Abstract
The aim was to characterize the neural breathing pattern in non-intubated preterm infants. The diaphragm electrical activity (EAdi) and heart rate were simultaneously measured repeatedly for 1 hour over several days using a modified feeding tube equipped with miniaturized sensors. The EAdi waveform was quantified for phasic and tonic activity, neural timings, and prevalence of recurring patterns, including central apnea. Ten infants with mean age 7 days (range 3–13) were studied. Their birth weight was 1512 g (1158–1800g), and gestational age (GA) at birth 31 weeks (28–36). Neural inspiratory and expiratory times were 278 ms (195–450 ms) and 867 ms (668–1436 ms), and correlated with GA (p<0.001). Tonic EAdi represented 29.5% of phasic EAdi (16–40%), and was related to GA (r=0.61, p<0.001). For the group, 68% of the time was regular phasic breathing (without tonic activity), and 29% of the time with elevated tonic activity. Central apneas >5s occurred on average 10 times per hour (2–29). Heart rate reductions were correlated to central apnea duration. In conclusion, esophageal recordings of the EAdi waveform demonstrate that neural breathing pattern is variable, with regards to timing, amplitude and pattern with a distinct amount of tonic diaphragm activity.
INTRODUCTION
Neonatal respiration has been described as being variable (1) with spontaneous changes in pattern between eupnea, apnea (2), sigh (3), and an irregular breathing pattern that is sometimes referred to as “periodic breathing” (4, 5). Measurements to characterize breathing patterns vary between investigators, and have yet to be standardized. Most have focussed on the final mechanical output at the airway, such as tidal volume or flow (6), or chest wall displacement by respiratory inductance plethysmography (7). Considering the immature development of the respiratory muscles and the chest wall in preterms, as well as the breath-to-breath variability in respiratory system compliance and resistance (8), these final “mechanical” signals may not reflect the true neural respiratory center output. In addition, standard mechanical measurements may not necessarily be able to discriminate between obstructive and true central apneas.
The neural signals for breathing can be inferred by measurements of the electrical activity of the diaphragm (EAdi) (9–11). A validated and standardized method for measuring EAdi signals has been described (12) in infants (13–15), and involves the use of microsensors placed on a conventional naso/orogastric feeding tube, thereby imposing no additional level of invasiveness on preterms who require tube feeding. Esophageal measurement of the EAdi allows assessment of neural respiratory pattern without requiring airway or abdominal sensors. The EAdi waveform provides information about the “phasic” (tidal) inspiratory effort made to overcome respiratory load. As well, it can quantify the amount of diaphragm activity present during end-expiration, the so-called “tonic EAdi” (14). The tonic EAdi is believed to be involved in the maintenance of end expiratory lung volume (16), as studied in intubated infants (14) and rabbits with acute lung injury (17). An absent EAdi signal (i.e. a flat EAdi waveform) is an indicator of central apnea. Additionally, the electrocardiogram (ECG) can be measured with the same microsensors, allowing changes in heart rate to be monitored simultaneously with the EAdi.
The aim of the present study was to characterize the neural breathing patterns in non-intubated preterm infants. Specifically, the aims were to (i) quantify the magnitude and prevalence of phasic and tonic EAdi (ii) evaluate the relationship between central apneas and changes in heart rate, and (iii) investigate the neural breathing strategies adopted before and after a central apnea. We anticipated that the tonic EAdi would be elevated in preterm infants, as it may be one mechanism to prevent de-recruitment of the lung in the face of alveolar instability and increased chest wall compliance.
METHODS
The protocol was approved by the Research and Ethics Board of Sunnybrook Health Sciences Centre, Toronto, Canada, and written informed consent was obtained from a parent or guardian according to the guidelines of Sunnybrook Health Sciences Centre.
Infants
Infants were eligible if they were born premature (<36 weeks) with a birth weight between 1000g and 2000g. Infants were eligible as long as they were <28 days post natal age and >1000g at the time of inclusion. Exclusion criteria: pneumothorax, neuromuscular or bleeding disorders, cardiovascular instability, congenital anomaly of the cardiorespiratory system, muscle relaxants or narcotics, phrenic nerve damage/diaphragm paralysis, or esophageal perforation.
