Abstract
The separations of small molecules using columns containing porous polymer monoliths invented two decades ago went a long way from the very modest beginnings to the current capillary columns with efficiencies approaching those featured by their silica-based counterparts. This review article presents a variety of techniques that have been used to form capillary formats of monolithic columns with enhanced separation performance in isocratic elutions. The following text first describes the traditional approaches used for the preparation of efficient monoliths comprising variations in polymerization conditions including temperature as well as composition of monomers and porogenic solvents. Encouraging results of these experiments fueled research of completely new preparation methods such as polymerization to an incomplete conversion, use of single crosslinker, hypercrosslinking, and incorporation of carbon nanotubes that are described in the second part of the text.
Keywords: Monolith, Polymerization, Chromatography, HPLC, Isocratic mode, Small molecules
1. Introduction
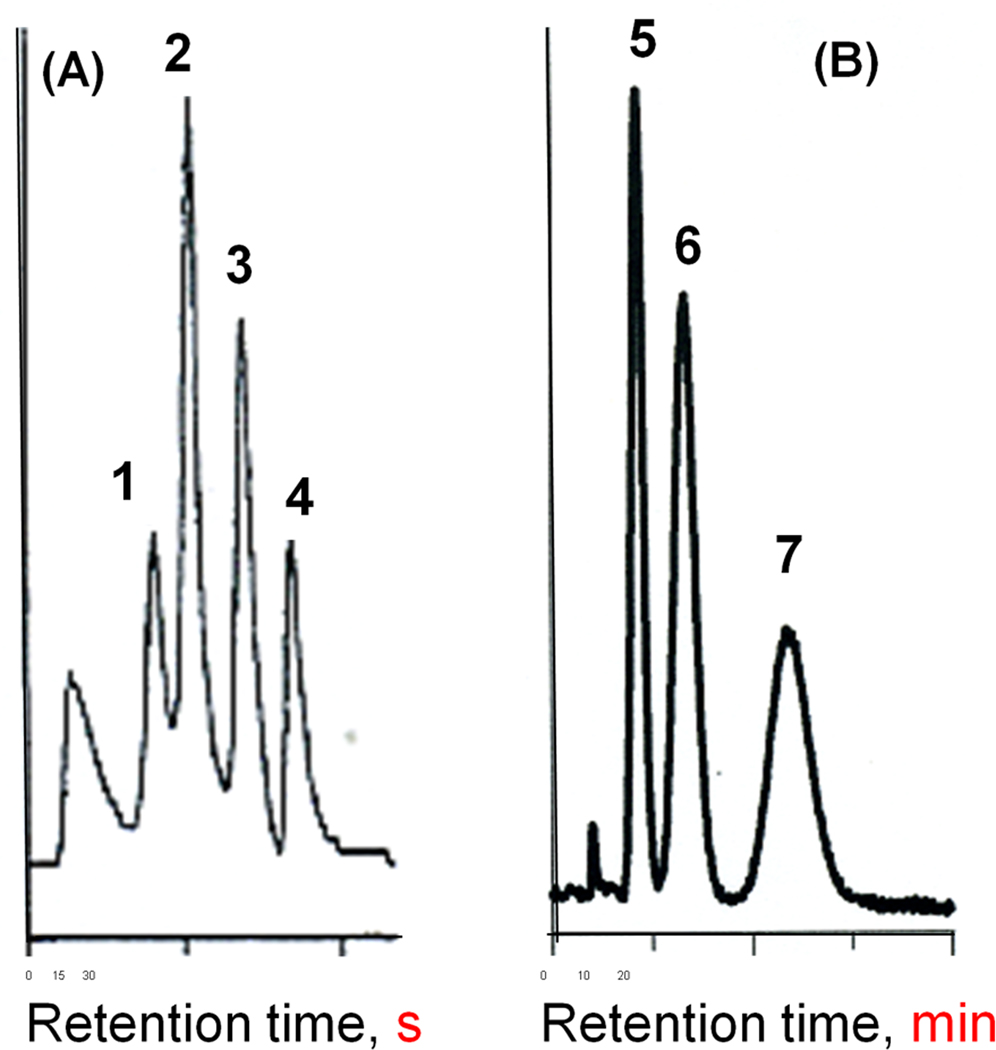
As of today, the modern monoliths are just out of their teens age since this new family of stationary phases was conceived at the verge of 1990. First monolithic columns were polymer-based discs and columns [1–3] followed by monolithic columns made of silica [4]. The former has proven to be an excellent stationary phase for the rapid separation large molecules such as proteins, nucleic acids and synthetic polymers while the latter enabled fast separations of small molecules. However, none of these monolithic columns worked well for both types of analytes. For example, Fig. 1 shows application monolithic poly(styrene-divinylbenzene) column for a rapid gradient elution of four proteins that was achieved in less than 30 s at a very high flow velocity of 10 mm/s. In contrast, the column efficiency in the isocratic separation of three alkylbenzenes that had to be carried out at a flow velocity of only 0.4 mm/s and required almost 15 min to be complete was poor and did not exceed 13 000 plates/m. This result was surprising since columns packed with porous poly(styrene-divinylbenzene) beads possessing the same chemistry performed reasonably well. We found that pore size distributions of both formats were actually entirely different [5].
Fig. 1.
Reversed-phase separations of proteins (A) and alkylbenzenes (B) using monolithic poly(styrene-co-divinylbenzene) columns. Conditions: (A) column 50 × 8 mm I.D., mobile phase linear gradient from 20 to 60 % acetonitrile in 0.1 % aqueous trifluoroacetic acid in 24 s, flow rate 25 mL/min; (B) column 100 × 8 mm I.D., mobile phase 70% aqueous acetonitrile, flow rate 1 mL/min. Peaks: ribonuclease A (1), cytochrome c (2), myoglobin (3), ovalbumin (4), benzene (5) ethylbenzene (6), butylbenzene (7).
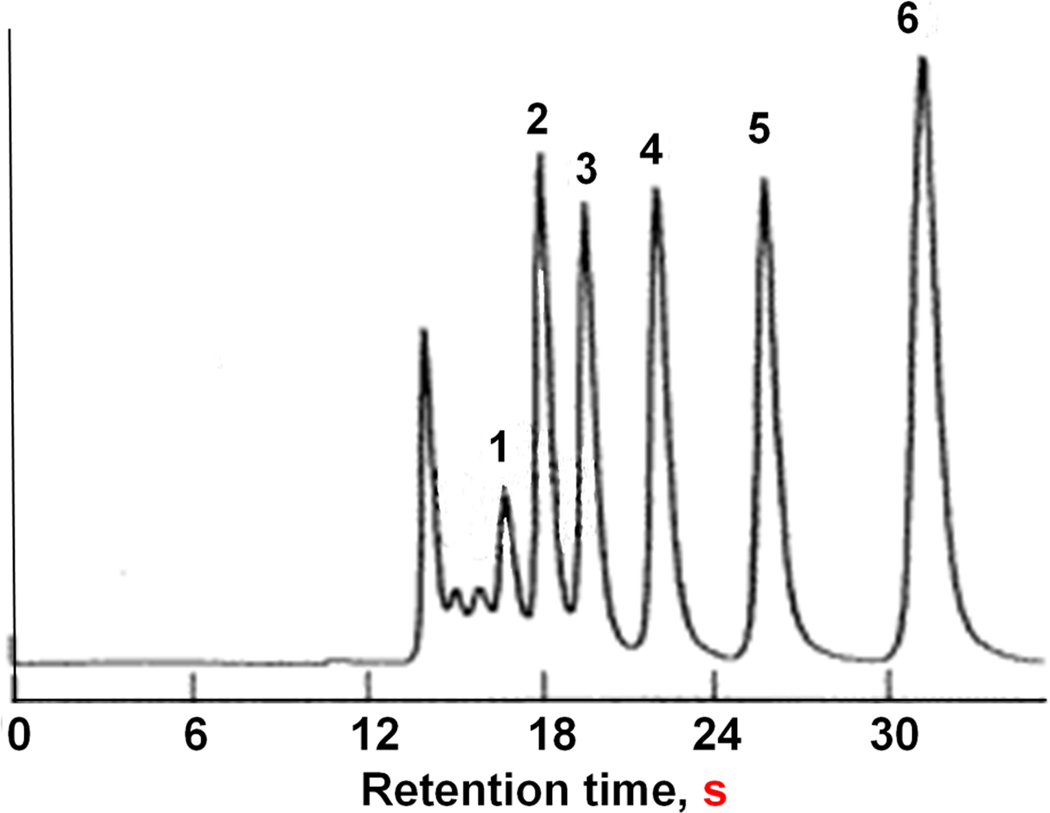
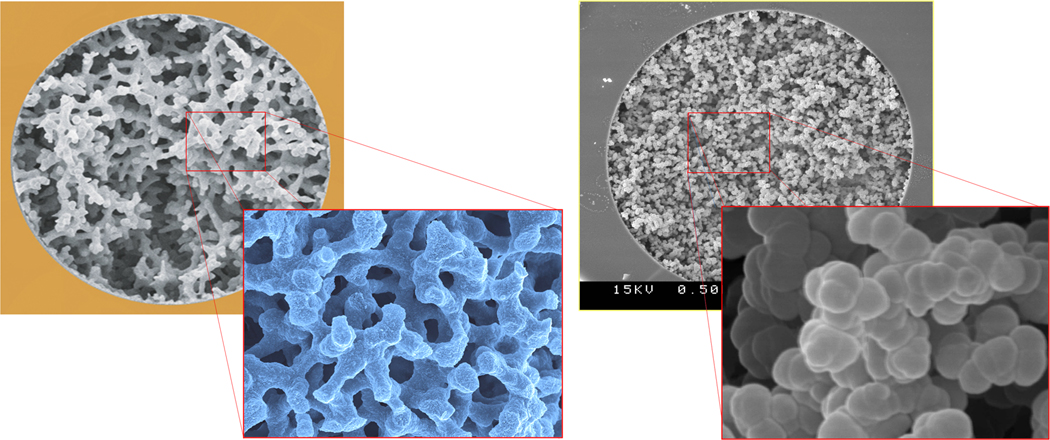
Soon after our failed experiments with the separation of small molecules using polymer-based monoliths, Tanaka’s group demonstrated the separation of five alkylbenzenes using C18 silica monolith shown in Fig. 2 that was easily achieved at a flow velocity of 5 mm/s in about 30 s and afforded a column efficiency of 100 000 plates/m [4]. The reason for the superior performance of silica-based monolithic columns appears to be again morphology of this monolith. While silica-based monolith with a bi-continuous structure was composed of mesoporous skeletons and possess a surface area of several hundred m2/g, polymer-based monolith were formed from interconnected non-porous microglobules and their surface area typically lies in the range one order of magnitude smaller. Fig. 3 compares morphologies of both types of monoliths and demonstrates their significant structural differences. However, both types of monoliths have one feature is common - presence of large through pores.
Fig. 2.
Reversed phase separation of alkylbenzenes using monolithic C18 silica column. Conditions: Column 8.3 × 7 mm I.D., mobile phase 80% aqueous methanol, linear flow velocity 4.99 mm/s. Peaks (1) benzene, (2) toluene, (3) ethylbenzene, (4) propylbenzene, (5) butylbenzene, (6) pentylbenzene. Adapted from ref. [4] with permission.
Fig. 3.
SEM micrographs showing difference in morphology of monolithic capillary columns prepared from silica (left, courtesy of E. Machtejevas, Merck KGaA, Germany) and poly(butyl methacrylate-co-ethylene dimethacrylate) (right).
The initial poor results have led to a conclusion that morphology of the microglobules must be changed to increase the surface area and to obtain organic polymer monoliths with a separation performance for small molecules matching that of silica monoliths. We knew that large through pores did not contribute dramatically to the overall surface area. However, these pores are essential in monoliths since they permit liquids to permeate through the material at a high flow velocity yet keeping the back pressure reasonably low. Therefore, procedures that have to be developed to create monoliths with desired porous properties must be selected in a way that the advantageous flow properties will be preserved while the surface area will be significantly enhanced. This easy sounding task represents a major challenge [6]. Numerous studies focused on development of polymer monoliths for efficient isocratic HPLC separations of small molecules and used multiplicity of methodologies that is discussed in detail in this review. It is worth noting that the best results have always been achieved with capillary columns in which the contribution of radial diffusion to the peak broadening is much smaller compared to the 4.6 and 8 mm I.D. analytical size columns.
2 Optimizing polymerization conditions
The early works aiming at the preparation of porous polymer beads identified key variables such as temperature, chemistry of monomers and content of crosslinking monomer, as well as type and composition of the pore-forming compounds (porogens) to be essential for control of porous properties [7]. All these variables are related to the polymerization conditions. They have also been found to enable tuning of porosity in monoliths [8, 9].
2.1 Temperature
We observed early on that temperature affects the kinetics of polymerization, thus being an efficient tool enabling the preparation of porous polymers widely varying in pore size distributions from a single polymerization mixture [10]. Fig. 4 shows the typical sharp pore size distribution profile for poly(styrene-co-divinylbenzene) monolith prepared at a temperature of 70 °C with a maximum centered around 1000 nm [11]. As we already know, this monolith is not suitable for the separation of small molecules. Using the same polymerization mixture, an increase in temperature to 130 °C affords monolith exhibiting a surface area of 300 m2/g with a very broad pore size distribution curve with no distinct maximum. This value is similar to that of silica monoliths. Unfortunately, these polymerization conditions also lead to disappearance of most of the large pores that are critical for flow of the mobile phase through the monolith. As a result, the high pressure resistance of the 8 mm I.D. column allows flow at a rate of only 0.2 mL/min, which makes this column useless for any rapid separations.
