Abstract
The ATP-binding cassette transporter-2 (ABCA2) has been identified as a possible regulator of lipid metabolism. ABCA2 is most highly expressed in the brain but its effects on cholesterol homeostasis in neuronal-type cells have not been characterized. It is important to study the role of ABCA2 in regulating cholesterol homeostasis in neuronal-type cells because ABCA2 has been identified as a possible genetic risk factor for Alzheimer’s disease. In this study, the effects of ABCA2 expression on cholesterol homeostasis were examined in mouse N2a neuroblastoma cells. ABCA2 reduced total, free- and esterified cholesterol levels as well as membrane cholesterol but did not perturb cholesterol distribution in organelle or lipid raft compartments. ABCA2 did not modulate de novo cholesterol biosynthesis from acetate. Cholesterol trafficking to the plasma membrane was not affected by ABCA2 but efflux to the physiological acceptor ApoE3 and mobilization of plasma membrane cholesterol to the endoplasmic reticulum for esterification were reduced by ABCA2. ABCA2 reduced esterification of serum and low-density lipoprotein-derived cholesterol but not 25-hydroxycholesterol. ABCA2 decreased low-density lipoprotein receptor (LDLR) mRNA and protein levels and increased its turnover rate. The surface expression of LDLR as well as the uptake of fluroresecent DiI-LDL was also reduced by ABCA2. Reduction of endogenous ABCA2 expression by RNAi treatment of N2a cells and rat primary cortical neurons produced the opposite effects of over-expression of ABCA2, increasing LDLR protein levels. This report identifies ABCA2 as a key regulator of cholesterol homeostasis and LDLR metabolism in neuronal cells.
1. Introduction
The ATP-binding cassette transporters use the energy of ATP hydrolysis to pump substrates across lipid bilayers and the ABC “A” subfamily of transporters has been functionally linked to intracellular lipid transport [1]. The most well-characterized member of this subfamily, ABCA1, functions in the efflux of cellular cholesterol to apolipoprotein carriers to generate high-density lipoproteins (HDL); a process called “reverse cholesterol transport [2].” Less is known about the ABCA2 transporter, although studies in cell lines and ABCA2 knockout mice have suggested a possible role in cholesterol and sphingolipid metabolism [3, 4]. ABCA2 is most highly expressed in the brain [5] and within neurons and oligodendrocytes of the central nervous system [6, 7]. ABCA2 is also expressed in Schwann cells of the peripheral nervous system [8]; however, its function in regulating cholesterol metabolism in these cells has not been investigated. The importance of a study of the role of ABCA2 in regulating cholesterol metabolism in neuronal cells is justified because of studies indicating ABCA2 may be a genetic risk factor for Alzheimer’s disease [9, 10]. Additional studies have linked ABCA2 to the expression of determinants of Alzheimer’s disease [11], including amyloid precursor protein (APP) and neurotoxic Abeta fragment generation [12]. In this report, the role of ABCA2 in regulating cholesterol metabolism in neuronal-type-like cells was investigated in mouse N2a neuroblastoma cells overexpressing ABCA2. ABCA2 reduced total, free- and esterified cholesterol levels as well as membrane cholesterol but did not alter its distribution in organelle or lipid raft compartments. ABCA2 decreased the mobility of plasma membrane cholesterol for efflux to ApoE3 acceptors as well as cholesterol trafficking from the plasma membrane to the endoplasmic reticulum for esterification. Serum and LDL-derived cholesterol esterification were also reduced by ABCA2. ABCA2 decreased LDLR mRNA and protein expression and increased its turnover rate. LDLR surface expression and fluorescent DiI-LDL uptake declined in ABCA2 overexpressing cells. The reduction of endogenous ABCA2 in N2a cells and rat primary cortical neurons reversed the effects of ABCA2 over-expression and elevated LDLR protein levels. This study indicates that ABCA2 is a key regulator of cholesterol homeostasis and LDLR metabolism in neuronal cells.
2. Materials and Methods
2.1. Materials
Dulbecco’s Modified Eagle medium (DMEM) and fetal bovine serum (FBS) were obtained from Hyclone. OptiMem I and DMEM methionine- and cysteine-free growth media were obtained from Invitrogen. Glutamine and Penstrep were obtained from Fisher. Lipoprotein deficient serum (LPDS) was prepared according to the traditional method [13] from fetal calf serum by ultracentrifugation to a density of 1.215 g/ml in potassium bromide and extensively dialyzed in 150 mM NaCl. Radiochemicals oleic acid, [1-14C] (40–60 mCi/mmol); cholesterol, [1,2-3H(N)] (40–60 Ci/mmol); acetic acid [1,2-14C] (45–60 mCi/mmol), [oleoyl-1-14C] coenzyme A (40–60 mCi/mmol) and methionine, L-[35S] (1000 Ci/mmol) were from Perkin Elmer. The cholesteryl linoleate, [cholesteryl-1,2,6,7-3H(N)] (60–100 Ci/mmol) was from American Radiolabeled Chemicals.
2.2. Cell lines and culture
The N2a mouse neuroblastoma cells were obtained from ATCC (CCL-131). Cells were grown in DMEM/Ham’s F12 (50:50) supplemented with 5% fetal bovine serum (FBS) 2 mM glutamine and 1% Penstrep at 37° C and 5% CO2.
The N2a cell line stably expressing ABCA2 was generated by transfection of a 6.5 kB pFRT/lacZeo2 FLP recombination target site vector designed for use with the Flp-In system and selection of resistant cells with Zeocin (0.7 mg/ml) to generate the N2a Flp-In host cell lines containing an integrated FRT site. N2a Flp-In cells were transfected with a 7.4 kilobase human ABCA2 cDNA construct that was subcloned into the 5.1 kB pcDNA5 FRT/TO vector (Invitrogen) designed for targeted integration into the FLP site in N2a cells stably expressing pFRT/lacZeo2 in the Flp-In system, followed by selection of resistant cells expressing ABCA2 with Hygromycin (0.5 mg/ml). The construct provided high-levels of constitutive ABCA2 expression under the control of the cytomegalovirus promoter (CMV). Individual clones were isolated and analyzed for relative ABCA2 expression level and two (A2.1, A2.4) were utilized for most of the experiments.
2.3. Western Blot
Cells were lysed in radioimmunoprecipitation (RIPA) buffer (150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 50 mM Tris-HCl, pH 7.5, 2 mM EDTA, RIPA) supplemented with HALT protease inhibitor cocktail (Pierce). Protein concentrations were determined using the DC-protein assay kit (Bio-Rad). For detection of ABCA2, 30 μg of protein were fractionated on 4–12% NuPAGE gels (Invitrogen). Proteins were transferred to nitrocellulose membranes and probed with primary rabbit polyclonal antibody to the ABCA2 c-terminal 20 amino acids (1:300), rabbit polyclonal antibody to LDLR (Cayman Chemical, 1:200, or Biovision 1:1000), mouse mononclonal antibody to β-Actin or glyceraldehyde phosphate dehydrogenase, GAPDH (Santa Cruz Biotechnology, 1:2000). Secondary antibodies were goat anti-rabbit- or anti-mouse horseradish peroxidase (HRP) (Thermo Scientific/Pierce, 1:1000). Blots were developed using the Dura-Signal enhanced chemiluminescence reagent (Thermo Scientific/Pierce) and imaged using the ImageStation IS2000R and Molecular Imaging software (Kodak).
2.4. Measurement of total cellular and membrane cholesterol
On day 0, 2 × 106 cells were plated in DMEM/F12 5% FBS medium. On day 2, cells were washed trypsinized and lysed in RIPA buffer and protein concentrations were determined. For membrane cholesterol, cells were scraped in 5 mM EDTA in phosphate buffered saline (PBS), pelleted by centrifugation and resupended in 200 μl of fractionation buffer (0.25 M sucrose, 1 mM MgCl2, 2 mM EGTA, 25 mM HEPES, pH 7.4) and lysed by three cycles of flash freezing in liquid nitrogen. Lysates were centrifuged at 100,000 × g for 30 min. Supernatants (cytosolic fraction, S100) were removed and the pellet (membrane fraction, P100) was resupended in 500 μl of fractionation buffer containing 5% Triton X-100 and briefly sonicated and protein concentrations were determined. Lipids were extracted by the method of Bligh and Dyer methanol-chloroform method [14]. Lipids were dried under nitrogen and resupended in 2-propanol/1% Triton X-100. Fluorescence was measured on an aliquot using the Amplex Red Cholesterol Assay kit (Invitrogen) and cholesterol mass (total cholesterol and free cholesterol) was calculated from a standard curve of cholesterol concentrations and normalized to total protein content (μg cholesterol/mg protein) in each sample. Cholesterol ester was calculated by subtracting the mass of free cholesterol from total cholesterol.
