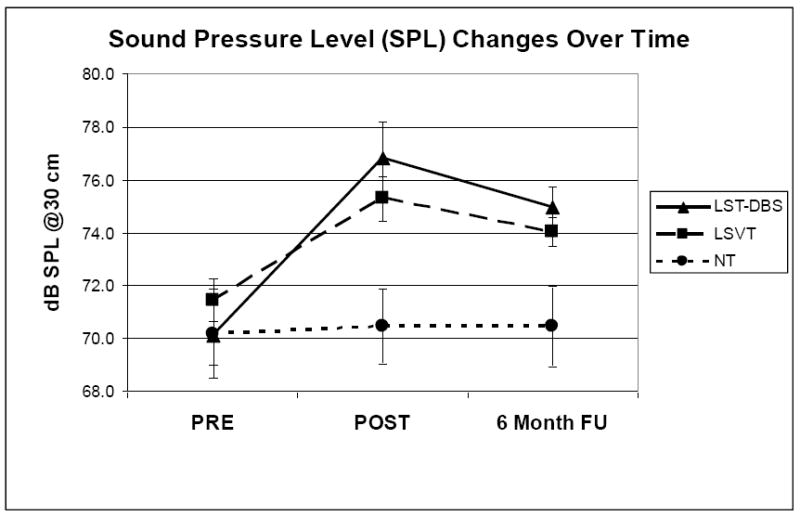
Sound Pressure Level (SPL) data (mean and standard error of measurement) at 30 cm for each group at PRE, POST, and six month FU.
Results of the RM-ANOVA revealed significant main effects for Group [F(2,139)=26.82, p < 0.000001], Task [F(3,139)=4.36, p < 0.007], and Time [F(2,139)=23.94, p < 0.000001], as well as a significant interaction effect for Group × Time [F(4,139)=6.12, p < 0.0002].
Post hoc planned comparisons (Tukey-Kramer HSD, p < 0.05) revealed a significant difference between the NT group and both LSVT-DBS and LSVT groups. These analyses also revealed significant increases in SPL from PRE to POST and PRE to FU for both the LSVT-DBS and LSVT groups, but no significant change for the NT group. When separated by Task, post hoc comparisons revealed that all speech tasks contributed to the significant increases in SPL from PRE to POST and PRE to FU. Taken together, these results suggest that the LSVT-DBS and LSVT groups increased SPL on all tasks following treatment to the point where they were significantly louder than the untreated group.
3.2.2 Vowel articulation
Data for each subject’s VAI at PRE, POST and FU can be found in Table 4. Figure 2 displays group averages (and SEM) at each time period.
Table 4.
Vowel Articulation Index (VAI) averages for each subject at PRE, POST and FU, along with PRE to POST and PRE to FU differences and group Effect Sizes. Increases in the VAI indicate reduced vowel centralization.
| Group/ Subject | PRE | POST | FU | POST-PRE* | FU-PRE* |
|---|---|---|---|---|---|
| DBS-1 | 0.900 | 0.952 | 0.951 | 0.051 | 0.051 |
| DBS-2 | 0.798 | 0.846 | 0.048 | ||
| DBS-3 | 0.896 | 0.906 | 0.941 | 0.009 | 0.045 |
| DBS-4 | 0.847 | 0.951 | 0.971 | 0.104 | 0.124 |
| Group mean | 0.86 | 0.91 | 0.95 | 0.053 | 0.073 |
| sd | 0.05 | 0.05 | 0.02 | 0.039 | 0.044 |
| Effect Size | 0.9 | 1.9 | |||
|
| |||||
| LSVT-5 | 0.933 | 0.974 | 0.904 | 0.041 | -0.030 |
| LSVT-6 | 0.843 | 0.867 | 0.909 | 0.024 | 0.066 |
| LSVT-54 | 0.908 | 0.951 | 0.973 | 0.042 | 0.064 |
| LSVT-65 | 1.035 | 1.010 | 1.009 | -0.025 | -0.026 |
| Group mean | 0.93 | 0.95 | 0.95 | 0.020 | 0.019 |
| sd | 0.08 | 0.06 | 0.05 | 0.031 | 0.054 |
| Effect Size | 0.28 | 0.28 | |||
|
| |||||
| NT-8 | 0.877 | 0.909 | 0.890 | 0.032 | 0.013 |
| NT-18 | 0.925 | 0.937 | 0.898 | 0.012 | -0.027 |
| NT-48 | 0.847 | 0.814 | 0.826 | -0.033 | -0.021 |
| NT-70 | 0.913 | 0.960 | 0.930 | 0.047 | 0.017 |
| Group mean | 0.89 | 0.91 | 0.89 | 0.01 | 0.00 |
| sd | 0.04 | 0.06 | 0.04 | 0.03 | 0.02 |
| Effect Size | 0.32 | 0.1 | |||
Mean PRE to POST and PRE to FU changes are significant for the LSVT-DBS (p < .01). Changes for individual subjects were not analyzed statistically. ES measures of .20, .50, and .80 are considered small, medium and large respectively.