Fig. 4.
Integral pore size distribution profiles of poly(styrene-co-divinylbenzene) monoliths prepared by a polymerization at different temperatures. Conditions: (A) polymerization mixture styrene 20 wt%, divinylbenzene 20 wt%, 1-dodecanol 40 wt%, toluene 20 wt%, benzoyl peroxide 0.5 wt% (with respect to monomers), temperature 70 °C, reaction time 24 h; (B) polymerization mixture styrene 20 wt%, divinylbenzene 20 wt%, 1-dodecanol 60 wt%, benzoyl peroxide 0.5 wt% (with respect to monomers), temperature 130 °C, reaction time 8 h.
Polymerizations carried out later at a temperature of 130 °C in the presence of stable free radicals such as functionalized 2,2,6,6-tetramethylpiperidinyl-1-oxy and 2,2,5,5-tetramethylpyrrolidinyl-1-oxy afforded monoliths with a large surface area and better permeability but their performance for the separation of small molecules have never been specifically tested [12]. However, some of these columns enabled decent separation in SEC mode with rather sharp peaks monitored for the smallest polystyrene standards.
While typical thermal initiators require high temperature to decompose and initiate the polymerization process, UV initiation can be carried out at any temperature even at temperatures below ambient. Szumski and Buszewski [13] used a polymerization mixture developed in our group previously [14] comprising butyl methacrylate, ethylene dimethacrylate, and 2-acrylamido-2-methyl-1-propanesulfonic acid (monomers), 1-propanol, 1,4-butanediol, water (porogens), and benzoin methyl ether (UV initiator) to prepare monolithic capillary columns at temperatures ranging from −15 to 75 °C. As expected from an earlier report [10], an increase in temperature led to a decrease in permeability. The best performance in the separation of small molecules characterized by an efficiency of 47 500 plates/m for unspecified analyte (most likely for unretained thiourea) was observed with monolithic column prepared at −15 °C. Unfortunately, both permeability and column efficiency shown in this work did not follow any specific patterns and some results do not make much sense. Thus, a clear picture of the effect of temperature could not be obtained. It is likely that a simpler system using a binary mixtures of both monomers and porogens producing well defined monolith such as poly(butyl methacrylate-co-ethylene dimethacrylate) would be more suitable for such study.
This is exactly what Hirano et al. did [15]. They used the polymerization mixture consisting of butyl methacrylate, ethylene dimethacrylate, 1-dodecanol, cyclohexanol, and 2,2-dimetoxyphenyl-2-acetophenone (UV initiator) to prepare columns at 0, 10 and 20 °C [15]. Although the authors claim that “the composition was decided empirically”, it is an almost verbatim copy of the mixtures used elsewhere [16, 17]. They found that a decrease in temperature affords monolithic column with enhanced permeability and efficiency. For example, a column prepared at 0 °C featured an efficiency of 45 000 plates/m for retained naphthalene. The low back pressure permitted use of very high flow velocities enabling the separation of five alkyl benzenes to be achieved in less than 8 s (Fig. 5)
Fig. 5.
Reversed phase separation of alkylbenzenes using monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) capillary column prepared by UV initiated polymerization at 0 °C. Conditions: Column 100 mm × 100 µm I.D.; mobile phase 50% aqueous acetonitrile, UV detection at 217 nm. For flow rate and back pressure see inserts. Peaks: uracil (1), toluene (2), ethylbenzene (3, propylbenzene (4), butylbenzene (5), pentylbenzene (6). Reproduced from ref. [15] with permission.
Quite different results were obtained by Li et al. [18]. They found that permeability of monolithic columns prepared from hydrophilic poly(ethylene glycol) diacrylates using UV initiated polymerization at 0 °C exhibited significantly higher back pressure than the counterparts prepared at room temperature. This might be the effect of entirely different polarity of the monomer compared to all previously studied systems.
2.2 Monomers
Use of different monomers including both monovinyl functional monomer and the crosslinker is another common approach to control of porous properties of monoliths [9]. Interestingly, the arsenal of monomers used for the preparation of monolithic columns for the separations of small molecules is limited by the fact that vast majority of these columns is designed for the separations using reversed phase chromatography. Therefore, hydrophobic monomers are mostly used with butyl methacrylate together with ethylene dimethacrylate [19–21] and styrene with divinylbenzene [22–24] appear to dominate the field (vide infra).
For example, Coufal et al. targeted the preparation of monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) column in a larger 320 µm I.D. capillary better suited for use with common HPLC systems [19]. After varying the percentages of both monomers in the polymerization mixture and the percentage of porogenic solvent (6:3:1 1-propanol, 1,4-butanediol, and water), they achieved a column efficiency of 37 000 plates/m for benzene at a flow rate of 1 µL/min.
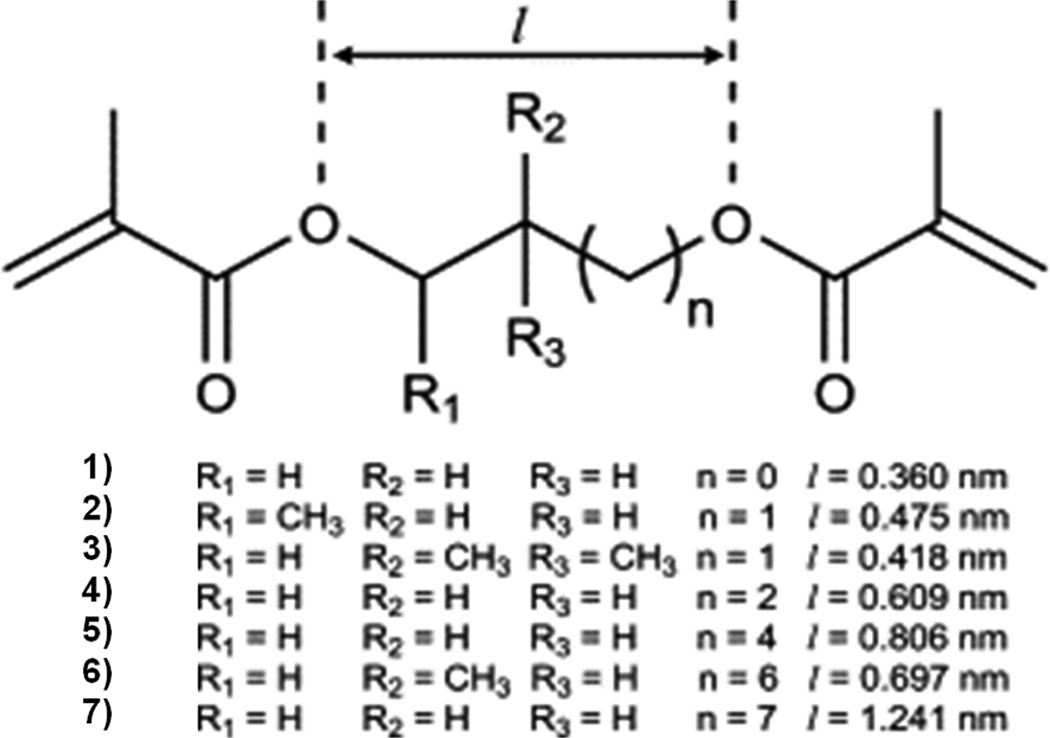
While the monovinyl monomer is most often varied during the search for monoliths designed for reversed phase separations [9], systematic studies of the effect of crosslinker are much less common. One of these exceptions targeting small molecules includes the copolymerization of hydrophobic lauryl methacrylate with dimethacrylates shown in Fig. 6 differing in the length and branching of the fragment connecting the polymerizable units [25]. Using a binary porogen consisting of tert. butanol and 1,4-butanediol, all the monoliths exhibited very small extent of surface area not exceeding 2 m2/g, a value that on the first sight would disqualify them from the group of monolith suitable for the separation of small molecules. Yet, monolithic poly(lauryl methacrylate-co-2-methyl-1,8-octanediol dimethacrylate) capillary column afforded a notable efficiency of 83 000 and 52 000 plates/m at a flow velocity of 1 mm/s for unretained thiourea and retained butylbenzene, respectively. Extending the length of the alkyl bridge between both methacrylate moieties of the crosslinker also leads to an increase in hydrophobicity as derived from the methylene selectivity. The highest value of 1.48 was found for 2-methyl-1,8-octanediol dimethacrylate and ascribed to the branching that exposes the methyl groups at the pore surface. This methylene selectivity value is similar to 1.46–1.54 found for C18 silica monoliths [21].
Fig. 6.
General chemical structure of a series of dimethacrylate crosslinkers and the lengths of the alkyl bridge. (1) Ethylene dimethacrylate, (2) 1,3-butanediol dimethacrylate, (3) 1,4-butanediol dimethacrylate, (4) 1,6-hexanediol dimethacrylate, (5) neopentyl glycol dimethacrylate, (6) 2-methyl-1,8-octanediol dimethacrylate, (7) 1.9-nonanediol dimethacrylate. Adapted from ref. [25] with permission.
While most of the recent monolithic columns for the separation of small molecules were prepared in capillaries, Smirnov et al. used 3 mm I.D. glass tube and studied effect of addition of 4–8 wt.% 2-hydroxyethyl methacrylate admixed to 34–30 wt.% divinylbenzene (80% grade with the rest being ethylstyrenes) and 62 wt% 1-dodecanol on the chromatographic performance of larger I.D. columns [26]. The azobisisobutyronitrile initiated polymerizations were completed at 60 °C in 22 h. All these monoliths exhibited large surface areas ranging from 490 to 370 m2/g due to the high percentage of divinylbenzene in the polymerization mixture. They found a remarkable effect of the 2-hydroxyethyl methacrylate on permeability to flow. For example, the calculated permeability for monolith prepared in the presence of 4 wt.% 2-hydroxyethyl methacrylate was three orders of magnitude higher than that found for monolith containing 8 wt.% of the hydrophilic monomer. Thus, the latter could not be used for the chromatographic separations, which in contrast could be easily carried out at a high flow velocity of 90 mm/s with the former. This significant effect of 2-hydroxyethyl methacrylate on porosity and permeability of monoliths was observed also in other studies [27–29]. The best isocratic separation of aromatic compounds at a flow velocity of 1.5 mm/s was observed with monolithic column containing 5.6 wt.% 2-hydroxyethyl methacrylate [26]. However, this separation was slow and less impressive with only 16 000 plates/m for benzene.
2.3 Porogens
The choice of porogens typically follows selection of monomers and varies significantly for monoliths prepared from aromatic monomers or methacrylates.
2.3.1 Poly(styrene-co-divinylbenzene) monoliths
Since poly(styrene-co-divinylbenzene) monoliths prepared in presence of porogen consisting of dodecanol-toluene mixtures did not perform well in the isocratic separations, Horvath’s group used a porogenic mixture of water, methanol, and ethanol to prepare 75 µm I.D. monolithic poly(styrene-co-divinylbenzene) capillary columns [22]. Although their target were columns for capillary electrochromatography, they also evaluated the performance in HPLC mode. The best efficiency of 43 000 plates/m for unretained compound dimethylsulfoxide was observed using a column crosslinked with 33% divinylbenzene. Monolithic capillary column prepared elsewhere from 20% styrene and 20% divinylbenzene in the presence of 40% 1-propanol and 20% formamide exhibited an efficiency of 91 000 plates/m for unretained uracil according to the vanDeemter plot [23]. However, values for retained compounds were not published in either of these reports.
Poly(styrene-co-divinylbenzene) monoliths have also been prepared in the presence of a mixture of toluene and isooctane [24]. As expected, these monoliths with a barely measurable surface area did not separate alkylbenzenes. All analytes were eluted in a single wide peak (Fig. 7). The situation changed dramatically after preparing the monolith from a 1:1:2 mixture of styrene, methacrylic acid, and divinylbenzene using the same porogenic mixture. Surprisingly, addition of the polymerizable carboxylic acid affected both selectivity and column efficiency. The bottom panel of Fig. 7 demonstrates the baseline separation of all compounds with an efficiency of up to 28 000 plates/m that could be achieved at a flow rate of 4 µL/min and a moderate back pressure of 4 MPa. The conclusions drawn from these dramatic changes can be twofold. First, addition of methacrylic acid afforded a monolith with a surface area of 261 m2/g. This increase indicates that the acid served as a micro co-porogen helping to form an ample volume of 0.3 mL/g mesopores. Second, the retention increases with increasing hydrophobicity of the separated compounds thus confirming that the reversed phase separation mechanism applies and the electrostatic interaction of carboxylic functionality is not operative. This is easily understandable since the weak acid is not ionized in the water-acetonitrile mobile phase.
Fig. 7.
Separation of small organic molecules using poly(styrene-co-divinylbenzene) (A) and poly(styrene-co-methacrylic acid-co-divinylbenzene) (B) columns. Conditions: mobile phase, acetonitrile/water (65/35, v/v); UV detection at 214 nm; flow rate, 4 µL/min; injection volume, 100 nL; column 170 mm × 320 µm I.D. Peaks: thiourea (1), phenol (2), aniline (3), benzene (4), toluene (5), ethylbenzene (6), propylbenzene (7), butylbenzene (8). Reproduced from ref. [24] with permission.
