Abstract
Biofilm infections are frequently caused by S. epidermidis, are resistant to antimicrobial agents and adversely affect patient outcomes. We evaluated farnesol, the Candida quorum sensing molecule, on S. epidermidis biofilms, in vitro and in vivo. We evaluated ED50, ED75 and ED90 –(drug concentrations causing 50%, 75% and 90% inhibition respectively) of farnesol and evaluated synergy with nafcillin and vancomycin. Farnesol’s effects on morphology of S. epidermidis biofilms were analyzed using confocal microscopy and real-time changes using a bioluminescent strain of S. epidermidis, Xen 43. In mice, effects of farnesol treatment on subcutaneous catheter biofilms, cultures of blood, kidney, catheter and peri-catheter tissues and bioluminescence in strain Xen 43 were evaluated. Farnesol inhibited biofilms (ED50 ranged from 0.625 to 2.5 mM) and was synergistic with nafcillin and vancomycin at most combination ratios. Farnesol significantly decreased biovolume, substratum coverage and mean thickness of S. epidermidis biofilms. In mice, farnesol significantly decreased viable colony counts of S. epidermidis from blood, kidney, catheter and peri-catheter tissues and decreased Xen 43 bioluminescence. We confirmed the anti-biofilm effects of farnesol both in vitro and in vivo, in a bioluminescent strain and its synergy with antibiotics. Farnesol may be effective against clinical S. epidermidis biofilm infections.
Introduction
Biofilms are three dimensional communities of microorganisms that are surface-associated and encased in an extracellular matrix, composed of polysaccharides, proteins and extracellular DNA (1). Biofilms contribute significantly to medical infections that include catheter and other device-associated infections(2). Catheter-related blood stream infections (CRBSI) significantly increase mortality, morbidity and healthcare costs (3). Approximately 250,000 cases of CRBSIs occur in the USA and it costs more than $50,000 to treat an episode of CRBSI (3).
S. epidermidis is the most common etiological agent isolated in device-associated biofilm-related infections (4). S. epidermidis is a normal skin commensal but as an opportunistic pathogen leads the list for healthcare-associated infections. While S. epidermidis lacks secreted virulence factors like the exotoxins produced by S. aureus, it is uniquely adapted to cause chronic biofilm infections. In addition, S. epidermidis is also the major cause for late-onset neonatal sepsis in infants born at < 1500 g (5). Neonatal sepsis is associated with significant mortality, morbidity and adverse neurodevelopmental outcomes (5, 6).
Microbial biofilms including those of S. epidermidis are inherently resistant to host defense and antimicrobial agents and hence difficult to eradicate. Clinical treatment strategies involve treatment with multiple antibiotics and removal of the life-saving catheters and devices. Novel strategies are necessary to combat biofilm-related infections to improve clinical outcomes. Farnesol, the Candida quorum sensing molecule has anti-biofilm activity. Farnesol is a sesquiterpene alcohol and is the first quorum sensing molecule described in eukaryotes (7) and produced by most candida species (8). Exogenous farnesol inhibits the development of Candida biofilms via inhibition of filamentation (9). Farnesol has also been reported to have efficacy against S. aureus biofilms and increases biofilm susceptibility to antibiotics (10). We evaluated the anti-microbial susceptibilities of S. epidermidis biofilms to farnesol and synergy with antistaphylococcal antibiotics in vitro. Quorum sensing mutants of S. epidermidis form larger biofilms and are implicated in chronic biofilm infections in humans and hence we evaluated mutants of the agr and luxS quorum sensing systems in addition to clinical isolates (11). We confirmed our findings in a clinically relevant mouse model of catheter infection in vivo.
METHODS
Strains and culture conditions
Organisms: S. epidermidis strains ATCC 55133, 1457, 1457 agr mutant, 1457 luxS mutant and Xen 43 were used. ATCC 55133 and 1457 are biofilm forming clinical isolates. Xen 43 (Caliper Life Sciences Inc., USA) is a bioluminescent strain derived from 1457 and was used to monitor infection in real-time (12). Growth media: Trypticose soy agar with 5% sheep blood was used for plating organisms and trypticose soy broth (TSB) for subcultures and antimicrobial susceptibility testing.
Farnesol preparation: A stock solution of trans-trans-farnesol (Sigma, USA) in DMSO was freshly prepared at 500 mM before use and diluted to required concentrations. Farnesol suspensions were prepared fresh to preserve the antioxidant effect. Farnesol was prepared in concentration ranges of 80 to 0.16 mM for biofilm susceptibility testing. Nafcillin and vancomycin were prepared in concentration ranges of 80 to 0.6 µg/ml for biofilm antimicrobial susceptibility testing.
Antimicrobial susceptibility testing of S. epidermidis biofilms
Biofilms were formed in 96-well microtiter plates by adding 100 µl of S. epidermidis at approximately 107 colony forming units/ml (CFU ml−1) in RPMI 1640 (pH of 7) and incubated for 24 h at 35° C. The supernatant was discarded and the biofilms washed with PBS to remove unadhered cells. Biofilm formation was confirmed by light microscopy. Antimicrobial susceptibility of biofilms to farnesol was performed in duplicate by adapting Clinical and Laboratory Standards Institute (CLSI) guidelines for planktonic antimicrobial testing (13). The S. epidermidis biofilms in the microtiter plates were exposed to serial dilutions of antimicrobial agent (farnesol from 0.2 mM to 80 mM, nafcillin and vancomycin from 80 to 0.6 µg/ml) for 24 h. The endpoint of inhibition of the biofilms by the antimicrobial agent was estimated by the colorimetric method using 2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenyl-amino) carbonyl]-2H-tetrazolium hydroxide (XTT) (14). A solution of XTT (0.5 g L−1) was prepared in phosphate buffered saline, filter sterilized (0.22 µm size) and stored at −80 °C. Menadione was prepared as 10 mM solution in acetone (99.9% HPLC grade) and stored at −80 °C and 100 µl of which added to 100 µl of XTT gives 1 µM concentration. Hundred µl of this combination is added to the wells of 96 well micro-titer plates with the biofilms with the antimicrobial and then incubated in the dark for 2 h. Microtiter plates were centrifuged for 5 min at 3000 rpm, 100 µl supernatant was transferred to a new microtiter plate and the color read at OD 490 nm (15). Growth and sterility controls on the same microtiter plate were used for comparison. The experiments were done in duplicate, repeated on 3 different days and the mean of the readings of XTT reduction were used to determine the biofilm inhibitory concentrations. We evaluated ED50, ED75 and ED90 (drug concentrations causing 50%, 75% and 90% inhibition respectively) of farnesol, nafcillin and vancomycin.
