Abstract
There has been enormous progress in the understanding of acute kidney injury (AKI) over the last five years. This article reviews some of the salient new findings, the challenges revealed by these findings, and new insights into the pathogenesis of ischemic AKI. Clinical studies have demonstrated that even a small, transient rise in serum creatinine increases the risk of mortality in hospitalized patients and that a single event of AKI increases the risk for developing chronic kidney disease. Although the overall mortality rate from AKI has improved over the last two decades, it continues to be significant. Current treatment is focused on maintaining renal perfusion and avoiding volume overload. However, new therapeutic targets are emerging for the treatment of AKI as our understanding of the pathogenesis of ischemic injury and inflammation increases. Early diagnosis, however, continues to be challenging as the search continues for sensitive and specific biomarkers.
Keywords: Acute kidney injury, Biomarkers, Pathogenesis
INTRODUCTION AND DEFINITIONS
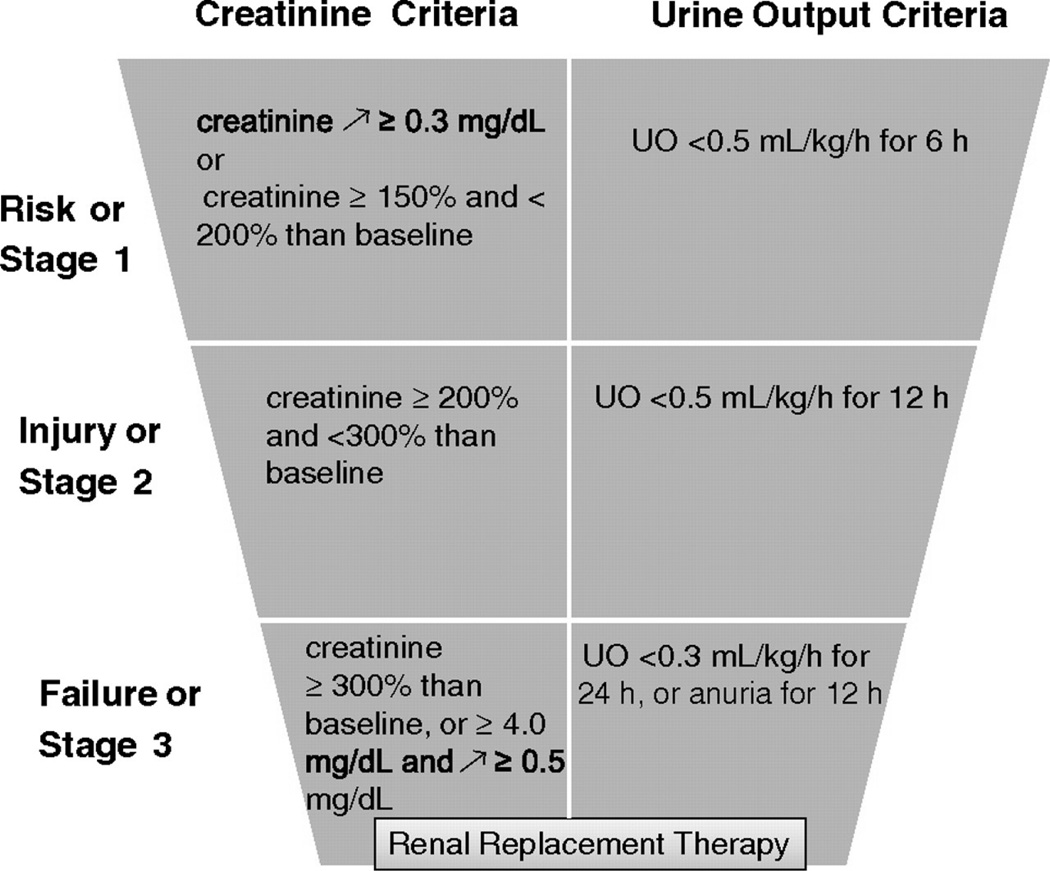
Early studies of “acute renal failure” (ARF) were compromised by inconsistent definitions of this disease by different investigators. Although all agreed that a decrease in renal function over the course of hours-to–days is the hallmark of ARF, there was no agreement on what constituted renal dysfunction. A major step forward was the formation of the Acute Dialysis Quality Initiative and the Acute Kidney Injury Network (AKIN).1,2 In 2005, these consensus groups of nephrologists and intensive care specialists proposed replacing the term “acute renal failure” with the new, strictly defined term “acute kidney injury” (AKI). This definition was further refined in 2007 and is often referred to as the RIFLE criteria (for “Risk, Injury, Failure, Loss of function, and Endstage renal disease”). The diagnostic criteria include increased serum creatinine and decreased urine output. Only the first three stages, shown in Figure 1, are in wide use.3
Figure 1.
RIFLE classification for AKI after modifications by the Acute Kidney Injury Network. Patients are classified according to the worst of creatinine or urine output criteria. Patients do not need to fulfil both creatinine and urine output criteria. Diagnosis of AKI on the basis of creatinine criteria is fulfilled when patients experience an increase in serum creatinine ≥0.3 mg/dL or >150% within a 48-h period. (Reprinted with permission from Hoste EA and Kellum JA. AKI severity class doesn't tell all: the case for transient AKI. Nephrol Dial Transplant 2010).
The subtle change of the word “failure” in the old term, “acute renal failure,” to “injury” in the new term, “acute kidney injury,” has profound implications. First, the concept of structural “injury” is differentiated from an adaptive physiologic response of an uninjured kidney to hypo-perfusion. Furthermore, as our understanding grows of how the kidney sustains injury, new therapies can be developed targeting these mechanisms. Finally, the staging of clinical measures is an early attempt to predict the likelihood of finding pathologic changes if the kidney were biopsied. Case in point is the Risk category (or Stage 1 AKI), which suggests that early detection and treatment may prevent severe damage and reduce the risk of progression to chronic kidney disease or end stage renal disease.
