Abstract
Although the pivotal role of platelet derived growth factor (PDGF)-mediated signaling in vascular diseases was demonstrated, the pathophysiological mechanisms driving its over-activation remain incompletely understood. Tissue transglutaminase (tTG) is a multifunctional protein expressed in the vasculature, including smooth muscle cells (SMCs), and implicated in several vascular pathologies. The goal of this study is to define the regulation of PDGF-BB/PDGFRβ-induced signaling pathways and cell responses by tTG in vascular SMCs. We find that in human aortic SMCs, shRNA-mediated depletion and over-expression of tTG reveals its ability to down-regulate PDGFRβ levels and induce receptor clustering. In these cells, tTG specifically amplifies the activation of PDGFRβ and its multiple downstream signaling targets in response to PDGF-BB. Furthermore, tTG promotes dedifferentiation and increases survival, proliferation and migration of human aortic SMCs mediated by this growth factor. Finally, PDGF-BB stimulates tTG expression in human aortic SMCs in culture and in the blood vessels in response to injury. Together, our results show that tTG in vascular SMCs acts as a principal enhancer within the PDGF-BB/PDGFRβ signaling axis involved in phenotypic modulation of these cells, thereby suggesting a novel role for this protein in the progression of vascular diseases.
Keywords: transglutaminase, PDGF, PDGFR, signaling, vascular SMCs
Introduction
In mammals, vascular smooth muscle cells (SMCs) maintain considerable plasticity throughout lifetime and exhibit a wide range of different phenotypes shaped by alterations in tissue microenvironment (Yoshida and Owens, 2005). Impaired control of the differentiation state of vascular SMCs contributes to a number of vascular pathologies, including restenosis and atherosclerosis (Owens et al., 2004; Cai, 2006). Differentiated SMCs in blood vessels undergo phenotypic modulation in response to tissue injury or inflammation, the process accompanied by increased cell proliferation, migration and ECM protein synthesis, and decreased expression of SMC-specific markers, including smooth muscle (SM) α-actin, myosin heavy chain, SM22α and others (Owens, 2007). SMC-specific gene expression was shown to be mediated by several transcription factors, such as serum response factor and myocardin (Yoshida and Owens, 2005; Owens et al., 2004). They mediate the transduction of regulatory extrinsic cues that impact on the plasticity of vascular SMC phenotype. Multiple growth factors, cytokines, and ECM proteins are involved in this regulation (Owens, 2007). Among them, platelet derived growth factor (PDGF) acting via its cognate receptor, PDGFR, has been established as a key survival factor, mitogen, and motogen for vascular SMCs, and principal regulator of vascular SMC phenotype (Heldin and Westermark, 1999; Raines, 2004; Andrae et al., 2008).
PDGF-BB and PDGFRβ are intimately involved in vascular development as their deficiency impairs the recruitment of vascular mural cell progenitors in the growing arteries (Andrae et al., 2008). Although PDGF-BB and PDGFRβ-deficient mouse embryos develop until E16–E19 they display a grossly abnormal vascular morphogenesis(Hellstrom et al., 2001). PDGF-AA and PDGF-BB expression was shown in various cell types in atherosclerotic arteries and their levels are elevated over those in the normal vessel wall(Raines, 2004). PDGFRα and PDGFRβ levels are also increased in vascular SMCs in atherosclerosis (Raines, 2004; Andrae et al., 2008). Neutralizing PDGFRβ antibodies and PDGFR kinase inhibitors were found to decrease atherosclerotic lesion formation in ApoE-deficient mice (Sano et al., 2001; Kozaki et al., 2002). Similarly, hyper-activation of the PDGF/PDGFR signaling axis was shown in animal models of acute arterial injury (Andrae et al., 2008) in which accumulation of neointimal SMCs was significantly attenuated by neutralizing PDGF or blocking PDGFR signaling(Ferns et al., 1991; Levitzki, 2005). Finally, a use of genetic approach in conjunction with carotid ligation model showed a critical role of PDGFRβ in the migration of vascular SMCs from media to neointima (Buetow et al., 2003), whereas no involvement of PDGFRα in neointima formation during acute or chronic arterial injury was found (Andrae et al., 2008).
An emerging theme in the field indicates a significant role for various modifiers of the PDGF/PDGFR signaling axis in vascular pathophysiology(Heldin and Westermark, 1999; Andrae et al., 2008). One important example comes from studies on the function of transmembrane endocytic and signaling receptor low-density lipoprotein receptor-related protein 1 (LRP1) in the vessel wall. While LRP1 was shown to interact with PDGFRβ, its SMC-specific knockout led to markedly increased atherosclerotic response in an LDL receptor-deficient background(Boucher et al., 2003). This response was mediated by robust elevation of the expression and autophosphorylation levels of PDGFRβ in vascular SMCs. These findings revealed atheroprotective role of the PDGFRβ interacting partner, LRP1, in controlling the PDGFRβ levels and activation in the vessel wall (Boucher et al., 2003). Likely, other proximal activators and inhibitors of PDGFR function in vascular SMCs remain to be identified.
tTG is a ubiquitously expressed member of transglutaminase family of protein cross-linking enzymes, which in addition to its enzymatic activity has important non-enzymatic functions(Lorand and Graham, 2003; Zemskov et al., 2006). On the cell surface, tTG binds to β 1 and β 3 integrins and mediates their interaction with fibronectin (Akimov et al., 2000; Zemskov et al., 2006). The adhesive/signaling function of cell surface tTG is based on its ability to regulate integrin-mediated adhesion, signaling, and downstream integrin-mediated cell responses such as cell survival, growth, migration, and ECM assembly(Zemskov et al., 2006). Importantly, recent studies point to emerging role of this protein in vascular pathologies (Sane et al., 2007; Bakker et al., 2008), including atherosclerosis (Haroon et al., 2001; Cho et al., 2008; Matlung et al., 2010), vascular calcification (Faverman et al., 2008; Johnson et al., 2008), small artery remodeling(Bakker et al., 2004; Pistea et al., 2008), and age-dependent aortic stiffening(Santhanam et al., 2010). Despite these advances, the molecular basis for the involvement of tTG in these processes remains largely unknown. Meanwhile, our recent work revealed a direct interaction of tTG with PDGFR on the cell surface and amplification of PDGF-dependent activation of this receptor and its signaling function in fibroblasts (Zemskov et al., 2009). Here we set to define the impact of tTG on the PDGF-BB/PDGFRβ-mediated signaling axis and its regulation of cellular phenotype in vascular SMCs.