Confocal imaging to assess biofilm morphology: Biofilms of S. epidermidis were formed on optical bottom microwell Petri dishes (Mattek Corp, USA) for 24 h and exposed to 0.5 mM of farnesol or DMSO control for another 24 hrs. The biofilms were washed with PBS, stained with LIVE/DEAD stain (Molecular probes, USA) and examined using Zeiss Meta confocal microscope. Serial sections of the biofilm were obtained at 1 µm intervals along the z-axis and the z-stack images were analyzed with the software PHLIP, for MATLAB toolbox, for biovolume (in µm3), substratum coverage (in %) and mean thickness (in µm) (16). At least 2 representative fields in the biofilm on any particular day were analyzed and measurements were averaged. Biofilm experiments were repeated on 3 different days. Biofilms exposed to farnesol were compared with the controls by t test and statistical significance was assumed at p < 0.05.
Synergy evaluation of farnesol antibiotic combinations
Biofilm inhibition by combinations of farnesol with nafcillin or vancomycin was evaluated in an 8 by 8 checkerboard format, in 96-well micro-titer plates, in 2-fold serial dilutions across rows and columns, and inhibitory endpoints were assessed by the XTT reduction assay. Inhibitory effects at equipotent drug-dose ratios (1:1 ratios of ED50, ED75 and ED90) and non-equipotent ratios (1:2, 1:4 or 2:1) of the combinations were determined. The median effects method described by Chou et al. was used to study interactions in drug combinations by the calculation of combination indices (CI) (17). A CI <1 indicates synergy, CI >1 antagonism and CI=1, an additive effect. Multiple drug dose-effect calculations were performed using Calcusyn software (Biosoft, Cambridge, U.K.) with constant ratios of drug combinations.
Mouse model of subcutaneous catheter infection
In 3 week old FVB albino mice (Charles River Laboratories Inc., USA) mice, 1.5 inch segment of 18 gauge Teflon catheter was inserted subcutaneously on the back of each animal and each animal receiving one catheter segment. Prior to insertion, catheters were immersed in suspensions of S. epidermidis (107 CFU/ml) for 2 h (catheter CFU/ml pre-insertion was 3 to 5 105 CFU/ml), to facilitate biofilm formation. Farnesol (100 µg/g in 0.1 ml DMSO, 6.7 mM, n=7) or DMSO (control, n=5) was injected for 6 consecutive days from day 2 of infection, once per day, s.c. near the catheter. The dose of farnesol was extrapolated from other animal studies and was higher than our estimated ED50 in vitro (18). The animals were euthanized on day 8 and cultures of blood, kidney homogenates, catheter (by sonication) and peri-catheter (skin and subcutaneous) tissues were plated in serial dilutions. Catheter biofilms were confirmed by confocal and electron microscopy of explanted catheter segments. Sample size calculations revealed that a sample size of 5 in each group gave a power of 93% in detecting a log difference in CFU/ml of catheter cultures. The differences in CFU/ml between farnesol and DMSO treatment were assessed for significance by the Kruskal Wallis test. The protocol for animal experiments was approved by The Institutional Animal Care and Use Committee at Baylor College of Medicine.
Scanning electron microscopy: A 5 mm sample was cut from each of the explanted catheter segments from mice with subcutaneous catheter infection. The catheter samples were cut in cross sections and fixed with 2% glutaraldehyde, followed by fixing with osmium tetroxide, tannic acid and uranyl acetate. Fixation was followed by a series of ethanol dehydration steps and samples were sputter-coated with gold palladium. The samples were then scanned by pathologists who were blinded for farnesol treatment.
Real-time imaging using the bioluminescent strain Xen 43
In vitro: Biofilms of Xen 43 were developed in 30 wells of an opaque 96-well microtiter plate (Corning, USA) and washed with PBS. At 48 h, 3 groups of ten wells each were exposed to DMSO, farnesol (FSL) (0.5 mM), or TSB for 24 h. Bioluminescence was monitored at 24, 48, 72 and 96 h and compared among the three exposures and the experiments were performed in duplicate. In vivo: Subcutaneous catheter infection was also established with the bioluminescent strain Xen 43, as described earlier. Subcutaneous injections of farnesol (100 µg/g in 0.1 ml DMSO, 6.7 mM, n=5) or DMSO (n=5) were administered only from days 2 to 5. Live animal imaging for bioluminescence was performed daily for 5 days.
Results
Antimicrobial susceptibilities of S. epidermidis biofilms
The antimicrobial susceptibilities of S. epidermidis biofilms at ED50, ED75 and ED90 for strains ATCC 55133, 1457, 1457 agr mutant and 1457 luxS mutant are shown in Table. 1. We were unable to establish ED90 of farnesol, nafcillin or vancomycin for strains 1457, 1457 agr mutant or the 1457 luxS mutant indicating the inherent resistance of the biofilm state. The agr and luxS quorum sensing mutants of 1457 were similar in susceptibilities to the parent strain 1457 (within two, 2-fold dilutions of the antimicrobial) except the luxS mutant, whose ED75 for nafcillin was more than 2 dilutions than the parent 1457 strain (5 µg/ml vs. 0.625 µg/ml).
Table 1.