2.5. Sucrose density gradient ultracentrifugation (Organelles)
On day 0, 4 × 106 cells were plated in 25 ml of DMEM/OptiMem I, 5% FBS in 175 mm flasks and grown to 90% confluency at 37° C 5% CO2. Cells were recovered by centrifugation and the pellets were resupended in 1 ml of homogenization buffer containing 0.25 sucrose, 10 mM Tris-HCl (pH 7.4), 1 mM magnesium acetate and HALT protease inhibitor cocktail. The cells were allowed to swell on ice for 20 min followed by Dounce homogenization. A post-nuclear supernatant was recovered after centrifugation at 10,000 × g for 3 min and protein concentrations were determined. The supernatant was centrifuged at 100,000 × g for 2.5 h and twelve 400 μl fractions were recovered from the top of the tube and 300 μl were precipitated by the methanol-chloroform method. Total precipitated proteins were fractionated on 4–12% NuPAGE gels, transferred to nitrocellulose and probed for plasma membrane (Na+/K+ ATPase, 1:1000, Cell Signaling Technology), early-endosome (EEA1, 1:2000, Santa Cruz Biotechnology), late-endosome (Rab 9, 1:1000, Cell Signaling Technology), endoplasmic reticulum (Calnexin, 1:2000, AssayDesigns), Golgi-apparatus (β-COP, 1:000, Enzo Life Sciences), and Trans-Golgi Network (TGN38, 1:1000 Santa Cruz Biotechnology) and Syntaxin-6 (1:1000, Cell Signaling Technology).
For [3H]cholesterol long-term radiolabeling to equilibrium experiments, cells were incubated in DMEM/F12, 5% FBS containing 1.0 μCi/ml [3H]cholesterol for 24 hours. The [3H]cholesterol in each fraction was analyzed by mixing 300 μl of sample with 5 ml of scintillation mixture and radioactivity was determined using a Beckman Coulter LS6500SC scintillation counter.
2.6. Sucrose density gradient ultracentrifugation (Lipid raft)
On day 0, 4 × 106 cells were plated in 25 ml of DMEM/OptiMem I, 5% FBS in 175 mm flasks and grown to 90% confluency at 37° C 5% CO2. The pellet was resupended in 1 ml of MBS lysis buffer and protein concentrations were determined using the DC protein assay (Bio-Rad). Approximately 1 mg of total protein in a gradient of 80%, 35% and 5% sucrose were centrifuged at 160,000 × g for 18 h at 4° C in AH650 rotor. Twelve 400 μl fractions were recovered from the top of the tube and 300 μl were precipitated by the methanol chloroform method. Total precipitated proteins were fractionated on 4–12% NuPAGE gels, transferred to nitrocellulose and probed for flotillin-1 (raft, 1:2000, BD Transduction Labs), and calnexin (non-raft). For [3H]cholesterol long-term radiolabeling experiments, cells were incubated in DMEM/F12, 5% FBS containing 0.5 μCi/ml [3H]cholesterol for 24 hours. The [3H]cholesterol in each fraction was analyzed by mixing 300 μl of sample with 5 ml of scintillation mixture and radioactivity was determined using a Beckman Coulter LS6500 scintillation counter.
2.7. De novo cholesterol synthesis
On day 0, 0.75 × 106 cells were plated in 6-well plates in 2 ml of DMEM/F12, 5% FBS. On day 1 the medium was replaced with fresh medium containing 5% LPDS and the cells were cultured for 48 hours. On day 3, the medium was replaced with fresh medium containing 5% LPDS and 0.5 mM [14C]acetate complexed with bovine serum albumin (BSA) and the cells were cultured for 4 hours. Each monolayer was washed twice with 1 ml of buffer B (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 2 mg/ml BSA and once with buffer C (buffer B without BSA). One ml of hexane-isopropanol (3:2) was added to the samples to extract lipids and the solvent was evaporated to dryness under nitrogen. The lipids and lipid chromatographic standards were resupended in hexane and spotted onto silica C thin layer chromatography plates. The chromatogram was developed in petroleum ether-diethyl ether-acetic acid (60:40:1). After lipid extraction the proteins were recovered in 0.2 N NaOH and protein concentrations were determined. The chromatogram was visualized using a Bioscan 2000 thin layer chromatography (TLC) scanner and the [14C] cholesterol counts per min (cpm) for each sample were determined.
2.8. LDL-derived free cholesterol [3H]CL-LDL movement to the plasma membrane
LDL labeled with [3H] cholesteryl linoleate ([3H]CL-LDL) was prepared as previously described [15]. On day 0, 0.5 × 106 cells were plated in 2 ml of DMEM/F12, 5% FBS. On day 1, the medium was replaced with 1 ml of DMEM/F12 in which FBS was replaced with LPDS and the cells were cultured for 48 h. On day 3, the cells were incubated at 4° C for 30 min, washed 2 times with phosphate buffered saline (PBS) and the medium was replaced with 1 ml of [3H]CL-LDL (50 μg/ml LDL) and 10 μg/ml of the ACAT inhibitor 58035 (Sigma) to block re-esterification of cholesterol released by hydrolysis of [3H]CL-LDL. The cells were cultured for 1, 2, 4 and 6 h at 37° C. The cells were washed in PBS and incubated in unlabeled medium containing 4% 2-hydroxypropyl-β-cyclodextrin (HPCD) for 10 min. The medium was recovered and centrifuged at 10,000 × g for 10 min. Lipids containing [3H]cholesterol in the media were extracted with by method of Bligh and Dyer. Cell-associated radioactivity was determined by extraction with hexane-isopropanol (3:2). The media and cellular lipids were dried under nitrogen, resuspended in hexane and spotted onto silica G TLC plates and developed in hexane-diethyl ether-acetic acid (160:40:3). Under the conditions of the experiment all of the [3H]CL-LDL was hydrolyzed to [3H]cholesterol and the ACAT inhibitor 58035 eliminated re-esterification of the hydrolyzed [3H]cholesterol. The % efflux of [3H]cholesterol to HPCD was calculated as 100 × cpm [3H]cholesterol medium/(cpm [3H]cholesterol medium + cpm cellular [3H]cholesterol).
2.9. Cholesterol efflux to ApoE3 acceptors
On day 0, 5 × 105 N2a and A2.1 cells were plated in 2 ml of DMEM/F12, 5% FBS, 2 mM glutamine and 1% Penstrep and cultured overnight. On day 1 the medium was replaced with DMEM/F12 containing 5% LPDS. On day 3, cells were washed with PBS and incubated with 50 μg/ml [3H]CL-LDL containing 10 μg/ml ACAT inhibitor 58035 and cultured overnight. On day 4, the cells were washed in PBS and equilibrated in DMEM/F12 0.1% BSA for two hours. Cells were washed in PBS and cultured in DMEM/F12 0.1% BSA containing ± 15 μg/ml apolipoprotein E3 (Sigma) for 6 hours. At the end of the incubation period, medium was collected and centrifuged to remove cellular debris. Cells were lysed in 0.2 M NaOH. Radioactivity was measured on a 300 μl aliquot and the percentage of efflux of [3H]cholesterol efflux to ApoE3 was measured as 100 × cpm medium/(cpm medium +cpm cells).
2.10. Mobilization of intracellular cholesterol to the endoplasmic reticulum for esterification
On day 0, 0.5 × 106 cells were plated in 2 ml of DMEM/F12 5% FBS. On day 2 the medium was replaced with fresh medium supplemented with 1 μCi/ml [3H]cholesterol and the cells were cultured for 24 hours. On day 3, the cells were washed with Hanks’s balanced salt solution. One ml of fresh unlabeled medium containing 0.5 units/ml of bacterial sphingomyelinase (Sigma) was added and cells were cultured for 0, 2, 4, and 8 hours. Each monolayer was washed twice with 1 ml of buffer B (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 2 mg/ml BSA) and once with buffer C (buffer B without BSA). One ml of hexane-isopropanol (3:2) was added to the samples to extract lipids and the solvent was evaporated to dryness under nitrogen. The lipids and lipid chromatographic standards were resupended in hexane and spotted onto silica C thin layer chromatography plates. The chromatogram was developed in hexane-diethyl ether-acetic acid (160:40:3). After lipid extraction the proteins were recovered in 0.2 N NaOH and protein concentrations were determined. The chromatogram was visualized using a Bioscan 2000 TLC scanner and the total [3H]cholesterol and [3H]cholesteryl ester cpm were determined. The data is expressed as the % cholesteryl ester formed and was normalized to total protein content in each sample.
2.11. Serum and LDL-stimulated cholesterol esterification
On day 0, 0.75 × 106 cells were plated in 2 ml of DMEM/F12, 5% FBS medium and cultured for overnight. On day 1, the medium was replaced with DMEM/F12 and 5% LPDS and cells were cultured for 48 hours. On day 3, the medium was replaced with DMEM/F12 containing 20% FBS or 5% LPDS and 50 μg/ml low-density lipoprotein (Biomedical Technologies) and cells were cultured for 5 hours at 37° C and 5% CO2. Monolayers were pulsed for two hours with 0.2 mM [14C] oleate bound to BSA (10,000 dpm/nmol). Each monolayer was washed twice with 1 ml of buffer B (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 2 mg/ml BSA) and once with buffer C (buffer B without BSA). One ml of hexane-isopropanol (3:2) was added to the samples to extract lipids and the solvent was evaporated to dryness under nitrogen. The lipids and lipid chromatographic standards were resupended in hexane and spotted onto silica C thin layer chromatography plates. The chromatogram was developed in hexane-diethyl ether-acetic acid (160:40:3). After lipid extraction the proteins were recovered in 0.2 N NaOH and protein concentrations were determined. The chromatogram was visualized using a Bioscan 2000 TLC scanner. The data is expressed as cpm [14C] cholesteryl ester and cpm [14C] triglycerides per mg protein.
2.12. 25-hydroxycholesterol stimulated cholesterol esterification
Cells were cultured as described above for LDL-stimulated cholesterol esterification except that medium was supplemented with 20 μg/ml 25-hydroxycholesterol/10 μg/ml cholesterol dissolved in ethanol. Cells were cultured for 5 hours before addition of [14C] oleate/BSA, lipid extraction and TLC as described above.