AKI is one major cause of acute renal dysfunction
One of the difficulties in treating acute renal dysfunction is making the diagnosis early enough to alter the course of the disease. The problem lies, in part, in differentiating AKI (structural injury to the kidney) from the other etiologies of acute renal dysfunction (rise in serum creatinine and/or decreased urine output). These etiologies have been divided into three broad categories, and have been discussed in many excellent standard textbooks and review articles.4 We summarize them briefly here.
Pre-renal causes occur in 55–60% of patients with renal dysfunction. In these situations, the renal parenchyma is intact, and the apparent decrease in renal function is due to decreased perfusion of the kidneys. Etiologies include decreased intravascular volume (e.g. from hemorrhage), decreased cardiac output (e.g. after excessive doses of antihypertensive medications or heart failure), renal vasoconstriction (e.g. following excessive cyclosporine doses), and impaired auto-regulation of renal blood flow (e.g. angiotensin-converting enzyme inhibitors or inhibitors of prostaglandin synthesis).
Post-renal causes occur in less than 5% of patients, but should not be missed because they are often treatable. Again, the renal parenchyma is intact, and the decreased renal function is caused by obstruction of urine flow. Obstruction may occur at the level of the ureter, bladder neck, or urethra.
Intrinsic-renal causes occur in the remainder of patients. Unlike pre-renal and post-renal causes of renal dysfunction, the initial pathophysiology lies within the kidney and is accompanied by early structural lesions in the parenchyma. Intrinsic-renal causes can be divided into four categories, and include diseases of the large renal vessels (e.g. bilateral thrombosis or dissection), glomeruli and renal microvasculature (e.g. glomerulonephritis or hemolytic uremic syndrome), tubules (e.g. ischemic ATN or toxic ATN) and of the tubulointerstitium (e.g. allergic interstitial nephritis or leukemic infiltration). Damage can be a result of cytotoxic, ischemic, or inflammatory insults.
In the clinical setting, multiple etiologies are often present concurrently. These often include decreased renal perfusion (due to hypovolemia, sepsis, or severe myocardial dysfunction) and toxins (either exogenous such as radio-contrast and other drugs, or endogenous such as hypermyoglobinemia from rhabdomyolysis or hyperuricemia from tumor lysis syndrome). Indeed a difficulty in treating acute kidney injury is the possibility of multiple etiologies, each of which may have a different pathophysiology. In addition, pre-renal azotemia and ischemic ATN lie on a spectrum of renal hypoperfusion states and may be coexistent.
This review will focus on ischemic AKI, which contributes to the majority of cases.
IMPACT OF AKI
AKI patients have a higher mortality than matched patients without AKI
The importance of AKI is often under-appreciated. In fact, even mild AKI has profound implications for mortality and progression of chronic kidney disease.
Two multicenter clinical studies, Program to Improve Care in Acute Renal Disease (PICARD) and Beginning and Ending Therapy for the Kidney (BEST Kidney), showed in-hospital mortality for patients with AKI in participating academic medical centers ranged from as low as 24% to as high as 75%.5
Studies of administrative databases have shown an increasing incidence of AKI but decreasing mortality during the 1990s.6–8 Despite the apparent decreasing trend in these studies, the overall mortality rate for patients with AKI remains unacceptably high at approximately 30–40%.9 Even after adjustment for co-morbidities, the mortality in patients with AKI remains higher that those without AKI.10,11
Even small increases in serum creatinine are associated with increased mortality
An increase in the serum creatinine by as little as 0.5 mg/dL has been associated with an increased mortality.11 A landmark study by Lassnigg, et al, analyzed over 4,000 patients who had cardiovascular surgery and found an increase in serum creatinine of 0–0.5 mg/dL was associated with an increased relative risk of death (hazard ratio 2.77).12 An increase in serum creatinine of >0.5 mg/dL signified a relative risk of death of 18.64. Similar increases in mortality have been reported in patients with AKI and stroke, radiocontrast nephropathy, and nonmyeloablative hematopoietic cell transplantation.13–15
Small, transient rises in serum creatinine are also associated with increased mortality
In an analysis of 2,469 patients with a variety of AKI and 16,485 patients without kidney injury, Uchino et al demonstrated that even a transient rise of serum creatinine (for 1–3 days) resulted in an increased odds ratio for in-hospital mortality.16 The authors did not differentiate "prerenal azotemia” from “transient AKI” because they did not think this could be done with confidence.