Antimicrobial Susceptibility of S. epidermidis Biofilms
| Strains | ATCC 55133 | 1457 | 1457 agr- | 1457 luxS- |
|---|---|---|---|---|
| Farnesol (mM) | ||||
| ED50 | 1.25 | 2.5 | 1.25 | 0.625 |
| ED75 | 10 | 10 | 10 | 10 |
| ED90 | 20 | >80 | >80 | >80 |
| Nafcillin (µg/ml) | ||||
| ED50 | 0.32 | 0.08 | 0.08 | 0.08 |
| ED75 | 0.625 | 0.625 | 1.25 | 5 |
| ED90 | 80 | >80 | >80 | >80 |
| Vancomycin (µg/ml) | ||||
| ED50 | 5 | 2.5 | 2.5 | 2.5 |
| ED75 | 10 | 10 | 10 | 10 |
| ED90 | 80 | >80 | >80 | >80 |
Biofilm inhibitory concentrations of the antimicrobial agent at 50%, 75% and 90% inhibition are reported as effective dose50 (ED50), ED75 and ED90 respectively.
In other experiments, 24 h biofilms of S. epidermidis strains were exposed to farnesol at 0.5 mM or DMSO (control). We chose 0.5 mM of farnesol, which was lower than the ED50 of all the strains to evaluate biofilm morphology. Confocal images of farnesol exposed biofilms and DMSO (control) obtained at 40 X magnification were examined at z intervals of 1 µm (Fig. 1) and analyzed. Farnesol significantly decreased biovolume, substratum coverage and mean thickness of S. epidermidis biofilms of all the four strains studied (p < 0.05) (Fig. 2).
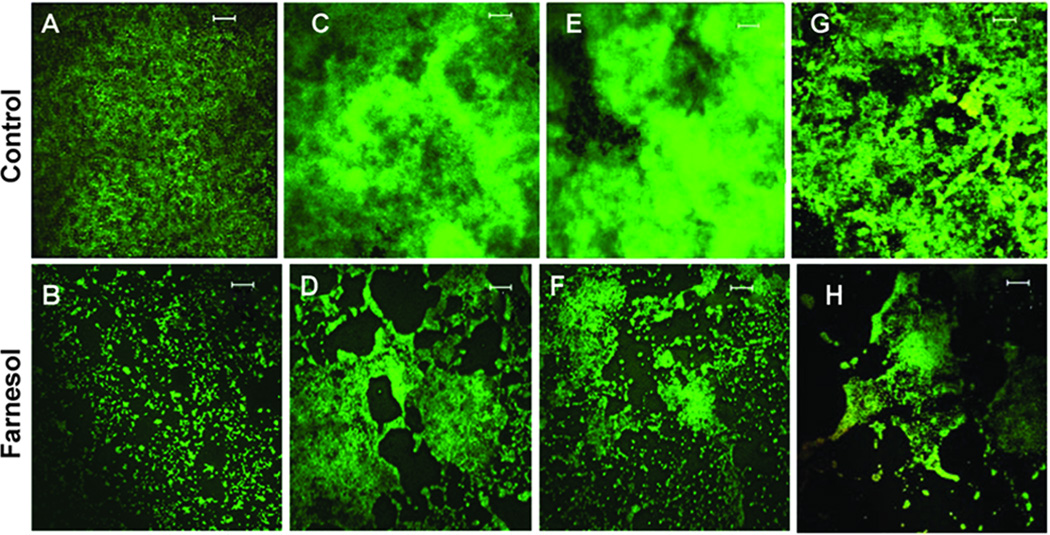
Fig. 1. Biofilm evaluation by confocal microscopy.
Representative images of biofilms of 4 strains of S. epidermidis exposed to DMSO (control) or farnesol (0.5 mM) for 24 h: ATCC 55133 (A and B), 1457 (C and D), 1457 agr mutant (E and F), and 1457 luxS mutant (G and H). Scale bars measure 20 µm. Biofilms were stained and examined by the Zeiss confocal microscope. Farnesol exposed biofilms are thinner and more sparse than controls.
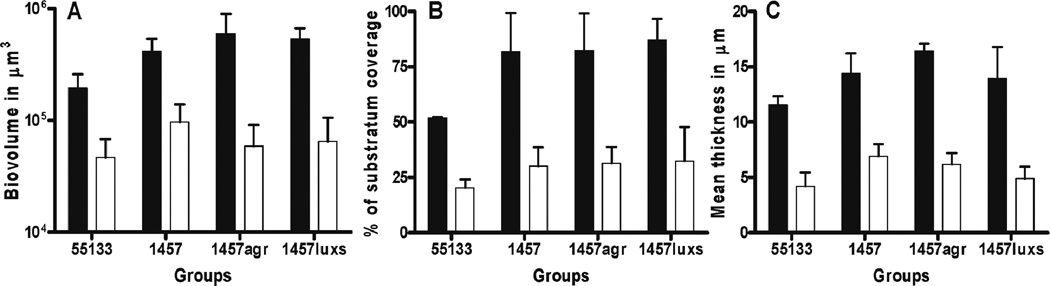
Fig. 2. Farnesol decreases biovolume, substratum coverage and mean thickness in biofilms in vitro.
The confocal images of the 24 h biofilms exposed to DMSO or farnesol were analyzed by PHLIP software, using MATLAB toolbox. The z stack images were analyzed for biovolume (µm3) (A) substratum coverage (%) (B) and mean thickness (µm) (C). Farnesol (□) decreased biovolume, substratum coverage and mean thickness significantly (p < 0.05), when compared to DMSO control (■) for all four S. epidermidis strains.
Evaluation of synergy of the antimicrobial combinations by combination indices
We evaluated farnesol synergy with nafcillin and vancomycin by calculating combination Indices (CI) against four strains of S. epidermidis (Table. 2). We estimated CIs at equipotency ratios (1:1 ratios of ED of farnesol and the antibiotic) of ED50, ED90 concentrations (ATCC 55133) and ED75 ratios for 1457, 1457 agr mutant and 1457 luxS mutant (where ED90 measurements were not available). We also evaluated non-equipotency ratios at 1:2, 1:4 and 2:1, where possible, from our checkerboard matrix of combinations. For farnesol and vancomycin combinations, ED50 1:1 combination ratios were the same as ED90 1:1 for strain 55133, and same as ED75 1:1 for strains 1457, 1457 agr mutant and 1457 luxS mutant. Farnesol was synergistic with nafcillin and vancomycin (CI <1) at most equipotency and non-equipotency drug-dose ratios at 75% and 90% combination inhibitory effects of the combination (CED75 and CED90) with few exceptions. The combinations that were not synergistic are bolded and italicized in the table (Table. 2). Strains 55133 and 1457 agr mutant were synergistic at all combinations tested and strains 1457 and 1457 luxS mutant had 2 and 1 exceptions respectively.