2.13. Measurement of in vitro ACAT activity
On day 0, cells were plated at 4 × 106 cells per T150 flask in 25 ml of DMEM/F12, 5% FBS medium and cultured overnight at 37° C 5% CO2. On day 1 the medium was replaced with DMEM/F12 and 5% LPDS and cells are cultured for 48 hours. On day 3, the medium was replaced with DMEM/F12, 5% LPDS supplemented with 50 μg/ml LDL and cultured for 5 hours. Cells were recovered by centrifugation and the pellet was resupended in 0.3 ml of buffer A containing 20 mM Tris-HCl pH 7.4 and 1 mM EDTA pH 8.0 and HALT protease inhibitor cocktail (Thermo/Pierce). Aliquots of whole cell extract (100 μg of protein) were incubated for 60 min at 37° C in 0.12 ml of solution containing 50 mM potassium phosphate pH 7.4, 2 mM dithiothreitol, 10 mg/ml BSA and 0.1 mM [oleoyl-1-14C] coenzyme A (55 mCi/mole, Perkin Elmer). The reaction was stopped by adding 1 ml of CHCl3 and 1 ml methanol. One ml of H20 and 1 ml of CHCl3 were added and mixed by vortexing. After centrifugation for 10 min at 1000 × g, the lower phase was recovered and evaporated to dryness under nitrogen. The dried lipids were dissolved in hexane and spotted onto silica C thin layer chromatography plates. The chromatogram was developed in hexane-diethyl ether-acetic acid (160:40:3). The chromatogram was visualized using a Bioscan 2000 TLC scanner.
2.14. Fluorescent DiI-LDL uptake
DiI-LDL uptake was performed as previously described [16]. On day 0, 0.75 × 106 cells were plated in 3 ml of DMEM/F12, 5% FBS medium in 60 mm plates. On day 1, the medium was replaced with DMEM/F12, 5% LPDS and cells were cultured for 48 hours. On day 3, the cells were treated with 10 μg/ml DiI-LDL and cultured for 6 hours. Control cells were not treated with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI-LDL, Biomedical Technologies) and were processed in parallel. Lipids were extracted in 1 ml of isopropanol for 15 min. The fluorescence was measured on an aliquot with a Turner fluorescence plate reader using excitation-emission of 520:580 nm. Proteins were extracted with 0.2 N NaOH and protein concentrations were determined. Data is expressed as DiI-fluorescence per mg protein.
2.15. Real-time PCR for ABCA2 and LDLR mRNA levels
Two micrograms of total RNA from each experimental sample were reverse transcribed using random primers for 90 min at 42° C with AffinityScript reverse transcriptase (Stratagene). One hundred nanograms of cDNA from each sample was amplified by real time PCR with ABCA2-specific primers, sense 5′ GCCCAGGTCTGGCTCAACATCTC 3′, antisense 5′ CTCACCTTGGACATGAACTGGAT 3′, LDLR-specific primers, sense 5′ TACTGGTCTGACCTGTCCCAGAGA 3′, antisense 5′ CGGTTGGCACTGAAAATGGCTTC 3′ and 18 S primers sense 5′ ATGCTCTTAGCTGAGTGTCC 3′, antisense 5′ AACTACGACGGTATCTGATC 3′. IQ SybrGreen PCR Supermix (Bio-Rad) was used for PCR reactions with the following program: 95° C for 30 sec, 60° C for 30 sec, 72° C for 1 min for 40 cycles. The concentration of expression for each sample was determined from the threshold cycle CT, which is the cycle where an increase in PCR product is first detected at a statistically significant level.
2.16. Measurement of LDLR synthesis and turnover
On day 0, 4 × 106 cells were plated in 10 ml of DMEM/F12, 5% FBS medium in 100 mm dishes. On day 1, the medium is replaced with DMEM/F12, 5% LPDS and the cells were cultured for 48 hours. The cells were starved for 30 min in DMEM methionine-free, 5% FBS medium. The medium was replaced with 3 ml of DMEM, 5% FBS medium containing 250 μCi of [35S] methionine (1000 Ci/mmol, 10.2 mCi/ml; Perkin-Elmer) and cultured for 2 hours (pulse). After the pulse, the cells were washed with phosphate buffered saline (PBS) and chased in unlabeled medium with DMEM methionine-free, 5% FBS and 1 mM methionine for 2, 4 and 6 hours before lysis in RIPA buffer. Equivalent masses of total protein were immunoprecipitated for 1 h with 2 μg of rabbit polyclonal LDL receptor antibody, then 30 μl of Protein A/G Plus agarose (Santa Cruz Biotechnology) was added, followed by overnight incubation at 4° C on a rocking platform. Proteins were fractionated on 4–12% NuPAGE Bis-Tris gels (Invitrogen), dried, exposed to storage phosphor screens and developed using a STORM Phosphorimager and quantified using ImageQuant software (GE Healthcare).
2.17. Biotinylation of cell surface LDL receptor
On day 0, cells 4 × 106 cells were plated per 100 mm plate in 10 ml of DMEM/F12, 5% FBS medium and cultured for overnight at 37° C 5% CO2. On day 1, the medium was replaced with DMEM/F12 and 5% LPDS and cells were cultured for 48 hours. On day 3, cells were washed twice with ice-cold PBS and treated with 1 mg/ml membrane-impermeant sulfo-NHS-biotin in PBS for 30 min at 4°. After washing twice with cold PBS and once with 3.75 mg/ml glycine in PBS, the medium was collected and the cells were lysed in RIPA buffer. Protein concentrations were determined and 300 μg of protein extract were incubated with 30 μl avidin-agarose beads overnight at 4° C on a rocking platform. Beads were centrifuged at 1000 × g for 5 min and washed 4 times in RIPA buffer. Proteins were separated by on 4–12% NuPAGE gels, transferred to nitrocellulose and analyzed by Western blot with rabbit polyclonal anti-LDL receptor primary antibody (1:200, Cayman chemical) and secondary antibody goat-anti-rabbit-HRP (1:1000, Thermo/Pierce). Total LDL receptor in cell extracts was determined by Western blot on 30 μg of total cell protein as described above. Control experiments using an anti-GAPDH antibody indicated that biotinylation of intracellular proteins was negligible under these experimental conditions. Blots were developed using the Dura-Signal reagent (Pierce) and imaged using the ImageStation IS2000R and Molecular Imaging software (Kodak).
2.18. RNAi silencing of ABCA2 expression in N2a cells and Rat Primary Neurons
On day 0, 0.5 × 106 N2a cells were plated in DMEM/F12, 5% FBS, 2 mM glutamine, 1% Penstrep and cultured overnight. On day 1 the medium was replaced with 1 ml of OptiMem I medium prior to transfection. Rat primary cortical neurons were obtained from Genlantis. On day 0, 2 × 106 cells were plated in Neurobasal medium supplemented with 2% B27 and 1% Glutamax (Invitrogen). To prepare RNAi transfection complexes, 25 μl of RNAiFect transfection reagent (Qiagen) was added to 50 μl of OptiMem I and incubated at room temperature for 5 min. Control RNAi (RISC-Free, Dharmacon) or ABCA2 mouse-specific RNAi, 5′GGAUGUGGCUAGUGAGCGA 3′ (Dharmacon) or ABCA2 rat-specific RNAi, 5′ GCACGGUUCUUCGACAGUA 3′ (Ambion) were added to 50 μl OptiMem I. The diluted RNAiFect transfection reagent was added to the diluted RNAi and incubated for 30 min at room temperature. The transfection complexes were added to the cells for a final concentration of 100 nM RNAi and cells were cultured for 48 hours before extraction of total RNA for reverse transcription and PCR for measurement of ABCA2 and LDLR mRNA levels or preparation of cell lysates for measurement of LDLR protein expression by Western blot.
3. Results
3.1. Generation of N2a cell lines stably expressing human ABCA2
Cell lines, CHO, HepG2, HEK293 have been utilized as acceptable model systems with well-established methodologies for studying intracellular cholesterol metabolism. In order to determine the effects of ABCA2 function on cholesterol metabolism in neuronal cells, several stable cell lines were generated in mouse N2a neuroblastoma cells that over-expressed human ABCA2. Stable expression of ABCA2 reduces variability in observable results that might be introduced by using transient transfection of an ABCA2 expressing plasmid. The relative protein expression of ABCA2 in the N2a stable A2.1 and the A2.4 lines was ~ 7–9- fold greater than the parental N2a cell line (Fig 1).
Fig. 1. Generation of N2a cell lines stably expressing human ABCA2.

A representative Western blot is shown of ABCA2 protein expression in parental N2a cells and two clones expressing human ABCA2 protein (A2.1, A2.4). The anti-ABCA2 rabbit polyclonal antibody detects both endogenous mouse ABCA2 as well as the human protein.