2.3.2 Polymethacrylate-based monoliths
In parallel with the poly(styrene-co-divinylbenzene), experiments were carried out to facilitate preparation of methacrylate monoliths suitable for the isocratic separation of small molecules. Optimization of the separation performance of these monolithic columns is often achieved by varying composition of both monomer and porogen mixtures. The work concerning monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) capillary columns published by Moravcova et al. is an exception [20]. This group used porogenic mixture consisting of water, 1-propanol, and 1,4-butanediol that have been developed earlier for the preparation of columns for capillary electrochromatography (CEC) [14, 30] and only varied percentage of 1-propanol and 1,4-butanediol in a narrow range of 60:30, 62:28 to 64:26 while keeping percentage of water at 10%. Despite these minuscule changes in the composition, the effect on the morphology presented in Fig. 8 was significant. This change was also reflected in column efficiency for benzene that increased from 5 100 to 33 000 and 35 000 plates/m, respectively. The difference in efficiency for higher alkylbenzenes was pronounced even more. Further refining efforts included changes in monomer and porogen composition as well as in the proportion of monomers to porogens but did not lead to any additional increase in column efficiency [21].
Fig. 8.
Scanning electron micrographs of monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) polymers prepared in a 320 µm I.D. capillary using mixtures consisting of 1-propanol 1,4-butanediol, and water as a porogen. Compositions of porogen: Water 10% 1-propanol-1,4-butanediol 60:30 (A), 62:28 (B), 64:26 (C). Adapted from ref. [20] with permission.
Eeltink et al. studied effects of percentage of porogenic solvent in the polymerization mixture on efficiency of a 100 µm I.D. capillary column [31]. They also used porogenic mixture of water, 1,4-butanediol, and 1-propanol. While poly(butyl methacrylate-co-(2-methacryloyloxyethyl) trimethylammonium chloride-co-ethylene dimethacrylate) “high density” monolith prepared in the presence of 60% porogen afforded about 13 000 plates/m, the column efficiency found for monolith with the same chemistry prepared from a mixture containing 80% porogenic solvent and featuring higher pore volume (“low density”) increased to 67 000 plates/m. Both values are reported for a non-disclosed analyte. Later, part of the butyl methacrylate in the “low density” monolith was replaced with lauryl methacrylate to achieve a 15% increase in retention in the reversed phase separations of aromatic hydrocarbons [32]. This column separated non-retained thiourea and retained naphthalene with efficiencies of 50 000 and 29 500 plates/m, respectively.
Use of polymers as porogens is known from the “ancient” times of the macroporous beads [7, 33]. They were also used for the preparation of monoliths [34–38]. However, use of polymeric porogen for the preparation of methacrylate-based monoliths is less frequent. A combination of high molecular mass polystyrene (Mw = 3 840 000) and chlorobenzene was used for the preparation of monoliths from glycerol dimethacrylate 1 with an unusual morphology [39]. This monolith contained certain volume of mesopores and was used for the separation of small molecules with an efficiency of up to 34 000 plates/m for retained benzene. Fig. 9 shows the isocratic separation of alkyl phenyl ketones as an example.
Fig. 9.
Separation of alkyl phenyl ketones using monolithic poly(glycerol dimethacrylate) capillary column prepared with ultra high molecular weight polystyrene/chlorobenzene porogen. Conditions: Column 400 mm × 200 µm I.D.; mobile phase 60:40 methanol-water; flow rate 2.5 µL/min. Peaks: thiourea (1), acetophenone (2), ethyl phenyl ketone (3), propyl phenyl ketone (4), butyl phenyl ketone (5), amyl phenyl ketone (6), hexyl phenyl ketone (7). Inlay SEM micrograph shows morphology of the monolith. Adapted from ref. [39] with permission.
All the studies presented above demonstrate that fine-tuning of the polymerization mixture together with the preparation of monoliths in capillaries helped to increase the efficiency in isocratic separations of small molecules. However, it appears that these efforts enable to reach efficiencies not exceeding about 50 000 plates/m, which remain inferior to those measured for both packed and monolithic silica-based columns [20, 21].
3 Other means of control
3.1 Polymerization time
While the typical means enabling modification of porous properties presented above did not afford monoliths with a significantly improved performance in the isocratic separation of small molecules, less common approaches have to be pursuit. For example, we observed a significant effect of polymerization time on porous properties of poly(glycidyl methacrylate-co-ethylene dimethacrylate) monoliths described in one of our early publications [40]. For example, a monolith resulting after polymerization for 1 h at a temperature of 55 °C exhibited a surface area of over 500 m2/g. Since the conversion was only 18%, most of the monomers remained unpolymerized and this initial monolith had a remarkably high pore volume of 3.8 mL/g. None of these monoliths was used for chromatography.
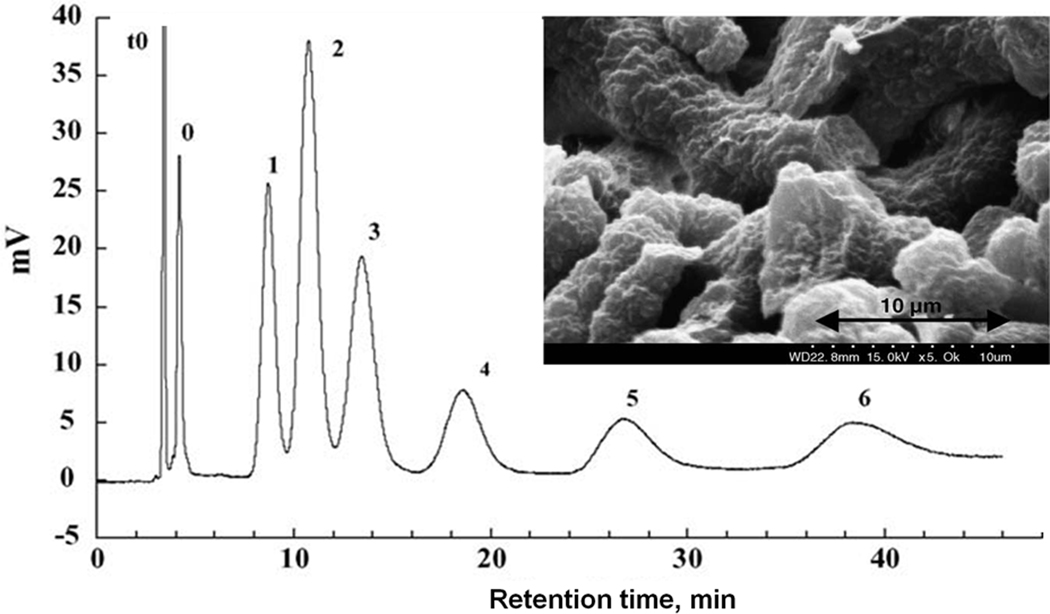
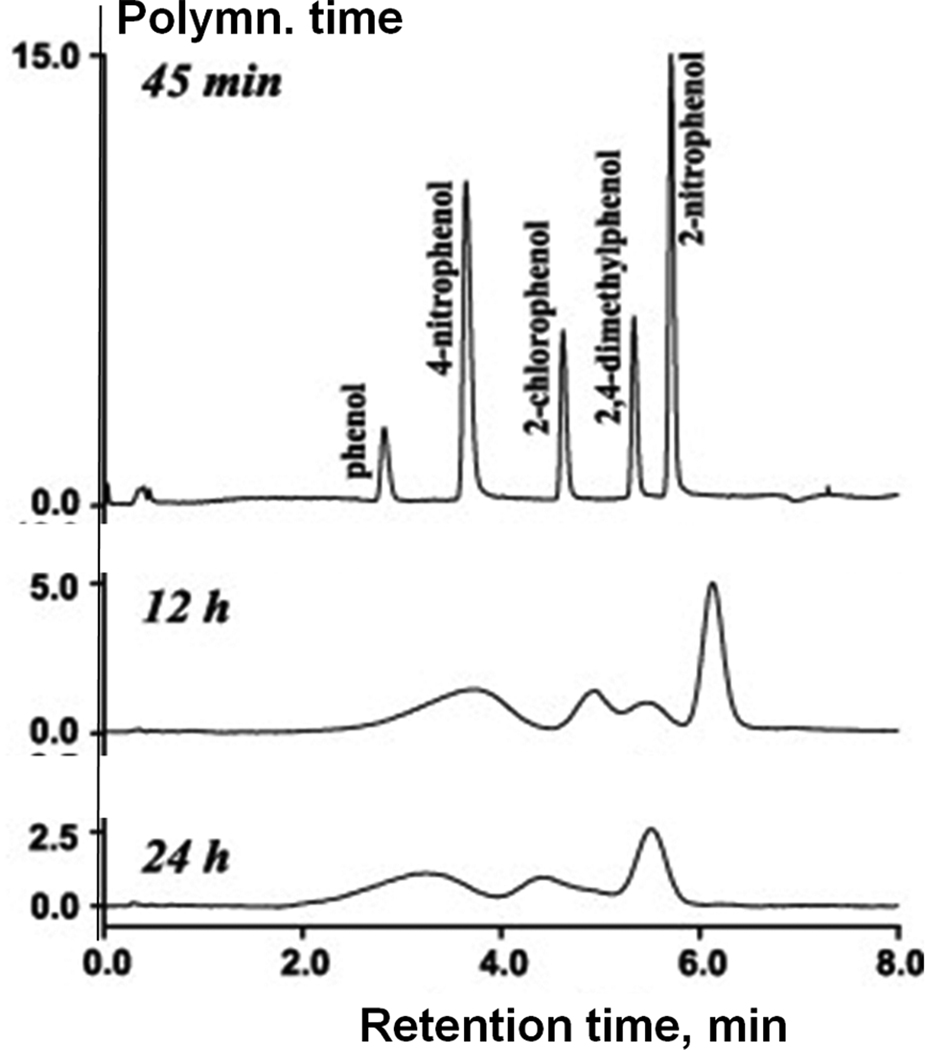
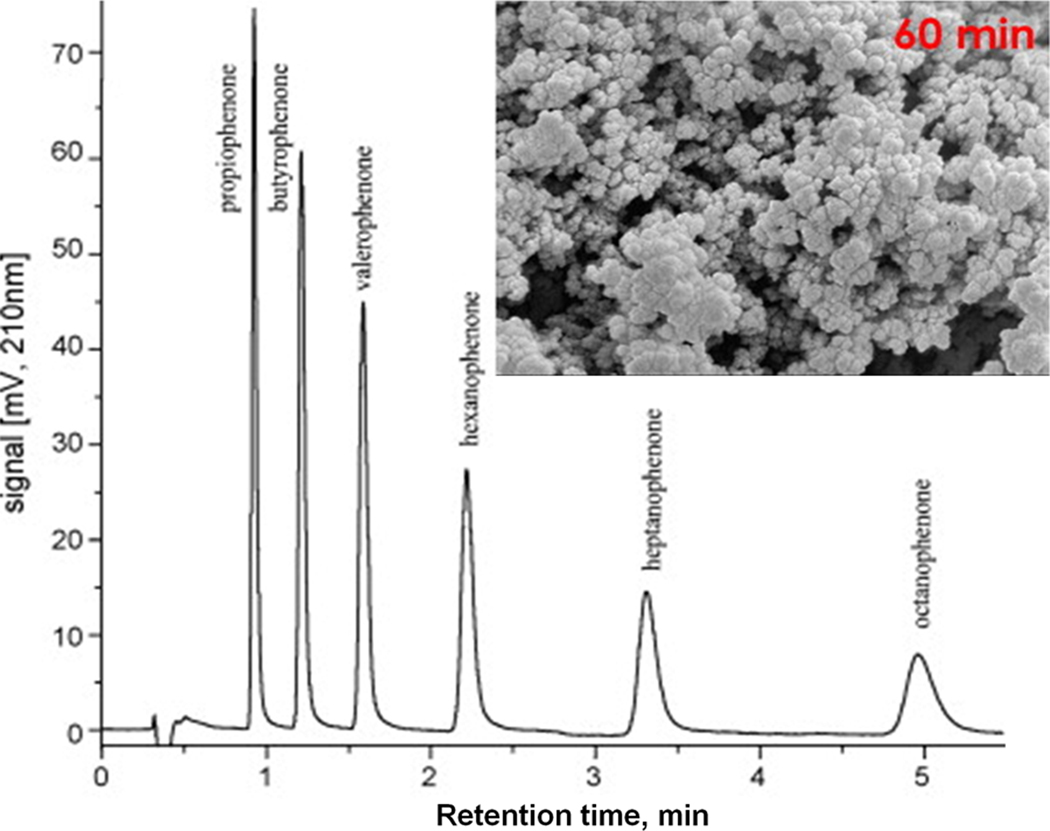
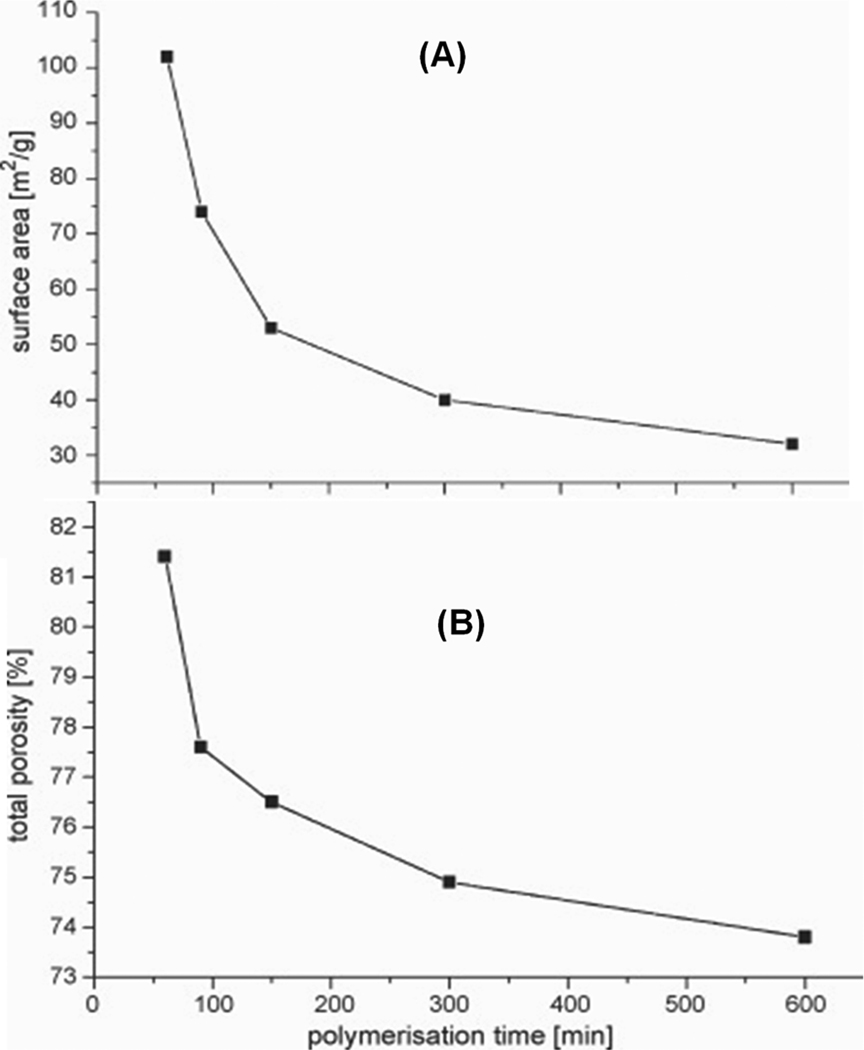
The control of porous properties via reaction time was recently adopted by other groups in order to prepare monolithic columns for the separation of small molecules. Trojer et al. prepared monolithic poly(4-methylstyrene-co-1,2-bis(4-vinylphenyl)ethane) capillary columns using polymerization times varied from 30 min to 24 h [41]. Most appealing is the high column efficiency of 65 000 plates/m calculated from results of the isocratic separation of alkylbenzoates using a column polymerized for only 45 min at which time the conversion was 39%. However, the quality of separation gradually deteriorated with the increase in polymerization time. It was poor after 2 h and completely unacceptable with columns polymerized for 12 and 24 h as shown in Fig. 10. The same group refined this approach by using the single crosslinker 1,2-bis(4-vinylphenyl)ethane 2 for the preparation of monoliths [42]. They found that monolith resulting from polymerization carried out for 60 min has a surface area of 102 m2/g while this value is only 32 m2/g after 10 h. Fig. 11 shows an excellent isocratic reversed phase separation of aromatic ketones with an efficiency reaching to 72 000 plates/m for butyrophenone on column prepared using 60 min long polymerization. Interestingly, separations of several other families of small molecules were carried out in the gradient mode.