Materials and Methods
Animals
The animal protocol used was approved by the Institutional Animal Care and Use Committee at the University of Maryland. 12 week-old male wild type C57BL/6J mice were used in these experiments. Complete ligation model of restenosis in the left carotid artery of mice (Kumar and Lindner, 1997) was employed to induce arterial remodeling and neointima formation due to cessation of blood flow during 4 weeks.
Cell culture and lentiviral infection
Human aortic SMCs were obtained from Lonza and cultured according to vendor’s protocol. The cells at 4th passage were infected with Trans-Lentiviral particles containing shRNA for human tTG, control non-silencing shRNA or human tTG cDNA (NM_004613) in pGIPZ™ vector with the use of Arrest-In™ or Express-In™ reagents (all from Thermo Scientific). The lentivirus-infected cells were selected with puromycin and used between 7th and 10th passages.
Reagents
mAbs CUB7402 and TG100 and rabbit polyclonal antibody against tTG were from Neomarkers. The following antibodies were from Santa Cruz Biotechnology (PDGFRβ, sc432; pTyr751-PDGFRβ, sc12906; pTyr716-PDGFRβ, sc19569; FGFR-1, sc123; epidermal growth factor receptor, sc03; pTyr861-FAK, sc16663; pTyr317-Shc, sc-18075; pp38MAPK, sc7975R; SM α-actin, sc130617;β-tubulin, sc9104); Cell Signaling Technology (PDGFRα, 3164; pTyr740-PDGFRβ, 3168; pTyr771-PDGFRβ, 3124; pTyr1021-PDGFRβ, 2227; pThr202-pTyr204-ERK1/2, 9101; pTyr418-src, 2113; pThr308-Akt1, 4056; pSer473-Akt1, 2337; pTyr542-Shp-2, 3751); R@D Systems (neutralizing antibody AF385 against the extracellular domains of human PDGFRβ is specific for the β isoform of the receptor and does not react with the α isoform; neutralizing antibody MAB765 against the extracellular domains of human FGFR-1); BD Pharmingen (rat anti-mouse/human β 1 integrin, clone 9EG7, 553715); Sigma (pTyr397-FAK); and Millipore (β 1 integrin cytoplasmic domain, AB1952). Horseradish peroxidase-conjugated secondary antibodies were from Pierce. Cross-species-adsorbed donkey anti-mouse AlexaFluor 594, donkey anti-rabbit AlexaFluor 488, and donkey anti-rat AlexaFluor 350 antibodies were from Molecular Probes.
Immunofluorescence and immunohistochemistry
To simultaneously visualize tTG, PDGFRβ, and β 1 integrins on the surface of SMCs, quiescent or PDGF-BB-treated live non-permeabilized cells were triple-labeled with mouse anti-tTG mAb CUB7402, goat antibody specific for the PDGFRβ receptor isoform (R@D Systems, AF385), and rat mAb 9EG7 againstβ 1 integrins (each at 20 μg/ml) for 30 min at 4°C. The cells were washed, fixed with 3% paraformaldehyde in PBS, and stained with a mixture of secondary anti-mouse AlexaFluor 350 IgG, donkey anti-goat AlexaFluor 488 IgG, and donkey anti-rat AlexaFluor 594 IgG. To test surface distribution of PDGFRβ in cells with normal or depleted levels of tTG, double staining for tTG and PDGFRβ was performed by co-incubation of PDGF-BB-treated live non-permeabilized cells with a combination of 20 μg/ml mouse anti-tTG mAb CUB7402, goat antibody to PDGFRβ for 30 min at 4°C, fixing with 3% paraformaldehyde in PBS, and staining with secondary anti-mouse AlexaFluor 594 IgG and donkey anti-goat AlexaFluor 488 IgG. Cells were viewed and photographed with 100× objective using Zeiss/Bio-Rad 200 confocal microscope. Images were acquired and digitally merged with Volocity software (Improvision).
For immunohistochemical detection of tTG and tTG-generated isopeptide cross-links, 5-μm paraffin sections of the entire necks were generated. Rabbit polyclonal antibody against tTG (Neomarkers) and mAb 81D2 against the tTG cross-links (Abcam) were used to identify the protein and its enzymatic footprints on tissue esctions. Staining for elastin was performed as described(Kumar and Lindner, 1997).
Immunoblotting
Quiescent or PDGF-BB-treated cells were lysed directly in SDS-PAGE sample buffer. Proteins (20 μg per sample) were separated on 4 12% Bis/Tris Novex gels (Invitrogen), electroblotted on polyvinylidene difluoride membranes, and probed with antibodies to the cytoplasmic domain of PDGFRα or PDGFRβ, their individual pTyr residues, or several downstream signaling targets of the receptor (ERK1/2, Akt1, Shp-2, Src, Shc, FAK, p38MAPK). Peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (ECL) were used for signal detection. The signals were quantified with NIH Image 1.63f software and averaged for each individual phospho-site.
Cell differentiation, survival, migration and proliferation assays
The levels of tTG, fibronectin, SM α-actin, and tubulin mRNAs in serum-starved and PDGF-BB-treated populations of human aortic SMCs were determined by isolation of total RNA with TRIzol™ (Invitrogen) and quantitative RT-PCR with specific primers. In parallel, the protein levels of tTG, fibronectin, SM α-actin, and tubulin were determined by immunoblotting with whole cell lysates.
Serum-starved populations of human aortic SMC were stimulated to undergo apoptosis with 100 μM H2O2 for 12 hours in the presence or absence of 10 nM PDGF-BB and 20 μg/ml AG1296 (Vantler et al., 2005). Cell death detection ELISA (Roche Diagnostics) was utilized to measure cell death by quantitation of cytoplasmic histone-associated DNA fragments (mono- and oligo-nucleosomes) in cell lysates.
To analyze the effects of tTG on PDGF-dependent cell proliferation, the populations of human aortic SMCs were plated at 5×103 cells per well in 96-well microtiter plates and starved for 48 hours. PDGF-BB (0 10 nM with or without 20 μg/ml PDGFR inhibitor AG1296) was added, and cell proliferation was determined 72 hours later by the addition of the CellTiter 96® AQueous MTS-based Reagent (Promega, G3580) during the last 4 hours of culture. The quantity of formazan product proportional to the number of metabolically active live cells was measured at 490 nm and converted to cell numbers.