3.2. ABCA2 expression decreases total and membrane cholesterol levels
The effects of ABCA2 expression on total cholesterol, free-cholesterol and esterified cholesterol levels as well as total cholesterol content in membrane compartments in N2a cells were determined. Total, free- and cholesteryl ester levels were ~ 91.2, 85.8 and 5.4 μg/mg protein in N2a cells and ~77.5, 72.7 and 5.0 μg/mg protein in A2.1 cells (2A). For membrane cholesterol, total cholesterol measurements were performed following ultracentrifugation to generate a P100 (membrane) fraction. Total cholesterol in the P100 fraction of N2a cells was ~ 16 μg/mg protein, whereas in A2.1 and A2.4 cells it was ~ 10 μg/mg protein (Fig. 2B). These results indicate that ABCA2 expression in N2a cells results in decreased levels of total cholesterol, free-cholesterol and cholesteryl ester levels as well as total cholesterol in membrane compartments.
Fig. 2. Cellular and membrane cholesterol are decreased in ABCA2 expressing cells.
A. Cell extracts were prepared and lipid extracts prepared. Total cholesterol and free cholesterol levels were determined as described in methods. B. For membrane cholesterol, cell extracts were subjected to ultracentrifugation at 100,000 × g and a P100 and S100 fraction were generated as described in Methods. Cholesterol levels were normalized to total protein content in each sample. The data are expressed as the mean of four experiments ± SD (A. * p < 0.001; B. * p < 0.001, ** p = 0.013, Students’ t test).
3.3. Subcellular cholesterol distribution in organelle and lipid raft compartments
To investigate further whether ABCA2 regulates the distribution of intracellular cholesterol, cells were metabolically radiolabeled to equilibrium for 24 hours in [3H]cholesterol and cell extracts were fractionated in a 0.5 M to 2 M sucrose gradient by ultracentrifugation. The [3H]cholesterol content in aliquots of each fraction was measured by scintillation counting. In parallel, Western blot was performed on protein fractions following precipitation, using antibodies to specific organelle markers; Na+/K+ATPase (plasma membrane), EEA1 (early-endosome), Rab-9 (late-endosome), Calnexin (endoplasmic reticulum), β-COP (Golgi Apparatus), Syntaxin 6 (Trans-Golgi Network), TGN38 (Trans-Golgi Network). For N2a cells light fractions 1–9, [3H]cholesterol levels ranged from 3.75% of total cholesterol (fraction 1, S.D. 0.28) to 6.22% (fraction 9, S.D. 0.25). For A2.1 cells light fractions 1–9, [3H]cholesterol levels ranged from 4.67% (fraction 1, S.D. 0.57) to 6.48% (fraction 9, S.D. 0.35). For N2a cells heavy fractions 10–12, [3H]cholesterol levels ranged from 9.0 % of total cholesterol (fraction 10, S.D. 1.18) to 22.2% (fraction 12, S.D. 0.41). For A2.1 cells heavy fractions 1–9, [3H]cholesterol levels ranged from 8.23% (fraction 10, S.D. 0.32) to 21.9% (fraction 12, S.D. 0.96). Statistical analysis indicated that there was no significant distribution in [3H]cholesterol content between N2a and A2.1 cells in any of the organelle compartments.
Lipid rafts are described as ordered regions of the lipid bilayer that are enriched in cholesterol and sphingolipids (sphingomyelin) and serve as organizing centers for protein signaling assemblies that modulate cellular behavior [17]. In order to evaluate if ABCA2 expression altered the cholesterol content of lipid raft compartments, cells were metabolically radiolabeled to equilibrium for 24 hours in [3H]cholesterol, lysed in MBS buffer and subject to discontinuous sucrose gradient ultracentrifugation. The [3H]cholesterol content in aliquots of each fraction was measured by scintillation counting. In parallel, Western blot was performed on protein fractions following precipitation, using antibodies to flotillin-1 (raft marker) and calnexin (non-raft marker). ABCA2 expression did not cause a significant alteration in cholesterol distribution in raft and non-raft compartments. ABCA2 appeared to slightly reduce the [3H]cholesterol content in the lightest lipid raft compartment (fraction 4) and slightly increase the [3H]cholesterol content in non-raft compartments (fractions 8–11) (Fig. 3B). These results show that ABCA2 is not a major regulator of the cholesterol content of lipid raft compartments.
Fig. 3.
Fig. 3A. Subcellular cholesterol distribution in organelle compartments. Cells were grown as described in Methods and cell extracts were fractionated on a sucrose step gradient followed by protein precipitation and Western blot with organelle-specific antibodies. For [3H]cholesterol distribution in fractions, radioactivity from aliquots was measured by scintillation counting. Data is expressed as the % of the total [3H]cholesterol in each fraction and are the mean of triplicate experiments (± SD).
Fig. 3B. Distribution of cholesterol in lipid raft compartments. Cell extracts were prepared by solubilization N2a and A2.1 cells in Triton X-100 and Lubrol in MBS followed by sucrose density gradient ultracentrifugation as described in Methods. Equal volumes of protein were precipitated and analyzed by Western blot with specific antibodies. For [3H]cholesterol distribution in fractions, radioactivity from aliquots was measured by scintillation counting. Data are expressed as the % of total [3H]cholesterol in each fraction and are the mean of duplicate experiments.
3.4. Cholesterol synthesis is not modulated by ABCA2 expression
Neurons acquire cholesterol from two major sources [18]. One source is through receptor-mediated endocytosis of lipoprotein-derived cholesterol that is synthesized in astrocytes and delivered in trans to neurons. This lipoprotein-derived cholesterol is acquired by neurons through the action of members of the low-density lipoprotein family (e.g. LDL receptor, low-density lipoprotein receptor-related protein-1/LRP1 and ApoER2 [19]. The second source of cholesterol by neurons is from endogenous synthesis from acetyl coenzyme A precursors. In order to determine if ABCA2 regulated cholesterol synthesis, cells were cultured in lipoprotein-deficient serum for 48 hours and subsequently radiolabeled with [14C] acetate for four hours before lipid extraction and TLC analysis. ABCA2 expression did not significantly perturb cholesterol biosynthesis in N2a cells (Fig. 4). These results suggest that ABCA2 is not a regulator of endogenous cholesterol synthesis in N2a neuroblastoma cells.
Fig. 4. ABCA2 does not perturb cholesterol synthesis.
Cells were grown in 5% LPDS supplemented with 0.5 mM [14C]acetate for four hours as described in Methods. Lipids were extracted and separated by TLC in petroleum ether-diethyl ether-acetic acid (60:40:1). The data are expressed as the mean cpm [14C]cholesterol/mg protein ± SD of three experiments (A2.1 p = 0.052, A2.4 p = 0.079, Students’ t test).
3.5. ABCA2 does not perturb trafficking of cholesterol to the plasma membrane or endoplasmic reticulum
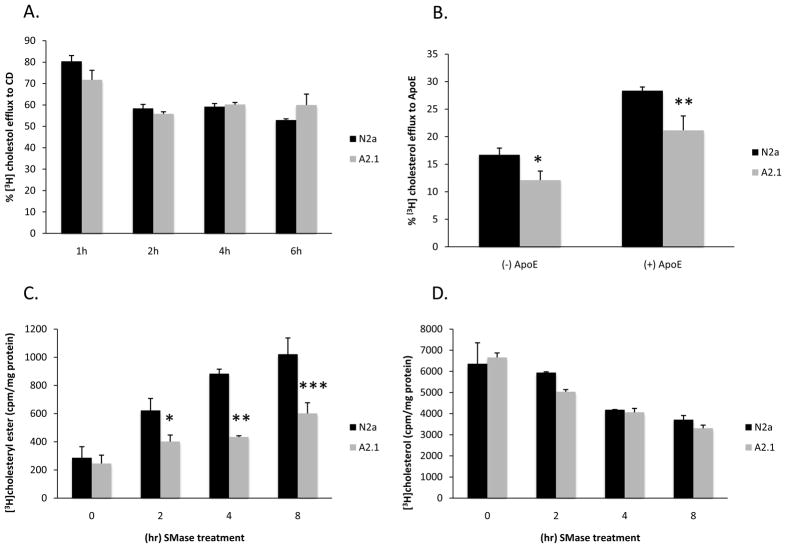
Since previous reports suggested that ABCA2 expression could modulate cholesterol trafficking, the movement of LDL-derived cholesterol to the plasma membrane was investigated. The cholesterol content in the plasma membrane available for efflux to extracellular acceptors can be monitored with a cyclodextrin cholesterol acceptor. LDL labeled with [3H] cholesteryl linoleate ([3H]CL-LDL), was generated by replacing the endogenous cholesteryl ester with radiolabeled [3H] cholesteryl linoleate. Cells were cultured in lipoprotein-deficient serum to up-regulate LDL receptors and then incubated with 50 μM [3H]CL-LDL for 1, 2, 4, and 6 hours. The [3H]CL-LDL was hydrolyzed in endosomal compartments releasing radiolabeled free- [3H]cholesterol and linoleate. The free [3H]cholesterol that trafficked to the plasma membrane was extracted for 10 min with 4% 2-hydroxypropyl β-cyclodextrin (HPCD) and [3H]cholesterol in medium and cells was measured by thin-layer chromatography and autoradiography. After 1 hour radiolabeling of N2a cells with [3H]CL-LDL, ~ 80.37% (S.D. 2.68%) of [3H]cholesterol was delivered to the plasma membrane and extracted by HPCD (Fig. 5A). In A2.1 cells, ~ 71.7% (S.D. 4.45) of [3H]cholesterol was extracted by HPCD. After 2 hours of radiolabeling, the amount of [3H]cholesterol extracted by HPCD declined to ~ 58.42 (S.D. 1.82) and 55.84 (S.D. 0.95) in N2a and A2.1 cell respectively and did not significantly change at later time points up to 6 hours. The reduction in HPCD extractable [3H]cholesterol observed during these time points may have been due to both efflux and equilibration in intracellular cholesterol pools between 2 and 6 hours. Since TLC analysis indicated that virtually all of the [3H]CL-LDL had been hydrolyzed to [3H]cholesterol after 1 hour radiolabeling, the data suggests that trafficking of lipoprotein-derived cholesterol to the plasma membrane may be perturbed in ABCA2 expressing cells. Alternatively, ABCA2 may alter the ability of the plasma membrane to release the cholesterol to cyclodextrin acceptors.