Measurements
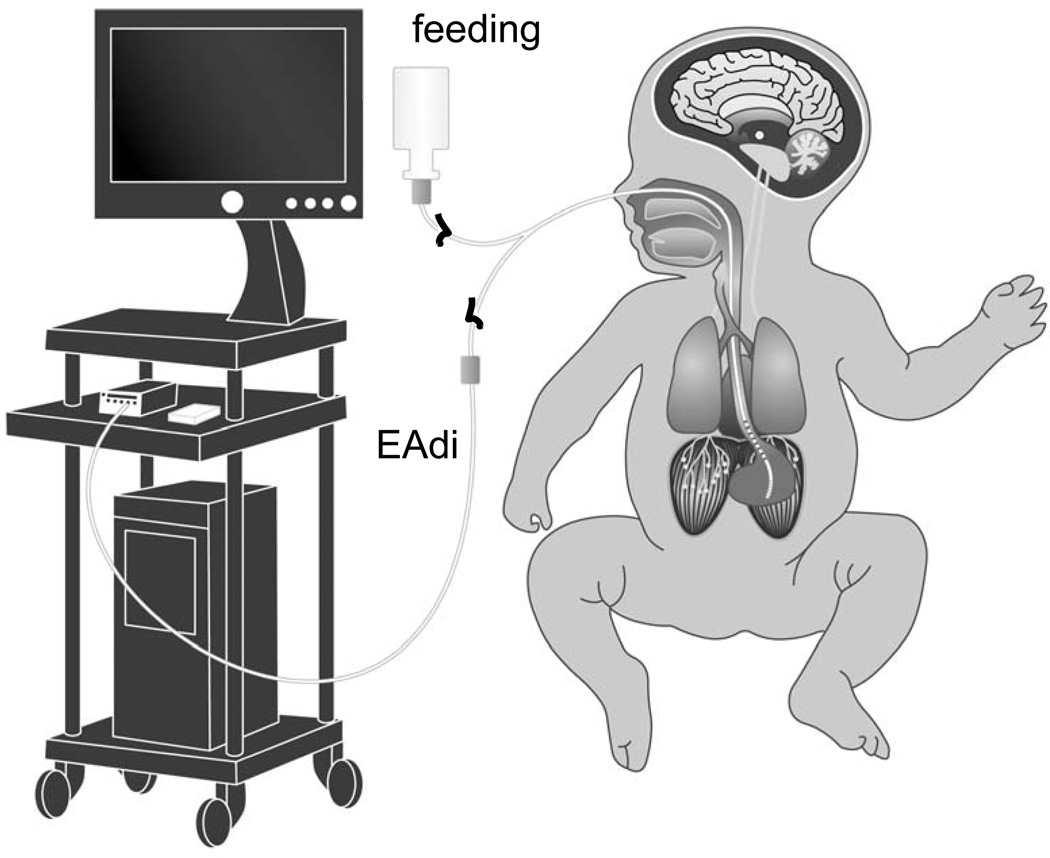
EAdi was obtained using an array of nine miniaturized electrodes (spaced 6 mm apart) mounted on a conventional (5.5F) feeding tube (Neurovent Research Inc, Toronto, Canada), and positioned so that the array would cross the lower esophagus at the level of the diaphragm. EAdi signals were acquired by computer, and displayed as a waveform throughout the study (FIGURE 1). Oxygen saturation was intermittently noted from the displayed monitor.
Figure 1. Experimental set-up.
A modified nasal or orogastric feeding tube for feeding and measuring EAdi was inserted and connected to a computer for data acquisition.
Experimental protocol
The day after obtaining consent, the study catheter was inserted via the nose or mouth and passed into the esophagus with the tip advancing into the stomach. The insertion route (oral or nasal) was decided by the clinical team (Table 1). The array was positioned at the level of the crural diaphragm (gastroesophageal junction) with the help of on-line feedback from the EAdi and ECG waveforms (18). The catheter was fixed with tape, and the position of the catheter was noted. The EAdi was recorded for 60 minutes (Day 1 am). Repeat 60-min measurements were made at 12 hours, 24 hours, 48 hours and 72 hours after the initial placement of the catheter. In between, the catheter remained in situ, and was used as an ordinary feeding tube.
Table 1.
Patient Characteristics
| Subject | sex | Diagnosis | GA at birth | Birth weight |
Age at study |
Weight day 1 |
Caffeine day 1? |
total hours studied |
Catheter |
|---|---|---|---|---|---|---|---|---|---|
| (weeks) | (g) | (days) | (g) | Y/N | Oral/nasal | ||||
| 1 | M | hypoglycemia | 36 | 1800 | 3 | 1778 | N | 4 | Nasal |
| 2 | M | RDS, apnea of prematurity | 31 | 1684 | 11 | 1500 | Y | 5 | Nasal |
| 3 | M | RDS mild, prematurity | 31 | 1634 | 11 | 1450 | Y | 5 | Nasal |
| 4 | M | RDS | 28 | 1486 | 11 | 1386 | Y | 5 | Nasal |
| 5 | M | RDS | 30 | 1158 | 5 | 1092 | Y | 5 | Nasal |
| 6 | M | RDS | 30 | 1736 | 6 | 1684 | Y | 5 | Oral |
| 7 | M | RDS | 33 | 1760 | 7 | 1602 | N | 5 | Oral |
| 8 | M | RDS | 31 | 1300 | 13 | 1334 | N | 3 | Nasal |
| 9 | F | apnea of prematurity | 30 | 1365 | 5 | 1165 | Y | 4 | Nasal |
| 10 | F | apnea of prematurity | 30 | 1205 | 6 | 1005 | Y | 5 | Nasal |
| Mean | 31 | 1513 | 8 | 1400 | 4.6 | ||||
| SD | 2 | 242 | 3 | 255 | 0.7 |
Signal Processing
EAdi signals were processed according to The American Thoracic Society recommendations (12) and displayed as a waveform (FIGURE 2) (19–21).
Figure 2. Breath-by-breath analysis method.
A: Raw signals from all eight electrode pairs.
B: Processed EAdi waveform. Cursors were manually placed at the onset, peak, and subsequent onset of the processed EAdi waveform for calculation of neural timings, and signal strength.
Analysis
Analysis was performed off-line.
Breath-by-breath analysis (on 1 hour of data)
Breath by breath analysis was performed on one hour of data from each infant. Three time cursors were placed for each breath on the displayed EAdi waveform (14, 15): (i) onset of neural inspiratory signal, (ii) peak of EAdi, and (iii) onset of next neural inspiratory signal (FIGURE 2).
The time period from onset to peak of EAdi was used to calculate the neural inspiratory time (Nti). The peak of the inspiratory EAdi waveform using this method was called the phasic EAdi (EAdiphasic). The period from peak EAdi to the onset of the next breath was identified as the neural expiration time (Nte). The EAdi value at the end of neural exhalation was called the tonic EAdi (EAditonic). Neural total breath time (Ntt) was calculated as Nti+Nte. Neural respiratory rate (Nrr) was calculated as 60/Ntt.