Chemotactic migration of human aortic SMC (5×104 cells/insert) toward PDGF-BB gradient under serum-free conditions was studied in Transwells with 8-μm pores (Costar). Before plating the cells into the inserts, they were metabolically labeled with Tran35S-label. PDGF-BB (0 10 nM) was added to the lower chambers and AG1296 (20 μg/ml) was added to both upper and lower chambers of some wells to inhibit PDGFR activation. After incubation for 8 hours at 37°C, cells transmigrated to the membrane undersurface were detached, radioactivity was counted in a scintillation counter and converted to cell numbers.
Other methods and statistics
Quantitative RT-PCR with specific primers was used to determine the levels of tTG, PDGFRα, PDGFRβ, SM α-actin and tubulin mRNAs in the populations of human aortic SMCs. Metabolic labeling of human aortic SMCs with Tran35S-label (MP Biomedicals) was performed as described(Zemskov et al., 2009). PDGFRβ immunoprecipitated from cell extracts was analyzed by SDS-PAGE and fluorography. To quantify the relative PDGFRβ amounts in each sample, gel slices containing the 35S-labeled protein bands were dissolved in 30% H2O2, and 35S radioactivity was determined by scintillation counting.
For flow cytometry, live populations of human aortic SMCs were stained for cell surface tTG as reported (Akimov et al., 2000). The cells were analyzed in FACScan™ flow cytometer (Becton Dickinson).
Statistical significances were determined using unpaired, two-tailed Student’s t tests. Differences were considered significant if the P value was <0.05.
Results
tTG down-regulates PDGFRβ levels by accelerating receptor turnover and induces receptor clustering in vascular SMCs
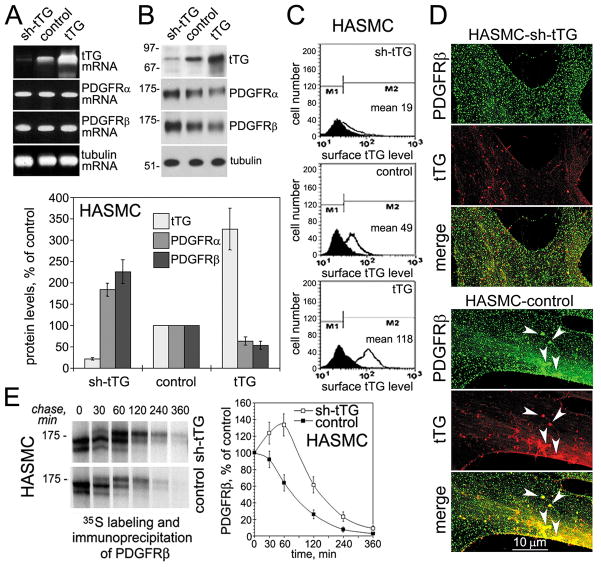
To determine the impact of tTG on the PDGFRβ function in vascular SMCs, we generated human aortic SMC populations stably expressing sh-tTG, non-silencing control, or overexpressing this protein (Fig. 1). Altering the levels of tTG in these cells did not affect those of PDGFRα and PDGFRβ mRNAs (Fig. 1A). However, tTG down-regulated the PDGFRα and PDGFRβ protein levels, suggesting that it acts posttransriptionally to modulate these receptors (Fig. 1B). The overall tTG levels in these populations correlated with those on the cell surface (Fig. 1C). Double staining for tTG and PDGFRβ revealed their partial colocalization on the surface of human aortic SMCs (Fig. 1D), which was further increased by PDGF-BB treatment (Supplemental Fig. 1). However, while the two proteins were often codistributed in large clusters throughout the lamellae, tTG depletion led to disappearance of PDGFRβ clusters in these cells. To study mechanisms of tTG-mediated PDGFRβ down-regulation, metabolic labeling and pulse-chase assays were performed with the cells expressing different tTG levels in the absence of exogenous PDGF-BB (Fig. 1E). Immunoprecipitation of 35S-labeled PDGFRβ from cell extracts showed that tTG decreased the amounts of de novo synthesized PDGFRβ 30 min after onset of the chase, and the receptor levels continued to decline faster thereafter. Additional experiments confirmed that PDGF-BB accelerated PDGFRβ internalization from the surface and revealed that tTG promotes this process (Supplemental Fig. 2). Therefore, tTG stimulates PDGFRβ clustering but reduces overall receptor levels by accelerating its turnover in vascular SMCs.
Figure 1. tTG regulates PDGFRβ receptor levels and localization in vascular SMCs.
(A) tTG does not alter PDGFRα and PDGFRβ mRNA levels in vascular SMCs. mRNA levels of tTG, PDGFRα, PDGFRβ, and tubulin were determined by quantitative RT-PCR in human aortic SMCs expressing sh-tTG, control vector, and tTG. (B) tTG down-regulates PDGFRα and PDGFRβ levels in vascular SMCs. The levels of tTG, PDGFRα, PDGFRβ, and tubulin were defined by immunoblotting. The amounts of tTG, PDGFRα, and PDGFRβ were quantified by densitometry, averaged, and expressed as % change compared to those in control cells. (C) Surface tTG levels were defined by immunostaining of live nonpermeabilized cells and flow cytometry. (D) tTG promotes PDGFRβ clustering in vascular SMCs. Human aortic SMCs that express non-silensing control (HASMC-control) or tTG (HASMC-shtTG) shRNA were double stained for PDGFRβ (green) and tTG (red). Note large PDGFRβ clusters containing tTG in the lamellae of HASMSC-control cells (arrowheads, bottom) and their lack in tTG-deficient HASMC-shtTG cells (top). (E) tTG promotes PDGFRβ turnover. HASMC-shtTG and HASMC-control cells were pulse-labeled with Tran35S-label™ for 30 min without PDGF-BB and then chased for indicated times in the absence of growth factors. PDGFRβ was immunoprecipitated from SDS-denatured cell extracts containing 200 μg total cell protein. The immune complexes were resolved by SDS-PAGE and detected by fluorography. The amounts of de novo synthesized PDGFRβ were defined by 35S scintillation counting and presented as percentages of those in the cells before start of the chase. (B,E) Shown are the means ± S.D. for three independent experiments.