Figure 2.
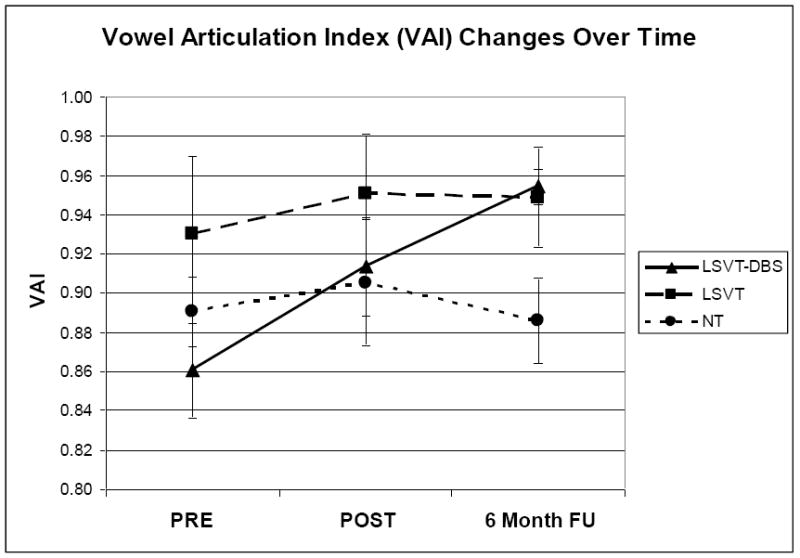
Vowel Articulation Index (VAI) (mean and standard error of measurement) for each group at PRE, POST, and six month FU.
Statistical analyses of VAI (RM-ANOVA) revealed a significant main effect of Group [F(2,18)=48.88, p<0.0001), and a significant interaction of Group × Time [F(4,36)=16.27, p<0.003)]. Post-hoc planned comparisons revealed a significant increase in VAI from PRE to POST (p<0.05), and PRE to FU (p<0.01) for the LSVT-DBS group but not for the LSVT or NT groups. VAI was significantly smaller for the LSVT-DBS compared to the LSVT group at PRE but not POST or FU. Additionally, FU VAI was significantly larger for both the LSVT-DBS and LSVT groups compared to the NT group (p<0.01).
3.3 Voice Handicap Index
Data for the VHI appear in Table 5. At the start of the study LSVT-DBS subjects rated themselves higher (worse) than all other subjects, with the remaining scores distributed in severity among the other two groups. Three of four LSVT-DBS subjects, and one LSVT subject, rated themselves as significantly improved at POST, and ratings for two LSVT-DBS subjects no longer fell in the top four for impairment. By FU, one person in each of the treated groups scored themselves significantly better than PRE, and the three remaining LSVT-DBS subjects once again rated themselves worse than all other subjects. Two NT subjects rated themselves almost significantly better at FU than PRE but neither reached the 18-point mark.
Table 5.
Voice Handicap Index (VHI) scores at PRE, POST and FU.
| Group/Subject | PRE | POST | FU | POST-PRE | POST-FU |
|---|---|---|---|---|---|
| DBS-1 | 84 | 35 | 66 | -49 | -18 |
| DBS-2 | 79 | 26 | n/a | -53 | n/a |
| DBS-3 | 72 | 52 | 60 | -20 | -12 |
| DBS-4 | 92 | 80 | 79 | -12 | -13 |
| Mean | 81.8 | 48.3 | 68.3 | -33.5 | -14.3 |
| sd | 8.4 | 23.8 | 9.7 | 20.5 | 3.2 |
| LSVT-5 | 37 | 35 | 40 | -2 | +3 |
| LSVT-6 | 64 | 58 | 45 | -6 | -19 |
| LSVT-54 | 33 | 7 | 28 | -26 | -5 |
| LSVT-65 | 22 | 10 | 12 | -12 | -10 |
| Mean | 39.0 | 27.5 | 31.3 | -11.5 | -7.8 |
| sd | 17.8 | 23.9 | 14.7 | 10.5 | 9.2 |
| NT-8 | 58 | 50 | 44 | -8 | -14 |
| NT-18 | 21 | 33 | 37 | +12 | +16 |
| NT-48 | 56 | 55 | 40 | -1 | -16 |
|
| |||||
| NT-70 | 12 | 10 | 13 | -2 | +1 |
| Mean | 36.8 | 37.0 | 33.5 | +0.3 | -3.3 |
| sd | 23.7 | 20.3 | 14.0 | 8.4 | 14.9 |
Note: Maximum (worst) score = 120. A reduction in score indicates improvement; a decrease of at least18 points is considered clinically meaningful.