The EAdi-time product (a surrogate measurement of diaphragmatic energy expenditure) was calculated as: ((EAdiphasic * Nti) + (EAditonic *Nte)) * Nrr.
Breaths for which Nte exceeded 5 seconds were considered to be central apneas (15), and hence were not included in this analysis. For each condition, an average of each variable was calculated.
Five-second epochs (analyzed for all hours of data)
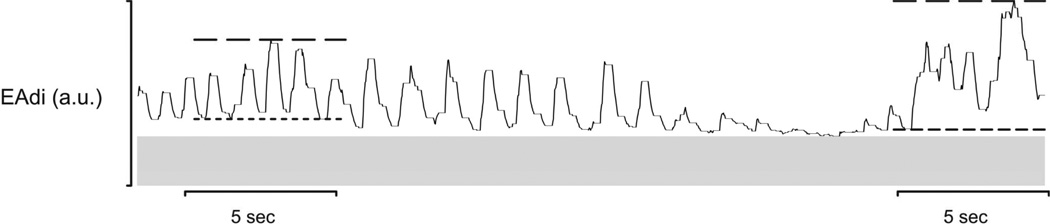
The repeated 1 hour periods of data were divided into 5-second epochs. The lowest (EAdimin5) and highest (EAdimax5) were identified for every epoch (FIGURE 3).
Figure 3. 5-second epoch analysis method.
Data was divided into 5 second-epochs, and a minimum EAdi, short dashed lines (EAdimin5), and maximum EAdi, long dashed lines (EAdimax5), were identified. The noise level is indicated by the grey horizontal bar.
Central apnea (all hours of data)
Central apneas were defined as periods where the EAdi was a flat tracing for more than 5 sec. Each file was visually displayed and time cursors were placed at the beginning and at the end of each apnea. Apneas were classified by their duration (5–10 sec, 10–15 sec, and >15 sec).
For each central apnea, heart rate was calculated (60/(time interval between two ECG peaks)), just prior to the apnea, and at the end of the apnea.
The noise level of the signal was obtained during all periods of central apnea that were >5 sec and the lowest levels during each 1 hour period were considered to be the noise level for that recording period. The lowest noise level was subtracted from all measurements of EAdi magnitudes (EAdiphasic, EAditonic, EAdimax5, and EAdimin5).
Classification and prevalence of EAdi patterns (all hours of data)
Repeating patterns in the EAdi signal could be observed during acquisition of the data at the bedside (FIGURE 4). In order to classify these patterns in off-line analysis, seven types of patterns were pre-defined. Customized software was developed to facilitate classification based on a visual display of 30-sec epochs of the EAdi waveform, with guidelines about the baseline noise level and timings. The investigator performing the analysis scrolled through the 30-sec epoch screens and tagged the different breathing patterns according to type 1 - type 7, as defined below:
Type 1: Central apnea (defined as the signal remaining at the noise level for > 5 sec)
Type 2: Regular phasic breathing returning to baseline: Phasic breathing with baseline at noise level (i.e. without tonic activity), and all breaths with Nti < 1 sec
Type 3: “Sigh” returning to baseline: Any single neural inspiration with Nti>1s, with subsequent baseline at noise level
Type 4: Regular phasic breathing with tonic activity: Phasic breathing with baseline above 20% of noise level (i.e. with tonic activity), and breaths < 1 sec duration (i.e. Nti<1 sec)
Type 5: “Sigh” not returning to baseline. Any single neural inspiration with Nti>1s, with subsequent return to a level of tonic activity (i.e. baseline above 20% of noise level)
Type 6: Tonic bursts: Maintained elevated baseline (>20% of noise level) without any phasic activity
Type 7: Unidentifiable (breathing pattern that does not fall into types 1–6)
Figure 4. Representative tracings of the different neural breathing patterns on the same day.
Each panel demonstrates the EAdi waveform obtained in one subject on the same recording day, and demonstrates examples of the different breathing pattern types (1–7, see Methods).
After the different time periods were tagged for type of pattern, in each infant, a sum of the time spent in each pattern was calculated and then divided by the total time of recorded data. This yielded a percentage spent within each type of breathing pattern.
Statistical analysis
Statistical analysis was performed with Sigmastat 3.2 (Jandel Scientific, San Rafael, CA, USA). Descriptive statistics were used to describe the neural breathing pattern (breath-by-breath analysis on 1 hour of data). Repeated measures ANOVA was used to calculate differences between days for EAdimax5, EAdimin5, and noise obtained with the 5-sec epoch technique, as well as for calculating differences in patterns on different days.
RESULTS
The characteristics of the infants are presented in TABLE 1. Ten infants (2 female) were studied. One infant was on nasal continuous positive airway pressure (CPAP) for the first hour. The other infants were not receiving any respiratory support. A total of 46 hours of data was analyzed (average 4.6±2.1 hours per infant).
Neural timings
Each hour of collected data produced an average of 3169 (range 2013 to 3845) breaths for analysis. Nti, Nte, Nrr, and the EAdi-time product are reported for each infant (TABLE 2). Nti was correlated to Nte (r=0.94, p<0.001). Nrr, Nti and Nte all correlated significantly with GA (for Nrr: r=0.90, p<0.001; for Nti r=0.79, p<0.001; for Nte r= 0.92, p<0.001).
Table 2.