Earlier work revealed a potentiation of PDGF-BB-induced signaling by PDGFRβ interaction with β 1 integrins(Sundberg and Rubin, 1998). Importantly, cell surface tTG interacts with β 1 integrins (Akimov et al., 2000) and binds to PDGFRβ in vitro and on the cell surface(Zemskov et al., 2009). We also found that all these three proteins co-localized in large clusters on the surface of PDGF-BB-treated human aortic SMCs and the increase in tTG levels elevated the amounts of PDGFRβ-associated tTG and PDGFRβ-β 1 integrin complexes (Supplemental Fig. 3). Thus, we proposed that tTG can bridge these receptors on the cell surface and amplify their signaling output in vascular SMCs.
tTG increases PDGF-induced PDGFRβ activation and amplifies downstream signaling in vascular SMCs
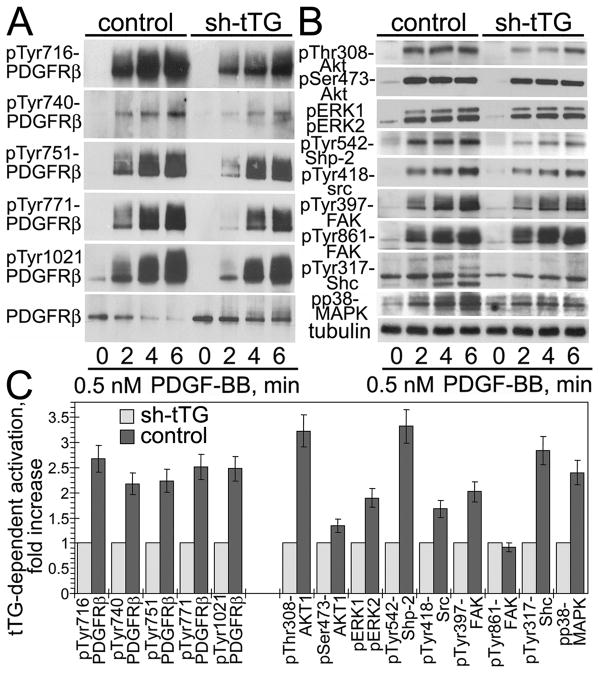
To examine the impact of tTG on the PDGF-BB/PDGFRβ signaling axis in human aortic SMCs, we tested the effect of its depletion on PDGF-BB-mediated activation of PDGFRβ and its targets (Fig. 2). Evaluation of the early time course of PDGFRβ autophosphorylation at Tyr residues 716, 740, 751, 716, and 1021 in its cytoplasmic tail revealed that tTG accelerated and amplified maximal receptor activation (Fig. 2A). The effect of tTG on the activation of multiple PDGFRβ downstream targets by PDGF-BB essentially mirrored that on the receptor activation (Fig. 2B). The PDGF-BB-mediated activation of selected PDGFRβ signaling targets Akt1, ERK1/2, Shp-2, Src, FAK, Shc, and p38 MAPK was attenuated and inhibited by tTG down-regulation. Quantification of phospho-site signals in these targets showed that some of them were particularly sensitive to the tTG levels, with the extent of phosphorylation of Shp-2 at Tyr542, Akt1 at Thr308, and Shc at Tyr317 declining the most upon depletion of tTG, indicating that the associated signaling pathways are primarily affected by this protein (Fig. 2C). At the same time, depletion of tTG in human aortic SMCs did not alter the overall protein levels of multiple PDGFRβ signaling targets (Supplemental Fig. 4). Therefore, tTG down-regulation in human aortic SMCs decreases PDGF-BB-dependent PDGFRβ activation and inhibits signaling by its downstream targets. In vascular SMCs, tTG raises the magnitude of PDGFRβ activation by the soluble ligand, PDGF-BB, and amplifies the receptor downstream signaling. These tTG-dependent alterations are specific for this signaling receptor, since the activation levels of FGFR-1 by FGF2 and of EGFR by HB-EGF were not affected by altering tTG levels in these cells (Supplemental Fig. 5).
Figure 2. tTG increases PDGF-BB-dependent activation of PDGFRβ and its downstream signaling targets in vascular SMCs.
Adherent quiescent human aortic SMCs expressing non-silencing shRNA (control) or tTG shRNA (shtTG) were treated with 0.5 nM PDGF-BB for 0 6 min. (A,B) Activation levels of PDGFRβ (A) and its downstream targets (B) were defined by immunoblotting with antibodies to pTyr716-PDGFRβ, pTyr740-PDGFRβ, pTyr751-PDGFRβ, pTyr771-PDGFRβ, pTyr1021-PDGFRβ, PDGFRβ, pThr308-Akt1, pSer473-Akt1, pERK1/2, pTyr542-Shp-2, pTyr418-src, pTyr397-FAK, pTyr861-FAK, pTyr317-Shc, and pp38MAPK. All samples were normalized for equal amounts of tubulin. (C) Phospho-site signals were quantified, averaged, and expressed for control cells as -fold activation over those in shtTG-expressing cells. Shown are the means ± S.D. for three independent experiments.
tTG promotes PDGF-BB-induced dedifferentiation of vascular SMCs
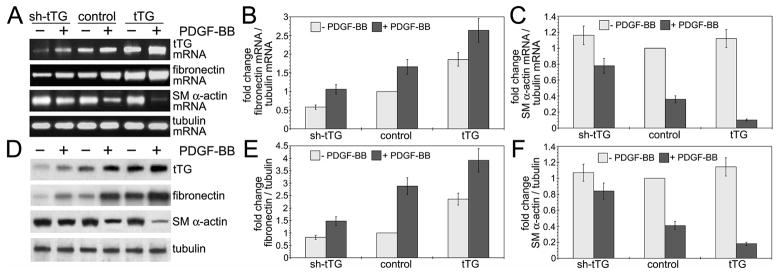
PDGF-BB is the most potent negative regulator of vascular SMC differentiation (Raines, 2004; Andrae et al., 2008), which also up-regulates the levels of fibronectin (Hedin et al., 1988), a major ECM binding partner of tTG (Akimov et al., 2000). In contrast, the PDGF-BB-induced transition of vascular SMCs from contractile to synthetic phenotype is accompanied by down-regulation SM α-actin and several other SMC differentiation markers by transcriptional mechanisms (Holycross et al., 1992). Meanwhile, the role of tTG in phenotypic modulation of vascular SMCs has not been explored. Hence, we employed quantitative RT-PCR and immunoblotting to determine the levels of fibronectin and SMα-actin mRNAs and proteins in human aortic SMCs with different tTG levels (Fig. 3). tTG expression increased the fibronectin mRNA levels in these cells both in the absence and presence of PDGF-BB (Fig. 3A,B). Conversely, the increase in tTG amplified the PDGF-BB-induced down-regulation of the SM α-actin mRNA levels, but did not alter those in the absence of this growth factor (Fig. 3A,C). These alterations were also mirrored in the tTG-dependent up-regulation of fibronectin (Fig. 3D,E) and PDGF-BB-mediated down-regulation of SM α-actin, which was further enhanced by tTG (Fig. 3D,F). Therefore, we concluded that tTG expression levels in vascular SMCs control those of the fibronectin mRNA and protein. Moreover, much like its key ECM partner, tTG serves as a marker of secretory phenotype by enhancing the PDGF-BB-mediated dedifferentiation of these cells via transcriptional down-regulation of SM α-actin expression levels.