TABLE 2.
Combination Indices for Farnesol-Antibiotic Combinations
| Ratios | CI values in mean (SD) | Ratios | CI values in mean (SD) | ||
|---|---|---|---|---|---|
| FSL: NAF | CED75 | CED90 | FSL:VAN | CED75 | CED90 |
| ATCC 55133 | |||||
| ED50 1:1 | 0 (0) | 0.26 (0.45) | ED50 1:1 = ED90 1:1 | 0.13 (0.11) | 0.1 (0.02) |
| ED90 1:1 | 0.5 (0.5) | 0.04 (0.01) | ED90 1:2 | 0.10 (0.07) | 0.07 (0.02) |
| ED90 1:2 | 0.13 (0.11) | 0.06 (0.01) | ED90 2:1 | 0.18 (0.17) | 0.1 (0.04) |
| ED90 2:1 | 0.22 (0.19) | 0.07 (0.03) | |||
| 1457 | |||||
| ED75 1:1 | 3.82 (0.53) | 0.05 (0) | ED50 1:1 = ED75 1:1 | 0.24 (0.05) | 0.08 (0.02) |
| ED75 1:2 | 2.41 (1.80) | 0.21 (0.13) | ED75 1:2 | 0.29 (0.09) | 0.15 (0.03) |
| ED75 1:4 | 0.46 (0.53) | 0.34 (0.13) | ED75 2:1 | 0.21 (0.03) | 0.07 (0.04) |
| 1457 agr- | |||||
| ED75 1:1 | 0.69 (0.26) | 0.41 (0.35) | ED50 1:1 = ED75 1:1 | 0.30 (0.29) | 0.2 (0.11) |
| ED75 1:2 | 0.55 (0.51) | 0.59 (0.13) | ED75 1:2 | 0.33 (0.28) | 0.35 (0.16) |
| ED75 1:4 | 0.48 (0.42) | 0.9 (0.79) | ED75 2:1 | 0.26 (0.25) | 0.14 (0.06) |
| 1457luxS- | |||||
| ED75 1:1 | 0.21 (0.30) | 0.03 (0.02) | ED50 1:1 = ED75 1:1 | 0.27 (0.06) | 0.05 (0.02) |
| ED75 1:2 | 0.01 (0.02) | 0.09 (0.07) | ED75 1:2 | 0.28 (0.06) | 0.06 (0.024) |
| ED75 1:4 | 0.08 (0.07) | 0.12 (0.09) | ED75 2:1 | 0.25 (0.1) | 0.05 (0.02) |
| ED50 1:1 | 0.29 (0.27) | 10.57 (182) | ED50 1:1 | 0.10 (0.03) | 0.03 (0.014) |
FSL- farnesol, NAF-nafcillin, VAN-vancomycin. ED50, ED75, and ED90 represent 50%, 75% and 90% inhibitory effects of the individual antimicrobial agent respectively. CED75 and CED90 represent 75% and 90% inhibitory effects of farnesol-antibiotic combination respectively. Combination indices (CI) derived by the median effects principle, indicate synergy if CI < 1, additive effect if CI=1 and antagonism if CI > 1. Non synergistic combinations are italicized and bolded.
Farnesol decreased viable colony counts of S. epidermidis in the mouse model of subcutaneous catheter infection
In mice after subcutaneous catheter infection, mice were euthanized on day 8 of infection, catheters explanted and cultures performed. Catheter biofilms were confirmed by confocal and electron microscopy (Figs 3A & 3B). Farnesol decreased catheter biofilms (Fig. 3C) compared to controls (Fig. 3B). Farnesol treatment decreased catheter and pericatheter infection of S. epidermidis as shown by significantly decreased viable colony counts (CFU/ml) from catheter (Fig 4A) and peri-catheter tissues (Fig. 4B). Farnesol treatment also decreased systemic dissemination of S. epidermidis as shown by decreased blood and kidney CFU/ml (Fig. 4C & 4D). There were not significant differences in weight gain, activity or local reactions in farnesol treated mice compared to controls (data not shown).
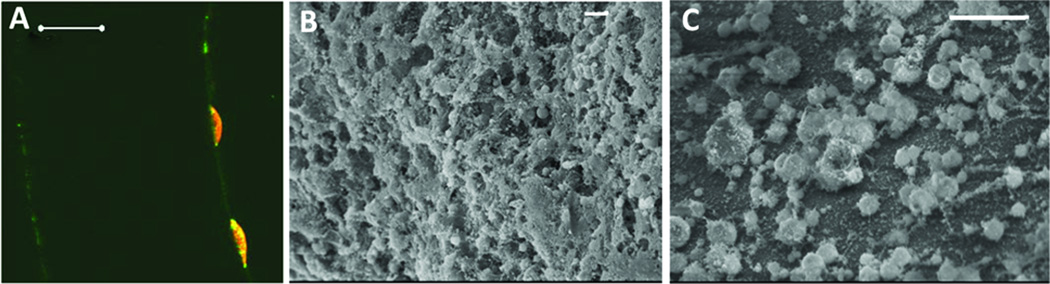
Fig. 3. Farnesol decreases catheter biofilms.
In 3 week old FVB mice, 1.5 inch Teflon catheter segments were inserted subcutaneously. Catheters were preincubated in S. epidermidis suspension for 2 h before insertion, to aid biofilm development. The catheter segments were explanted on day 8 of implantation, sectioned into thin slices, stained with LIVE/DEAD stain and examined under the Zeiss confocal microscope (A). Scale bar measures 50 µm in A. Some catheter segments were examined by scanning electron microscopy (B & C). Confocal imaging and electron microscopy confirmed S. epidermidis biofilm formation (A & B). Farnesol treatment (100 µg/g, s.c. for 6 days) decreased S. epidermidis catheter biofilms (C). Scale bars measure 10 µm in B and C.