Fig. 10.
Separation of phenol derivatives using monolithic poly(4-methylstyrene-co-1,2-bis-(4-vinylphenyl)ethane) capillary column polymerized for varying times. Conditions: column 80 × 0.2 mm I.D., mobile phase A: 0.1% aqueous trifluoroacetic acid, B: 0.1% trifluoroacetic acid in acetonitrile, gradient 0–50% B in A in 5 min, flow rate 10 µL/min, UV detection at 210 nm. Adapted from ref. [41] with permission.
Fig. 11.
Isocratic separation of phenol derivatives using monolithic poly(1,2-bis-(4-vinylphenyl)ethane) capillary column polymerized for 60 min. Conditions: column 80 mm × 200 µm I.D., mobile phase 66% acetonitrile in water flow rate 10 µL/min, UV detection at 210 nm. Inlay SEM micrograph shows morphology of the monolith. Adapted from ref. [41] with permission.
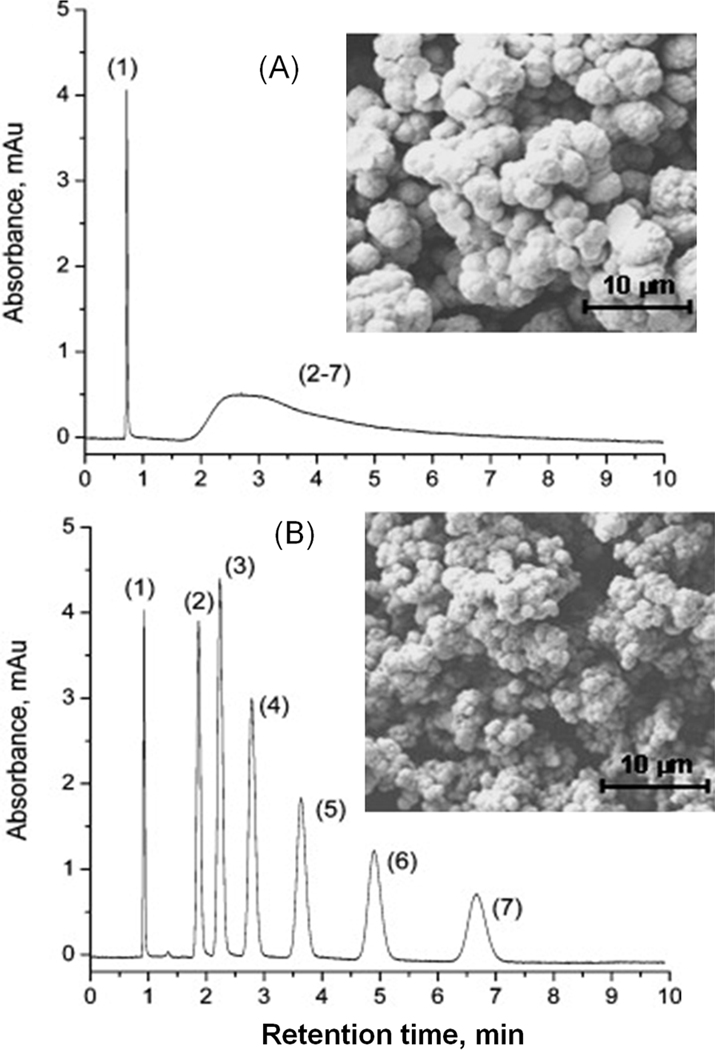
Inspired by the above results, Nischang and Bruggemann tested the effect of polymerization time on performance of monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) columns [43]. Fig. 12 demonstrates again that monolithic column prepared under conditions of incomplete conversion separates alkylbenzenes with sufficient resolution and exhibits an efficiency of about 67 000 plates/m at the minimum of the vanDeemter plot while similar column polymerized for 48 h completely fails. The authors also observed a significant decrease in permeability with increasing polymerization time which is supported by SEM micrographs also shown in the figure that point to some changes in the morphology.
Fig. 12.
Isocratic separation of alkylbenzenes using monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) capillary columns polymerized for 48 (A) and 0.5 h (B). in a 100 µm I.D.. Conditions: column 200 mm × 100 µm I.D.; mobile phase: 50% aqueous acetonitrile; flow rate: 1.6 µL/min; linear flow velocity 4.6 mm/s (A) and 3.6 mm/s (B); back pressure 3.92 (A) and 1.14 MPa (B). Peaks: (1) uracil, (2) benzene, (3) toluene, (4) ethylbenzene, (5) propylbenzene, (6) butylbenzene, (7) pentylbenzene. Inlay SEM micrographs show morphology of the monoliths. Adapted from ref. [43] with permission.
The less common approach to control of porous properties described in this section excels in its simplicity. Certain problem is seen in fact that the conversion of polymerization is not controlled directly but indirectly through the time of polymerization. This makes termination of the polymerization process at specific conversion less accurate since the reaction kinetics is affected by many other factors. For example, the temperature ramp to a value at which the initiation and polymerization proceeds, cooling speed to terminate the reaction, as well as the presence of compounds acting as inhibitors are difficult to control exactly. All these effects may decrease the batch-to-batch repeatability. However, a short batch-to-batch study with three experiments carried out by Bonn’s group indicated good repeatability of their process [44].
All these studies have some features in common. The authors always observed a decrease in both surface area and pore volume (Fig. 13). For example, the surface area of poly(4-methylstyrene-co-1,2-bis(4-vinylphenyl)ethane) monoliths dropped from 76 to 23 m2/g and pore volume from 70 to 40% with polymerization time varied from 30 min to 24 h, respectively. Likewise, poly(1,2-bis(4-vinylphenyl)ethane) monolith resulting from polymerization carried out for 60 min has a surface area of 102 m2/g while this value is only 32 m2/g after 10 h. Similarly, the surface area of poly(butyl methacrylate-co-ethylene dimethacrylate) decreased from 5.9 to 1.2 m2/g upon extension in polymerization time from 0.5 to 48 h. These typically three-fold changes are surprisingly small and indicate absence of any large volume of mesopores. This is not unexpected since the initial monolithic structure is less crosslinked despite the higher rates of incorporation of crosslinker in the polymer. For example, the higher rate of incorporation of ethylene dimethacrylate [43] results from its higher molar concentration of double bonds compared to butyl methacrylate. The fact that the crosslinker is incorporated in the monolithic structure does not mean that both of its double bond reacted forming a crosslink. Just in opposite, most of the polymer chains are not crosslinked to any significant extent yet and therefore collapse on drying the monoliths prior to the nitrogen adsorption measurement. However, these chains can swell with the mobile phase. This solvation/swelling opens the structure, liberates pores providing the desired surface area that is then instrumental for the separation of small molecules. As the polymerization time increases, more monomers and/or pending double bonds polymerize thus creating more crosslinks. The rigid structure formed cannot swell any longer and the only accessible external surface of the microglobules is not large enough to enable good separation of small molecules. Experimental evidence supporting these assumptions could be probably obtained using inverse size exclusion chromatography.
Fig. 13.
Effect of the polymerization time on total porosity of poly(1,2-bis-(4-vinylphenyl)ethane) monolith determined using mercury intrusion porosimetry (A) and on specific surface area calculated from nitrogen adsorption/desorption isotherms (B). Adapted from ref. [42] with permission.
3.2. Single crosslinker
Use of single crosslinker for the preparation of porous polymer monoliths has been reported by several groups. Li et al. claimed recently in this journal that using a single crosslinker provides for several advantages including straightforward optimization of the polymerization mixture, improved column-to-column reproducibility, better mechanical stability, and higher surface area due to the highly crosslinked network [45].
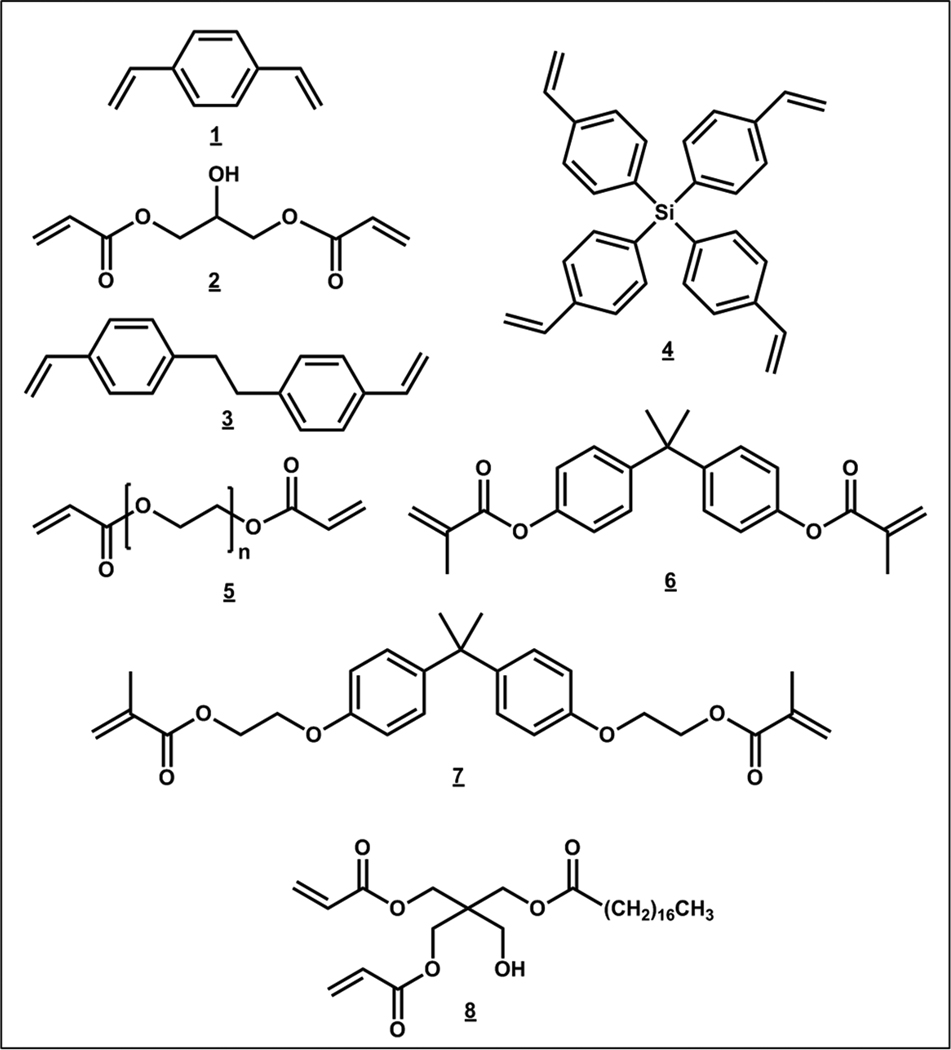
The first two studies of this kind emerged in 2006. One of them concerned preparation of monoliths from divinylbenzene 1 [46]. However, the technical grade of this crosslinker contains 45% of monovinyl monomers and disqualifies this work from the “single crosslinker” category. Although the second report from the same group describes use of the 80% grade, the resulting product is still a copolymer [47]. Thus, the Hosoya’s group was the first to prepare monoliths from a single crosslinker. In their two initial papers, they used glycerol dimethacrylate 2 (for structures see Fig. 14), nonaethylene glycol diacrylate and 2,18-dihydroxy-4,7,10,13,16-pentaoxanonadecane-1,19-diacrylate and polymers as a part of the porogenic systems [35, 39]. The poly(glycerol dimethacrylate) monolith was used for the separation of small molecules and an efficiency of 34 000 plates/m was observed for retained benzene (vide supra).