Figure 3. tTG promotes PDGF-BB-mediated dedifferentiation of vascular SMCs.
(A,B) Adherent quiescent human aortic SMCs expressing tTG shRNA (shtTG), non-silencing shRNA (control) or tTG were treated with 10 nM PDGF-BB for 72 hours. (A) tTG increases fibronectin mRNA levels and enhances PDGF-BB-dependent down-regulation of SM α-actin mRNA. mRNA levels of tTG, fibronectin, SM α-actin, and tubulin were determined by quantitative RT-PCR. (D) tTG increases the levels of fibronectin and promotes PDGF-BB-induced down-regulation of SM α actin. The levels of tTG, fibronectin, SM α-actin, and tubulin were defined by immunoblotting. The amounts of fibronectin and SM α-actin mRNAs (B,C) and proteins (E,F) were quantified by densitometry, averaged, and expressed as -fold increase over those in control cells without PDGF-BB treatment. Shown are the means ± S.D. for three independent experiments.
tTG stimulates PDGF-BB-dependent survival, proliferation, and chemotactic migration of vascular SMCs
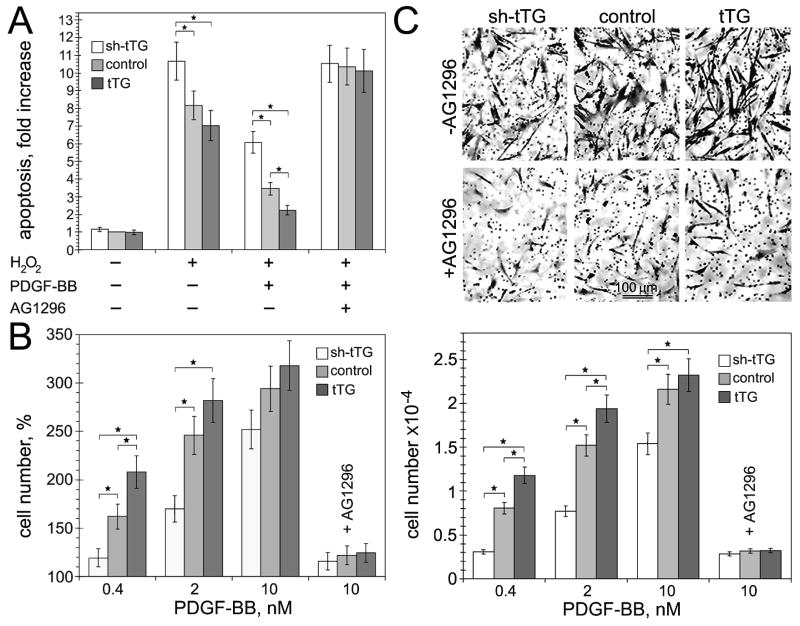
PDGF-BB was shown to act as a principal survival factor, mitogen, and motogen for vascular SMCs(Heldin and Westermark, 1999; Raines, 2004; Andrae et al., 2008), whereas the impact of tTG on these processes has not been studied. Hence, we examined the effects of tTG on PDGF-BB-induced survival, growth, and migration of human aortic SMCs with various levels of this protein (Fig. 4). tTG had only marginal effects on the extent of peroxide-induced apoptosis of these cells in the absence of PDGF-BB (Fig. 4A). While this growth factor increased the resistance of cells to peroxide-induced apoptosis for all the cell populations, its effect was proportional to the cellular tTG levels. Likewise, whereas PDGF-BB displayed a potent concentration-dependent mitogenic effect in human aortic SMCs, its impact on cell proliferation was significantly amplified by tTG (Fig. 4B). Similarly, PDGF-BB-dependent chemotactic migration of human aortic SMCs was enhanced by the cells expressing increased tTG levels (Fig. 4C). Notably, the observed stimulatory effects of tTG on the survival, proliferation, and migration of human aortic SMCs were abolished by specific and potent inhibitor of PDGFR tyrosine kinase activity, AG1296 (Fig. 4A–C). Therefore, tTG specifically promotes the survival, growth, and chemotactic migration of vascular SMCs driven by the PDGF-BB/PDGFRβ-mediated signaling.
Figure 4. tTG stimulates PDGF-BB-induced survival, proliferation, and migration of vascular SMCs.
(A) tTG promotes PDGF-BB-mediated vascular SMC survival. Apoptosis of adherent quiescent human aortic SMCs expressing tTG shRNA (shtTG), non-silencing shRNA (control) or tTG was induced by treatment with 100 μM H2O2 for 12 hours with or without 10 nM PDGF-BB and 20 μg/ml AG1296. The numbers of apoptotic cells were quantified and expressed as -fold increase over those in the control cells without H2O2 and PDGF-BB. (B) tTG promotes PDGF-BB-mediated vascular SMC proliferation. Proliferation of adherent serum-starved human aortic SMCs expressing tTG shRNA (shtTG), non-silencing shRNA (control) or tTG was measured after induction with 0–10 nM PDGF-BB for 72 hours. Cell numbers were quantified and expressed as % change compared to those in the population of control cells without PDGF-BB. (C) tTG stimulates PDGF-BB-mediated migration of vascular SMCs. Chemotactic migration of 5×104 35S-labeled adherent quiescent human aortic SMCs expressing tTG shRNA (shtTG), non-silencing shRNA (control) or tTG through 8-μm membrane pores was studied in Transwells™ with 0–10 nM PDGF-BB in lower chambers. After 12 hours, transmigrated cells were removed from the filter bottoms; radioactivity was counted and converted to cell numbers. The upper panels show membrane undersides stained with crystal violet. Bar - 100 μm. (A–C) Some samples contained PDGFR inhibitor AG1296. Shown are the means ± S.D. for three independent experiments with measurements performed in triplicate, *P< 0.05.