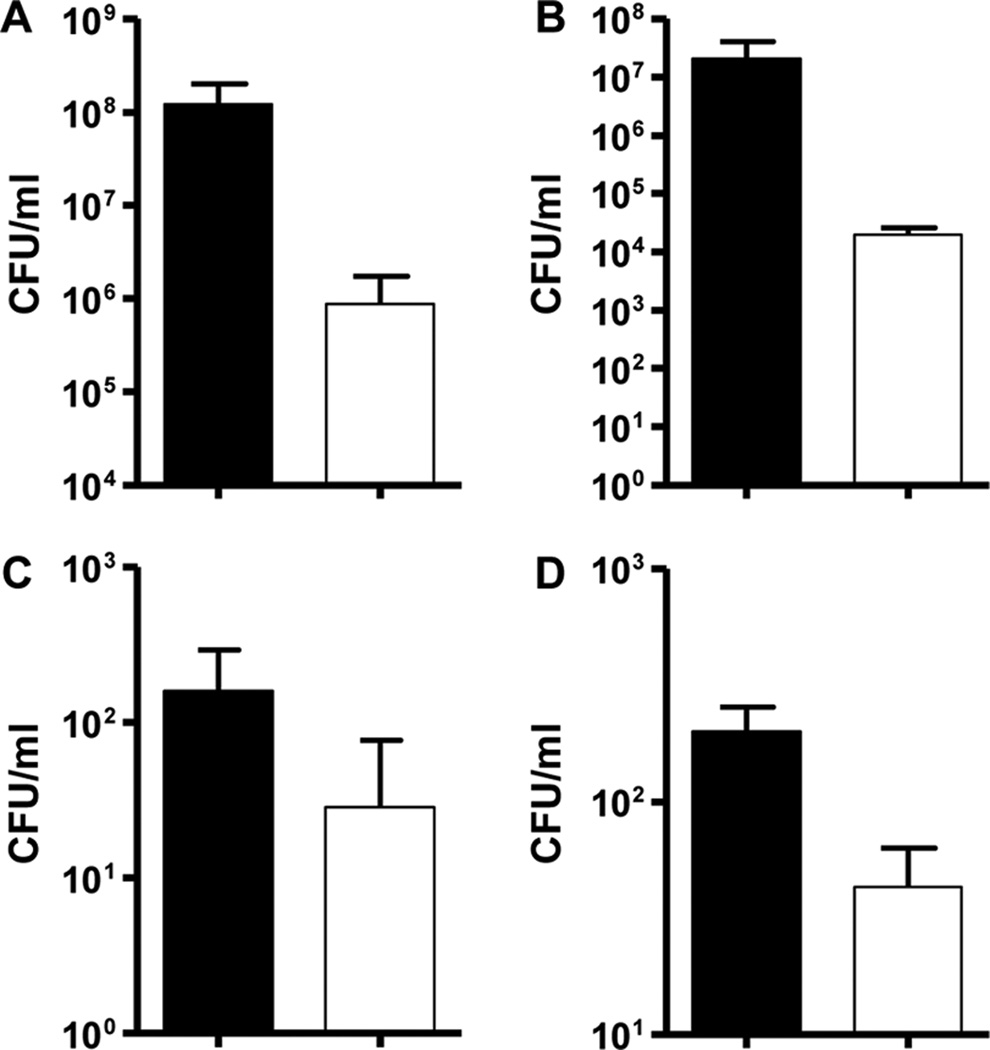
Fig. 4. Farnesol decreases viable cell counts of S. epidermidis during catheter infection in mice.
Mice were euthanized on day 8 of catheter implantation and cultures of the catheters (A) and skin and tissue around the catheter (peri-catheter) (B) were performed. Systemic dissemination was assessed by cultures of blood (C) and kidney homogenate (kidneys of one animal homogenized in 1 ml of PBS) (C). Farnesol treatment (n=7) was compared to DMSO control (n=5). Farnesol treatment significantly decreased catheter CFU/ml, peri-catheter infection and systemic dissemination (p < 0.05). All panels: DMSO control (■);Farnesol treatment (□).
Farnesol inhibits biofilms of bioluminescent S. epidermidis Xen 43 in vitro and in vivo
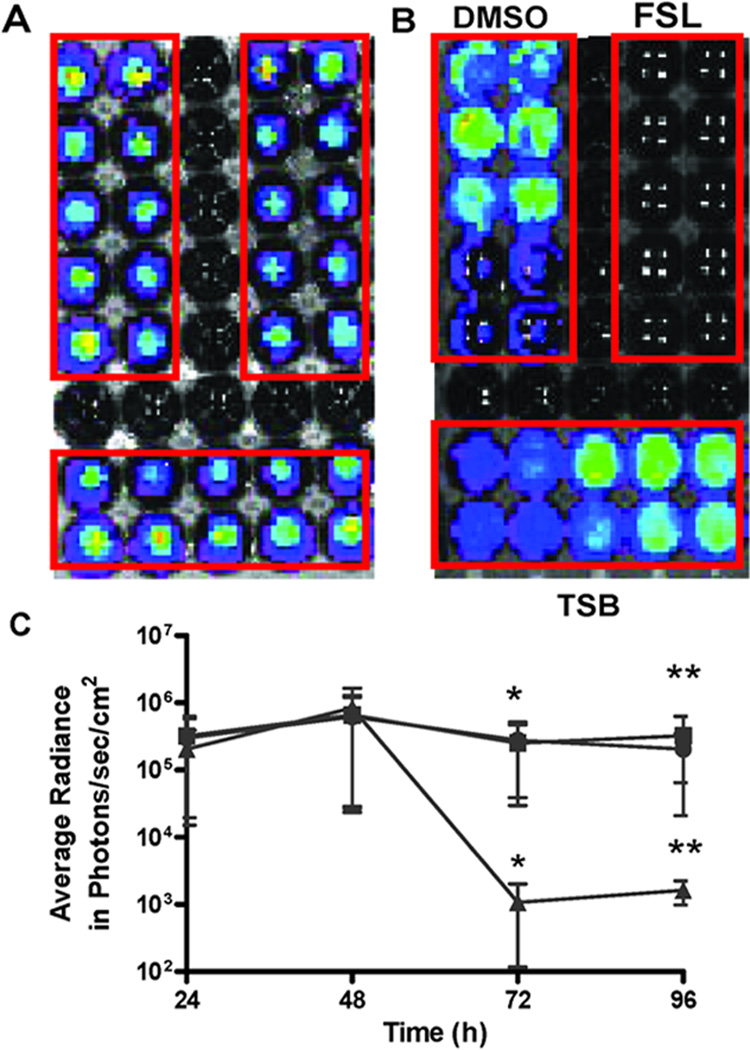
As an alternative approach to assess the effects of farnesol on S. epidermidis biofilms in vivo, a bioluminescent strain was used. The utility of this strain was validated in vitro. After 48 h, Xen 43 biofilms were divided into three groups of 10 wells and treated with farnesol, DMSO or fresh medium. In vitro, Xen 43 biofilms, bioluminescence was not significantly different among the 3 groups of 10 wells at 48 h, before exposure to farnesol (Fig. 5A). After exposure to DMSO, farnesol (FSL) (0.5 mM), or Trypticose soy broth (TSB) for 24 h, farnesol significantly reduced bioluminescence compared to DMSO or TSB (* and ** p < 0.05) (Fig. 5B and 5C). Bioluminescence did not differ significantly between DMSO and TSB exposed biofilms.