4. Discussion
The present study evaluated four individuals with PD whose motor symptoms were treated with bilateral STN-DBS to determine how they would respond to behavioral voice and speech treatment, LSVT LOUD, compared to treated and untreated subjects with PD but without STN-DBS. Following LSVT LOUD, both the LSVT and LSVT-DBS groups significantly increased SPL from PRE to POST and FU. SPL changes were comparable to previously published outcomes (Ramig et al., 2001b) and indicate that individuals may successfully learn to speak with increased loudness following STN-DBS surgery. Previously published perceptual data have also reported improvement in judgments of voice quality and loudness following LSVT LOUD (Baumgartner, Sapir, & Ramig, 2001; Sapir et al., 2002), suggesting that the current outcomes may be considered clinically significant.
Both treated groups also demonstrated positive changes from PRE to POST and FU on a measure of vowel articulation (VAI), suggesting a spreading of LSVT LOUD effects to articulation consistent with previous research (Sapir et al., 2007). Individual data appearing in Table 4 show that all LSVT-DBS subjects increased VAI from PRE to POST, and the remaining three subjects continued to increase through FU. Three of four LSVT subjects improved from PRE to POST and two of those three increased further at FU. Three NT subjects also showed gains from PRE to POST but these were largely lost by FU. Both treated groups had significantly higher VAI measures at FU compared to the untreated group, although only the LSVT-DBS group significantly improved from PRE to POST and also PRE to FU. Previous research documenting improvement in listener judgments of “vowel goodness” following LSVT LOUD supports an interpretation that the vowel formant changes reported here may reflect audible improvement in speech articulation (Sapir et al., 2007), particularly for the LSVT-DBS group.
Given the potential effects of STN-DBS stimulation on articulation, it is reasonable to question whether changes in stimulator settings from POST to FU (see Methods) had an effect on this measure, accounting for the significant increase in VAI for the LSVT-DBS group from PRE to POST and FU. However, evaluation of individual data shows continued increases in VAI for two treated non-surgical participants from POST to FU which could not be explained by stimulator changes. One simple explanation is that because the LSVT-DBS group started with lower VAI scores at PRE it had more room to make larger gains and eventually approach the LSVT group at FU. Another explanation could be that speakers will maintain changes in behaviors most directly related to their ability to communicate. In this case, the more severe articulatory problems measured prior to treatment might have motivated speakers to maintain improved articulatory behaviors gained as a result of treatment, or even continue to improve them. Either hypothesis is supported by individual data in the current study. Specifically, of the subjects followed out to six months DBS-4 and LSVT-6 had the most compact VAI prior to treatment (0.847 and 0.843 respectively), and maintained the two largest increases at FU (+ 0.124 and + 0.066 respectively).
A qualitative measure of communication (VHI) was also completed to determine whether there was evidence of self-rated improvement in voice, speech, and functional communication. Across the three groups prior to treatment, the mean LSVT-DBS score was more than twice that of each of the other groups, indicating a more severe perception of impairment. This appeared to be driven by higher scores from all four LSVT-DBS subjects and supports published observations of degraded speech and voice following STN-DBS (see Introduction). Three of the four LSVT-DBS subjects showed clinically significant gains at POST compared to one LSVT and no NT subjects, suggesting a positive effect of voice therapy on self-ratings of voice-related handicap. By FU, however, the three LSVT-DBS subjects once again had all of the highest scores and only one individual maintained a (borderline) significant improvement, a possible indicator of limited long-term maintenance. All three LSVT-DBS subjects also reported on FU interview that people sometimes had a hard time understanding them, while none of the LSVT subjects reported such a problem.
When evaluating the relationships between acoustic and self-rating measures in this study, no clear picture emerges. Changes in VHI and SPL from PRE to POST were moderately correlated (Spearman rank correlation, ρ = -0.61, p < .05) but less so from PRE to FU (ρ = -0.35, n.s.). VHI/VAI correlations were both weak to moderate but insignificant (-0.48 PRE to POST and -0.56 PRE to FU). These inconsistent correlations (see also Spielman et al., 2010) support a continued need to use a platter of measures – both objective and subjective – when studying treatment outcomes, rather than rely on a single index of improvement as has sometimes been the case when evaluating the effects of STN-DBS on speech (see Introduction).