Similar results were found in a study of 6,033 patients admitted to a Yale community hospital, 735 of whom developed an increase of serum creatinine of 0.3 mg/dL or greater. These patients had a 14.2% greater mortality rate even if the serum creatinine returned to normal within 48 hours.17
In an analysis of over 40,000 transplant recipients, transient AKI in the renal allograft immediately after transplantation was associated with increased mortality. This increased risk of mortality occurs even if the allograft recovers completely from the post-transplant AKI and subsequent renal allograft function is excellent. For example, an eGFR (MDRD) of 60–69 mL/min/1.72m2 after full recovery from AKI was associated with a hazard ratio for death of 1.35.18
AKI has long-term detrimental effects on renal function and contributes to the “epidemic” of chronic kidney disease and end-stage kidney disease
Historically, conventional wisdom suggested that AKI resulted in temporary injury to the kidney and that following resolution of the acute event, there was no permanent damage to the kidney. However, it is now clear that AKI has a long-term detrimental effect on renal function. In one study of 3,769 adults with AKI requiring dialysis, the incidence rate of chronic dialysis over the ensuing 3 years was 2.63 per 100 person-years compared to only 0.91 per 100 person-years in the control patients. The control patients were matched for age, propensity score for developing AKI, and mechanical ventilation. Unlike the studies above, there was no difference in mortality in the AKI patient and controls. This was most likely due to the fact that patients were not included in the study unless they survived for at least 30 days after discharge.19 This and similar studies raise the possibility that AKI may be a significant contributor to the growing epidemic of chronic kidney disease and end-stage renal disease (ESRD).20–22
A growing proportion of patients on the medical service have pre-existing chronic kidney disease. These patients are more susceptible to AKI and the associated increased mortality and risk of progression of their chronic kidney disease.23,24
Distant effects of AKI may explain increased mortality
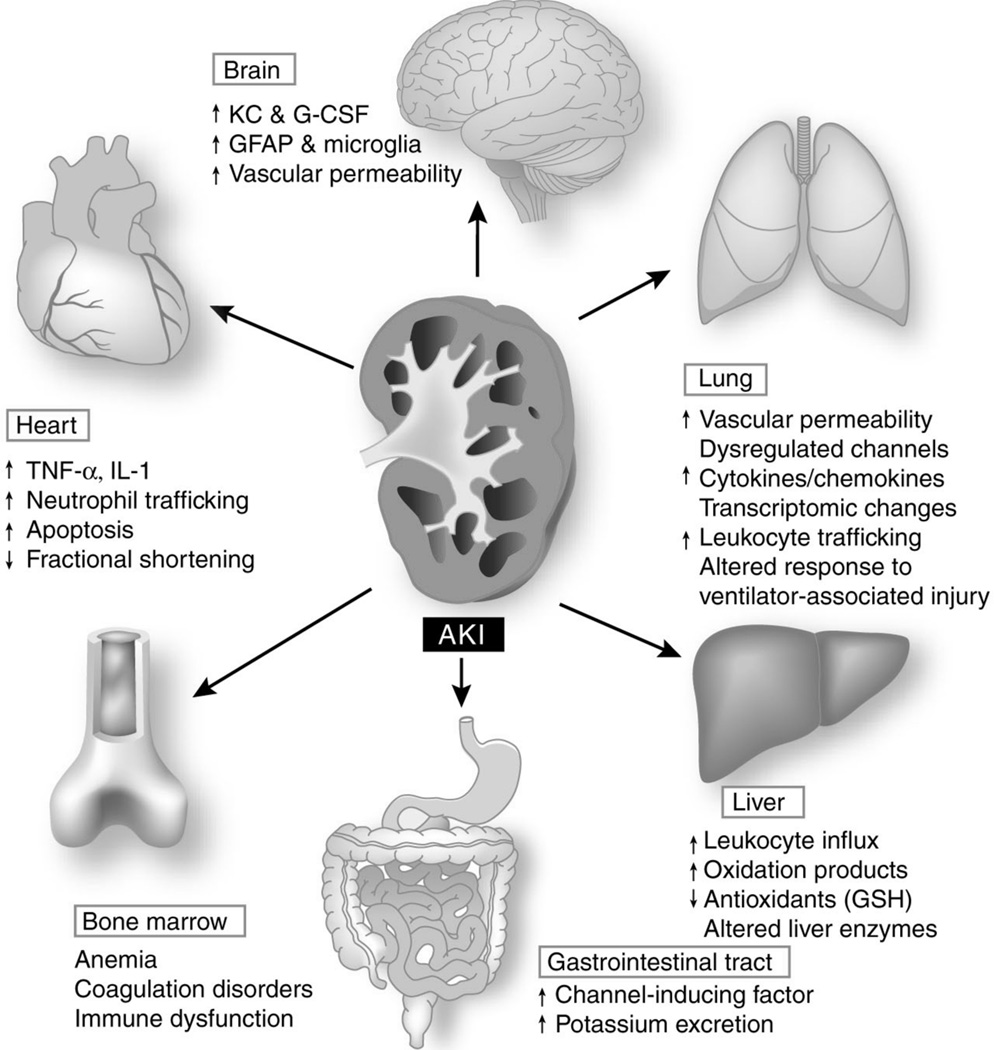
The increased mortality during the acute phase of AKI cannot be solely explained by the fluid, electrolyte, and acid-base abnormalities associated with renal failure since mortality remains high despite advances in modern dialysis. Research from rodents suggests that AKI is an inflammatory disease that affects multiple organ systems including the cardiovascular and respiratory systems, among others. These insights arise from rodent models that allow for kidney injury to occur in isolation (e.g. surgically clamping the renal artery) followed by evaluation of inflammation and dysfunction in non-renal organs that have not been directly injured.25
Cardiac function is diminished in AKI, not only from volume overload, but also from increased inflammation and cytokine secretion within the heart.25–27 Similarly, the lung suffers increased permeability, and inflammation during ischemic AKI.28–34 Cardiac dysfunction and pulmonary inflammation together with volume overload from “fluid resuscitation” may exacerbate an ARDS picture (Figure 2).
Figure 2.
Extra-renal effects of AKI. AKI leads to changes in distant organs, including brain, lungs, heart, liver, gastrointestinal tract, and bone marrow. Changes have been described in organ function, microvascular inflammation and coagulation, cell apoptosis, transporter activity, oxidative stress, and transcriptional responses. Abbreviations: AKI, acute kidney injury; G-CSF, granular colony-stimulating factor; GFAP, glial fibrillary acidic protein; GSH, glutathione; IL-1, interleukin-1; KC, keratinocyte-derived chemokine; TNF-α, tumor necrosis factor-α. (Reprinted by permission from Macmillan Publishers Ltd: Kidney International, Scheel PJ, Liu M, Rabb H. Uremic lung: new insights into a forgotten condition. 74:849–51, copyright 2008).