Breath-by-breath analysis of the EAdi waveform for 1 hour of data
| Subject | EAdiphasic (a.u.) |
EAditonic (a.u.) |
Nti (ms) | Nte (ms) | Nti/Ntt | Nrr | EAdi-time product (au*s/min) |
# breaths analyzed |
|---|---|---|---|---|---|---|---|---|
| 1 | 21.3 | 5 | 450 | 1436 | 0.24 | 43 | 737 | 2013 |
| 2 | 11.7 | 6.1 | 278 | 910 | 0.24 | 69 | 644 | 3071 |
| 3 | 17.5 | 10.1 | 261 | 772 | 0.26 | 73 | 1291 | 3354 |
| 4 | 22.8 | 15.6 | 260 | 668 | 0.28 | 79 | 1329 | 3834 |
| 5 | 29.5 | 5.8 | 281 | 898 | 0.25 | 81 | 754 | 2900 |
| 6 | 19.5 | 6.8 | 240 | 756 | 0.25 | 76 | 985 | 3471 |
| 7 | 13.1 | 7.3 | 294 | 1006 | 0.24 | 71 | 826 | 2418 |
| 8 | 16.3 | 5.1 | 194 | 707 | 0.22 | 75 | 686 | 3846 |
| 9 | 27.1 | 11.4 | 268 | 759 | 0.26 | 82 | 1376 | 3410 |
| 10 | 27.9 | 13.7 | 250 | 759 | 0.25 | 83 | 1695 | 3372 |
| Mean | 20.7 | 8.7 | 278 | 867 | 0.25 | 73 | 1032 | 3169 |
| SD | 6.2 | 3.8 | 67 | 225 | 0.02 | 12 | 364 | 588 |
a.u.: arbitrary units
Strength of EAdi Signal
The amount of EAditonic represented on average 29.5% of EAdiphasic (range 16–40%). The magnitude of EAditonic was significantly related to GA (r=0.61, p<0.001), but was not related to Nrr (p=0.128). Using the breath-by-breath analysis, the within-subject variability for the magnitude of EAdiphasic and EAditonic were high (mean coefficient of variation (CV) for EAdiphasic 125%, CV for EAditonic 89%). The between subject variability was highest for EAditonic (CV=44%) and lowest for Nti/Ntt (CV=6%).
EAdiphasic calculated breath-by-breath correlated well with EAdimax5 obtained with the 5-sec epoch method (r=0.94, p<0.001). Similarly, EAditonic calculated breath-by-breath correlated well with EAdimin5 (r=0.91, p<0.001). There were no significant differences in EAdimax5 or EAdimin5 over days. Within a subject, EAdimax5 and EAdimin5 were variable over the one hour of recording (CV EAdimax5 = 95%; CV EAdimin5 = 94%), and between days (mean variability between days, CV EAdimax5 =24%; CV EAdimin5 = 26%). The between subject variability was 31% for EAdimax5 and 15% for EAdimin5.
The EAdi signal was stable over the duration of the study period (days). The absolute noise level was constant between study days within a subject (mean variability in noise level day-to-day, CV =1.6%) and variability was low between subjects (CV =7.9%). Figure 5 demonstrates in one subject examples of the EAdi signal recorded for five hours over 4 sequential days.
Figure 5. Representative tracings of the different neural breathing patterns on different days.
Each panel demonstrates the EAdi waveform obtained in one subject on the different recording days. A= Day 1 AM, B= Day 1 PM, C= Day 2 AM, D= Day 3 AM, E = Day 4 AM
The prevalence of breathing pattern types (types 1–7) for each infant was analyzed for every hour of recorded data (n = 46 hours) and is presented in Table 3. For the group average, 68% of the time was spent with a relatively “normal” breathing pattern (regular phasic breathing without tonic activity), and 29% of the time breathing was phasic with elevated tonic activity.
Table 3.
Prevalence of neural breathing pattern types
| subject | Type 1 | Type 2 | Type 3 | Type 4 | Type 5 | Type 6 | Type 7 |
|---|---|---|---|---|---|---|---|
| apnea | Phasic no tonic |
Sigh no tonic |
Phasic with tonic |
Sigh with tonic |
Tonic “bursts” |
unidentifiable | |
| 1 | 0.97 | 83.05 | 1.38 | 13.63 | 0.69 | 0.18 | 0.11 |
| 2 | 2.07 | 68.41 | 0.64 | 27.30 | 1.17 | 0.23 | 0.19 |
| 3 | 1.17 | 68.79 | 0.63 | 28.52 | 0.70 | 0.08 | 0.10 |
| 4 | 1.39 | 65.48 | 1.08 | 30.96 | 0.95 | 0.07 | 0.03 |
| 5 | 0.91 | 79.23 | 1.81 | 16.29 | 1.52 | 0.12 | 0.12 |
| 6 | 0.40 | 70.88 | 1.22 | 26.30 | 0.91 | 0.11 | 0.17 |
| 7 | 0.20 | 77.43 | 1.09 | 20.26 | 0.85 | 0.07 | 0.10 |
| 8 | 0.42 | 71.29 | 0.41 | 27.75 | 0.11 | 0.03 | 0.00 |
| 9 | 0.13 | 44.05 | 0.68 | 53.58 | 1.45 | 0.02 | 0.09 |
| 10 | 0.19 | 54.15 | 0.67 | 44.36 | 0.55 | 0.08 | 0.00 |
| mean | 0.79 | 68.28 | 0.96 | 28.90 | 0.89 | 0.10 | 0.09 |
| SD | 0.63 | 11.70 | 0.43 | 12.16 | 0.42 | 0.06 | 0.06 |
| min | 0.13 | 44.05 | 0.41 | 13.63 | 0.11 | 0.02 | 0.00 |
| max | 2.07 | 83.05 | 1.81 | 53.58 | 1.52 | 0.23 | 0.19 |
Central apnea and heart rate
Despite representing a small percentage of the hour, the maximum number of Type 1 (central apnea>5s) events occurred on average 10 times per hour (range 2–29 per hour). Figure 6 demonstrates in one infant the EAdi waveforms (bottom tracings in each panel) with the simultaneous measurement of ECG. The slowing of the heart rate can be observed in Figure 6B during the apneic period. For the group of infants (all hours), there was a significant reduction in heart rate for central apneas 5–10 sec in duration, which became stronger for central apneas 10–20 s in duration and for central apneas >20 sec duration (FIGURE 7).