In general the outcomes of this study should be interpreted with caution, as the small sample size limits generalization. Other limitations include partial rather than full randomization of group assignment, since all four LSVT-DBS subjects were immediately placed into a treatment group. Furthermore, because surgery was not performed by the same individual, there may have been less consistent surgical outcomes. Finally, one DBS-LSVT subject received treatment on a slightly modified schedule (note DBS-2), one was lost to follow up (same subject), and one (DBS-1) was discovered to have received voice therapy two years before, creating a rather heterogeneous group. Requirements for future research should include full randomization of a larger, more homogeneous subject population, none of whom has ever received any kind of speech therapy.
Future research should also further evaluate the long-term impact of treatment on functional communication following STN-DBS. In addition to the VHI, functional rating scales in current use or under development, such as the Modified Communicative Effectiveness Index (Ball, Beukelman, & Pattee, 2004), the Dysarthria Impact Profile (Walshe, Peach, & Miller, 2009), and the Communicative Participation Item Bank (Baylor, Yorkston, Eadie, Miller, & Amtmann, 2009), have the potential to provide valuable information regarding changes in communicative effectiveness and dysarthria impact following therapy. A greater variety of acoustic measures – including contrastivity measures as described by Rosen et al. (Rosen, Kent, Delaney, & Duffy, 2006) – may also offer more insight into the kinds of pre-treatment voice and articulation difficulties typical for this population, and help evaluate whether improvements in specific behaviors contribute more to improved intelligibility and/or are easier to maintain.
Finally, it may be desirable to examine modifications of LSVT LOUD that could contribute to better long-term maintenance of treatment effects following STN-DBS. High VHI scores and reports of decreased intelligibility at FU reflect a need to consider additional training. A study is currently underway to evaluate modifications to treatment, including two additional weeks of traditional LSVT LOUD as well as two additional weeks of treatment that maintains vocal loudness while specifically addressing the articulation problems that may be caused by STN-DBS. Finally, future research should also evaluate the effectiveness of administering treatment before or after surgical intervention to determine whether this can significantly affect communication following surgery.
Highlights.
We compared 12 subjects with Parkinson disease before and after LSVT®LOUD
4 treated subjects had subthalamic nucleus (STN) DBS, 8 other treated or untreated subjects did not.
Both treated groups responded well to LSVT®LOUD based on acoustic and perceptual measures
LSVT®LOUD may be a viable speech treatment option for people with PD and STN-DBS
Additional research is needed to evaluate long-term maintenance of speech and voice improvements
Acknowledgments
This research was made possible by funding from National Institutes of Health (NIH) grant R01 DC1150 (National Institutes of Deafness and Other Communication Disorders), the Parkinson Alliance, and the Davis Phinney Foundation. Funding sources were not active in study design, data collection, analysis, interpretation, or in the writing and submission of this manuscript. We would like to thank Jill Cable for help with recruitment and treatment, and the clients and families who participated in this study.
Footnotes
Author Contributions: LR, JS, AH and LM participated in the conception, organization and execution of the research project. JS, LM, and AH wrote the first draft of the manuscript. LR and OK further reviewed, critiqued and edited the manuscript. PG provided statistical analysis and consultation.