The interaction between the kidney and liver during ischemic AKI may actually be beneficial for recovery of renal function. The growth factor, hepatocyte growth factor (HGF), produced in the liver and lung appears to facilitate repair of the kidney following ischemic injury. Similarly, interleukin 6 (IL-6) produced by the ischemic kidney, stimulates hepatic production of interleukin 10 (IL-10), which, in return, acts on the kidney to inhibit maladaptive intra-renal inflammation.35
These investigations illustrate that there is a complex “crosstalk” between the kidney and other organs during ischemic AKI.
Progression from “pre-renal failure” to structural renal damage: The concept of “renal angina”
To understand how “pre-renal failure”, which is a decrease in GFR caused by hypoperfusion without structural injury to the kidney, progresses to outright structural injury, one needs to consider the unique structure and physiology of the kidney. These unique structural and physiologic features also explain those patients where ischemic AKI occurs, yet perfusion to the brain, heart, and other vital organs remains intact (i.e. normotensive renal failure).
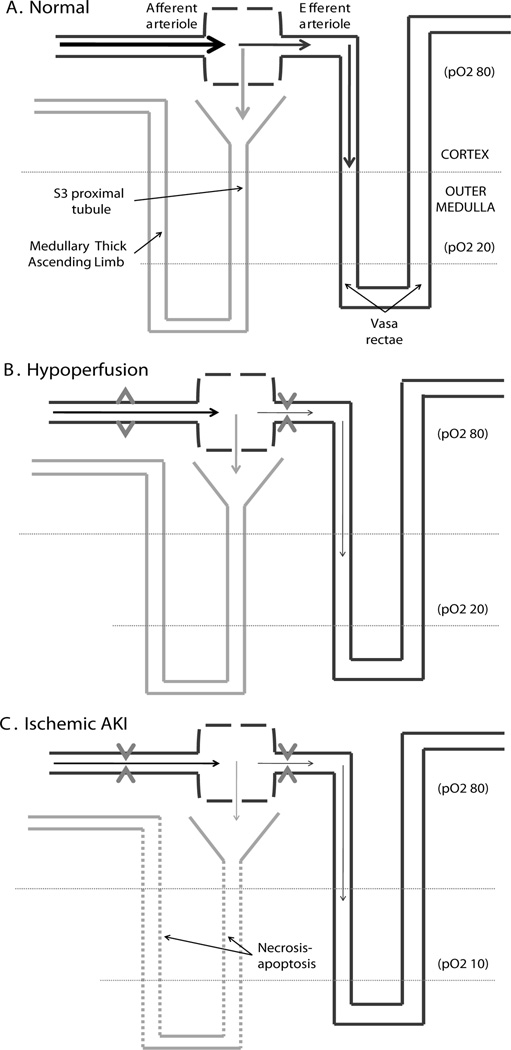
As shown in Figure 3, there are two features of the kidney that make it uniquely susceptible to hypoperfusion. As a result of the counter current mechanism, which allows the formation of highly concentrated urine, the oxygen tension in the medulla is low at approximately 20 torr. Thus tubular cells in the medulla live in an environment that is just barely compatible with survival. Furthermore, the unique blood flow to the kidney is structured such that perfusion of the tubules is in series, directly downstream, of perfusion of the glomerulus. When blood flow to the kidney is decreased, renal auto-regulation ensues to preserve GFR, and a large fraction of blood volume is diverted to the urinary space, resulting in less blood flow reaching the medulla. In the medulla, tubular cells which are already surviving on marginal levels of oxygen, die when their oxygen supply is decreased even further.36,37 Once renal blood flow falls below the auto-regulation range, endogenous vasoconstrictors increase vascular resistance in the afferent arterioles leading to decreased GFR.
Figure 3.
Features of the vascular supply to the medulla predispose the tubular cells of this region to hypoxic injury. A) The counter current mechanism, which allows the formation of concentrated urine, results in a low oxygen tension (about 20 torr) within the medulla. The blood supply to this area is directly downstream from the perfusion of the glomerulus (via the vasa rectae). B) With hypoperfusion, auto-regulation of the afferent and efferent arterioles initially preserves GFR. A large fraction of blood volume is diverted to the urinary space, resulting in decreased blood flow to the medulla. C) Over time, the tubular cells in the medulla, which are already surviving under marginal oxygen levels, begin to die when their oxygen supply is decreased further. If blood pressure drops below the auto-regulation range, endogenous vasoconstrictors are released and the GFR and urine production fall with constriction of the afferrent arterioles. This is thought to be protective because it lowers the solute load delivered to the injured tubules.
The above explanation of how “pre-renal azotemia” turns into structural renal damage makes two predictions – both of which are confirmed by data. The first prediction is that there should be pathology in the renal medulla. As mentioned above, this challenges the conventional wisdom that there is no structural injury during what was called acute renal failure. This mistaken belief is based on the rare renal biopsy of ischemic AKI in the modern era. Such biopsies generally do not interrogate the outer medulla where the injury should occur. However, autopsy studies, many from the 1940s, confirm the predicted lesions in the human outer medulla.38,39 Indeed, the long-forgotten name for AKI was “lower nephron nephrosis” in reference to necrosis of renal tubules seen in the outer medulla. This is the localization of the distal tubules (“lower nephron”).