Figure 6. Representative tracings of EAdi and ECG in one subject.
Time sequence is Figure 6A to 6D. Each panel demonstrates the EAdi waveform (bottom in each panel) and the ECG. A = thirty seconds prior to the apnea, heart rate was 153, and saturation 98%. B = slowing in heart rate at the same time as the central apnea. C= Heart rate 66, saturation 82%, and the nurse aroused the baby (D). Three minultes later, HR = heart rate 159, saturation 99%.
Figure 7. Relationship between central apnea duration and heart rate.
Group data demonstrating that longer apneas are associated with greater reductions in heart rate. * indicates statistical significance from beginning to end of apnea.
Sighs
The maximum number of sighs (types 3 and 5 combined) ranged between 12 and 59/hour, among infants.
Neural breathing pattern pre and post-apnea
Of all the central apneas identified (n=188), 31.3% were followed by Type 3, 4, 5, or 6 (sighs or patterns with tonic EAdi) within 10 seconds of resolution of the apnea. 86% of the central apneas were preceded by Type 2 (regular phasic breathing without tonic). Only 9 of all the central apneas detected were preceded by a type 3 or 5 (sigh) (4.8 %). Infants receiving caffeine (n=7) had more central apneas (>5sec) than those that did not receive caffeine (13 vs. 6 per hour), but had more type 3 and 5 (sighs) (38 vs 24 per hour). The number of central apneas did not correlate with the EAdi-time product.
DISCUSSION
In the present study, we present a new method for simultaneous monitoring of neural respiratory drive and heart rate using a standardized and relatively non-invasive method. The EAdi signal was found to be stable over 4 days of recording. In this group of preterm infants, the EAdi signal was characterized as variable, with regards to timing, amplitude and pattern with, in many cases, a distinct amount of tonic diaphragm activity. Pattern recognition analysis revealed that more than one third of the neural breathing pattern is not “regular” phasic breathing. Central apneas >5sec were frequent and were associated with reductions in heart rate that were greater for longer respiratory pauses. Either “sighs” and/or tonic activity followed one third of these apneic events.
Phasic and Tonic EAdi
The output from the respiratory centers is determined through a complex set of functions including responses to inputs from many receptors. The Hering-Breuer (HB) reflexes play a key role in the control of breathing in infants (22–24). The HB reflexes are vagally-mediated mechano-reflexes, and respond to inflation beyond a critical volume, as well as deflation below end-expiratory lung volume (25).
In the present study, the amplitude and prevalence of tonic activity of the diaphragm was found to be elevated compared to adults, whose neural breathing pattern can be characterized by regular phasic breathing with a return of the EAdi waveform to baseline between breaths (26). The tonic EAdi is believed by several authors to be one of the mechanisms to prevent lung de-recruitment (14, 16, 27, 28). D’Angelo (11) showed with rib cage compression that there is an instantaneous augmentation in the post-inspiratory activity in all regions of the rabbit diaphragm. Allo et al (17) and Emeriaud (14) demonstrated that removing positive ned-expiratory pressure (PEEP) increases tonic EAdi. It is thought that tonic EAdi “brakes” against the passive recoil of the lung and chest wall (16, 28).
In the face of alveolar instability and low compliance, it could be hypothesized that those infants with lower GA would demonstrate higher levels of tonic EAdi to maintain end expiratory lung volume (EELV). Several other mechanisms may play a role in maintaining EELV, such as increased respiratory rate (14, 16, 28), which correlated with GA in the present study. The braking of expiratory flow with laryngeal muscles also has a role in preventing de-recruitment (28, 29), however, this was not evaluated in the present study. We observed up to 59 large neural inspirations per hour; these “sighs” have been identified in preterm infants (30), and are hypothesized to be a mechanism to recruit the lung, especially after an apnea (3). Whether or not the type 3 and type 5 patterns occurred in order to recruit the lung - or if they were inspiratory efforts during obstructive apneas – could not be determined. We can only speculate that these neural breathing patterns (tonic EAdi and sighs) were adopted to regulate EELV, since no direct measurements of lung volume were performed.
Central apneas
Premature infants often demonstrate recurring apneas, which is more frequent in infants of lower GA and birth weight (2, 31). In the present study, we did not observe this relationship perhaps due to the lack of distinction made between central and obstructive apneas, both types being associated with absence of airflow. In the present study, central apneas were evaluated and defined as no diaphragm activity> 5 sec. We are confident that the loss of EAdi signal was not due to malfunctioning of the electrodes since the ECG could still be observed.
One possibility to explain the occurrence of central apnea is that the diaphragm may be overexerted by the continuous and elevated activity of the diaphragm. In this case, central apneas could actually be considered as a strategy for diaphragmatic rest. However, our results of no relationship between the EAdi-time product and the number of central apneas contradict this hypothesis.