Disclosure: Dr. Lorraine Ramig receives a lecture honorarium and has ownership interest in LSVT Global, a for-profit organization that runs training courses and sells products related to LSVT®LOUD treatment. Leslie Mahler and Angela Halpern are consultants for LSVT Global and receive an honorarium for conducting certification workshops.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ball LJ, Beukelman DR, Pattee GL. Communication effectiveness of individuals with amyotrophic lateral sclerosis. Journal of Communication Disorders. 2004;37(3):197–215. doi: 10.1016/j.jcomdis.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Baumgartner CA, Sapir S, Ramig TO. Voice quality changes following phonatory-respiratory effort treatment (LSVT) versus respiratory effort treatment for individuals with Parkinson disease. J Voice. 2001;15(1):105–114. doi: 10.1016/s0892-1997(01)00010-8. [DOI] [PubMed] [Google Scholar]
- Baylor CR, Yorkston KM, Eadie TL, Miller RM, Amtmann D. Developing the communicative participation item bank: Rasch analysis results from a spasmodic dysphonia sample. Journal of Speech, Langugage, and Hearing Research. 2009;52(5):1302–1320. doi: 10.1044/1092-4388(2009/07-0275). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Beck Depression Inventory(R)-II. San Antonio, Texas: Pearson Education, Inc; 1996. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, N.J.: Erlbaum; 1988. [Google Scholar]
- Countryman S, Ramig L, Pawlas A. Speech and voice deficits in Parkinsonian Plus Syndromes: can they be treated? Journal of Medical Speech-Language Pathology. 1994;2(3):211–225. [Google Scholar]
- D’Alatri L, Paludetti G, Contarino MF, Galla S, Marchese MR, Bentivoglio AR. Effects of bilateral subthalamic nucleus stimulation and medication on parkinsonian speech impairment. Journal of Voice. 2008;22(3):365–372. doi: 10.1016/j.jvoice.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Darley F, Aronson A, Brown J. Motor speech disorders. Philadelphia: Saunders; 1975. [Google Scholar]
- Dromey C, Kumar R, Lang AE, Lozano AM. An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Movement Disorders. 2000;15(6):1132–1138. doi: 10.1002/1531-8257(200011)15:6<1132::aid-mds1011>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- El-Sharkawi A, Ramig L, Logemann JA, Pauloski BR, Rademaker AW, Smith CH, et al. Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT): a pilot study. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72(1):31–36. doi: 10.1136/jnnp.72.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G. Voice and articulation drillbook. New York: Harper & Brothers; 1960. [Google Scholar]
- Forrest K, Weismer G, Turner GS. Kinematic, acoustic, and perceptual analyses of connected speech produced by parkinsonian and normal geriatric adults. Journal of the Acoustical Society of America. 1989;85(6):2608–2622. doi: 10.1121/1.397755. [DOI] [PubMed] [Google Scholar]
- Fox C, Ramig L. Vocal sound pressure level and self-perception of speech and voice in men and women with idiopathic Parkinson disease. American Journal of Speech-Language Pathology. 1997;6:85–94. [Google Scholar]
- Fox CM, Ramig LO, Ciucci MR, Sapir S, McFarland DH, Farley BG. The science and practice of LSVT/LOUD: neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders. Seminars in Speech and Language. 2006;27(4):283–299. doi: 10.1055/s-2006-955118. [DOI] [PubMed] [Google Scholar]
- Gentil M, Garcia-Ruiz P, Pollak P, Benabid AL. Effect of bilateral deep-brain stimulation on oral control of patients with parkinsonism. European Neurology. 2000;44(3):147–152. doi: 10.1159/000008224. [DOI] [PubMed] [Google Scholar]
- Halpern A, Spielman J, Ramig L, Sharpley A, Panzer I. The Effects of Loudness and Noise on Speech Intelligibility in Parkinson disease. Presented at 14th Biennial Conference on Motor Speech; Monterey, California. 3-6-2008. [Google Scholar]
- Hammer MJ, Barlow SM, Lyons KE, Pahwa R. Subthalamic nucleus deep brain stimulation changes speech respiratory and laryngeal control in Parkinson’s disease. Journal of Neurology. 2010;257(10):1692–1702. doi: 10.1007/s00415-010-5605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer MJ, Barlow SM, Lyons KE, Pahwa R. Subthalamic nucleus deep brain stimulation changes velopharyngeal control in Parkinson’s disease. Journal of Communication Disorders. 2011;44(1):37–48. doi: 10.1016/j.jcomdis.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartelius L, Svensson P. Speech and swallowing symptoms associated with Parkinson’s disease and multiple sclerosis: a survey. Folia Phoniatrica et Logopaedica. 1994;46(1):9–17. doi: 10.1159/000266286. [DOI] [PubMed] [Google Scholar]