Fig. 5. Trafficking of cholesterol to the plasma membrane and endoplasmic reticulum.
A. For lipoprotein-derived cholesterol trafficking to the plasma membrane, cells were cultured in medium containing LPDS for 48 hours. Then the medium was supplemented with 50 μg/ml [3H]CL-LDL and 10 μg/ml 58035 and culture continued for 1, 2, 4, and 6 h followed by incubation in unlabeled medium containing 4% HPCD for 10 min. B. For efflux of lipoprotein-derived cholesterol to ApoE3 receptors, medium was supplemented with 50 μg/ml [3H]CL-LDL and 10 μg/ml 58035 and culture continued overnight followed by incubation in medium containing 15 μg/ml ApoE3 for 6 h. Medium- and cell- associated radioactivity was measured as described in Methods. The % efflux to CD or ApoE3 was calculated as 100 × cpm medium/(cpm medium + cpm cells). Data are expressed as the mean ± SD of three experiments (A. 1 h, p = 0.055, 6 h, p = 0.062; B. * p = 0.033, ** p = 0.023; Students’ t test).
C. For movement of plasma membrane cholesterol to the endoplasmic reticulum for esterification, cells were labeled to equilibrium for 24 hours in 1 μCi/ml [3H]cholesterol. Following treatment with 0.5 units/ml bacterial sphingomyelinase for 0, 2, 4, 8 hours, lipids were extracted and TLC was performed in hexane-diethyl ether-acetic acid (160:40:3). Data are expressed as the mean % [3H]cholesteryl ester formed normalized to total protein in each sample ± SD of three experiments (0 h p = 0.329; * 2 h p = 0.049, ** 4 h, p = 0.02, *** 8 h p = 0.031; Students’ t test).
Since cyclodextrin induces cholesterol efflux independently of the molecule over-expressed in a cell, the efflux of lipoprotein-derived cholesterol to physiological apolipoprotein E acceptors (ApoE3) was determined. N2a and A2.1 cells were radiolabeled with [3H]CL-LDL for 24 hours and efflux of [3H]cholesterol to ApoE3 into cell culture medium was measured for 6 hours. In the absence of ApoE3 acceptor in N2a cells, ~ 16.7% (S.D. 1.2) of total [3H]cholesterol was recovered in the culture medium, whereas in A2.1 cells it was ~ 12.1% of total cholesterol (S.D. 1.65, p = 0.033). Addition of 15 μg/ml ApoE3 in N2a cells resulted in an increase in [3H]cholesterol recovery to ~ 28.35% of total cholesterol (S.D. 0.67) and in A2.1 cells 21.16% of total cholesterol (S.D. 2.6, p = 0.023). These results suggest that ABCA2 expression in N2a cells decreases the efflux of lipoprotein-derived cholesterol to physiological ApoE3 acceptors.
In order to evaluate if ABCA2 modulated movement of plasma membrane cholesterol to the endoplasmic reticulum, cells were metabolically radiolabeled to equilibrium with [3H]cholesterol and esterification to [3H]cholesteryl esters by ACAT was determined. Since cholesterol is in equilibrium with sphingomyelin in lipid bilayers, hydrolysis of plasma membrane sphingomyelin to ceramide by the addition of sphingomyelinase mobilizes plasma membrane cholesterol movement to the endoplasmic reticulum for esterification [20]. Following radiolabeling, cells were treated with 0.5 U/ml bacterial sphingomyelinase for 0, 2, 4 and 8 hours before lipid extraction and analysis by thin layer chromatography. At 0 h, N2a cells, ~ 286.2 cpm/mg protein (S.D. 78.5) and A2.1 cells, ~ 245 cpm/mg (S.D. 59.1) did not demonstrate a significant difference in [3H]cholesteryl ester formation (Fig. 5C). However, at 2, 4, and 8 h, N2a cells formed ~ 622.53 (S.D. 84.8), ~ 883.07 (S.D. 35.1), and 1021.14 (S.D. 115.7) [3H]cholesteryl ester, respectively, whereas A2.1 cells formed ~ 401.04 (S.D. 46.4 p = 0.049), ~ 433.44 (S.D. 8.9 p = 0.02), ~ 601.09 (S.D. 75.9 p = 0.031). The total cholesterol content declined by similar amounts during the time course in N2a and A2.1 cells (Fig. 5D). These results show than in N2a cells ABCA2 expression perturbs movement of plasma membrane cholesterol to the endoplasmic reticulum for esterfication.
3.6. ABCA2 decreases serum and LDL-stimulated cholesterol esterification
In a previous report by this lab, it was demonstrated that in Chinese hamster ovary cells (CHO), ABCA2 expression decreased the esterification of LDL-derived cholesterol by ACAT in the endoplasmic reticulum [3]. The goal in these experiments was to confirm and extend the findings observed in non-neuronal CHO cells to neuronal cells, since ABCA2 is most highly expressed in the central and peripheral nervous systems. To determine whether ABCA2 reduced esterification of LDL-derived cholesterol, N2a and A2.1 cells were grown in lipoprotein-deficient serum for 48 h before addition of either 20% FBS or 50 μg/ml LDL and culture for an additional 5 hours. Following a two-hour pulse with [14C] oleate, lipids were extracted and analyzed by TLC. When cells were cultured in 5% lipoprotein-deficient serum alone, [14C] cholesteryl oleate formation was ~ 1700 cpm/mg protein in control N2a cells and ~ 770 cpm/mg protein in A2.1 cells (Fig. 6A). Addition of 20% serum increased [14C] cholesteryl ester formation to ~ 3700 cpm/mg protein in control N2a cells and to ~ 1220 cpm/mg protein in A2.1 cells. The lower level of [14C] cholesteryl oleate formation in A2.1 cells was not due to a deficiency in uptake of [14C] oleate, since [14C] triglyceride formation in cells cultured in lipoprotein-deficient serum was 1.66 × 105 cpm/mg protein in N2a cells and 2.44 × 105 cpm/mg protein in A2.1 cells (Fig. 6B). In N2a cells cultured in 20% FBS, [14C] triglyceride formation was 1.27 × 105 cpm/mg protein in N2a cells and 2.06 × 105 cpm/mg protein in A2.1 cells (Fig. 6B).
Fig. 6. ABCA2 expression decreases serum and LDL-stimulated cholesterol esterification.
A. Cells were cultured in medium containing LPDS for 48 hours followed by incubation in medium containing 20% FBS (A) or 50 μg/ml LDL (B) for 5 hours. Cells were pulsed with 0.2 mM [14C] oleate/BSA for 2 hours before lipid extraction and TLC was performed in hexane-diethyl ether-acetic acid (160:40:3). Data are expressed as the mean cpm [14C] cholesteryl ester and [14C] triglycerides/mg protein ± SD of three experiments (A. LPDS CE * p = 0.048 FBS CE ** p < 0.001; B. LPDS TG *** p < 0.001, FBS TG **** p = 0.0014; C. LPDS CE * p = 0.007 LDL, CE ** p = 0.0026, D. LPDS TG p = 0.109, LDL TG p = 0.36, Students’ t test). C. ABCA2 expression increases 25-hydroxycholesterol-stimulated cholesterol esterification. Cells were cultured in medium containing LPDS as described above and then the medium was supplemented with 20 μg/ml 25-hydroxycholesterol/10 μg/ml cholesterol in ethanol and culture continued for 5 hours before addition of [14C] oleate/BSA, lipid extraction and TLC as described above. Data are expressed as the mean cpm [14C] cholesteryl ester and [14C] triglycerides/mg protein ± SD of three experiments (CE A2.1, A2.4 * p < 0.001; TG A2.1 p = 0.061, A2.4 p = 0.047, Students’ t test).
Similar results were observed with LDL-stimulated cholesterol esterification. Addition of 50 μg/ml LDL resulted in [14C] cholesteryl ester formation from ~ 3000 cpm/mg protein in control N2a cells and ~ 740 cpm/mg protein in A2.1 cells (Fig. 6C). The [14C] triglyceride formation was relatively unchanged in N2a, A2.1 and A2.4 cells and ranged from 1.76–1.86 × 105 cpm/mg protein (Fig. 6D). These results are consistent with the results observed in CHO cells and show that ABCA2 expression results in reduced levels of esterification of lipoprotein-derived cholesterol by ACAT in the endoplasmic reticulum.