It is well known that apnea can lead to oxygen desaturation and bradycardia (32, 33). If prolonged, the apnea and bradycardia episodes can have a negative impact on cerebral circulation (34). These episodes are usually detected when the heart rate or saturation decline to a level where they trigger alarms, alerting personnel about the apneic event. The present study may provide a method for detecting milder forms of apnea prior to the occurrence of detrimental physiological changes.
Techniques for monitoring in the neonatal intensive care unit
In intubated and non-intubated preterm infants, monitoring is crucial for early detection of clinical instability and response to therapy. If the goal is to monitor respiratory drive, in the non-intubated preterm infant, breathing pattern can be monitored non-invasively with the use of pneumotachs, which provide information about tidal volume and flow; however, this instrument may in itself affect the breathing pattern (35) as it imposes an added dead space. Recently, Te Pas et al (6) examined flow patterns using a mask and hot wire anemometer in preterm and term infants immediately after birth, and needed to apply a bias flow to compensate for the added dead-space. Displacement of the chest wall using Respiratory Impedance Plethysmography has also been used for non-invasive measurement of breathing pattern, but requires calibration to a measured tidal volume, and loses accuracy during ventilator assistance and over time (7).
Surface electromyography (EMG) of the respiratory muscles has been used in several physiological studies in infants (5, 23, 24, 28, 29). Surface EMG recordings are susceptible to changes in muscle-to-electrode distance (12) and cross-talk from other muscles (12), for example postural muscles. In light of the fact that premature infants are often not receiving sedation and remain active, surface EMG recordings of EAdi may not represent respiratory activity reliably.
In contrast to surface EMG recordings, the signal processing of the esophageal EAdi “tracks” the movement of the diaphragm along the array (19) every 16 ms, and eliminates common mode signals such as cross-talk signals from postural muscles. This is due to the “double-subtraction” signal processing technique (19). Disturbances induced by swallowing are detected and filtered. The method used in the present study does not interfere with breathing pattern, and does not require calibration, as would measurements of airway flow. As well, the signal properties were found to be stable over 4 days, as demonstrated by the stable noise level. The EAdi measured in the esophagus is thus a measurement of the crural (hiatal) diaphragm activity. Although it has been recognized that the costal and crural behave as separate muscles (36), the crural diaphragm EMG correlates to phrenic nerve activity (10), and to global diaphragm activity (11, 20, 21, 37).
Critique
The present study was designed to characterize neural breathing pattern in preterm neonates under general and routine clinical conditions by utilizing a new method to capture EAdi. Evaluating the effectiveness of this technique in larger, relatively stable infants was felt to be prudent prior to testing in smaller neonates and/or those who have significant respiratory disease. Interventions such as feeding, caffeine administration, sleep/behavioral state and gross body movements – many of which are known to influence apnea and tonic EAdi (36, 38) - were not controlled for. This may explain the high intra-subject variability in neural breathing parameters observed.
In the present study, it was only possible to note the oxygen saturation intermittently. Had a simultaneous recording of oxygen saturation been possible, the interplay between central apnea, bradycardia and desaturation could have been further examined. As well, due to the lack of simultaneous and continuous recordings of oxygen saturation, we could not investigate the possible role between hypoxia and tonic EAdi, a link previously described in animals (39).
Besides measurements of oxygen saturation, it is important to recognize the influence of arterial carbon dioxide (39–41) on diaphragm activity and upper airway muscle activities. In our institution, transcutaneous carbon dioxide measurements, and arterial blood gases are not routinely performed in non-intubated infants, and we cannot comment about the role of carbon dioxide in our findings.
This study demonstrates that the neural breathing pattern in preterm infants is more complex than previously believed. Thirty percent of the time is spent breathing with breathing pattern consisting of a continuously active diaphragm. Monitoring the EAdi provides insight into the neural respiratory pattern of premature infants and may be useful in the assessment of therapeutic interventions to treat apnea.
Acknowledgments
Financial support: J.B. and C.S. were supported by the NIH # 1 R21 HD45047-01; C.S. was supported by the McLaughlin Foundation.
ABBREVIATIONS
- CV
coefficient of variation
- EAdi
electrical activity of the diaphragm
- EAdiphasic
using breath by breath analysis, the peak of the inspiratory EAdi waveform
- EAditonic
using breath by breath analysis, the EAdi value at end-exhalation
- EAdimax5
using 5-sec epochs method, the maximum EAdi value
- EAdimin5
using 5-sec epochs method, the minimum EAdi value
- EELV
end-expiratory lung volume
- EMG
electromygram
- Nrr
using breath-by-breath analysis, neural respiratory rate
- Nte
using breath-by-breath analysis, neural expiratory time
- Nti
using breath-by-breath analysis, neural inspiratory time
- Ntt
Neural total breath time calculated as Nti+Nte
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Drs. Beck and Sinderby have made inventions related to neural control of mechanical ventilation that are patented. The license for these patents belongs to Maquet Critical Care. Future commercial uses of this technology may provide financial benefit to Drs. Beck and Sinderby through royalties. Dr Beck and Dr Sinderby each own 50% of Neurovent Research Inc (NVR). NVR is a research and development company that builds the equipment and catheters for research studies. NVR has a consulting agreement with Maquet Critical Care. A. Slutsky is a paid consultant to Maquet Critical Care.
Part of the data was presented in abstract form at the American Thoracic Society/American Lung Association and the PAS/SPR meetings in 2006.