- Hintze J. NCSS (Version NCSS 2007) [Computer software] Kaysville, Utah: NCSS, LLC; 2007. [Google Scholar]
- Ho AK, Bradshaw JL, Iansek R. For better or worse: The effect of levodopa on speech in Parkinson’s disease. Movement Disorders. 2008;23(4):574–580. doi: 10.1002/mds.21899. [DOI] [PubMed] [Google Scholar]
- Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson’s disease. Behavioral Neurology. 1998;11(3):131–137. [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Holmes RJ, Oates JM, Phyland DJ, Hughes AJ. Voice characteristics in the progression of Parkinson’s disease. International Journal of Language & Communication Disorders. 2000;35(3):407–418. doi: 10.1080/136828200410654. [DOI] [PubMed] [Google Scholar]
- Iulianella I, Adams SG, Gow AK. Effects of sub-thalamic deep brain stimulation on speech production in Parkinson’s Disease: A critical review of the literature. Canadian Journal of Speech-Language Pathology & Audiology. 2008;32(2):85–91. [Google Scholar]
- Jacobson B, Johnson A, Grywalski C, Silbergleit J, Jacobson G, Benninger M, et al. The Voice Handicap Index (VHI): Development and validation. American Journal of Speech-Language Pathology. 1997;6(3):66–70. [Google Scholar]
- Jones HN, Kendall DL, Sudhyadhom A, Rosenbek JC. The effects of lesion therapy and deep brain stimulation on speech function in patients with Parkinson’s disease. Communicative Disorders Review. 2007;1(3-4):133–173. [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. Journal of Speech, Langugage, and Hearing Research. 2008;51(1):S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Klostermann F, Ehlen F, Vesper J, Nubel K, Gross M, Marzinzik F, et al. Effects of subthalamic deep brain stimulation on dysarthrophonia in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79(5):522–529. doi: 10.1136/jnnp.2007.123323. [DOI] [PubMed] [Google Scholar]
- Liotti M, Ramig LO, Vogel D, New P, Cook CI, Ingham RJ, et al. Hypophonia in Parkinson’s disease: neural correlates of voice treatment revealed by PET. Neurology. 2003;60(3):432–440. doi: 10.1212/wnl.60.3.432. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. Journal of Speech and Hearing Disorders. 1978;43(1):47–57. doi: 10.1044/jshd.4301.47. [DOI] [PubMed] [Google Scholar]
- Mahler L, Spielman J, Sapir S, Ramig L, Halpern A. Effects of LSVT® LOUD on Four Participants with Parkinson disease Who Received Deep Brain Stimulation of the Subthalamic Nucleus. Presented at 12th International Congress of Parkinson’s Disease and Movement Disorders; Chicago, IL. 6-22-2008. [Google Scholar]
- Matos C. SPL Extractor [Computer software] Salvador, Brazil: 2003. [Google Scholar]
- Milenkovic P. TF32 [Computer software] 2004 [Google Scholar]
- Miller N, Allcock L, Jones D, Noble E, Hildreth AJ, Burn DJ. Prevalence and pattern of perceived intelligibility changes in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78(11):1188–1190. doi: 10.1136/jnnp.2006.110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Noble E, Jones D, Burn D. Life with communication changes in Parkinson’s disease. Age and Ageing. 2006;35(3):235–239. doi: 10.1093/ageing/afj053. [DOI] [PubMed] [Google Scholar]
- Narayana S, Fox PT, Zhang W, Franklin C, Robin DA, Vogel D, et al. Neural correlates of efficacy of voice therapy in Parkinson’s disease identified by performance-correlation analysis. Human Brian Mapping. 2010;31(2):222–236. doi: 10.1002/hbm.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana S, Jacks A, Robin DA, Poizner H, Zhang W, Franklin C, et al. A noninvasive imaging approach to understanding speech changes following deep brain stimulation in Parkinson’s disease. American Journal of Speech-Language Pathology. 2009;18(2):146–161. doi: 10.1044/1058-0360(2008/08-0004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Ozsancak C, Tripoliti E, Thobois S, Limousin-Dowsey P, Auzou P. Treatments for dysarthria in Parkinson’s disease. Lancet Neurology. 2004a;3(9):547–556. doi: 10.1016/S1474-4422(04)00854-3. [DOI] [PubMed] [Google Scholar]