tTG levels are increased in blood vessels in response to injury and by PDGF-BB in cultured vascular SMCs
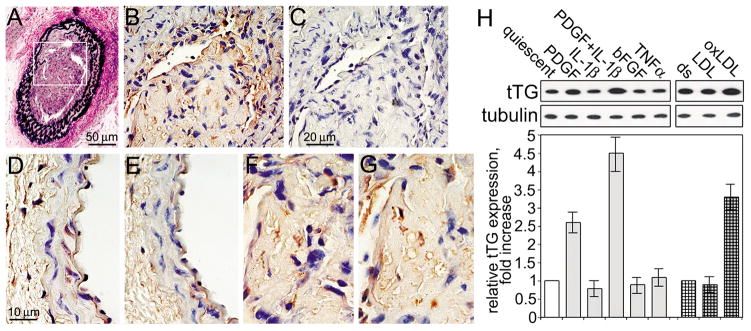
To further explore the potential role of tTG in modulation of the PDGF-BB/PDGFRβ autocrine/paracrine signaling in the vasculature, we evaluated its protein levels and extracellular enzymatic activity in uninjured mouse carotid artery and in the neointima after injury to the vessel wall (Fig. 5A–G). tTG and the tTG-generated isopeptide cross-links were detected primarily in the endothelium of uninjured artery, while their levels appeared to be low in the normal media. However, elevated levels of tTG and the enzymatic tTG-generated cross-links were observed in the neointima of carotid artery 4 weeks after vessel wall injury. We also evaluated the tTG levels in human aortic SMC cultures treated with PDGF-BB, proinflammatory cytokines, or proatherogenic LDLs (Fig. 5H). Stimulation of quiescent cells with PDGF-BB increased the amounts of tTG, whereas IL-1β further amplified this effect (left panels). Likewise, proatherogenic oxidized LDLs, but not native unmodified LDLs, up-regulated the tTG levels (right panels). Hence, PDGF-BB and proinflammatory/proatherogenic factors present at the site of vessel wall injury increase the levels of tTG in vascular SMCs. Together, our findings indicate that tTG acts as a potent enhancer within the PDGF-BB/PDGFRβ signaling axis in the vasculature (Fig. 6).
Figure 5. PDGF-BB and injury increase tTG levels in vascular SMCs.
(A–G) Left mouse carotid artery was wire-injured (Lindner et al., 1993) and neointima was allowed to form for 4 weeks. (A) Transverse section of the injury area was stained to visualize elastin. The area marked by rectangle shows neointima formation. (B,C) Extracellular tTG-generated Gln-Lys isopeptides are present in the neointima. Sections were labeled with mAb reacting with tTG-generated Gln-Lys isopeptide (B), or the same antibody preincubated with excess free Gln-Lys isopeptide (C). (D–G) The levels of tTG and extracellular tTG-generated cross-links are increased in neointima. Transverse serial sections of uninjured contralateral (D,E) and neointima in the injured (F,G) carotid arteries were labeled with antibodies to tTG (D,F) or tTG-generated Gln-Lys isopeptides (G,E). The staining was developed with peroxidase-conjugated secondary IgG, DAB substrate, and counter-staining with hematoxylin and eosin. Bars - 50 μm (A), 20 μm (B,C), and 10 μm (D–G). (H) PDGF-BB and oxidized LDLs increase tTG levels in human aortic SMCs. Quiescent adherent cells were treated with 2 nM PDGF-BB, 1 nM IL-1β, their combination, 2 nM basic FGF, or 1 nM TNFα for 48 hours (left panel). Adherent cells in delipidated 10% serum (ds) were treated with 100 μg/ml native or oxidized LDLs for 24 hours (right panel). The levels of tTG and tubulin were defined by immunoblotting. tTG amounts were quantified by densitometry, averaged, normalized for tubulin loadings and expressed as -fold increase over those in cells in quiescence (left panel) or in delipidated serum (right panel). Shown in (H) are the means ± S.D. for three independent experiments.
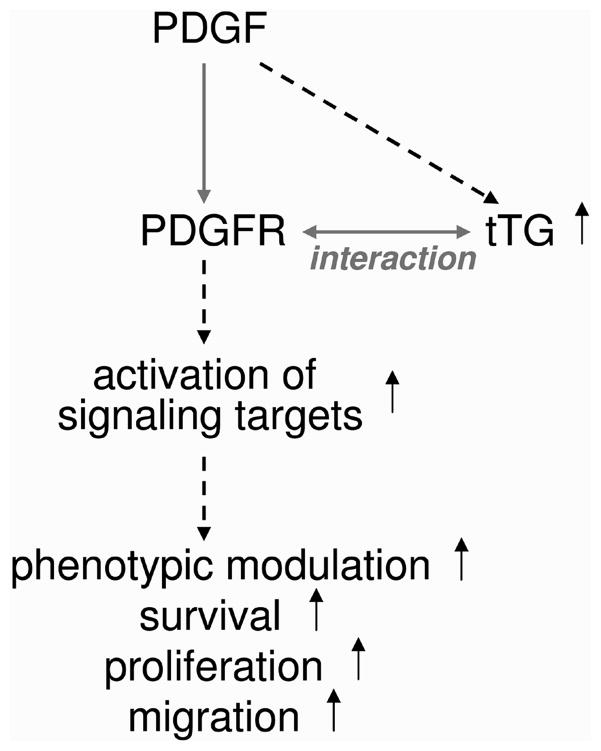
Figure 6. The enhancer role of tTG within the PDGF/PDGFR signaling axis in vascular SMCs.
tTG amplifies PDGF-BB-mediated signaling and downstream responses due to binding PDGFRβ and promoting the receptor activation, whereas tTG own levels are increased by PDGF-BB. See additional comments in the text.
Discussion
Whereas the central role of the PDGF/PDGFR signaling axis and its over-activation has been recognized in atherosclerosis, restenosis, and vascular proliferative diseases (Ferns et al., 1991; Buetow et al., 2003; Raines, 2004), the factors and mechanisms that regulate the activities of this ligand/receptor pair in driving pathophysiological alterations of vascular SMC phenotype remain incompletely understood(Heldin and Westermark, 1999; Andrae et al., 2008). Here we describe the regulation of PDGF-BB/PDGFRβ signaling and associated responses in vascular SMCs by the PDGFR-binding protein, tTG (Zemskov et al., 2009). Our results show the ability of tTG to control the levels and localization of PDGFRβ, and increase the PDGF-BB-induced receptor activation and downstream signaling in vascular SMCs (Fig. 6). The tTG-dependent reduction in PDGFRβ levels, combined with an increase in signaling output shows that tTG sensitizes this receptor to its soluble ligand. tTG also amplifies the PDGF-BB-elicited responses of vascular SMCs, including their phenotypic modulation, survival, growth, and chemotactic migration. Finally, tTG own levels are elevated in these cells by injury and inflammation with PDGF-BB having a robust stimulatory effect. Together, these findings indicate a novel role for tTG as a signaling amplifier within the PDGF-BB/PDGFRβ pathway in vascular SMCs.