The second prediction forms the basis for the only rationale therapy currently available for preventing the progression of pre-renal azotemia into ischemic AKI. That prediction is that optimization of renal perfusion should prevent progression to injury.40 In a patient with an intact cardiovascular system, who is not receiving nephrotoxic medications, renal perfusion is increased by optimizing cardiac filling pressures. We discuss this in greater detail below.
TREATMENT OF ISCHEMIC AKI IN 2010 - BEFORE DIALYSIS IS NEEDED
The management of patients with ischemic AKI may be divided into two different objectives: one is the treatment of the kidney; the other is the treatment of the patient. Confusing these two different objectives will result in serious errors in management and complications.
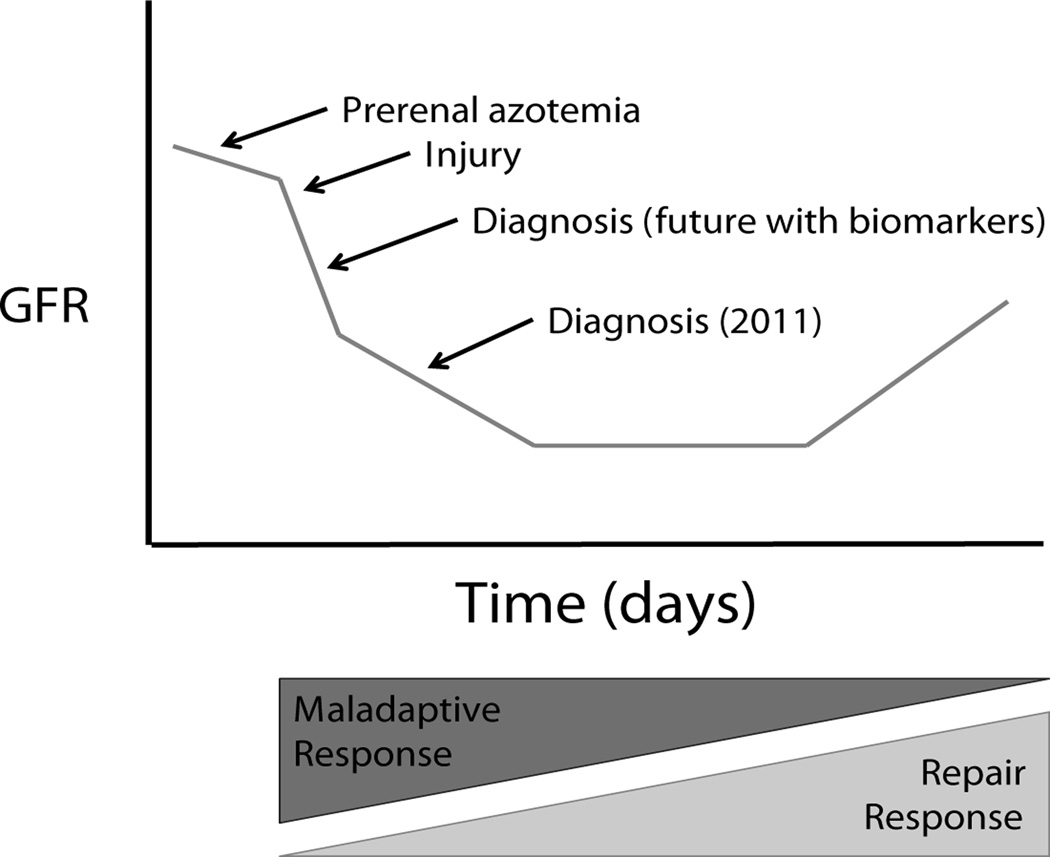
Successful management of ischemic AKI rests on a firm understanding of the pathophysiology of renal failure. Following the initial precipitous drop in GFR during hypoperfusion, secondary effects such as exposure to nephrotoxins and the inflammatory response to ischemia can serve to exacerbate the injury (Figure 4). Altering the maladaptive immune responses to ischemia may serve as a future therapeutic objective in the future if reliable markers of early injury are developed. Another striking feature of the course of AKI is that the injured kidney does not immediately recover even after renal blood flow is restored. This simple, yet important fact may result in significant complications if ignored in patient management.
Figure 4.
Course of AKI. Prerenal azotemia can progress to overt kidney injury. Following the initial sharp drop in GFR from ischemia, there is thought to be further injury as a result of secondary effects. The early immune response to ischemia can actually be maladaptive and contributes to further injury. After injury, the GFR does not recover immediately following return of renal blood flow. Our current diagnostic testing identifies injury late in the process which likely has contributed to disappointing results of therapies that appeared promising in animal models.
USE AND MISUSE OF IV FLUIDS – EARLY VERSUS LATE IN THE COURSE OF ISCHEMIC AKI
Optimization of cardiac filling pressures, and thus, improved renal perfusion is the mainstay of treating and preventing ischemic AKI in 2010. This is usually accomplished by a combination of intravenous fluids and vasopressors. It is becoming increasingly apparent that early, appropriate administration of IV crystalloid solution may prevent AKI, particularly in patients with septic shock.42–44 Early goal-directed therapy for sepsis as originally described by Rivers et al, has been shown to not only decrease in-hospital mortality, but also decrease the risk of developing renal dysfunction.43 These patients received more fluids in the first 6 hours following diagnosis of sepsis, had a shorter time to initiation of vasopressors and shorter time to resolution of shock state than patients off-protocol.
Although early fluid resuscitation and vasopressor support has improved outcomes for patients with sepsis, there is also evidence that fluid excess, particularly late in the course of AKI, is associated with increased mortality. Volume overload is associated with increased mortality in patients with AKI and sepsis with an odds ratio of 1.1 for each liter increase in volume.45–48 Early aggressive IV fluids with later conservative fluid administration may be appropriate.49–51
Use and misuse of diuretics
Recall our earlier division of the therapy of ischemic AKI into two different objectives: treatment of the kidney and treatment of the patient.