REFERENCES
- 1.Patzak A, Foitzik B, Mrowka R, Schmalisch G. Time of measurement influences the variability of tidal breathing parameters in healthy and sick infants. Respir Physiol. 2001;128:187–194. doi: 10.1016/s0034-5687(01)00277-8. [DOI] [PubMed] [Google Scholar]
- 2.Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the apnea-of-prematurity group. Pediatrics. 2006;117:S47–S51. doi: 10.1542/peds.2005-0620H. [DOI] [PubMed] [Google Scholar]
- 3.Poets CF, Rau GA, Neuber K, Gappa M, Seidenberg J. Determinants of lung volume in spontaneously breathing preterm infants. Am J Respir Crit Care Med. 1997;155:649–653. doi: 10.1164/ajrccm.155.2.9032208. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Shaweesh JM. Maturation of respiratory reflex responses in the fetus and neonate. Semin Neonatol. 2004;9:169–180. doi: 10.1016/j.siny.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Ochoa JM, Osorio JS, Torres R, McLeod CN. Development of an apnea detector for neonates using diaphragmatic surface electromyography; Conf Proc IEEE Eng Med Biol Soc; 2009. pp. 7095–7098. [DOI] [PubMed] [Google Scholar]
- 6.te Pas AB, Wong C, Kamlin CO, Dawson JA, Morley CJ, Davis PG. Breathing patterns in preterm and term infants immediately after birth. Pediatr Res. 2009;65:352–356. doi: 10.1203/PDR.0b013e318193f117. [DOI] [PubMed] [Google Scholar]
- 7.Emeriaud G, Eberhard A, Benchetrit G, Debillon T, Baconnier P. Calibration of respiratory inductance plethysmograph in preterm infants with different respiratory conditions. Pediatr Pulmonol. 2008;43:1135–1141. doi: 10.1002/ppul.20923. [DOI] [PubMed] [Google Scholar]
- 8.Pandit PB, Pyon KH, Courtney SE, England SE, Habib RH. Lung resistance and elastance in spontaneously breathing preterm infants: effects of breathing pattern and demographics. J Appl Physiol. 2000;88:997–1005. doi: 10.1152/jappl.2000.88.3.997. [DOI] [PubMed] [Google Scholar]
- 9.Parthasarathy S, Jubran A, Tobin MJ. Assessment of neural inspiratory time in ventilator-supported patients. Am J Respir Crit Care Med. 2000;162:546–552. doi: 10.1164/ajrccm.162.2.9901024. [DOI] [PubMed] [Google Scholar]
- 10.Lourenço RV, Cherniack NS, Malm JR, Fishman AP. Nervous output from the respiratory center during obstructed breathing. J Appl Physiol. 1966;21:527–533. doi: 10.1152/jappl.1966.21.2.527. [DOI] [PubMed] [Google Scholar]
- 11.D'Angelo E, Monaco A, D'Angelo E, Pecchiari M. Motor control of the diaphragm in anesthetized rabbits. Respir Physiol Neurobiol. 2010;170:141–149. doi: 10.1016/j.resp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Aldrich TK, Sinderby C, McKenzie DK, Estenne M, Gandevia SC. Electrophysiologic techniques for the assessment of respiratory muscle function. American Thoracic Society/European Respiratory Society 2002 ATS/ERS Statement on Respiratory Muscle Testing. Am J Respir Crit Care Med. 2002;166:518–624. [Google Scholar]
- 13.Beck J, Tucci M, Emeriaud G, Lacroix J, Sinderby C. Prolonged neural expiratory time induced by mechanical ventilation in infants. Pediatr Res. 2004;55:747–754. doi: 10.1203/01.PDR.0000119368.21770.33. [DOI] [PubMed] [Google Scholar]
- 14.Emeriaud G, Beck J, Tucci M, Lacroix J, Sinderby C. Diaphragm electrical activity during expiration in mechanically ventilated infants. Pediatr Res. 2006;59:705–710. doi: 10.1203/01.pdr.0000214986.82862.57. [DOI] [PubMed] [Google Scholar]
- 15.Beck J, Reilly M, Grasselli G, Mirabella L, Slutsky AS, Dunn MS, Sinderby C. Patient-ventilator interaction during neurally adjusted ventilator assist in very low birth weight infants. Pediatr Res. 2009;65:663–668. doi: 10.1203/PDR.0b013e31819e72ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopes J, Muller NL, Bryan MH, Bryan AC. Importance of inspiratory muscle tone in maintenance of FRC in the newborn. J Appl Physiol. 1981;51:830–834. doi: 10.1152/jappl.1981.51.4.830. [DOI] [PubMed] [Google Scholar]
- 17.Allo JC, Beck JC, Brander L, Brunet F, Slutsky AS, Sinderby CA. Influence of neurally adjusted ventilatory assist and positive end-expiratory pressure on breathing pattern in rabbits with acute lung injury. Crit Care Med. 2006;34:2997–3004. doi: 10.1097/01.CCM.0000242520.50665.9F. [DOI] [PubMed] [Google Scholar]
- 18.Barwing J, Ambold M, Linden N, Quintel M, Moerer O. Evaluation of the catheter positioning for neurally adjusted ventilatory assist. Intensive Care Med. 2009;35:1809–1814. doi: 10.1007/s00134-009-1587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinderby CA, Beck JC, Lindström LH, Grassino AE. Enhancement of signal quality in esophageal recordings of diaphragm EMG. J Appl Physiol. 1997;82:1370–1377. doi: 10.1152/jappl.1997.82.4.1370. [DOI] [PubMed] [Google Scholar]