Fig. 14.
Chemical structures of crosslinkers. Divinylbenzene 1, glycerol methacrylate 2, 1,2-bis(4-vinylphenyl)ethane 3, tetrakis(4-vinylbenzyl)silane 4, poly(ethylene glycol) diacrylates 5, bisphenol A dimethacrylate 6, bisphenol A ethoxylate diacrylate 7, and pentaerythritol diacrylate monostearate 8.
Multiplicity of papers describing the preparation of monoliths using a single crosslinker were published in the last two years. For example, thermally and photoinitiated free radical polymerization was used to prepare monoliths from methylene-bis-acrylamide [48] and poly(ethylene) diacrylate [49] with poly(ethylene glycol) or poly(propylene glycol) as respective porogens. The authors demonstrated specific morphological features of their monoliths however without showing any chromatographic applications.
Greiderer at al. polymerized 1,2-bis(4-vinylphenyl)ethane 3 using thermally initiated polymerization in the presence of 1-decanol and toluene as porogen [42, 44]. While a complete polymerization of all of the crosslinker afforded monolithic columns with a poor performance, reducing the polymerization time to mere 60 min, at which the conversion of monomer to polymer was not complete, excellent monolithic capillary columns were obtained (vide supra).
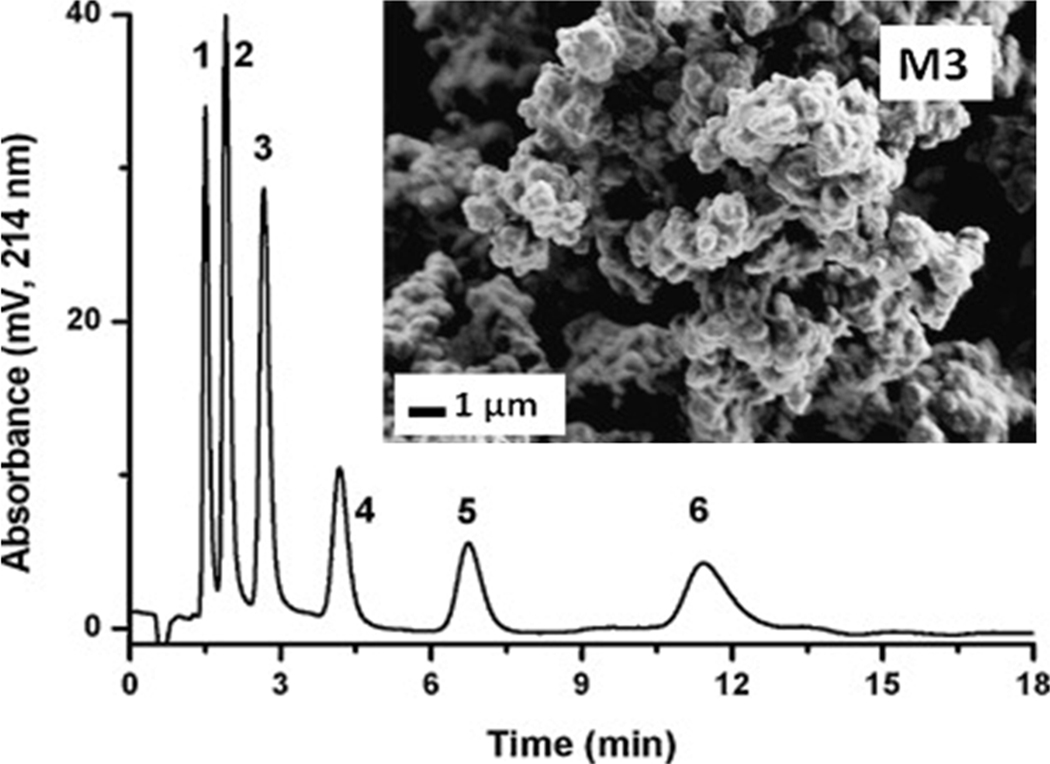
Lubbad and Buchmeiser also used a less common crosslinker tetrakis(4-vinylbenzyl)silane 4 they prepared in a good yield from chloromethylstyrene [50, 51]. Its mixtures with 1-dodecanol and toluene was thermally polymerized for 24 h to complete conversion at which about 75% of all double bonds were incorporated in the monolithic polymer. The surface area of the monolithic polymer ranged from 350 to 79 m2/g depending on the percentage of crosslinker in the polymerization mixture and polymerization temperature. Capillary columns comprising monolith with the highest surface area exhibited excessively high resistance to flow. The optimal polymerization mixture contained 17.5% tetrakis(4-vinylbenzyl)silane. The back pressure for water pumped through this 50 mm × 200 µm I.D. monolithic capillary column at a flow rate of 2 µL/min was only 1.1 MPa. This column was then used for the separation of a variety of small molecules such as alkylbenzenes, amines, carboxylic acids, phenols, phenones, and drugs as well as peptides and proteins in reversed phase using gradient elution. However, only one example demonstrates separation of alkylbenzenes in isocratic mode (Fig. 15) with both modest speed (12 min) and efficiency ranging from 17 000 to 23 000 plates/m.
Fig. 15.
Separation of alkyl benzenes using 50 mm × 0.2 mm i.d. monolithic capillary prepared from tetrakis(4-vinylbenzyl)silane and morphology of the monolith. Conditions: mobile phase 50% aqueous acetonitrile, flow rate not available; UV detection 214 nm. Peaks: (1) toluene, (2) ethylbenzene, (3) propylbenzene, (4) butylbenzene, (5) pentylbenzene, (6) hexylbenzene. Adapted from ref. [51] with permission.
The newest contribution to the monoliths prepared from a single crosslinker emerged from Lee’s group. First they prepared monolithic columns from poly(ethylene glycol) diacrylates 5 differing in the length of the PEG bridge [18]. They experimented with porogens using pairs cyclohexanol-decanol and methanol-diethyl ether and a temperature of 0 and 20 °C. The optimized photopolymerized monoliths were excellent stationary phases for the separation of peptides and proteins using hydrophobic interaction mechanism. They recently extended their study to three more crosslinkers bisphenol A dimethacrylate 6, bisphenol A ethoxylate diacrylate 7, and pentaerythritol diacrylate monostearate 8 [45]. Once again, this study started with selection of binary porogens from mixtures of tetrahydrofuran and dimethylformamide with decanol and dodecanol for monoliths prepared from bisphenol A derived crosslinkers. The best results in isocratic separation of benzene derivatives characterized with a column efficiency of 61 500 plates/m for butylbenzene were achieved with monolith prepared from bisphenol A dimethacrylate in the presence of dimethylformamide/dodecanol porogen.
According to the authors “the selection of suitable porogenic solvents to form a rigid monolith from pentaerythritol diacrylate monostearate proved to be challenging” [45]. The conditions published previously [52, 53] did not lead to desirable monoliths and numerous solvents and hydrophilic polymers just produced gel-like structures. Eventually, they found a combination of tetrahydrofuran, 2-propanol, and triblock poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) that afforded rigid monolith. However, these monoliths were less retentive in the reversed phase separations most likely due to the presence of hydroxyl group in the crosslinker structure. Also, the column efficiency did not reach the values achieved with poly(bisphenol A dimethacrylate) monolith and only slightly exceeded 21 000 plates/m.
A closer look at all approaches to monolithic columns using a single crosslinker results in the following inference. Use of only one monomer in the polymerization mixture simplifies the system. On the other hand, variability in the composition of the monoliths is lost. This disadvantage can be counteracted (i) via grafting of pore surface with monomers including the desired chemistry provided the monolith is UV transparent or (ii) more tediously, by designing and synthesizing crosslinkers with specific functionalities. Monolithic columns prepared from single crosslinker exhibit significantly larger surface area compared to “classical” monomer+crosslinker monoliths. This property is assigned to the larger volume of small pores. This in turn leads to enhanced column efficiencies observed for separations of small molecules. Interestingly, most of these columns also perform well in the separations of peptides, proteins, and nucleic acids [18, 44, 50]. Low percentage of crosslinker in the polymerization mixture, typically less than 30%, must be used in order to obtain monoliths with acceptable permeability. These mixtures then result in monoliths with high porosity. Alternatively, polymerization is terminated before the complete conversion of crosslinker to polymer which again results in high porosity. Although all these monoliths are designed to enable good separation of small molecules, vast majority of reported separations is carried out in the gradient elution mode that helps to reduce the peak width and controls retention. In contrast, isocratic separations are rarely shown. Also these isocratic separations are typically rather slow, which can be caused by limited permeability to flow or poor performance at higher flow velocities due to unfavorable shape of the vanDeemter plot and need to work using flow rates at its minimum.
3.3 Hypercrosslinking
Hypercrosslinking is actually an “old news” since this process has been pioneered by Davankov several decades ago [54–58]. His approach enabled the preparation of large surface area materials from pre-formed polymer precursors such as high molecular weight polystyrene as well as crosslinked poly(styrene-divinylbenzene) gels and porous particles [59–62]. Hypercrosslinked macroporous particles then contained both the original large pores and an extensive network of additional small pores. Despite this opportunity that would permit formation of monoliths with a large surface area, hypercrosslinking has until recently not been applied to that polymer format.
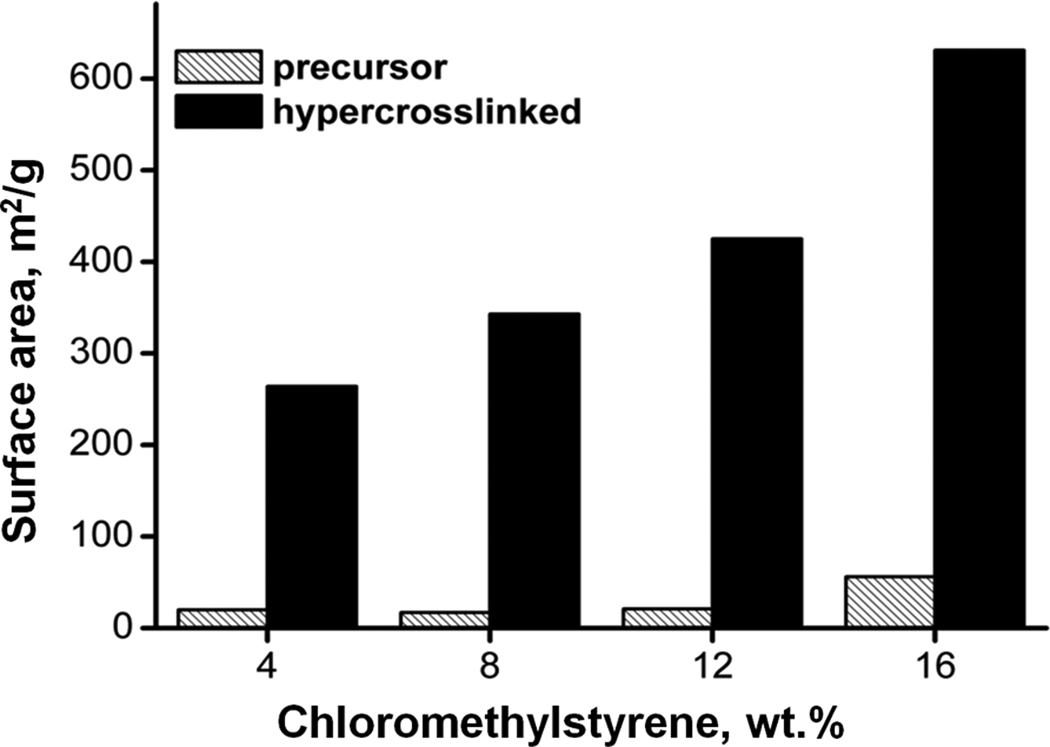
We have demonstrated the power of this approach with hypercrosslinking of monoliths formed from styrene, vinylbenzyl chloride, and divinylbenzene [63, 64]. The unfavorable reactivity ratios for the monomer pairs styrene-divinylbenzene and vinylbenzyl chloride-divinylbenzene result in faster polymerization of the divinyl monomer that depletes from the system while the remaining monomer mixture becomes significantly richer in the monovinyl monomers. As the polymerization reaction approaches completion, only slightly crosslinked chains attached to the surface of highly crosslinked microglobular scaffolds are formed. The presence of such layers in porous poly(styrene-divinylbenzene) polymers was suggested by Jerabek who used inverse size exclusion chromatography measurements [65]. This structure is amenable to hypercrosslinking via Friedel-Crafts alkylation reaction. To carry out this reaction, pores of the monolith are filled with a solvent, typically dichloroethane that swells the surface layer followed by addition of the ferric chloride catalyst, which initiates the hypercrosslinking process immobilizing the polymer chains in their solvated state and forming small pores that persist even after removal of the solvent. It is the creation of these new pores that leads to a high surface area monolith well suited for the separation of small molecules. Varying percentage of chloromethylstyrene in the monomer mixture we obtained the precursor monoliths exhibiting a specific surface area of only 17–56 m2/g. Fig. 16 shows the considerable increase in the surface area to values exceeding 600 m2/g. This value compares very favorably to other polymer-based monoliths [42, 66–69] and even exceeds the surface area of 300 m2/g measured for the silica monoliths [4, 70]. It is worth noting, that these high surface areas measured in the dry state confirm rigidity of the newly formed structure that does not collapse on drying.