Treatment of the kidney
The classical thought that loop diuretics could “protect” tubule cells in the thick ascending loop of Henle by reducing energy requirements during hypoxia has not proven true in clinical practice. In modern practice, diuretics should only be used to optimize cardiac output and thus renal perfusion. Thus, if the patient has pulmonary edema, diuretics will improve cardiac output, renal perfusion, and help prevent the progression of “pre-renal azotemia” to ischemic AKI. Otherwise, unless given at the time of a single limited ischemic insult, for example in the OR during aortic surgery or at the creation of vascular anastomoses during transplantation, diuretics are not recommended for treatment of the kidney during AKI.52–55
Misuse of urine output as a primary goal, instead of as a surrogate for renal perfusion
In an azotemic patient with a hypoperfused kidney, it may be possible to force a diuresis with high dose combination diuretics. If this treatment exacerbates hypovolumia and thus renal perfusion, then the renal outcome will be worsened after diuretic administration. The goal is renal perfusion, and not urine output. In the absence of diuretics, increased renal perfusion will lead to increase urine output.
Treatment of the patient
After AKI is established, diminished urine output is common. In the face of obligate fluid intake for nutrition, and medications (such as antibiotics), the goal is maintenance of fluid balance. In this setting diuretics are helpful. This is not for treatment of the kidney, but for treatment of the patient.
Effects of dialysis on renal recovery
“Conventional wisdom” states that intermittent hemodialysis impairs recovery of the kidney from ischemic AKI. This is thought to occur because of hemodynamic instability during dialysis, vascular catheter-related infection, and/or cytokine release after blood interacts with the dialyzer membrane. Based on these issues, continuous renal replacement therapy (CRRT) has been proposed to be a better dialysis modality for AKI. In fact, there is limited data that any of these factors have a negative impact on renal recovery.56 Some data suggest that with careful dialysis, and in the absence of catheter-related infections, hemodialysis may not have a negative impact on renal recovery.
PROBLEMS IN MEASURING RENAL FUNCTION AND RENAL INJURY
Since we lack an easy, direct method of measuring renal injury, our current diagnosis of AKI relies on assaying two major renal functions and assuming that decreased functions are directly correlated with injury. Unfortunately, this may not be true, particularly early after injury.57,58
One function is the glomerular filtration rate (GFR). Even if we were able to easily and reliably measure the GFR, this would be a poor indicator of injury because, in many patients, the renal mass must be markedly reduced before the GFR decreases. An excellent example is the preserved GFR of normal people who lose half their renal mass. These normal people are living kidney donors who give one of their two kidneys to a loved one with end stage renal disease.
Furthermore, we have problems directly measuring the GFR. Instead, we rely on the serum creatinine as a surrogate. Unfortunately, it takes time (in many cases, days) for the serum creatinine to rise after renal injury. In addition, the same increment in serum creatinine may have different clinical implications depending the patient’s baseline creatinine. Furthermore, the serum creatinine level is also affected by the patient’s diet, muscle mass, and metabolic state.
The other major renal function used to diagnose renal injury is the urine output and composition (urine volume, FENa, specific gravity, etc). However, the normal kidney may produce anywhere from 0.5 liters to nearly 50 liters of urine in a 24hr period. The normal urine may be concentrated and contain almost no sodium, or be dilute and rich in sodium depending upon the patient’s physiologic state. Interpreting the quality of urine the kidney should be producing in a given patient at a given time may be a formidable challenge, and requires a detailed history and excellent serial physical exams. For example, a high FENa and low urine osmolality may be found in a patient with hypotension and oliguria, or in a person with normal renal function who has just ingested an fast-food meal with excessive sodium content. For all of these reasons, the FENa is not used as a criterion for AKI in the recent guidelines.59
An important and easily available test is the urine analysis; unfortunately it is often underutilized. If “renal failure casts” are present, the likelihood of AKI is very high.60 Unfortunately, many patients with acute renal failure will not have “renal failure casts”, and many centers lack experienced personnel to interpret the urine microscopy. Indeed, some have suggested that the severity of AKI may be predicted by quantifying the number renal failure casts.61
Needed: a renal troponin
As mentioned previously, ischemic AKI passes through the phases of pre-renal azotemia to initiation, extension, maintenance, and recovery. The diagnosis is often missed until the maintenance phase because, unlike the cardiologists, we do not have a sensitive and specific marker of early injury (e.g. a renal “troponin”). An active effort is currently underway to identify biomarkers in the blood and urine for earlier diagnosis of AKI.62 Cardiac surgery has often been used to study these markers since the time of renal insult is known.