3.7. ABCA2 expression increases 25-hydroxycholesterol-stimulated cholesterol esterification
Since it is possible that the decrease in lipoprotein-derived cholesterol esterification in N2a cells was not due to a cholesterol trafficking defect as investigated in the previously described experiments in this report, but due to a deficiency in ACAT activity on cholesterol substrates, the effect of ABCA2 expression on esterification of 25-hydroxycholesterol (25-HC) was examined. N2a and A2.1 cells were grown in lipoprotein-deficient serum for 48 h before the addition of 20 μg/ml 25-hydroxycholesterol substrate dissolved in ethanol and culture for 5 hours. Following a two-hour pulse with [14C] oleate, lipids were extracted and analyzed by TLC. Addition of 20 μg/ml 25-HC resulted [14C] cholesteryl ester formation ~ 1830 cpm/mg protein in N2a cells and ~ 3600 cpm/mg protein in A2.1 and A2.4 cells (Fig. 6E). Addition of 20 μg/ml 25-HC resulted [14C] triglyceride formation of ~ 1.42 × 105 cpm/mg protein in N2a cells and ~ 1.55 × 105 cpm/mg protein in A2.1 and A2.4 cells (Fig. 6F). These results indicate that cellular ACAT enzymatic activity on non-lipoprotein cholesterol substrates to generate cholesteryl esters is not perturbed by ABCA2 but results in elevated levels in 25-HC-treated cells.
3.8. In vitro ACAT activity is not perturbed by ABCA2 expression
As a further direct demonstration of the effect of ABCA2 expression on ACAT activity, an in vitro ACAT assay was performed on cell extracts from N2a, A2.1 and A2.4 cells. Cells were cultured for 48 hours in 5% lipoprotein-deficient serum before addition of ± 50 μg/ml LDL and the cells were cultured for an additional 5 hours before preparation of cell extracts. The in vitro ACAT assays were performed on 100 μg of protein and 0.1 mM [oleoyl-1-14C] coenzyme A followed by lipid extraction and TLC analysis. In vitro ACAT activity was not altered by ABCA2 in N2a cells. The [14C] cholesteryl ester formation averaged ~ 275–285 cpm/mg protein.
3.9. ABCA2 reduces LDLR mRNA and protein expression and t ½ life
The effects of ABCA2 expression on LDLR mRNA and LDLR protein levels were evaluated. For determination of LDLR mRNA levels, N2a and A2.1 cells were cultured in lipoprotein-deficient serum for 48 hours before total RNA extraction, reverse transcription and real-time PCR using LDLR-specific primers. LDLR mRNA expression in N2a cells was ~ 50% (S.D. 0.01 p = 0.0048) of the level in A2.1 cells (Fig. 7A). Similarly, LDLR protein expression (Fig. 7B) in N2a cells was ~ 61% (S.D. 0.01 p = 0.06) of the level in A2.1 cells (Fig. 7C).
Fig. 7. ABCA2 expression decreases LDLR mRNA and protein level.
A. Real-time PCR measurement of LDLR mRNA level. N2a and A2.1 cells were cultured for 48 hours in medium containing 5% LPDS before extraction of total RNA reverse transcription and PCR with LDLR-specific primers. LDLR expression levels were normalized to 18S levels as described in Methods. Data are expressed as the mean ± SD of six experiments (* p = 0.0048, Students’ t test). B. Western blot measurement of LDLR protein level. Cells were cultured as above and Western blot was performed on cell extracts from N2a and A2.1 cells using LDLR-specific antibodies. GAPDH served as a protein loading control. C. Data are expressed as the mean ± SD of four experiments (* p = 0.01, Students’ t test). D. Kinetics of ABCA2 effects on LDLR synthesis and turnover. Cells were cultured in medium containing LPDS for 48 hours. Afterwards, cells were starved in methionine- and cysteine-free DMEM for 30 min, then metabolically radiolabeled with 250 μCi/ml [35S] methionine for 2 hours. Cells were chased in methionine- and cysteine-free DMEM supplemented with unlabeled methionine for 2–6 hour intervals before preparation of cell lysates, immunoprecipitation with the anti-LDLR antibody as described in Methods. E. The data are expressed as the mean of duplicate experiments.
Since the total mass of LDLR protein was decreased by ABCA2 expression, experiments were performed to evaluate if the synthesis and stability of the LDLR protein were modulated by ABCA2. Cells were cultured in lipoprotein-deficient serum for 48 hours, starved in methionine-free medium for 30 min, followed by a pulse with methionine-free medium containing 250 μCi [35S] methionine for 2 hours. Cells were chased in unlabeled medium for periods between 2 and 6 hours before immunoprecipitation with LDLR-specific antibodies, gel electrophoresis and autoradiography to measure LDLR protein expression levels. After the two hour radiolabeling period (0 h chase), ABCA2 increased the synthesis of the [35S] LDLR protein by ~ 1.6 fold (Fig. 7D). During the chase period in A2.1 cells, the level of the [35S] LDLR protein declined linearly with a t ½ of ~ 6 h. In N2a cells, the level of the [35S] LDLR protein was more stable, declining only ~ 20% after 6 h of chase (Fig. 7E). These results indicate that ABCA2 expression increases the initial synthesis of the LDLR but also promotes its rapid decay relative to control cells.
3.10. ABCA2 decreases LDLR surface expression and reduces uptake of fluorescent DiI-LDL
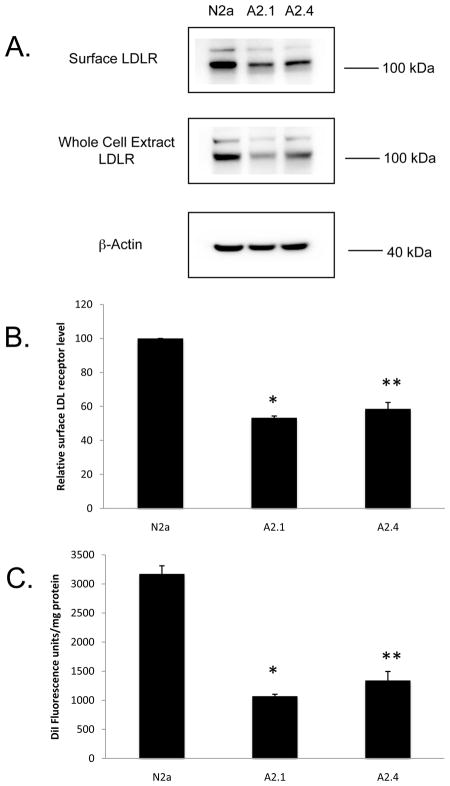
To investigate further whether ABCA2 reduced the expression of functional LDL receptors, cell surface expression of LDL receptors was evaluated. The cells were cultured in lipoprotein-deficient serum for 48 hours before incubation with membrane-impermeant sulfo-NHS-biotin and immunoprecipitation of biotinylated surface proteins with avidin-agarose. Western blot was performed with LDLR-specific antibody and LDLR expression level was quantified by densitometry. ABCA2 expression reduced surface LDLR levels by ~ 45% to 50% in A2.1 and A2.4 cells, relative to N2a cells (Fig. 8A). Western blot of LDL receptor level in whole cell extracts confirmed that total LDLR levels were decreased by a similar amount (Fig. 8B). These results indicate that ABCA2 decreases cell surface LDLR abundance.
Fig. 8. Cell surface LDLR protein expression and fluorescent DiI-LDL uptake are decreased in ABCA2 expressing cells.
A. Cells were treated with 1 mg/ml of membrane impermeant sulfo-NHS-biotin in PBS for 30 min at 4° C as described in Methods. Cell surface biotinylated proteins were collected with avidin-agarose beads. Following PAGE and transfer to nitrocellulose filters, blots were probed with an anti-LDLR antibody and protein levels were measured by densitometry. Total cellular LDLR was measured in cell lysates following Western blot with anti-LDLR receptor antibody. B. The data are expressed as the mean % surface LDLR protein expressed relative to control N2a cells ± SD of three experiments (A2.1 * p < 0.001, A2.4 ** p = 0.0013, Students’ t test). C. ABCA2 expression decreases uptake of fluorescent DiI-LDL. Cells were cultured in medium containing LPDS for 48 hours followed by supplementation with 10 μg/ml DiI-LDL. Culture was continued for 6 hours followed by lipid extraction and DiI fluorescence was measured on an aliquot at Ex:Em 520:580 nm. Data are expressed as the mean DiI fluorescence units per mg protein ± SD of three experiments (A2.1, A2.4 * p < 0.001, Students’ t test).
To evaluate if the decline in cell surface abundance of LDLR protein affected uptake of LDL-derived lipoproteins, The uptake of the fluorescent LDL analog, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate-LDL (DiI-LDL) was investigated. Cells were cultured in lipoprotein-deficient serum for 48 hours followed by the addition of 10 μg/ml DiI-LDL and culture for six hours before lipid extraction and fluorescence measurements of DiI-LDL uptake. In N2a cells DiI fluorescence was ~ 3200 units/mg protein (S.D. 168.1), in A2.1 cells ~ 1076.2 units/mg protein (S.D. 40.34 p < 0.001) and in A2.4 cells 1438.7 units/mg protein (S.D. 59.3 p < 0.001) (Fig. 8C). From these series of experiments, the results indicate that ABCA2 expression decreases uptake of lipoproteins as consequence of a decline in the abundance of functional LDL receptors at the cell surface.