Fig. 16.
Effect of hypercrosslinking of poly(styrene-co-chloromethylstyrene-co-divinylbenzene) precursor monolith on the specific surface area. Conditions: Polymerization mixture used for the preparation of precursor monolith: 16% styrene + chloromethylstyrene, 24% divinylbenzene, 18% toluene, 42% 1-dodecanol, 1% (with respect to monomers) azobisisobutyronitrile; hypercrosslinking 24 h at 80°C. Reproduced from ref. [64].
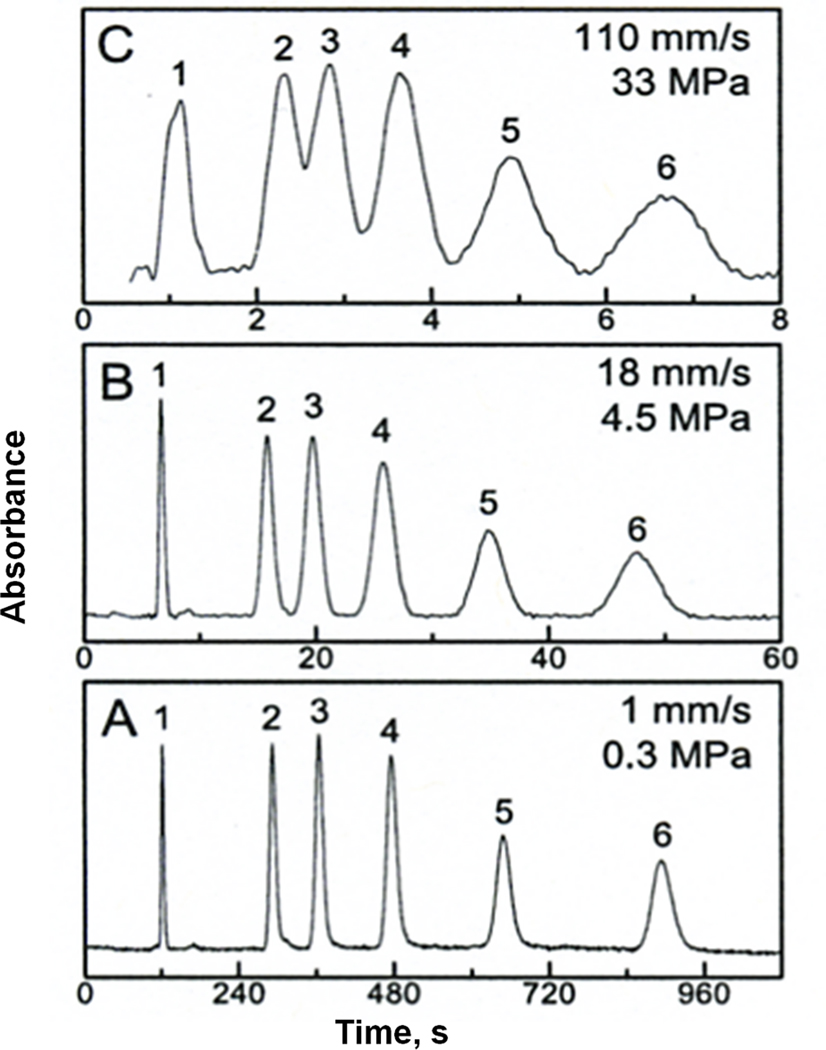
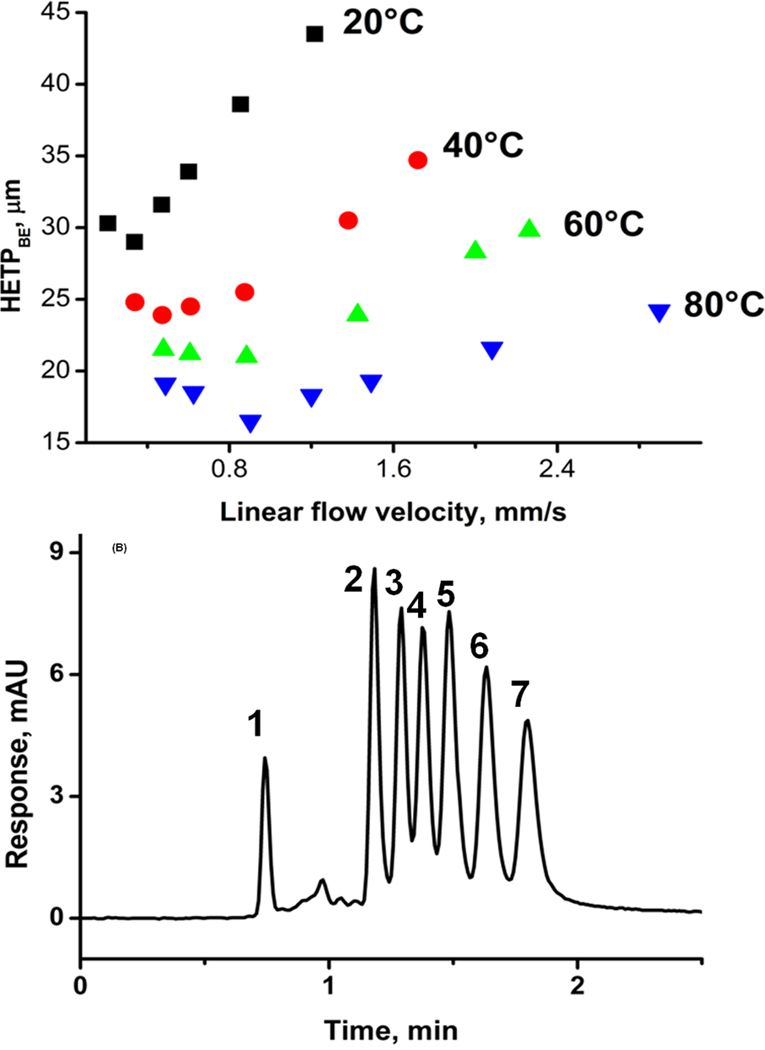
An extensive study using design of experiments targeting column efficiency included variations in percentages of components of the polymerization mixture, i.e. styrene, vinylbenzyl chloride, divinylbenzene, toluene, and 1-dodecanol. We found that two main factors affect the column efficiency: synergistic percentage of the vinylbenzyl chloride and antagonistic percentage of divinylbenzene [64]. An increase in temperature at which the hypercrosslinking reaction is carried out is also an important factor affecting column efficiency from the material point of view. The chromatographic conditions such as composition of the mobile phase, flow velocity, temperature at which the separation is carried out, and sample loading represent another set of variables that were also optimized. For example, temperature has a significant effect on column efficiency. At higher temperature, the vanDeemter plot in Fig. 17 has its minimum at a smaller height equivalent to the theoretical plate (HETP) and, more importantly, this minimum is shifted to a higher flow velocity thus enabling faster separations. This figure also demonstrates that using both optimized monolith and hypercrosslinking procedure together with ternary mobile phase consisting water, tetrahydrofuran, and acetonitrile at 80°C, seven compounds are separated in less than 2 min with a resolution of at least 1.0. The column efficiency for retained benzene is 83 200 plates/m.
Fig. 17.
Effect of temperature on van Deemter plots for benzene (A) and separation of small molecules (B) using hypercrosslinked monolithic column Conditions: column 130 mm × 100 µm I.D., ternary mobile phase 20% water, 20% tetrahydrofuran, 60% acetonitrile, (B) flow rate 0.5 µL/min; temperature 80°C; UV detection 254 nm; back pressure 26 MPa. Analytes: (1) uracil, (2) benzene, (3) toluene, (4) ethylbenzene, (5) propylbenzene, (6) butylbenzene, (7) pentylbenzene. Adapted from ref. [64].
Another interesting feature of hypercrosslinked monoliths is their ability to separate large molecules such as proteins. We compared the separation of five proteins achieved under identical conditions using both the precursor and hypercrosslinked column. The separation was slightly better with the precursor column as expected from the negative effect of mesopores on the gradient elution of large molecules. The small size of the mesopores allows proteins to probe the pores and the slow diffusional transport in and out of the entrance to the small pores reduces the separation performance and impairs resolution [63].
So far, the hypercrosslinking has only been demonstrated with styrene-based monoliths. This chemistry has proven useful for the separations of small molecules in the reversed phase mode. However, there is a need for chemistries that can serve other separation modes. Several other polymers such as polyaniline, polypyrrole, polyarylates, polyxylylene, polyamides, and polypyridine have already been hypercrosslinked [62] but none of them has been prepared in the monolithic format yet. Also, polyacrylates and polymethacrylates that are typically used for the preparation of monoliths wait for the development of methods enabling their hypercrosslinking and formation of monolithic columns exhibiting porous structure desirable for highly efficient separations using other chromatographic mechanisms such as enantioseparations in normal phase.
3.4 Use of carbon nanostructures
While the method described in the previous section represent a significant enhancement over the prior state of the art, it is not readily applied to methacrylate-based monolithic columns. Thus, the newest contribution to methods seeking to enhance performance of porous polymer monoliths in isocratic separations of small molecules emanates from nanoscience. This development can be expected since the recent directions in many scientific fields focus on progress in nanotechnologies. Due to unique characteristics of nanoparticles, such as their large surface-to-volume ratio and their properties that differ from those of corresponding bulk materials, the application of nanomaterials in separation science is also growing [71–73]. Several groups used nanostructures, such as polymer latex nanoparticles, fullerene derivatives, metal oxides, and carbon nanotubes for the modifications of capillary walls and separation media for application in gas and liquid chromatography, capillary electrophoresis, and electrochromatography [74–84]. For example, methacrylate-based monoliths with attached latex nanoparticles were used for the separation of saccharides [85] and ions [86–88]. Both our and Paull’s groups have recently prepared monoliths with pore surface coated with gold nanoparticles [89–91] suitable for the pre-concentration of thiol containing peptides and the separation of proteins. Monoliths with embedded hydroxyapatite nanoparticles proved useful in the fishing-out of phosphorylated peptides [92].
Surprisingly, only little has been done to accommodate carbon nanostructures in liquid chromatography. To our best knowledge, only Horváth’s group published a single paper concerned with carbon nanotubes entrapped into a monolithic poly(chloromethylstyrene-co-ethylene dimethacrylate) capillary columns and tested them mostly in capillary electrochromatography and briefly in HPLC applications [93]. We have very recently demonstrated the use multiwalled carbon nanotubes entrapped within or attached to the pore surface of poly(glycidyl methacrylate-co-ethylene dimethacrylate) monoliths [94].
The simplest approach to a monolith containing carbon nanotubes comprises their admixing in the polymerization mixture followed by polymerization [94]. A homogeneous system could only be obtained if the nanotubes were added to the complete mixture containing both monomers and alcohol-based porogen. Although addition of mere 0.25 wt% of nanotubes did not result in any apparent change in the porous properties, the column efficiency increased from 1800 plates/m found for the parent monolith to 15 400 plates/m for benzene under the same separation conditions. This encouraging result demonstrated the enormous effect of carbon nanotubes on performance of the monolithic column.