The two broad categories of biomarkers include constitutive proteins (e.g. those that are released by dying tubule cells) and inducible markers (proteins that are upregulated following insult but are not normally expressed in tubule cells or excreted in the urine). Inducible biomarkers include neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1).63,64 Cystatin (CyC) is a constitutive protein produced by all nucleated cells that is freely filtered at the glomerulus without secretion from the tubules, and therefore the serum level serves as a functional marker of GFR. Unlike creatinine, the serum level of cystatin C is not affected by muscular mass. The α-glutathione-S-transferase (GSTs) proteins (of which there are different isotypes expressed in proximal versus distal tubules) are intracellular proteins that are released into the urinary space during tubule cell damage. Each biomarker has its individual strengths and weaknesses in accurately diagnosing AKI from different etiologies. Therefore, the most powerful clinical application of biomarkers in the diagnosis of AKI will likely be in a panel format.65
Unfortunately, assays for these biomarkers are not widely available today and most of them have not been broadly validated for clinical use. In addition, for these assays to be useful for early treatment of AKI, clinicians will need to identify patients most at risk of AKI. Unlike patients with myocardial ischemia, patients with renal ischemia are often asymptomatic. Goldstein and Chawla have proposed criteria for a “renal angina syndrome” to identify patients that should be further assessed with biomarkers in an effort to increase specificity of these tests and eventually pave the way for novel interventions.66
CONCLUSION
Clinical research during the past decade has advanced our understanding of the implications of acute kidney injury and redefined the goals of fluid management. Despite these advances, the mortality rate of patients with acute kidney injury remains high. We also appreciate that renal injury is an inflammatory condition that affects multiple organ systems. Basic research in the inflammatory response to acute kidney injury has the potential of identifying novel therapeutic targets, however, until we have accurate and sensitive tests that can detect injury early, it is unlikely that these interventions will be helpful. The upcoming decade promises to hold exciting advances in the diagnosis, prevention, and treatment of AKI, and hopefully will deliver the field from “the dark ages”.
Acknowledgments
Sources of Funding: Supported by NIH RO-1 DK069633 grant to CYL, NIH T32DK07257 to PDW, NIH DK079328 UT Southwestern O’Brien Kidney Research Core Center, and a Beecherl grant to CYL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None
REFERENCES
- 1.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kellum JA, Ronco C, Mehta R, et al. Consensus development in acute renal failure: The Acute Dialysis Quality Initiative. CurrOpinCrit Care. 2005;11:527–532. doi: 10.1097/01.ccx.0000179935.14271.22. [DOI] [PubMed] [Google Scholar]
- 3.Hoste EA, Kellum JA. AKI severity class doesn't tell all: the case for transient AKI. Nephrol Dial Transplant. 2010;25:1738–1739. doi: 10.1093/ndt/gfq133. [DOI] [PubMed] [Google Scholar]
- 4.Brady HR, Clarkson MR, Lieberthal W, et al. The Kidney. St Louis: Saunders; 2004. Acute renal failure; pp. 1215–1270. [Google Scholar]
- 5.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 6.Hsu CY, McCulloch CE, Fan D, et al. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 8.Waikar SS, Curhan GC, Wald R, et al. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 9.Lameire N, Van Biesen W, Vanholder R. The rise of prevalence and the fall of mortality of patients with acute renal failure: what the analysis of two databases does and does not tell us. J Am Soc Nephrol. 2006;17:923–925. doi: 10.1681/ASN.2006020152. [DOI] [PubMed] [Google Scholar]
- 10.Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 12.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 13.Parikh CR, Yarlagadda SG, Storer B, et al. Impact of acute kidney injury on long-term mortality after nonmyeloablative hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:309–315. doi: 10.1016/j.bbmt.2007.12.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489–1494. [PubMed] [Google Scholar]
- 15.Tsagalis G, Akrivos T, Alevizaki M, et al. Long-term prognosis of acute kidney injury after first acute stroke. Clin J Am Soc Nephrol. 2009;4:616–622. doi: 10.2215/CJN.04110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchino S, Bellomo R, Bagshaw SM, et al. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010 doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- 17.Tian J, Barrantes F, Amoateng-Adjepong Y, et al. Rapid reversal of acute kidney injury and hospital outcomes: a retrospective cohort study. Am J Kidney Dis. 2009;53:974–981. doi: 10.1053/j.ajkd.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Tapiawala SN, Tinckam KJ, Cardella CJ, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21:153–161. doi: 10.1681/ASN.2009040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 20.Venkatachalam MA, Griffin KA, Lan R, et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1078. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waikar SS, Winkelmayer WC. Chronic on acute renal failure: long-term implications of severe acute kidney injury. JAMA. 2009;302:1227–1229. doi: 10.1001/jama.2009.1364. [DOI] [PubMed] [Google Scholar]
- 23.Hsu CY, Ordonez JD, Chertow GM, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khosla N, Soroko SB, Chertow GM, et al. Preexisting chronic kidney disease: a potential for improved outcomes from acute kidney injury. Clin J Am Soc Nephrol. 2009;4:1914–1919. doi: 10.2215/CJN.01690309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly KJ. Acute renal failure: much more than a kidney disease. Semin Nephrol. 2006;26:105–113. doi: 10.1016/j.semnephrol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Tokuyama H, Kelly DJ, Zhang Y, et al. Macrophage infiltration and cellular proliferation in the non-ischemic kidney and heart following prolonged unilateral renal ischemia. Nephron Physiol. 2007;106:54–62. doi: 10.1159/000103910. [DOI] [PubMed] [Google Scholar]