Fig. 5. Biofilm bioluminescence of Xen 43 decreased by farnesol.
Xen 43 biofilms were developed in 30 wells of an opaque 96-well microtiter plate (A). At 48 h, supernatants were removed and unadhered cells were washed with PBS. Three groups of ten wells each were exposed to DMSO, farnesol (FSL) (0.5 mM), or Trypticose soy broth (TSB) media for 24 h (B). Bioluminescence was monitored at 24, 48, 72 and 96 h and compared among the three exposures. Farnesol exposure significantly reduced average radiance compared with DMSO or TSB exposed wells (* and ** p < 0.05) (C). ■: DMSO; ●: TSB; ▲: Farnesol.
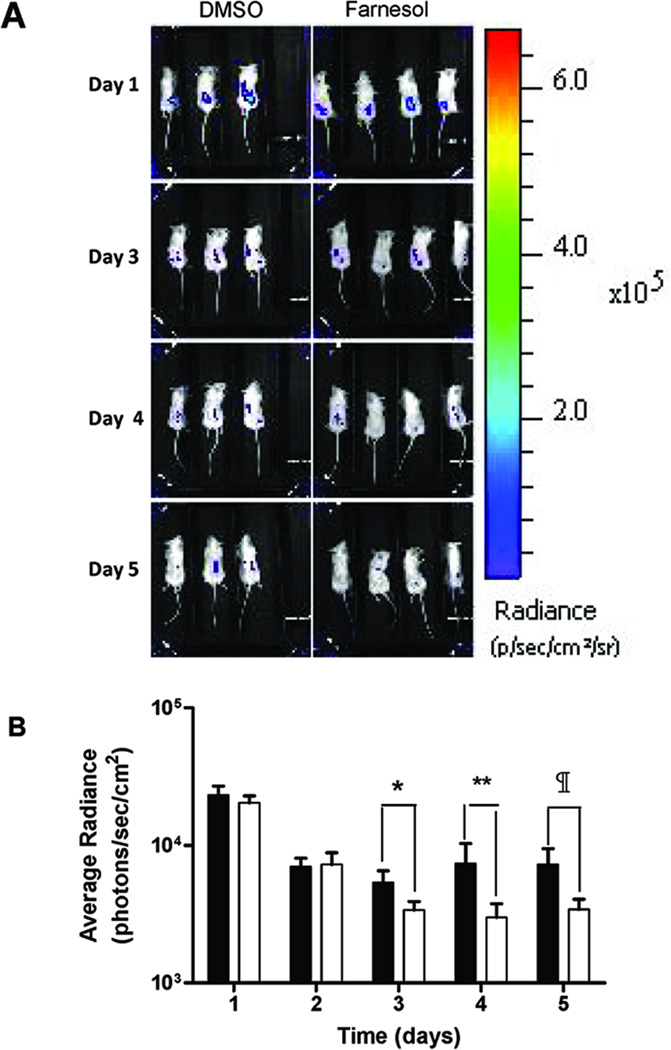
In vivo, subcutaneous catheter biofilm infection of Xen 43, bioluminescence over the infected catheters was monitored daily for 5 days in live animals (Fig 6A & 6B). Average radiance was similar on day 1 and day 2, before exposure to farnesol. After farnesol treatment, a significant decrease in bioluminescence was observed on day 3, 4 and 5 of infection. Farnesol treatment significantly decreased biofilm infection in vivo.
Fig. 6. Farnesol decreases bioluminescence in S. epidermidis catheter infection in vivo.
A bioluminescent strain of S. epidermidis 1457 Xen 43 was used in the subcutaneous catheter infection model in mice and bioluminescence followed serially for 5 days (A). Mice were treated with farnesol at 100 µg/g/day or DMSO (control) from day 2 of infection for 4 days. Bioluminescence was quantified and expressed as average radiance in photons/sec/cm2 (B). Farnesol treatment (□) decreased bioluminescence significantly (*, ** and #, p < 0.05) on days 3, 4 and 5 compared to DMSO control (■).
Discussion
Farnesol inhibited biofilms of S. epidermidis biofilms both in vitro and in vivo and was synergistic with nafcillin and vancomycin at most combination ratios. In our model of subcutaneous catheter infection in mice that is clinically relevant, farnesol treatment decreased catheter infection and systemic dissemination. We also confirmed the biofilm inhibiting effects of farnesol in real-time, using a bioluminescent strain of S. epidermidis.