- Pinto S, Thobois S, Costes N, Le BD, Benabid AL, Broussolle E, et al. Subthalamic nucleus stimulation and dysarthria in Parkinson’s disease: a PET study. Brain. 2004b;127(Pt 3):602–615. doi: 10.1093/brain/awh074. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Countryman S, Thompson LL, Horii Y. Comparison of two forms of intensive speech treatment for Parkinson disease. Journal of Speech and Hearing Research. 1995;38(6):1232–1251. doi: 10.1044/jshr.3806.1232. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Countryman S, Pawlas AA, O’Brien C, Hoehn M, et al. Intensive voice treatment (LSVT) for patients with Parkinson’s disease: a 2 year follow up. Journal of Neurology, Neurosurgery, and Psychiatry. 2001a;71(4):493–498. doi: 10.1136/jnnp.71.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Fox C, Countryman S. Changes in vocal loudness following intensive voice treatment (LSVT) in individuals with Parkinson’s disease: a comparison with untreated patients and normal age-matched controls. Movement Disorders. 2001b;16(1):79–83. doi: 10.1002/1531-8257(200101)16:1<79::aid-mds1013>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Rosen KM, Kent RD, Delaney AL, Duffy JR. Parametric quantitative acoustic analysis of conversation produced by speakers with dysarthria and healthy speakers. Journal of Speech, Langugage, and Hearing Research. 2006;49(2):395–411. doi: 10.1044/1092-4388(2006/031). [DOI] [PubMed] [Google Scholar]
- Rousseaux M, Krystkowiak P, Kozlowski O, Ozsancak C, Blond S, Destee A. Effects of subthalamic nucleus stimulation on parkinsonian dysarthria and speech intelligibility. Journal of Neurology. 2004;251(3):327–334. doi: 10.1007/s00415-004-0327-1. [DOI] [PubMed] [Google Scholar]
- Roy N, Nissen SL, Dromey C, Sapir S. Articulatory changes in muscle tension dysphonia: evidence of vowel space expansion following manual circumlaryngeal therapy. J Commun Disord. 2009;42(2):124–135. doi: 10.1016/j.jcomdis.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Santens P, De Letter M, Van Borsel J, De Reuck J, Caemaert J. Lateralized effects of subthalamic nucleus stimulation on different aspects of speech in Parkinson’s disease. Brain and Language. 2003;87(2):253–258. doi: 10.1016/s0093-934x(03)00142-1. [DOI] [PubMed] [Google Scholar]
- Sapir S. Vowel Articulation Index as a robust acoustic index of dysarthric vowel articulation: Evidence from the speech of individuals with and without idiopathic Parkinson’s disease. Presented at the Annual conference of the Israeli Association of Physical and Rehabilitation Medicine; Tel Aviv, Israel. 2007. [Google Scholar]
- Sapir S, Ramig LO, Hoyt P, Countryman S, O’Brien C, Hoehn M. Speech loudness and quality 12 months after intensive voice treatment (LSVT) for Parkinson’s disease: a comparison with an alternative speech treatment. Folia Phoniatr Logop. 2002;54(6):296–303. doi: 10.1159/000066148. [DOI] [PubMed] [Google Scholar]
- Sapir S, Ramig LO, Spielman JL, Fox C. Formant centralization ratio: a proposal for a new acoustic measure of dysarthric speech. Journal of Speech, Langugage, and Hearing Research. 2010;53(1):114–125. doi: 10.1044/1092-4388(2009/08-0184). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir S, Spielman J, Ramig LO, Hinds SL, Countryman S, Fox C, et al. Effects of intensive voice treatment (the Lee Silverman Voice Treatment [LSVT]) on ataxic dysarthria: a case study. American Journal of Speech-Language Pathology. 2003;12(4):387–399. doi: 10.1044/1058-0360(2003/085). [DOI] [PubMed] [Google Scholar]
- Sapir S, Spielman JL, Ramig LO, Story BH, Fox C. Effects of intensive voice treatment (the Lee Silverman Voice Treatment [LSVT]) on vowel articulation in dysarthric individuals with idiopathic Parkinson disease: acoustic and perceptual findings. Journal of Speech, Langugage, and Hearing Research. 2007;50(4):899–912. doi: 10.1044/1092-4388(2007/064). [DOI] [PubMed] [Google Scholar]
- Sarno MT. Speech impairment in Parkinson’s disease. Archives of Physical Medicine and Rehabilitation. 1968;49(5):269–275. [PubMed] [Google Scholar]
- Schulz GM. The effects of speech therapy and pharmacological treatments on voice and speech in Parkinson s disease: a review of the literature. Current Medicinal Chemistry. 2002;9(14):1359–1366. doi: 10.2174/0929867023369808. [DOI] [PubMed] [Google Scholar]
- Skodda S, Visser W, Schlegel U. Vowel Articulation in Parkinson’s disease. Journal of Voice. 2010 doi: 10.1016/j.jvoice.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Hixon TJ. Speech breathing in Parkinson’s disease. Journal of Speech and Hearing Research. 1993;36(2):294–310. doi: 10.1044/jshr.3602.294. [DOI] [PubMed] [Google Scholar]