- 20.Beck J, Sinderby C, Lindström L, Grassino A. Effects of lung volume on diaphragm EMG signal strength during voluntary contractions. J Appl Physiol. 1998;85:1123–1134. doi: 10.1152/jappl.1998.85.3.1123. [DOI] [PubMed] [Google Scholar]
- 21.Sinderby C, Beck J, Weinberg J, Spahija J, Grassino A. Voluntary activation of the human diaphragm in health and disease. J Appl Physiol. 1998;85:2146–2158. doi: 10.1152/jappl.1998.85.6.2146. [DOI] [PubMed] [Google Scholar]
- 22.Cross KW, Klaus M, Tooley WH, Weisser K. The response of the new-born baby to inflation of the lungs. J Physiol. 1960;151:551–565. doi: 10.1113/jphysiol.1960.sp006459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerhardt T, Bancalari E. Maturational changes of reflexes influencing inspiratory timing in newborns. J Appl Physiol. 1981;50:1282–1285. doi: 10.1152/jappl.1981.50.6.1282. [DOI] [PubMed] [Google Scholar]
- 24.Kosch PC, Davenport PW, Wozniak JA, Stark AR. Reflex control of expiratory duration in newborn infants. J Appl Physiol. 1985;58:575–581. doi: 10.1152/jappl.1985.58.2.575. [DOI] [PubMed] [Google Scholar]
- 25.Hering E, Breuer J. Reflex of respiration through the vagus nerves. Sitzber Deut Akad Wiss Wein. 1868;57:672–677. [Google Scholar]
- 26.Sinderby C, Beck J, Spahija J, de Marchie M, Lacroix J, Navalesi P, Slutsky AS. Inspiratory muscle unloading by neurally adjusted ventilatory assist during maximal inspiratory efforts in healthy subjects. Chest. 2007;131:711–717. doi: 10.1378/chest.06-1909. [DOI] [PubMed] [Google Scholar]
- 27.Meessen NE, van der Grinten CP, Folgering HT, Luijendijk SC. Tonic activity in inspiratory muscles during continuous negative airway pressure. Respir Physiol. 1993;92:151–166. doi: 10.1016/0034-5687(93)90035-9. [DOI] [PubMed] [Google Scholar]
- 28.Kosch PC, Stark AR. Dynamic maintenance of end-expiratory lung volume in full-term infants. J Appl Physiol. 1984;57:1126–1133. doi: 10.1152/jappl.1984.57.4.1126. [DOI] [PubMed] [Google Scholar]
- 29.Kosch PC, Hutchison AA, Wozniack JA, Carlo WA, Stark A. Posterior cricoarytenoid and diaphragm activities during tidal breathing in neonates. J Appl Physiol. 1988;64:1968–1978. doi: 10.1152/jappl.1988.64.5.1968. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez JE, Bodani J, Fajardo CA, Kwiatkowski K, Cates DB, Rigatto H. Sighs and their relationship to apnea in the newborn infant. Biol Neonate. 1993;63:139–146. doi: 10.1159/000243923. [DOI] [PubMed] [Google Scholar]
- 31.Martin RJ, Abu-Shaweesh JM. Control of breathing and neonatal apnea. Biol Neonate. 2005;87:288–295. doi: 10.1159/000084876. [DOI] [PubMed] [Google Scholar]
- 32.Henderson-Smart DJ, Butcher-Puech MC, Edwards DA. Incidence and mechanism of bradycardia during apnoea in preterm infants. Arch Dis Child. 1986;61:227–232. doi: 10.1136/adc.61.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Fiore JM, Arko MK, Miller MJ, Krauss A, Betkerur A, Zadell A, Kenney SR, Martin RJ. Cardiorespiratory events in preterm infants referred for apnea monitoring studies. Pediatrics. 2001;108:1304–1308. doi: 10.1542/peds.108.6.1304. [DOI] [PubMed] [Google Scholar]
- 34.Perlman JM, Volpe JJ. Episodes of apnea and bradycardia in the preterm newborn: impact on cerebral circulation. Pediatrics. 1985;76:333–338. [PubMed] [Google Scholar]
- 35.Marsh MJ, Ingram D, Milner AD. The effect of instrumental dead space on measurement of breathing pattern and pulmonary mechanics in the newborn. Pediatr Pulmonol. 1993;16:316–322. doi: 10.1002/ppul.1950160508. [DOI] [PubMed] [Google Scholar]
- 36.Henderson-Smart DJ, Johnson P, McClelland ME. Asynchronous respiratory activity of the diaphragm during spontaneous breathing in the lamb. J Physiol. 1982;327:377–391. doi: 10.1113/jphysiol.1982.sp014237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol. 2010;173:101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stokowski LA. A primer of apnea of prematurity. Adv Neonatal Care. 2005;5:155–170. doi: 10.1016/j.adnc.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Bonora M, Boule M, Gautier H. Diaphragmatic and ventilatory responses to alveolar hypoxia and hypercapnia in conscious kittens. J Appl Physiol. 1992;72:203–210. doi: 10.1152/jappl.1992.72.1.203. [DOI] [PubMed] [Google Scholar]
- 40.Wozniak JA, Hutchison AA, Kosch PC. Laryngeal and pump muscle activities during CO2 breathing in neonates. J Appl Physiol. 1993;75:416–423. doi: 10.1152/jappl.1993.75.1.416. [DOI] [PubMed] [Google Scholar]
- 41.Eichenwald EC, Ungarelli RA, Stark AR. Hypercapnia increases expiratory braking in preterm infants. J Appl Physiol. 1993;75:2665–2670. doi: 10.1152/jappl.1993.75.6.2665. [DOI] [PubMed] [Google Scholar]