We report ED50, ED75 and ED90 of farnesol, nafcillin and vancomycin against biofilms of 2 clinical isolates and 3 laboratory strains of S. epidermidis, all of which were sensitive to nafcillin (minimum inhibitory concentration50 (MIC50) < 0.5 µg/ml) and vancomycin (MIC50 < 2 µg/ml) in the planktonic state (data not shown). We evaluated quorum sensing mutants (agr and luxS mutants) of S. epidermidis 1457, as these mutant strains form thicker biofilms than WT strains and spontaneous agr mutants predominate in chronic biofilm infections (11). Quorum sensing mechanisms determine antibiotic and biocide susceptibility in Pseudomonas aeruginosa and we sought to clarify farnesol susceptibility of these quorum sensing mutants in S. epidermidis (19). The agr and luxS quorum sensing mutants were similar in susceptibilities to the parent strain 1457 (within two, 2-fold dilutions of the antimicrobial) except the luxS mutant, whose ED75 for nafcillin was more than 2 dilutions than the parent strain (5 µg/ml vs. 0.625 µg/ml).
Gomes et al reported the antibacterial effects of farnesol on planktonic cells of S. epidermidis at concentrations up to 300 µM and reported S. epidermidis susceptibility to farnesol at 100 µM (20). However, biofilms were tolerant to farnesol in vitro. Gomes et al did not report MICs or EDs performed according to standardized guidelines or the effects of farnesol on biofilms in vivo. Antibacterial effects of farnesol on S. aureus at 150 µM concentration and synergy with gentamicin at 100 µM on S. aureus biofilms have been reported (10). Possible mechanisms for the antibacterial effects of farnesol have been explored. Farnesol at 100 µg/ml inhibits the pro-coagulant effect, production of S. aureus exotoxins and potentiated the effects of cell wall acting ampicillin (21). Inoue et al demonstrated that K+ ion leakage from S. aureus induced by the terpene alcohols such as farnesol correlated with the antibacterial effects (22). Thus, the antibacterial effects of farnesol may be due to its effects on membrane integrity.
We evaluated the morphology of S. epidermidis biofilms exposed to farnesol at 0.5 mM and observed a significant inhibiting effect. Detailed evaluation on confocal imaging revealed significant decrease in biovolume, mean thickness and substratum coverage of farnesol exposed biofilms, of both WT and quorum sensing mutants. The efficacy of farnesol at concentrations much lower than the estimated ED50 (0.625 to 2.5 mM) against S. epidermidis biofilms may have clinical implications in the treatment of biofilm-related catheter and device-associated healthcare infections.
Biofilms are inherently resistant to antibiotics, and antimicrobial combinations may be an important strategy against biofilm infections. Antimicrobial combinations against biofilms may enhance efficacy, reduce drug dosages and minimize the development of drug resistance. Therefore, we evaluated combinations of farnesol with the commonly used antistaphylococcal antibiotics; nafcillin and vancomycin, by discerning inhibitory endpoints by the XTT assay. We used the median-effects principle expounded by Chou et al, to evaluate synergy for the antimicrobial combinations (17). Evaluation of antimicrobial combinations by the median-effects method is widely used in cancer and infectious diseases research (17). Advantages of this method include surmounting the assumption that drug interactions are linear across drug dosages and effects. No general equation fits all the dose-response curves because mechanisms of drug actions differ. Dose-response curves evaluated at various dose effects may overcome this problem. Therefore, we evaluated drug combinations in a systematic manner at 2 different dose-effects, CED75 and CED90 at constant drug ratios, including equipotency ratios of the drug combinations. We observed synergy at most combination ratios with few exceptions.
We also evaluated the effects of farnesol in vivo in a mouse model of subcutaneous catheter infection that is a clinically relevant model of device-associated infection. We confirmed the formation of biofilms on the subcutaneously implanted catheters in mice, by electron and confocal laser microscopy. Farnesol treatment significantly decreased catheter infection and systemic dissemination. The dose of farnesol was extrapolated from animal studies and that was higher than our estimated ED50 in vitro (18). Other investigators have evaluated higher doses of farnesol (1 ml of 20 mM i.p.) in systemic candidiasis in mice (23, 24). Farnesol treatment by injections once a day at 100 µg/g did not cause any local or systemic adverse effects in mice. We are not aware of other studies evaluating the anti-biofilm effects of farnesol on S. epidermidis biofilms in vivo.
Real-time photonic imaging of animals offers advantages over conventional animal infection models. Real-time monitoring has the advantage of serial monitoring of infection and may decrease the sample size of animals (25). Growth curves of Xen 43 are similar to the parent strain 1457 and the bioluminescence is directly proportional to the CFU/ml (data not shown). The feasibility of monitoring catheter infection in mice using a bioluminescent strain of S. epidermidis Xen 43 has been reported (12). We evaluated the effects of farnesol on biofilms in vitro and on subcutaneous catheter infection using the bioluminescent strain of S. epidermidis Xen43, in vivo. Farnesol significantly decreased Xen 43 biofilm bioluminescence in vitro and in our catheter infection model in vivo.
Standard guidelines for biofilm antimicrobial susceptibility do not exist and we adapted CLSI guidelines for planktonic cells, to evaluate EDs against biofilms. We evaluated farnesol (0.5 mM) on biofilms formed in vitro and a daily dose of 100 µg/g subcutaneously, for 6 days in the animal model. It is possible that several other concentrations or doses are effective and the optimum effective dose that has clinical applicability needs to be established. The mouse model of subcutaneous catheter infection mimics a foreign device infection and to evaluate the effects on intravascular catheter biofilm infection, our future experiments will focus on a vascular indwelling catheter infection in mice.
We have evaluated and confirmed the efficacy of farnesol against biofilms of S. epidermidis by multiple approaches, both in vitro and in a clinically relevant subcutaneous catheter biofilm infection in mice in vivo. No adverse effects of farnesol treatment were observed in this animal model. We also observed synergy of farnesol with nafcillin and vancomycin. We conclude that farnesol alone or in combination with nafcillin or vancomycin, may be effective against device-associated biofilm infections and improve clinical outcomes.