- 27.Burchill L, Velkoska E, Dean RG, et al. Acute kidney injury in the rat causes cardiac remodelling and increases angiotensin-converting enzyme 2 expression. Exp Physiol. 2008;93:622–630. doi: 10.1113/expphysiol.2007.040386. [DOI] [PubMed] [Google Scholar]
- 28.Hassoun HT, Grigoryev DN, Lie ML, et al. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Renal Physiol. 2007;293:F30–F40. doi: 10.1152/ajprenal.00023.2007. [DOI] [PubMed] [Google Scholar]
- 29.Ko GJ, Rabb H, Hassoun HT. Kidney-lung crosstalk in the critically ill patient. Blood Purif. 2009;28:75–83. doi: 10.1159/000218087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Hassoun HT, Santora R, et al. Organ crosstalk: the role of the kidney. Curr Opin Crit Care. 2009;15:481–487. doi: 10.1097/MCC.0b013e328332f69e. [DOI] [PubMed] [Google Scholar]
- 31.Deng J, Hu X, Yuen PS, et al. Alpha-melanocyte-stimulating hormone inhibits lung injury after renal ischemia/reperfusion. Am J Respir Crit Care Med. 2004;169:749–756. doi: 10.1164/rccm.200303-372OC. [DOI] [PubMed] [Google Scholar]
- 32.Klein CL, Hoke TS, Fang WF, et al. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008;74:901–909. doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 33.Kramer AA, Postler G, Salhab KF, et al. Renal ischemia/ reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;53:2362–2367. doi: 10.1046/j.1523-1755.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- 34.Scheel PJ, Liu M, Rabb H. Uremic lung: new insights into a forgotten condition. Kidney Int. 2008;74:849–851. doi: 10.1038/ki.2008.390. [DOI] [PubMed] [Google Scholar]
- 35.Kielar ML, Jeyarajah DR, Lu CY. The regulation of ischemic acute renal failure by extrarenal organs. CurrOpinNephrolHypertens. 2002;11:451–457. doi: 10.1097/00041552-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 37.Brezis M, Rosen S. Hypoxia of the renal medulla - its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 38.Lucke B. Lower nephron nephrosis. Mil Surg. 1946;99:371–396. [PubMed] [Google Scholar]
- 39.Solez K. Pathogenesis of acute renal failure. Int Rev Exp Pathol. 1983;24:277–333. [PubMed] [Google Scholar]
- 40.Himmelfarb J, Joannidis M, Molitoris B, et al. Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:962–967. doi: 10.2215/CJN.04971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molitoris BA. Transitioning to therapy in ischemic acute renal failure. J Am SocNephrol. 2003;14:265–267. doi: 10.1097/01.asn.0000048852.53881.d9. [DOI] [PubMed] [Google Scholar]
- 42.Jones AE, Focht A, Horton JM, et al. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin SM, Huang CD, Lin HC, et al. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock. 2006;26:551–557. doi: 10.1097/01.shk.0000232271.09440.8f. [DOI] [PubMed] [Google Scholar]
- 44.Rhodes A, Bennett ED. Early goal-directed therapy: an evidence-based review. Crit Care Med. 2004;32:S448–S450. doi: 10.1097/01.ccm.0000145945.39002.8d. [DOI] [PubMed] [Google Scholar]
- 45.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 46.Mehta RL. Fluid balance and acute kidney injury: the missing link for predicting adverse outcomes? Nat Clin Pract Nephrol. 2009;5:10–11. doi: 10.1038/ncpneph0988. [DOI] [PubMed] [Google Scholar]
- 47.Payen D, de Pont AC, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 49.Rivers EP. Fluid-Management Strategies in Acute Lung Injury -- Liberal, Conservative, or Both? N Engl J Med. 2006 doi: 10.1056/NEJMe068105. [DOI] [PubMed] [Google Scholar]
- 50.Stewart RM, Park PK, Hunt JP, et al. Less is more: improved outcomes in surgical patients with conservative fluid administration and central venous catheter monitoring. J Am Coll Surg. 2009;208:725–735. doi: 10.1016/j.jamcollsurg.2009.01.026. discussion 35-7. [DOI] [PubMed] [Google Scholar]
- 51.Schrier RW. Fluid administration in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2010;5:733–739. doi: 10.2215/CJN.00060110. [DOI] [PubMed] [Google Scholar]
- 52.Bennett-Jones DN. Early intervention in acute renal failure. BMJ. 2006;333:406–407. doi: 10.1136/bmj.38945.596215.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karajala V, Mansour W, Kellum JA. Diuretics in acute kidney injury. Minerva Anestesiol. 2008 [PubMed] [Google Scholar]
- 54.Mehta RL, Pascual MT, Soroko S, et al. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288:2547–2553. doi: 10.1001/jama.288.20.2547. [DOI] [PubMed] [Google Scholar]
- 55.Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ. 2006;333:420. doi: 10.1136/bmj.38902.605347.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palevsky PM, Baldwin I, Davenport A, et al. Renal replacement therapy and the kidney: minimizing the impact of renal replacement therapy on recovery of acute renal failure. CurrOpinCrit Care. 2005;11:548–554. doi: 10.1097/01.ccx.0000179936.21895.a3. [DOI] [PubMed] [Google Scholar]
- 57.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 58.Palevsky PM, Murray PT. Acute kidney injury and critical care nephrology. NephSAP. 2006;5:63–129. [Google Scholar]
- 59.Nguyen MT, Maynard SE, Kimmel PL. Misapplications of commonly used kidney equations: renal physiology in practice. Clin J Am Soc Nephrol. 2009;4:528–534. doi: 10.2215/CJN.05731108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esson ML, Schrier RW. Diagnosis and treatment of acute tubular necrosis. AnnInternMed. 2002;137:744–752. doi: 10.7326/0003-4819-137-9-200211050-00010. [DOI] [PubMed] [Google Scholar]
- 61.Perazella MA, Coca SG, Hall IE, et al. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5:402–408. doi: 10.2215/CJN.06960909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 64.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 65.Ho EF, Arrash, Maisel, Alan Evolving use of biomarkers for kidney injury in acute care settings. Current Opinion in Critical Care. 2010;16:399–407. doi: 10.1097/MCC.0b013e32833e10bc. [DOI] [PubMed] [Google Scholar]
- 66.Goldstein SL, Chawla LS. Renal angina. Clin J Am Soc Nephrol. 2010;5:943–949. doi: 10.2215/CJN.07201009. [DOI] [PubMed] [Google Scholar]