3.11. RNAi silencing of endogenous ABCA2 levels in N2a cells increases LDLR protein expression
The significance of the preceding work, indicating that over-expression of ABCA2 in N2a cells reduces LDLR mRNA and protein expression, would be enhanced by demonstrating that silencing of endogenous ABCA2 expression in N2a cells or primary neurons results in the opposite effects, i.e. silencing of ABCA2 increases LDLR expression levels. To address this question, N2a cells or rat primary cortical neurons were transiently transfected with control RNAi or ABCA2-specific RNAi for 48 hours, total RNA was isolated, reverse transcribed and real-time PCR was performed with ABCA2-specific primers. ABCA2 expression in RNAi-treated N2a cells was ~ 35% (S.D. 0.06 p =0.02) of the level in N2a cells. To evaluate the effect of ABCA2 silencing on LDLR protein expression, Western blot was performed with LDLR-specific antibodies. LDLR protein expression was ~ 1.55-fold greater (S.D. 0.048 p = 0.02) in RNAi-transfected N2a cells compared to control RNAi-transfected cells. In rat primary cortical neurons, LDLR protein expression was ~ 1.43-fold greater (S.D. 0.136) compared to control RNAi-transfected rat primary cortical neurons.
4. Discussion
In this report, the regulation of cholesterol metabolism by ABCA2 was examined in N2a neuroblastoma cells. Although the evidence presented in this study suggests that unlike other ABC proteins that are established cholesterol transporters e.g. ABCA1, ABCA7, ABCG1, ABCG4, ABCG5/8) [21], ABCA2 may not function directly as a transporter of cholesterol but may modulate the mobilization of plasma membrane cholesterol for efflux to apolipoprotein acceptors and trafficking of plasma membrane cholesterol to the endoplasmic reticulum for esterification.
Additionally, evidence presented in this report indicates that ABCA2 may modulate cholesterol homeostasis in neuronal cells through regulation of the expression of the low-density lipoprotein receptor. ABCA2 decreased LDLR receptor mRNA and protein levels and increased its turnover rate. The decrease in LDLR steady-state protein mass may be due to the observed decrease in the half-life of the LDLR protein in ABCA2 expressing cells. The cell surface expression of the LDLR as well as the uptake of fluroresecent DiI-LDL was reduced by ABCA2. As a further demonstration of the role of ABCA2 in regulation of LDLR expression, the reduction of endogenous ABCA2 expression by RNAi treatment of N2a cells and rat primary cortical neurons reversed the effects of over-expression of ABCA2, increasing LDLR protein levels.
The LDLR gene is transcribed upon sterol deprivation. Transcription is activated by the sterol-responsive element binding protein-2 (SREBP-2), which is itself transcribed and activated by proteolytic cleavage upon sterol deprivation to its transcriptionally-active form that binds and activates the sterol responsive elements in the SREBP2 and LDLR promoters [13, 22]. The abundance of LDLR protein is also regulated by post-translational mechanisms that regulate its internalization and degradation [23]. The internalization of the LDLR is regulated by the Autosomal Recessive Hypercholesterolemia adaptor protein (ARH) [24]. ARH serves as an endocytic sorting adaptor protein that associates with additional members of the endocytic machinery to promote packaging of the LDLR into endocytic vesicles. The LDLR is also regulated post-translationally by the proprotein convertase PCSK9 [25, 26]. PCSK9 is also known as the neural apoptosis-regulated convertase-1 (NARC-1) and is implicated in the differentiation of neural progenitor cells into cortical neurons [27]. A recent report suggested that PCSK9 is not involved in the degradation of the LDLR in the brain [28]; however, the LDLR levels in individual types of neurons in the brain were not reported. In addition, a recent review proposed that LDL binding to the LDLR favors the internalization and recycling of the LDLR, whereas secreted PCSK9 and subsequent binding to the LDLR favors the internalization and degradation of the receptor [29]. Therefore, circulating LDL levels may be significant in the alternative fates of the LDLR following internalization. Preliminary experiments in this laboratory indicate that ARH protein levels are increased, which would favor LDLR internalization and intracellular PCSK9 levels are decreased (LDLR and bound PCSK9 are both degraded upon internalization) in ABCA2 overexpressing N2a cells. Future work will determine if these key regulators of LDLR internalization and degradation or other mechanisms mediate the increase in LDLR turnover and decrease the uptake of lipoprotein-derived cholesterol reported in these studies.
Uptake of cholesterol from lipoproteins by the LDLR is important in regulating cholesterol homeostasis, neurite development and axon regeneration. Lipoproteins that are generated from axon and myelin degeneration supply cholesterol for axonal growth in regenerating axons of sympathetic neurons [30]. Low-density lipoprotein is taken up by LDLR on distal axons and transported to cell bodies or it is internalized by LDLR on proximal axons and cell bodies.
Lipoproteins are also internalized in neurons by additional members of the LDL receptor family of proteins, including the low-density lipoprotein receptor-related protein (LRP) [31], LR7/8B (ApoER2) [21] and LR11/(SorLA)[32], that have been implicated in hippocampal neurite development [33], as well as in Alzheimer’s disease [34]. Studies with LDLR knockout mice have demonstrated that it is a direct regulator of the cellular uptake and central nervous system levels of astroctye-derived ApoE [35]. ApoE is the major carrier of cholesterol in the brain and the elevated levels that are a result of LDL deficiency may contribute to the pathology of Alzheimer’s disease [36, 37]. An interesting question is whether LDLR levels are reduced and ApoE levels are increased in brains when ABCA2 expression is elevated and whether the elevated ApoE contributes to Alzheimer’s disease pathology.
A possible role for ABCA2 has been proposed in the etiology of Alzheimer’s disease. Microarray analysis of gene expression patterns in HEK293 cells expressing ABCA2 identified a number of gene products that have been implicated in Alzheimer’s disease [11]. Two large-scale case-controlled epidemiological studies have proposed a genetic link between ABCA2 and Alzheimer’s disease. Both studies identified the same single nucleotide polymorphism (SNP), rs908832 that was found to be significantly associated with both early-onset [9] and sporadic Alzheimer’s disease [10]; however, in a small subset of early-onset AD subjects, no association was detected between this SNP and AD [38]. In a recent study performed in this laboratory, it was demonstrated that in N2a cells ABCA2 increases endogenous amyloid precursor protein (APP) expression and neurotoxic Abeta fragment generation [12].
In summary, this report has presented evidence that indicates that ABCA2 is a key regulator of cholesterol homeostasis in neuronal cells and of the expression of the LDLR. Future work will examine the mechanism of ABCA2 regulation of LDLR expression, trafficking and degradation in primary neurons and also whether ApoE levels and brain cholesterol homeostasis in the mouse brain are modulated by ABCA2 function.
Fig. 9. RNAi silencing of endogenous ABCA2 levels in N2a cells and primary rat cortical neurons increases LDLR protein expression.
A. RNAi silencing of endogenous ABCA2. N2a cells or primary rat cortical neurons were transiently transfected with control RNAi or ABCA2 mouse-specific RNAi for 48 hours, total RNA was isolated and reverse transcribed and real-time PCR was performed with ABCA2-specific primers. ABCA2 expression levels were normalized to 18S levels as described in Methods. Data are expressed as the mean ± SD of six experiments (* p = 0.02, Students’ t test). B. Western blot of LDLR protein levels in control RNAi and ABCA2-specific RNAi treated N2a cells and rat primary cortical neurons. N2a cells or rat primary neurons were treated with control RNAi, ABCA2 mouse-specific, or ABCA2 rat-specific RNAi for 48 hours, and Western blot was performed on cell extracts with an LDLR-specific antibody. C. Data are expressed as the mean ± SD of four experiments (* p = 0.02, Students’ t test), N2a cells or ± SD of two experiments, rat primary cortical neurons.
Research Highlights.
ABCA2 expression in N2a neuroblastoma cells reduced total, free- and cholesterol ester levels as well as membrane cholesterol.
ABCA2 reduced efflux of cholesterol to the physiological acceptor ApoE3 and reduced mobilization of plasma membrane cholesterol to the endoplasmic reticulum for esterification.
ABCA2 reduced esterification of serum and low-density lipoprotein-derived cholesterol but not 25-hydroxycholesterol.
ABCA2 reduced low-density lipoprotein receptor (LDLR) mRNA and protein and increased its protein turnover rate.
The surface expression of the LDLR as well as the uptake of fluorescent DiI-LDL was reduced by ABCA2 expression.