- Spielman J, Gilley P, Halpern A, Ramig L. Relationship of Communicative Effectiveness to Voice Handicap, Acoustics, and Behavioral Characteristics in People with Parkinson Disease. Presented at 15th Biennial Conference on Motor Speech; Savannah, Georgia. 3-4-2010. [Google Scholar]
- Spielman J, Petska J, Halpern A, Ramig L. LSVT® Following Deep Brain Stimulation of the Subthalamic Nucleus: Preliminary Findings. Presented at 13th Biennial Conference on Motor Speech; Austin, Texas. 3-23-2006. [Google Scholar]
- Spielman JL, Borod JC, Ramig LO. The effects of intensive voice treatment on facial expressiveness in Parkinson disease: preliminary data. Cognitive and Behavioral Neurology. 2003;16(3):177–188. doi: 10.1097/00146965-200309000-00005. [DOI] [PubMed] [Google Scholar]
- Svec JG, Popolo PS, Titze IR. Measurement of vocal doses in speech: experimental procedure and signal processing. Logopedics, Phoniatrics, Vocology. 2003;28(4):181–192. doi: 10.1080/14015430310018892. [DOI] [PubMed] [Google Scholar]
- Tjaden K, Wilding GE. Rate and loudness manipulations in dysarthria: acoustic and perceptual findings. Journal of Speech, Langugage, and Hearing Research. 2004;47(4):766–783. doi: 10.1044/1092-4388(2004/058). [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Tornqvist AL, Schalen L, Rehncrona S. Effects of different electrical parameter settings on the intelligibility of speech in patients with Parkinson’s disease treated with subthalamic deep brain stimulation. Movement Disorders. 2005;20(4):416–423. doi: 10.1002/mds.20348. [DOI] [PubMed] [Google Scholar]
- Tripoliti E, Limousin-Dowsey P, Tisch S, Borrell E, Hariz ML. Speech in Parkinson’s disease following subthalamic nucleus deep brain stimulation: Preliminary results. Journal of Medical Speech-Language Pathology. 2006;14:309–325. [Google Scholar]
- Tripoliti E, Zrinzo L, Martinez-Torres I, Frost E, Pinto S, Foltynie T, et al. Effects of subthalamic stimulation on speech of consecutive patients with Parkinson disease. Neurology. 2011;76(1):80–86. doi: 10.1212/WNL.0b013e318203e7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripoliti E, Zrinzo L, Martinez-Torres I, Tisch S, Frost E, Borrell E, et al. Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Movement Disorders. 2008;23(16):2377–2383. doi: 10.1002/mds.22296. [DOI] [PubMed] [Google Scholar]
- Walker HC, Phillips DE, Boswell DB, Guthrie BL, Guthrie SL, Nicholas AP, et al. Relief of acquired stuttering associated with Parkinson’s disease by unilateral left subthalamic brain stimulation. Journal of Speech, Langugage, and Hearing Research. 2009;52(6):1652–1657. doi: 10.1044/1092-4388(2009/08-0089). [DOI] [PubMed] [Google Scholar]
- Walshe M, Peach RK, Miller N. Dysarthria impact profile: development of a scale to measure psychosocial effects. International Journal of Language & Communication Disorders. 2009;44(5):693–715. doi: 10.1080/13682820802317536. [DOI] [PubMed] [Google Scholar]
- Wang E, Verhagen ML, Bakay R, Arzbaecher J, Bernard B. The effect of unilateral electrostimulation of the subthalamic nucleus on respiratory/phonatory subsystems of speech production in Parkinson’s disease--a preliminary report. Clinical Linguistics and Phonetics. 2003;17(4-5):283–289. doi: 10.1080/0269920031000080064. [DOI] [PubMed] [Google Scholar]
- Wang EQ, Verhagen Metman L, Bakay RAE, Arzbaecher J, Bernard B, Corcos DM. Hemispheric-specific effects of subthalamic nucleus deep brain stimulation on speaking rate and articulatory accuracy of syllable repetitions in Parkinson’s disease. Journal of Medical Speech-Language Pathology. 2006;14:323–333. [PMC free article] [PubMed] [Google Scholar]
- Weismer G, Jeng JY, Laures JS, Kent RD, Kent JF. Acoustic and intelligibility characteristics of sentence production in neurogenic speech disorders. Folia Phoniatrica et Logopaedica. 2001;53(1):1–18. doi: 10.1159/000052649. [DOI] [PubMed] [Google Scholar]
- Wenke RJ, Theodoros D, Cornwell P. The short- and long-term effectiveness of the LSVT for dysarthria following TBI and stroke. Brain Injury. 2008;22(4):339–352. doi: 10.1080/02699050801960987. [DOI] [PubMed] [Google Scholar]
- Yorkston KM, Beukelman DR. Communication efficiency of dysarthric speakers as measured by sentence intelligibility and speaking rate. Journal of Speech and Hearing Disorders. 1981;46(3):296–301. doi: 10.1044/jshd.4603.296. [DOI] [PubMed] [Google Scholar]
- Yorkston KM, Spencer KA, Duffy JR. Behavioral management of respiratory/phonatory dysfunction from dysarthria: A systematic review of the evidence. Journal of Medical Speech-Language Pathology. 2003;11(2):12–38. [Google Scholar]