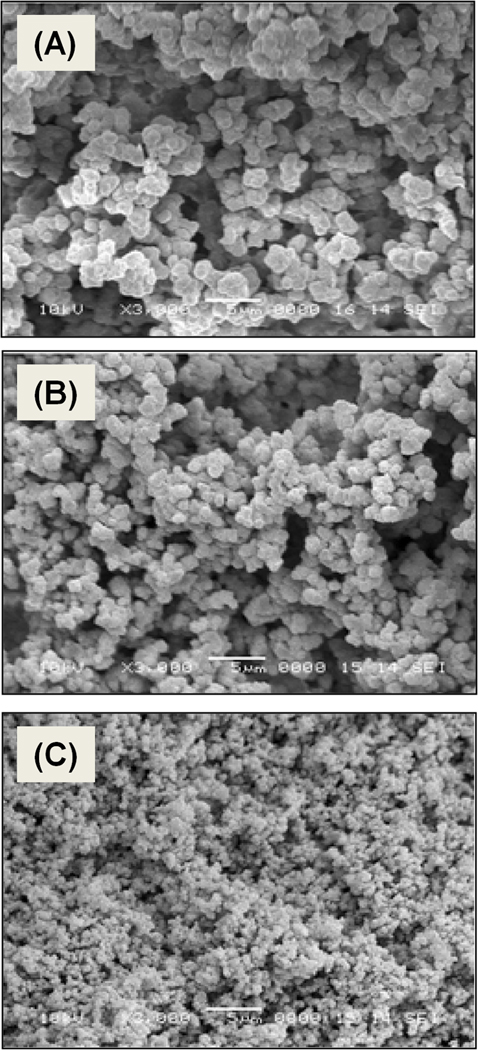
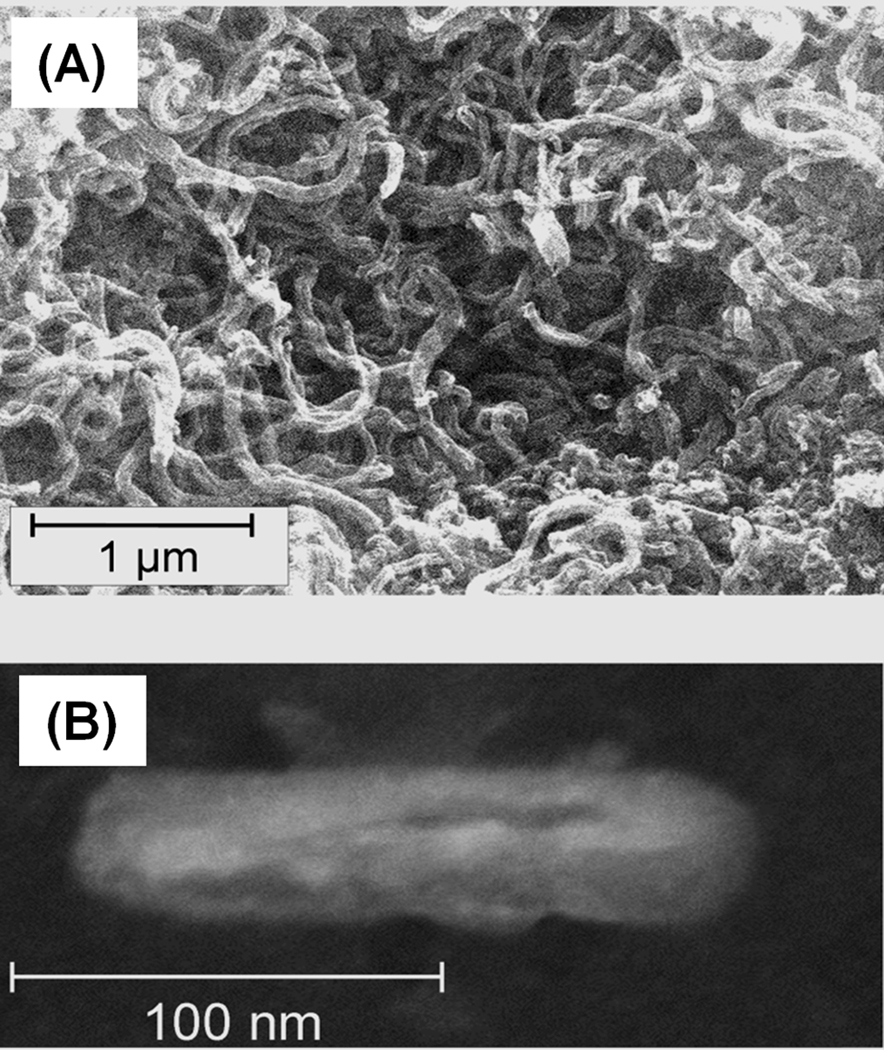
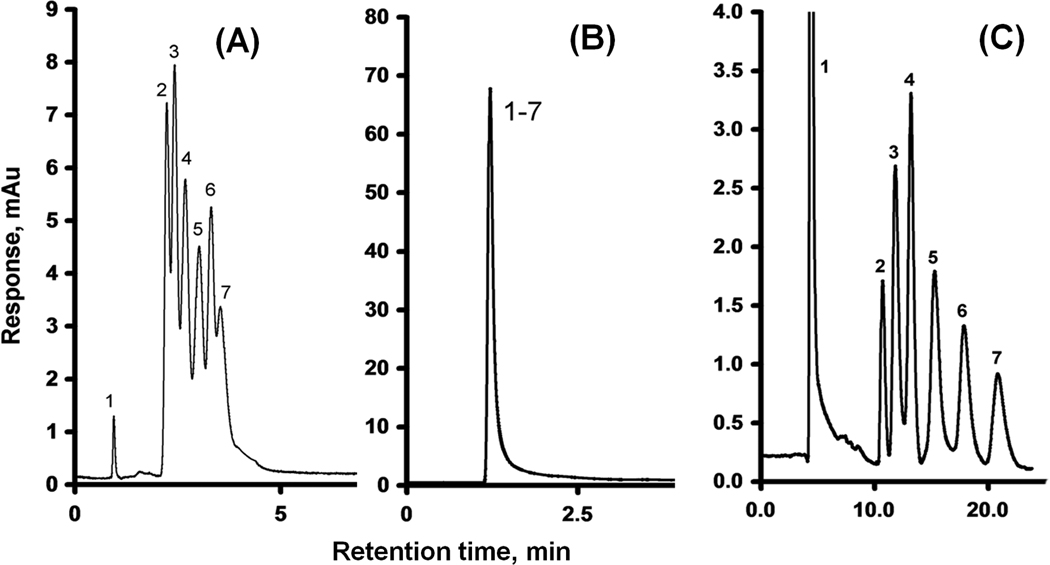
Alternatively, we tried to attach the pristine carbon nanotubes to the pore surface using pumping their dispersion through the monolith. However, the length of the native 1–2 µm nanotubes shown in Fig. 18 prevented them from perfusing through the tortuous pores of the monoliths, which had pore sizes not exceeding 1.9 µm. Shorter fragments were then prepared via oxidation of tubes using a mixture of sulfuric and nitric acids [95, 96] that in addition to the reduction in length also provides their tips with carboxylic acid functionalities. Simultaneously, the epoxy groups of the poly(glycidyl methacrylate-co-ethylene dimethacrylate) monolith were treated with ammonia to afford primary amine functionalities [97]. This monolith is useless for isocratic separations (Fig. 19). However, the ionizable functionalities can interact with the oxidized nanotubes through electrostatic interactions between their carboxylic acid moieties and the surface amino groups. The oxidized nanotubes were strongly retained within the monolith and did not elute even when pure acetonitrile was used as a mobile phase. Fig. 19 illustrates the significant improvement in performance of the monolithic column on separation of alkylbenzenes with an efficiency of 44 000 plates/m. The mechanism responsible for these effects is not completely clear. Previous studies with silica beads suggest that the cause may be a high affinity of the immobilized nanotubes for aromatic compounds [98] and/or π−π interaction at the large contact area [83].
Fig. 18.
SEM image of pristine 1–2 µm long dry carbon nanotubes aggregated after exposure to water (A) and SEM micrograph of nanotubes oxidatively cut using treatment with a mixture of concentrated sulfuric and nitric acids (B). Adapted from ref. [94].
Fig. 19.
Separation of uracil and alkylbenzenes using a monolithic poly(glycidyl methacrylate-co-ethylene dimethacrylate) capillary column (A), the previous column reacted with ammonia (B), and column modified with oxidized carbon nanotubes (C). Conditions: (A) Column, 180 mm × 100 µm i.d., mobile phase 45% acetonitrile-5% THF-50% water, flow rate 1.00 µL/min, back pressure 16 MPa; (B) Column, 200 mm × 100 µm i.d., mobile phase 50% acetonitrile-50% water, flow rate 1.00 µL/min, back pressure 17 MPa; (C) Column, 170 mm × 100 µm i.d., mobile phase 47.5% acetonitrile-2.5% THF-50% water- mixture; flow rate 0.25 µL/min, back pressure 30 MPa; Peaks: uracil (1), benzene (2), toluene (3), ethylbenzene (4), propylbenzene (5), butylbenzene (6), and amylbenzene (7). Adapted from ref. [94].
4 Conclusions
The research presented above clearly demonstrates that the challenge of preparing porous polymer monoliths for the rapid and efficient separation of small molecules in isocratic mode is taken seriously. The traditional means of manipulation of porous structure of monoliths such as modulation of polymerization conditions improved the performance. Yet they did not afford monolithic columns with efficiencies competing with those featured by silica-based monoliths. Therefore, several novel approaches have been developed including polymerization to an incomplete conversion, use of single crosslinker, and hypercrosslinking. Our attempts to fabricate monoliths with incorporated carbon nanotubes were also successful. All these studies led to monoliths with some significantly enhanced efficiency exceeding 80 000 plates/m. We are currently experimenting with monomers based on functionalized C60 fullerenes. The monoliths appear to be very promising since we are reproducibly preparing capillary columns with an efficiency of almost 110 000 plates/m that is on par or better compared to the typical commercial silica-based monolithic columns.
It is worth noting that the less efficient early columns had an internal diameter of 8 or 4.6 mm while all the monoliths with enhanced characteristics were prepared in capillaries. This finding indicates that the decrease in radial diffusion of separated compounds plays indeed an important role in the chromatographic process carried out in monolithic columns.
It is likely that new discoveries will appear in the future providing polymer-based monolithic columns with even higher efficiency in isocratic elution mode. For example, reactive gelation process recently introduced by Morbidelli’s group [99, 100] for separation of proteins may, after some modifications, have a potential for the separation of small molecules. All reports summarized in this review concern monolithic columns for separations utilizing reversed phase mechanism. Monolithic columns enabling highly efficient separations via other mechanisms are also emerging. For example, Dionex Corp. launched recently monolithic capillary columns for the separation of ions. In contrast, monoliths for the isocratic separation of small molecules using other modes such as normal phase or HILIC have yet to be demonstrated.