Reduction of endogenous ABCA2 expression in N2a cells and rat primary cortical neurons produced the opposite effects of ABCA2 overexpression and increased LDLR protein levels
Acknowledgments
This research was supported by Grant Number 1K01NS062113-01A2 from the National Institute of Neurological Disorders and Stroke and Grant Number P20 RR017677 from the National Center for Research Resources (NCRR) a component of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aye IL, Singh AT, Keelan JA. Transport of lipids by ABC proteins: interactions and implications for cellular toxicity, viability and function. Chem Biol Interact. 2009;180:327–339. doi: 10.1016/j.cbi.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 3.Davis W, Jr, Boyd JT, Ile KE, Tew KD. Human ATP-binding cassette transporter-2 (ABCA2) positively regulates low-density lipoprotein receptor expression and negatively regulates cholesterol esterification in Chinese hamster ovary cells. Biochim Biophys Acta. 2004;1683:89–100. doi: 10.1016/j.bbalip.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Sakai H, Tanaka Y, Tanaka M, Ban N, Yamada K, Matsumura Y, Watanabe D, Sasaki M, Kita T, Inagaki N. ABCA2 deficiency results in abnormal sphingolipid metabolism in mouse brain. J Biol Chem. 2007;282:19692–19699. doi: 10.1074/jbc.M611056200. [DOI] [PubMed] [Google Scholar]
- 5.Vulevic B, Chen Z, Boyd JT, Davis W, Jr, Walsh ES, Belinsky MG, Tew KD. Cloning and characterization of human adenosine 5′-triphosphate-binding cassette, subfamily A, transporter 2 (ABCA2) Cancer Res. 2001;61:3339–3347. [PubMed] [Google Scholar]
- 6.Zhou CJ, Inagaki N, Pleasure SJ, Zhao LX, Kikuyama S, Shioda S. ATP-binding cassette transporter ABCA2 (ABC2) expression in the developing spinal cord and PNS during myelination. J Comp Neurol. 2002;451:334–345. doi: 10.1002/cne.10354. [DOI] [PubMed] [Google Scholar]
- 7.Kim WS, Guillemin GJ, Glaros EN, Lim CK, Garner B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport. 2006;17:891–896. doi: 10.1097/01.wnr.0000221833.41340.cd. [DOI] [PubMed] [Google Scholar]
- 8.Saito T, Yamada K, Wang Y, Tanaka Y, Ohtomo K, Ishikawa K, Inagaki N. Expression of ABCA2 protein in both non-myelin-forming and myelin-forming Schwann cells in the rodent peripheral nerve. Neurosci Lett. 2007;414:35–40. doi: 10.1016/j.neulet.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Mace S, Cousin E, Ricard S, Genin E, Spanakis E, Lafargue-Soubigou C, Genin B, Fournel R, Roche S, Haussy G, Massey F, Soubigou S, Brefort G, Benoit P, Brice A, Campion D, Hollis M, Pradier L, Benavides J, Deleuze JF. ABCA2 is a strong genetic risk factor for early-onset Alzheimer’s disease. Neurobiol Dis. 2005;18:119–125. doi: 10.1016/j.nbd.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Wollmer MA, Kapaki E, Hersberger M, Muntwyler J, Brunner F, Tsolaki M, Akatsu H, Kosaka K, Michikawa M, Molyva D, Paraskevas GP, Lutjohann D, von Eckardstein A, Hock C, Nitsch RM, Papassotiropoulos A. Ethnicity-dependent genetic association of ABCA2 with sporadic Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:534–536. doi: 10.1002/ajmg.b.30345. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZJ, Vulevic B, Ile KE, Soulika A, Davis W, Jr, Reiner PB, Connop BP, Nathwani P, Trojanowski JQ, Tew KD. Association of ABCA2 expression with determinants of Alzheimer’s disease. FASEB J. 2004;18:1129–1131. doi: 10.1096/fj.03-1490fje. [DOI] [PubMed] [Google Scholar]
- 12.Davis W., Jr The ATP-binding Cassette Transporter-2 (ABCA2) Increases Endogenous Amyloid Precursor Protein Expression and Abeta Fragment Generation. Curr Alzheimer Res. 2010;7:566–577. doi: 10.2174/156720510793499002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JR, Osborne TF, Goldstein JL, Brown MS. Identification of nucleotides responsible for enhancer activity of sterol regulatory element in low density lipoprotein receptor gene. J Biol Chem. 1990;265:2306–2310. [PubMed] [Google Scholar]
- 14.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Sugii S, Reid PC, Ohgami N, Du H, Chang TY. Distinct endosomal compartments in early trafficking of low density lipoprotein-derived cholesterol. J Biol Chem. 2003;278:27180–27189. doi: 10.1074/jbc.M300542200. [DOI] [PubMed] [Google Scholar]
- 16.Stephan ZF, Yurachek EC. Rapid fluorometric assay of LDL receptor activity by DiI-labeled LDL. J Lipid Res. 1993;34:325–330. [PubMed] [Google Scholar]
- 17.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 18.Mulder M. Sterols in the central nervous system. Curr Opin Clin Nutr Metab Care. 2009;12:152–158. doi: 10.1097/MCO.0b013e32832182da. [DOI] [PubMed] [Google Scholar]
- 19.Vance JE, Hayashi H. Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim Biophys Acta. 2010;1801:806–818. doi: 10.1016/j.bbalip.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Luker GD, Nilsson KR, Covey DF, Piwnica-Worms D. Multidrug resistance (MDR1) P-glycoprotein enhances esterification of plasma membrane cholesterol. J Biol Chem. 1999;274:6979–6991. doi: 10.1074/jbc.274.11.6979. [DOI] [PubMed] [Google Scholar]
- 21.Kim WS, Weickert CS, Garner B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J Neurochem. 2008;104:1145–1166. doi: 10.1111/j.1471-4159.2007.05099.x. [DOI] [PubMed] [Google Scholar]
- 22.Sato R, Inoue J, Kawabe Y, Kodama T, Takano T, Maeda M. Sterol-dependent transcriptional regulation of sterol regulatory element-binding protein-2. J Biol Chem. 1996;271:26461–26464. doi: 10.1074/jbc.271.43.26461. [DOI] [PubMed] [Google Scholar]
- 23.Seidah NG, Khatib AM, Prat A. The proprotein convertases and their implication in sterol and/or lipid metabolism. Biol Chem. 2006;387:871–877. doi: 10.1515/BC.2006.110. [DOI] [PubMed] [Google Scholar]
- 24.Sirinian MI, Belleudi F, Campagna F, Ceridono M, Garofalo T, Quagliarini F, Verna R, Calandra S, Bertolini S, Sorice M, Torrisi MR, Arca M. Adaptor protein ARH is recruited to the plasma membrane by low density lipoprotein (LDL) binding and modulates endocytosis of the LDL/LDL receptor complex in hepatocytes. J Biol Chem. 2005;280:38416–38423. doi: 10.1074/jbc.M504343200. [DOI] [PubMed] [Google Scholar]
- 25.Park SW, Moon YA, Horton JD. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem. 2004;279:50630–50638. doi: 10.1074/jbc.M410077200. [DOI] [PubMed] [Google Scholar]
- 26.Poirier S, Mayer G, Benjannet S, Bergeron E, Marcinkiewicz J, Nassoury N, Mayer H, Nimpf J, Prat A, Seidah NG. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J Biol Chem. 2008;283:2363–2372. doi: 10.1074/jbc.M708098200. [DOI] [PubMed] [Google Scholar]
- 27.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M, Wu G, Baysarowich J, Kavana M, Addona GH, Bierilo KK, Mudgett JS, Pavlovic G, Sitlani A, Renger JJ, Hubbard BK, Fisher TS, Zerbinatti CV. PCSK9 is not involved in the degradation of LDL receptors and BACE1 in the adult mouse brain. J Lipid Res. 2010;51:2611–2618. doi: 10.1194/jlr.M006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akram ON, Bernier A, Petrides F, Wong G, Lambert G. Beyond LDL cholesterol, a new role for PCSK9. Arterioscler Thromb Vasc Biol. 2010;30:1279–1281. doi: 10.1161/ATVBAHA.110.209007. [DOI] [PubMed] [Google Scholar]
- 30.Posse De Chaves EI, Vance DE, Campenot RB, Kiss RS, Vance JE. Uptake of lipoproteins for axonal growth of sympathetic neurons. J Biol Chem. 2000;275:19883–19890. doi: 10.1074/jbc.275.26.19883. [DOI] [PubMed] [Google Scholar]
- 31.Bu G, Maksymovitch EA, Nerbonne JM, Schwartz AL. Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neurons. J Biol Chem. 1994;269:18521–18528. [PubMed] [Google Scholar]
- 32.Yamazaki H, Bujo H, Kusunoki J, Seimiya K, Kanaki T, Morisaki N, Schneider WJ, Saito Y. Elements of neural adhesion molecules and a yeast vacuolar protein sorting receptor are present in a novel mammalian low density lipoprotein receptor family member. J Biol Chem. 1996;271:24761–24768. doi: 10.1074/jbc.271.40.24761. [DOI] [PubMed] [Google Scholar]
- 33.Narita M, Bu G, Holtzman DM, Schwartz AL. The low-density lipoprotein receptor-related protein, a multifunctional apolipoprotein E receptor, modulates hippocampal neurite development. J Neurochem. 1997;68:587–595. doi: 10.1046/j.1471-4159.1997.68020587.x. [DOI] [PubMed] [Google Scholar]
- 34.Marzolo MP, Bu G. Lipoprotein receptors and cholesterol in APP trafficking and proteolytic processing, implications for Alzheimer’s disease. Semin Cell Dev Biol. 2009;20:191–200. doi: 10.1016/j.semcdb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fryer JD, Demattos RB, McCormick LM, O’Dell MA, Spinner ML, Bales KR, Paul SM, Sullivan PM, Parsadanian M, Bu G, Holtzman DM. The low density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J Biol Chem. 2005;280:25754–25759. doi: 10.1074/jbc.M502143200. [DOI] [PubMed] [Google Scholar]
- 36.Leduc V, Jasmin-Belanger S, Poirier J. APOE and cholesterol homeostasis in Alzheimer’s disease. Trends Mol Med. 2010;16:469–477. doi: 10.1016/j.molmed.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Martins IJ, Berger T, Sharman MJ, Verdile G, Fuller SJ, Martins RN. Cholesterol metabolism and transport in the pathogenesis of Alzheimer’s disease. J Neurochem. 2009;111:1275–1308. doi: 10.1111/j.1471-4159.2009.06408.x. [DOI] [PubMed] [Google Scholar]
- 38.Minster RL, DeKosky ST, Kamboh MI. No association of DAPK1 and ABCA2 SNPs on chromosome 9 with Alzheimer’s disease. Neurobiol Aging. 2009;30:1890–1891. doi: 10.1016/j.neurobiolaging.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]