Recent studies implicated tTG in the development and progression of vascular diseases(Haroon et al., 2001; Bakker et al., 2004; Cho et al., 2008; Faverman et al., 2008; Johnson et al; 2008; Pistea et al., 2008; Matlung et al., 2010; Santhanam et al., 2010) and collectively suggested that the classical enzymatic (cross-linking) function of this protein contributes to these pathologies (Iismaa et al., 2009). In contrast, our findings indicate the involvement of non-enzymatic tTG functions in vascular pathologies as its cross-linking function is dispensable for PDGFR binding and receptor activation (Zemskov et al., 2009). Notably, two alternatively spliced tTG forms with truncated C terminus, tTGV1 and tTGV2, are co-expressed with full-length tTG in human vascular SMCs(Lai et al., 2007). While tTGV1 and tTGV2 were shown to retain <10% of the cross-linking activity of the major tTG isoform (Lai et al., 2007), they are likely to interact with PDGFR as the second tTG domain is involved in binding this receptor (Zemskov et al., 2009).
Previous studies implicated the gatekeeper function of LRP1 in restricting the PDGFRβ activity in the vasculature as SMC-specific LRP1 knockout enhanced PDGFRβ activation and atherosclerotic response in LDL receptor-deficient mice (Boucher et al., 2003). Yet, cell surface tTG is efficiently down-regulated via LRP1-mediated endocytosis and its levels increase in the LRP1-deficient cells (Zemskov et al., 2007). Hence, one can envision a cross-talk between the negative regulator of PDGFRβ function, LRP1, and its activator, tTG, in the modulation of signaling by this receptor on the surface of vascular SMCs. In addition, the LRP1 function in vascular SMCs is antagonized by the membrane-anchored matrix metalloproteinase MT1-MMP, which cleaves this endocytic/signaling receptor, thereby facilitating the PDGF-BB/PDGFRβ signaling and concomitant dedifferentiation of these cells (Lehti et al., 2009). Although cell surface tTG also serves as proteolytic substrate of MT1-MMP, its degradation is blocked by the major ECM ligand of tTG, fibronectin (Belkin et al., 2001).
Our findings also suggest that cell surface tTG cooperates with fibronectin in the PDGF-BB-induced modulation of vascular SMC phenotype. Indeed, tTG serves as an integrin-binding receptor for fibronectin on the cell surface (Akimov et al., 2000), and expression of these proteins is co-regulated on the transcriptional level in several cell types (Thomas-Ecker et al., 2007; Chen et al., 2010). Fibronectin enhances PDGF-BB-mediated signaling in fibroblasts (Sundberg and Rubin, 1998), serves as a key ECM component that stimulates the PDGF-BB-induced transition of vascular SMCs from contractile to synthetic phenotype (Hedin et al., 1988), and promotes their survival, growth and migration (Grainger et al., 1994; Nelson et al., 1997). Moreover, fibronectin gene expression is induced by PDGF-BB in vascular SMCs (Lo et al., 1995 and this study). Finally, the alterations in tTG expression in these cells parallel the changes in the levels of fibronectin mRNA and protein. Hence, the tTG-fibronectin adhesive/signaling complexes in the ECM can amplify the PDGF-BB/PDGFRβ signaling axis and associated cell responses acting as potent modulators of vascular SMC phenotype. Future mechanistic studies on the cross-talk among tTG, fibronectin, LRP1 and MT1-MMP on the surface of vascular SMCs should help to define the individual roles of these proteins as well as their interactions in the regulation of PDGF-mediated processes in the normal vasculature and vascular diseases.
Supplementary Material
Acknowledgments
Contract grant sponsor: NIH; Contract number: GM 062895
Contract grant sponsor: MSCRF; Contract number: E028-2009
Contract grant sponsor: AHA; Contract number: 11GRNT7200007
This work was supported by National Institutes of Health grant RO1 GM062895, American Heart Association Grant-in-Aid 11GRNT7200007, and Maryland Stem Cell Research Fund exploratory grant E028-2009 (to A.M.B.).
Literature Cited
- Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol. 2000;148:825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker EN, Buus CL, Spaan JAE, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, van Bavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2004;96:119–126. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]
- Bakker EN, Pistea A, Vanbavel E. Transglutaminase in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res. 2008;22:271–278. doi: 10.1159/000113599. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Akimov SS, Zaritskaya LS, Ratnikov BI, Deryugina EI, Strongin AY. Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J Biol Chem. 2001;276:18415–18422. doi: 10.1074/jbc.M010135200. [DOI] [PubMed] [Google Scholar]
- Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- Buetow BS, Tappan KA, Crosby JR, Seifert RA, Bowen-Pope DF. Chimera analysis supports a predominant role of PDGFRbeta in promoting smooth-muscle cell chemotaxis after arterial injury. Am J Pathol. 2003;163:979–984. doi: 10.1016/s0002-9440(10)63457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. Regulation of smooth muscle cells in development and vascular disease: current therapeutic strategies. Expert Rev Cardiovasc Ther. 2006;4:789–800. doi: 10.1586/14779072.4.6.789. [DOI] [PubMed] [Google Scholar]
- Chen SH, Lin CY, Lee LT, Chang GD, Lee PP, Hung CC, Kao WT, Tsai PH, Schally AV, Hwang JJ, Lee MT. Up-regulation of fibronectin and tissue transglutaminase promotes cell invasion involving increased association with integrin and MMP expression in A431 cells. Anticancer Res. 2010;30:4177–4186. [PubMed] [Google Scholar]
- Cho BR, Kim MK, Suh DH, Hahn JH, Lee BG, Choi YC, Kwon TJ, Kim SY, Kim DJ. Increased tissue transglutaminase expression in human atherosclerotic coronary arteries. Coron Artery Dis. 2008;19:459–468. doi: 10.1097/MCA.0b013e3283108fc3. [DOI] [PubMed] [Google Scholar]
- Faverman L, Mikhaylova L, Malmquist J, Nurminskaya M. Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett. 2008;582:1552–1557. doi: 10.1016/j.febslet.2008.03.053. [DOI] [PubMed] [Google Scholar]
- Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;253:1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Grainger DJ, Witchell CM, Weissberg PL, Metcalfe JC. Mitogens for adult rat aortic vascular smooth muscle cells in serum-free primary culture. Cardiovasc Res. 1994;28:1238–1242. doi: 10.1093/cvr/28.8.1238. [DOI] [PubMed] [Google Scholar]
- Haroon ZA, Wannenburg T, Gupta M, Greenberg CS, Wallin R, Sane DC. Localization of tissue transglutaminase in human carotid and coronary artery atherosclerosis: implications for plaque stability and progression. Lab Invest. 2001;81:83–93. doi: 10.1038/labinvest.3780214. [DOI] [PubMed] [Google Scholar]
- Hedin U, Bottger BA, Forsberg E, Johansson S, Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988;107:307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C-H, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res. 1992;71:1525–1532. doi: 10.1161/01.res.71.6.1525. [DOI] [PubMed] [Google Scholar]
- Iismaa SE, Mearns BM, Lorand L, Graham RM. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009;89:991–1023. doi: 10.1152/physrev.00044.2008. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki K, Kaminski WE, Tang J, Hollenbach S, Lindahl P, Sullivan C, Yu JC, Abe K, Martin PJ, Ross R, Betsholtz C, Giese NA, Raines EW. Blockade of platelet-derived growth factor or its receptors transiently delays but does not prevent fibrous cap formation in ApoE null mice. Am J Pathol. 2002;161:1395–1407. doi: 10.1016/S0002-9440(10)64415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- Lai TS, Liu Y, Li W, Greenberg CS. Identification of two GTP-independent alternatively spliced forms of tissue transglutaminase in human leukocytes, vascular smooth muscle, and endothelial cells. FASEB J. 2007;21:4131–4143. doi: 10.1096/fj.06-7598com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti K, Rose NF, Valavaara S, Weiss SJ, Keski-Oja J. MT1-MMP promotes vascular smooth muscle dedifferentiation through LRP1 processing. J Cell Sci. 2009;122:126–135. doi: 10.1242/jcs.035279. [DOI] [PubMed] [Google Scholar]
- Levitzki A. PDGF receptor kinase inhibitors for the treatment of restenosis. Cardiovasc Res. 2005;65:581–586. doi: 10.1016/j.cardiores.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Lindner V, Fingerle J, Reidy M. Mouse model of arterial injury. Circ Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- Lo CS, Tamaroglio T, Zhang J. Regulation of fibronectin by platelet-derived growth factors in cultured rat thoracic aortic smooth muscle cells. J Biomed Sci. 1995;2:63–69. doi: 10.1007/BF02257927. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Matlung HL, van Bavel E, van den Akker J, de Vries CJ, Bakker EN. Role of trans-glutaminases in cuff-induced atherosclerotic lesion formation in femoral arteries of ApoE3 Leiden mice. Atherosclerosis. 2010;213:77–84. doi: 10.1016/j.atherosclerosis.2010.07.054. [DOI] [PubMed] [Google Scholar]
- Nelson PR, Yamamura S, Kent KC. Platelet-derived growth factor and extracellular matrix proteins provide a synergistic stimulus for human vascular smooth muscle cell migration. J Vasc Surg. 1997;26:104–112. doi: 10.1016/s0741-5214(97)70153-8. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Owens GK. Molecular control of vascular smooth muscle cell differentiation and phenotypic plasticity. Novartis Found Symp. 2007;283:174–191. doi: 10.1002/9780470319413.ch14. [DOI] [PubMed] [Google Scholar]
- Pistea A, Bakker EN, Spaan JA, Hardeman MR, van Rooijen N, van Bavel E. Small artery remodeling and erythrocyte deformability in L-NAME-induced hypertension: role of transglutaminases. J Vasc Res. 2008;45:271–278. doi: 10.1159/000109073. [DOI] [PubMed] [Google Scholar]
- Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Sane DC, Kontos JL, Greenberg CS. Roles of transglutaminases in vascular and cardiac diseases. Front Biosci. 2007;12:2530–2545. doi: 10.2741/2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Sudo T, Yokode M, Murayama T, Kataoka H, Takakura N, Nishikawa S, Nishikawa SI, Kita T. Functional blockade of platelet-derived growth factor receptor-beta but not of receptor-alpha prevents vascular smooth muscle cell accumulation in fibrous cap lesions in apolipoprotein E-deficient mice. Circulation. 2001;103:2955–2960. doi: 10.1161/01.cir.103.24.2955. [DOI] [PubMed] [Google Scholar]
- Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, Dunn J, Gutbrod S, Yin D, Shoukas A, Nyhan D, Flavahan NA, Belkin AM, Berkowitz DE. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res. 2010;107:117–125. doi: 10.1161/CIRCRESAHA.109.215228. [DOI] [PubMed] [Google Scholar]
- Sundberg C, Rubin K. Stimulation of β 1 integrins on fibroblasts induces PDGF independent phosphorylation of PDGF β-receptors. J Cell Biol. 1998;132:741–752. doi: 10.1083/jcb.132.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Ecker S, Lindecke A, Hatzmann W, Kaltschmidt C, Zanker KS, Dittmar T. Alteration in the gene expression pattern of primary monocytes after adhesion to endothelial cells. Proc Natl Acad Sci USA. 2007;104:5539–5544. doi: 10.1073/pnas.0700732104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantler M, Caglayan E, Zimmermann WH, Baumer AT, Rosenkranz S. Systematic evaluation of anti-apoptotic growth factor signaling in vascular smooth muscle cells. Only phosphatidylinositol 3′-kinase is important. J Biol Chem. 2005;280:14168–14176. doi: 10.1074/jbc.M413310200. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96:280–291. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- Zemskov EA, Janiak A, Hang J, Waghray A, Belkin AM. The role of tissue transglutaminase in cell-matrix interactions. Front Biosci. 2006;11:1057–1076. doi: 10.2741/1863. [DOI] [PubMed] [Google Scholar]
- Zemskov EA, Mikhailenko I, Strickland DK, Belkin AM. Cell-surface transglutaminase undergoes internalization and lysosomal degradation: an essential role of LRP1. J Cell Sci. 2007;120:3188–3199. doi: 10.1242/jcs.010397. [DOI] [PubMed] [Google Scholar]
- Zemskov EA, Loukinova E, Mikhailenko I, Coleman RA, Strickland DK, Belkin AM. Regulation of platelet-derived growth factor receptor function by integrin-associated cell surface transglutaminase. J Biol Chem. 2009;284:16693–16703. doi: 10.1074/jbc.M109.010769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.