Acknowledgement
Work on this paper was supported by the Office of Science, Office of Basic Energy Sciences, U.S. Department of Energy, under Contract No. DE-AC02-05CH11231. Support of experimental work cited in the text by grants of the National Institute Institutes of Health (GM-48364 and EB-006133) is also gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hjertén S, Liao JL, Zhang R. J. Chromatogr. 1989;473:273. [Google Scholar]
- 2.Tennikova TB, Svec F, Belenkii BG. J. Liquid Chromatogr. 1990;13:63. [Google Scholar]
- 3.Svec F, Fréchet JMJ. Anal. Chem. 1992;54:820. [Google Scholar]
- 4.Minakuchi H, Nakanishi K, Soga N, Ishizuka N, Tanaka N. Anal. Chem. 1996;68:3498. doi: 10.1021/ac960281m. [DOI] [PubMed] [Google Scholar]
- 5.Svec F, Fréchet JMJ. Chem. Mater. 1995;7:707. [Google Scholar]
- 6.Eeltink S, Decrop WMC, Rozing GP, Schoenmakers PJ, Kok WT. J. Sep. Sci. 2004;27:1431. doi: 10.1002/jssc.200401826. [DOI] [PubMed] [Google Scholar]
- 7.Seidl J, Malinsky J, Dusek K, Heitz W. Adv. Polym. Sci. 1967;5:113. [Google Scholar]
- 8.Viklund C, Svec F, Fréchet JMJ, Irgum K. Chem. Mater. 1996;8:744. [Google Scholar]
- 9.Svec F. J. Chromatogr. A. 2010;1217:902. doi: 10.1016/j.chroma.2009.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svec F, Fréchet JMJ. Macromolecules. 1995;28:7580. [Google Scholar]
- 11.Peters EC, Svec F, Fréchet JMJ, Viklund C, Irgum K. Macromolecules. 1999;32:6377. [Google Scholar]
- 12.Viklund C, Irgum K, Svec F, Fréchet JMJ. Macromolecules. 2001;34:4361. [Google Scholar]
- 13.Szumski M, Buszewski B. J. Sep. Sci. 2009;32:2574. doi: 10.1002/jssc.200900220. [DOI] [PubMed] [Google Scholar]
- 14.Peters EC, Petro M, Svec F, Fréchet JMJ. Anal. Chem. 1997;69:3646. doi: 10.1021/ac970377w. [DOI] [PubMed] [Google Scholar]
- 15.Hirano T, Kitagawa S, Ohtani H. Anal Sci. 2009;25:1107. doi: 10.2116/analsci.25.1107. [DOI] [PubMed] [Google Scholar]
- 16.Hilder EF, Svec F, Fréchet JMJ. Anal. Chem. 2004;76:3887. doi: 10.1021/ac049732q. [DOI] [PubMed] [Google Scholar]
- 17.Lee D, Svec F, Fréchet JMJ. J. Chromatogr. A. 2004;1051:53. doi: 10.1016/j.chroma.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Tolley HD, Lee ML. J. Chromatogr. A. 2010;1217:4934. doi: 10.1016/j.chroma.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 19.Coufal P, Cihak M, Suchankova J, Tesarova E, Bosakova Z, Stulik K. J. Chromatogr. A. 2002;946:99. doi: 10.1016/s0021-9673(01)01570-9. [DOI] [PubMed] [Google Scholar]
- 20.Moravcova D, Jandera P, Urban J, Planeta J. J. Sep. Sci. 2003;26:1005. doi: 10.1002/jssc.200401778. [DOI] [PubMed] [Google Scholar]
- 21.Moravcova D, Jandera P, Urban J, Planeta J. J. Sep. Sci. 2004;27:789. doi: 10.1002/jssc.200401778. [DOI] [PubMed] [Google Scholar]
- 22.Gusev I, Huang X, Horváth C. J. Chromatogr. 1999;855:273. doi: 10.1016/s0021-9673(99)00697-4. [DOI] [PubMed] [Google Scholar]
- 23.Kucerova Z, Szumski M, Buszewski B, Jandera P. J. Sep. Sci. 2007;30:3018. doi: 10.1002/jssc.200700346. [DOI] [PubMed] [Google Scholar]
- 24.Svobodova A, Krizek T, Sirc J, Salek P, Tesarova E, Coufal P, Stulik K. J. Chromatogr. A. 2011;1218:1544. doi: 10.1016/j.chroma.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, Yang L, Wang Q. J. Chromatogr. A. 2009;1216:3098. doi: 10.1016/j.chroma.2009.01.089. [DOI] [PubMed] [Google Scholar]
- 26.Smirnov KN, Dyatchkov IA, Telnov MV, Pirogov AV, Shpigun OA. J. Chromatogr. A. doi: 10.1016/j.chroma.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Hradil J, Jelinkova M, Ilavsky M, Svec F. Angew. Macromol. Chem. 1991;185/186:175. [Google Scholar]
- 28.Xie SF, Svec F, Fréchet JMJ. Chem. Mater. 1998;10:4072. [Google Scholar]
- 29.Lammerhofer M, Peters EC, Yu C, Svec F, Fréchet JMJ, Lindner W. Anal. Chem. 2000;72:4614. doi: 10.1021/ac000322l. [DOI] [PubMed] [Google Scholar]
- 30.Peters EC, Petro M, Svec F, Fréchet JMJ. Anal. Chem. 1998;70:2288. doi: 10.1021/ac9713518. [DOI] [PubMed] [Google Scholar]
- 31.Eeltink S, Herrero-Martinez JM, Rozing GP, Schoenmakers PJ, Kok WT. Anal. Chem. 2005;77:7342. doi: 10.1021/ac051093b. [DOI] [PubMed] [Google Scholar]
- 32.Huo Y, Schoenmakers PJ, Kok WT. J. Chromatogr. A. 2007;1175:81. doi: 10.1016/j.chroma.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 33.Abrams IM, Millar JR. React. Funct. Polym. 1997;35:7. [Google Scholar]
- 34.Palm A, Novotny MV. Anal. Chem. 1997;69:4499. [Google Scholar]
- 35.Kubo T, Kimura N, Hosoya K, Kaya K. J. Polym. Sci., Polym. Chem. 2007;45:3811. [Google Scholar]
- 36.Li Y, Tolley HD, Lee ML. Anal. Chem. 2009;81:4406. doi: 10.1021/ac900364d. [DOI] [PubMed] [Google Scholar]
- 37.Sinitsyna ES, Sergeeva Y, Vlakh EG, Saprikina NN, Tennikova TB. React. Funct. Polym. 2009;69:385. [Google Scholar]
- 38.Sinitsyna ES, Vlakh EG, Rober MY, Tennikova TB. Polymer. 2011;52:2132. [Google Scholar]
- 39.Aoki H, Kubo T, Ikegami T, Tanaka N, Hosoya K, Tokuda D, Ishizuka N. J. Chromatogr. A. 2006;1119:66. doi: 10.1016/j.chroma.2006.01.133. [DOI] [PubMed] [Google Scholar]
- 40.Svec F, Fréchet JMJ. Chem. Mater. 1995;7:707. [Google Scholar]
- 41.Trojer L, Bisjak CP, Wieder W, Bonn GK. J. Chromatogr. A. 2009;1216:6303. doi: 10.1016/j.chroma.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Greiderer A, Trojer L, Huck CW, Bonn GK. J. Chromatogr. A. 2009;1216:7747. doi: 10.1016/j.chroma.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 43.Nischang I, Bruggemann O. J. Chromatogr. A. 2010;1211:5389. doi: 10.1016/j.chroma.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Greiderer A, Ligon SC, Jr, Huck CW, Bonn GK. J. Sep. Sci. 2009;32:2510. doi: 10.1002/jssc.200900211. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Tolley HD, Lee ML. J. Chromatogr. A. 2011;1218:1399. doi: 10.1016/j.chroma.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 46.Kanamori K, Nakanishi K, Hanada T. Adv. Mater. 2006;18:2407. [Google Scholar]
- 47.Hasegawa J, Kanamori K, Nakanishi K, Hanada T, Yamago S. Macromolecules. 2009;42:1270. [Google Scholar]
- 48.Hasegawa J, Kanamori K, Nakanishi K, Hanada T, Yamago S. Macromol. Rapid Commun. 2009;30:986. doi: 10.1002/marc.200900066. [DOI] [PubMed] [Google Scholar]
- 49.Wu Z, Frederic KJ, Talarico M, De Kee D. Can. J. Chem. Eng. 2009;87:579. [Google Scholar]
- 50.Lubbad SH, Buchmeiser MR. J. Sep. Sci. 2009;32:2521. doi: 10.1002/jssc.200900188. [DOI] [PubMed] [Google Scholar]
- 51.Lubbad SH, Buchmeiser MR. J. Chromatogr. A. 2010;1217:3223. doi: 10.1016/j.chroma.2009.10.090. [DOI] [PubMed] [Google Scholar]
- 52.Okanda FM, El Rassi Z. Electrophoresis. 2005;26:1988. doi: 10.1002/elps.200500073. [DOI] [PubMed] [Google Scholar]
- 53.Jiang Z, Smith NW, Ferguson PD, Taylor MR. J. Sep. Sci. 2009;31:2774. doi: 10.1002/jssc.200800124. [DOI] [PubMed] [Google Scholar]
- 54.Davankov VA, Rogozhin SV, Tsyurupa MP. US. 1971 [Google Scholar]
- 55.Davankov VA, Tsyurupa MP. React. Polym. 1990;13:27. [Google Scholar]
- 56.Pastukhov AV, Tsyurupa MP, Davankov VA, Polym J. Sci., Polym. Phys. 1999;37:2324. [Google Scholar]
- 57.Davankov VA, Tsyurupa M, Ilyin M, Pavlova L. J. Chromatogr. A. 2002;965:65. doi: 10.1016/s0021-9673(01)01583-7. [DOI] [PubMed] [Google Scholar]
- 58.Tsyurupa MP, Davankov VA. React. Funct. Polym. 2006;66:768. [Google Scholar]
- 59.Veverka P, Jerabek K. React. Funct. Polym. 2004;59:71. [Google Scholar]
- 60.Ahn JH, Jang JE, Oh CG, Ihm SK, Cortez J, Sherrington DC. Macromolecules. 2006;39:627. [Google Scholar]
- 61.Germain J, Hradil J, Svec F, Frechet JMJ. Chem. Mater. 2006;18:4430. [Google Scholar]
- 62.Davankov VA, Tsyurupa M. Hypercrosslinked polymeric networks and adsorbing materials. Amsterdam: Elsevier; 2011. [Google Scholar]
- 63.Urban J, Svec F, Fréchet JMJ. Anal. Chem. 2010;82:1621. doi: 10.1021/ac100008n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urban J, Svec F, Fréchet JMJ. J. Chromatogr. A. 2010;1217:8212. doi: 10.1016/j.chroma.2010.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jerabek K. Anal. Chem. 1985;57:1598. [Google Scholar]
- 66.Aoki H, Tanaka N, Kubo T, Hosoya K. J. Polym. Sci., Polym. Chem. 2008;46:4651. [Google Scholar]
- 67.Lubbad SH, Buchmeiser MR. Macromol. Rapid Commun. 2002;23:617. [Google Scholar]
- 68.Santora BP, Gagne MR, Moloy KG, Radu NS. Macromolecules. 2001;34:658. [Google Scholar]
- 69.Wieder W, Lubbad SH, Trojer L, Bisjak CP, Bonn GK. J. Chromatogr. A. 2008;1191:253. doi: 10.1016/j.chroma.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 70.Cabrera K, Lubda D, Eggenweiler HM, Minakuchi H, Nakanishi K. J. High Resolut. Chromatogr. 2000;23:93. [Google Scholar]
- 71.Zhang Z, Wang Z, Liao Y, Liu H. J. Sep. Sci. 2006;29:1872. doi: 10.1002/jssc.200600154. [DOI] [PubMed] [Google Scholar]
- 72.Nilsson C, Birnbaum S, Nilsson S. J. Chromatogr. A. 2007;1168:212. doi: 10.1016/j.chroma.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 73.Nilsson C, Nilsson S. Electrophoresis. 2006;27:76. doi: 10.1002/elps.200500535. [DOI] [PubMed] [Google Scholar]
- 74.Small H, Stevens PW, Bauman WC. Anal. Chem. 1975;47:1801. [Google Scholar]
- 75.Stevens TS, Langhorst MA. Anal. Chem. 1982;54:950. [Google Scholar]
- 76.Guihen E, Glennon JD. Anal. Lett. 2003;36:3309. [Google Scholar]
- 77.Zhang Z, Yan B, Liao Y, Liu H. Anal. Bioanal. Chem. 2008;391:925. doi: 10.1007/s00216-008-1930-2. [DOI] [PubMed] [Google Scholar]
- 78.Qu QS, Shen F, Shen M, Hu XY, Yang GJ, Wang CY, Yan C, Zhang YK. Anal. Chim. Acta. 2008;609:76. doi: 10.1016/j.aca.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 79.Tran CD, Challa S. Analyst. 2008;133:455. doi: 10.1039/b716443b. [DOI] [PubMed] [Google Scholar]
- 80.Liu FK, Hsu YT, Wu CH. J. Chromatogr. A. 2005;1083:205. doi: 10.1016/j.chroma.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 81.Jimenez-Soto JM, Moliner-Martinez Y, Cardenas S, Valcarcel M. Electrophoresis. 2010;31:1681. doi: 10.1002/elps.200900628. [DOI] [PubMed] [Google Scholar]
- 82.Qu QS, Zhang XX, Zhao ZZ, Hu XY, Yan C. J. Chromatogr. A. 2008;1198–1199:95. doi: 10.1016/j.chroma.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 83.Andre C, Gharbi T, Guillaume YC. J. Sep. Sci. 2009;32:1757. doi: 10.1002/jssc.200800683. [DOI] [PubMed] [Google Scholar]
- 84.Zhong Y, Zhou W, Zhang P, Zhu Y. Talanta. 2010;82:1439. doi: 10.1016/j.talanta.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 85.Hilder EF, Svec F, Fréchet JMJ. J. Chromatogr. A. 2004;1053:101. [PubMed] [Google Scholar]
- 86.Hutchinson JP, Zakaria P, Bowie AR, Macka M, Avdalovic N, Haddad PR. Anal. Chem. 2005;77:407. doi: 10.1021/ac048748d. [DOI] [PubMed] [Google Scholar]
- 87.Zakaria P, Hutchinson JP, Avdalovic N, Liu Y, Haddad PR. Anal. Chem. 2005;77:417. doi: 10.1021/ac048747l. [DOI] [PubMed] [Google Scholar]
- 88.Glenn KM, Lucy CA, Haddad PR. J. Chromatogr. A. 2007;1155:8. doi: 10.1016/j.chroma.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 89.Xu Y, Cao Q, Svec F, Fréchet JMJ. Anal. Chem. 2010;82:3352. doi: 10.1021/ac1002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao Q, Xu Y, Liu F, Svec F, Fréchet JMJ. Anal. Chem. 2010;82:7416. doi: 10.1021/ac1015613. [DOI] [PubMed] [Google Scholar]
- 91.Connolly D, Twamley B, Paull B. Chem. Commun. 2010;46:2109. doi: 10.1039/b924152c. [DOI] [PubMed] [Google Scholar]
- 92.Krenkova J, Lacher NA, Svec F. Anal. Chem. 2010;82 doi: 10.1021/ac1018815. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Chen Y, Xiang R, Ciuparu D, Pfefferle LD, Horváth C, Wilkins JA. Anal. Chem. 2005;77:1398. doi: 10.1021/ac048299h. [DOI] [PubMed] [Google Scholar]
- 94.Chambers SD, Svec F, Fréchet JMJ. J. Chromatogr. A. 2011;1218:2546. doi: 10.1016/j.chroma.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Forrest GA, Alexander AJ. J. Phys. Chem. C. 2007;111:10792. [Google Scholar]
- 96.Liu P, Wang T. Appl. Phys. A: Mater. Sci. Process. 2009;97:771. [Google Scholar]
- 97.Svec F, Hrudkova H, Horak D, Kalal J. Angew. Macromol. Chem. 1977;63:37. [Google Scholar]
- 98.Menna E, la Negra F, Prato M, Tagmatarchis N, Ciogli A, Gasparrini F, Misiti D, Villani C. Carbon. 2006;44:1609. [Google Scholar]
- 99.Bechtle M, Butte A, Storti G, Morbidelli M. J. Chromatogr. A. 2010;1217:4675. doi: 10.1016/j.chroma.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 100.Marti N, Quattrini F, Butte A, Morbidelli M. Macromol. Mater. Eng. 2005;290:221. [Google Scholar]