Acknowledgements
We appreciate the advice and help of Waleed Gaber Ph.D., at the small animal imaging facility at Texas Children’s Hospital. We acknowledge the productive discussions with Paul Fey Ph.D. and Deborah Hogan Ph.D. We also thank all members of the Versalovic lab for their insightful discussion and critiques.
Statement of financial support: This work is supported by funding from NIH (CHRCDA, K12 HD41648) to M.P.
Abbreviations
- CRBSI
Catheter-related Bloodstream Infection
- CFU/ml
Colony Forming Units/ml
- CI
Combination index
- CED50 and CED90
Combination effective dose causing 50% and 90% inhibition respectively
- ED50, ED75 and ED90
Drug concentrations causing 50%, 75% and 90% inhibition respectively
- FSL
Farnesol
- TSB
Trypticose Soy Broth
- XTT
2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenyl-amino) carbonyl]-2H-tetrazolium hydroxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fey PD. Modality of bacterial growth presents unique targets: how do we treat biofilm-mediated infections? Curr Opin Microbiol. 2010;13:610–615. doi: 10.1016/j.mib.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potera C. Forging a link between biofilms and disease. Science. 1999;283:1837–1839. doi: 10.1126/science.283.5409.1837. [DOI] [PubMed] [Google Scholar]
- 3.Raad I, Hanna H, Maki D. Intravascular catheter-related infections: advances in diagnosis, prevention, and management. Lancet Infect Dis. 2007;7:645–657. doi: 10.1016/S1473-3099(07)70235-9. [DOI] [PubMed] [Google Scholar]
- 4.Fey PD, Olson ME. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010;5:917–933. doi: 10.2217/fmb.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 6.Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis. 2006;19:290–297. doi: 10.1097/01.qco.0000224825.57976.87. [DOI] [PubMed] [Google Scholar]
- 7.Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001;67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber K, Sohr R, Schulz B, Fleischhacker M, Ruhnke M. Secretion of E,E-farnesol and biofilm formation in eight different Candida species. Antimicrob Agents Chemother. 2008;52:1859–1861. doi: 10.1128/AAC.01646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabra-Rizk MA, Meiller TF, James CE, Shirtliff ME. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob Agents Chemother. 2006;50:1463–1469. doi: 10.1128/AAC.50.4.1463-1469.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuong C, Kocianova S, Yao Y, Carmody AB, Otto M. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J Infect Dis. 2004;190:1498–1505. doi: 10.1086/424487. [DOI] [PubMed] [Google Scholar]
- 12.Vuong C, Kocianova S, Yu J, Kadurugamuwa JL, Otto M. Development of real-time in vivo imaging of device-related Staphylococcus epidermidis infection in mice and influence of animal immune status on susceptibility to infection. J Infect Dis. 2008;198:258–261. doi: 10.1086/589307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wikler MA. Clinical and Laboratory Standards Institute 2009 Methods for dilution antimcrobial susceptibility tests for bacteria that grow aerobically; approved standard, eighth edition. CLSI document M07-A8. Wayne: Clinical and Laboratory Standards Institute; [Accessed, June 10, 2011]. Available at: http://www.clsi.org/source/orders/free/m07-a8.pdf. [Google Scholar]
- 14.Hawser SP, Norris H, Jessup CJ, Ghannoum MA. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-t etrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998;36:1450–1452. doi: 10.1128/jcm.36.5.1450-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerca N, Martins S, Cerca F, Jefferson KK, Pier GB, Oliveira R, Azeredo J. Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J Antimicrob Chemother. 2005;56:331–336. doi: 10.1093/jac/dki217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller LN, de Brouwer JFC, Almeida JS, Stal LJ, Xavier JB. Analysis of a marine phototrophic biofilm by confocal laser scanning microscopy using the new image quantification software PHLIP. BMC Ecol. 2006;6:1. doi: 10.1186/1472-6785-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhary SC, Alam MS, Siddiqui MS, Athar M. Chemopreventive effect of farnesol on DMBA/TPA-induced skin tumorigenesis: involvement of inflammation, Ras-ERK pathway and apoptosis. Life Sci. 2009;85:196–205. doi: 10.1016/j.lfs.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Bjarnsholt T, Jensen PO, Burmolle M, Hentzer M, Haagensen JA, Hougen HP, Calum H, Madsen KG, Moser C, Molin S, Hoiby N, Givskov M. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology. 2005;151:373–383. doi: 10.1099/mic.0.27463-0. [DOI] [PubMed] [Google Scholar]
- 20.Gomes FI, Teixeira P, Azeredo J, Oliveira R. Effect of farnesol on planktonic and biofilm cells of Staphylococcus epidermidis. Curr Microbiol. 2009;59:118–122. doi: 10.1007/s00284-009-9408-9. [DOI] [PubMed] [Google Scholar]
- 21.Akiyama H, Oono T, Huh WK, Yamasaki O, Ogawa S, Katsuyama M, Ichikawa H, Iwatsuki K. Actions of farnesol and xylitol against Staphylococcus aureus. Chemotherapy. 2002;48:122–128. doi: 10.1159/000064916. [DOI] [PubMed] [Google Scholar]
- 22.Inoue Y, Shiraishi A, Hada T, Hirose K, Hamashima H, Shimada J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol Lett. 2004;237:325–331. doi: 10.1016/j.femsle.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 23.Navarathna DH, Nickerson KW, Duhamel GE, Jerrels TR, Petro TM. Exogenous farnesol interferes with the normal progression of cytokine expression during candidiasis in a mouse model. Infect Immun. 2007;75:4006–4011. doi: 10.1128/IAI.00397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarathna DH, Hornby JM, Krishnan N, Parkhurst A, Duhamel GE, Nickerson KW. Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans. Infect Immun. 2007;75:1609–1618. doi: 10.1128/IAI.01182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis KP, Yu J, Bellinger-Kawahara C, Joh D, Hawkinson MJ, Xiao G, Purchio TF, Caparon MG, Lipsitch M, Contag PR. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect Immun. 2001;69:3350–3358. doi: 10.1128/IAI.69.5.3350-3358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]