Abstract
Leguminous plants regulate the number of Bradyrhizobium- or Rhizobium-infected sites that develop into nitrogen-fixing root nodules. Ethylene has been implicated in the regulation of nodule formation in some species, but this role has remained in question for soybean (Glycine max). The present study used soybean mutants with decreased responsiveness to ethylene, soybean mutants with defective regulation of nodule number, and Ag+ inhibition of ethylene perception to examine the role of ethylene in the regulation of nodule number. Nodule numbers on ethylene-insensitive mutants and plants treated with Ag+ were similar to those on wild-type plants and untreated plants, respectively. Hypernodulating mutants displayed wild-type ethylene sensitivity. Suppression of nodule numbers by high nitrate was also similar between ethylene-insensitive plants, wild-type plants, and plants treated with Ag+. Ethylene insensitivity of the roots of etr1-1 mutants was confirmed using assays for sensitivity to 1-aminocyclopropane-1-carboxylic acid and for ethylene-stimulated root-hair formation. Additional phenotypes of etr1-1 roots were also characterized. Ethylene-dependent pathways regulate the number of nodules that form on species such as pea and Medicago truncatula, but our data indicate that ethylene is less significant in regulating the number of nodules that form on soybean.
Legumes form root nodules as part of a symbiotic association with Bradyrhizobium or Rhizobium (Sinorhizobium) bacteria (Hirsch and LaRue, 1997). These structures allow fixation of atmospheric dinitrogen but are energetically expensive to develop and maintain (Shantharam and Mattoo, 1997). The growth of most potential root nodules is suppressed by the host soon after the initial bacterial invasion of root hairs (Pierce and Bauer, 1983; Caetano-Anolles and Gresshoff, 1991; Spaink, 1997). The host plant further regulates nodule number in response to environmental factors such as the presence of nitrate or other sources of fixed nitrogen in the soil (Harper, 1987; Streeter, 1988). Plant mutants altered in the regulation of nodule formation have been characterized. These include hypernodulating and nonnodulating mutants of soybean (Glycine max), low-nodulating mutants of pea, and a hypernodulating mutant of Medicago truncatula (Kneen and LaRue, 1984; Carroll et al., 1985a, 1985b; Gremaud and Harper, 1989; Caetano-Anolles and Gresshoff, 1991; Akao and Kouchi, 1992; Kneen et al., 1994; Penmetsa and Cook, 1997).
Many aspects of plant growth and development are regulated by the gaseous plant hormone ethylene (Matoo and Suttle, 1991; Abeles et al., 1992). Several studies have shown that ethylene production can have a negative effect on nodule formation. For example, ethylene production significantly increases in roots infected by Rhizobium or Bradyrhizobium, and added exogenous ethylene can decrease the number of nodules that form on infected plants (Grobbelaar et al., 1971; Drennan and Norton, 1972; Goodlass and Smith, 1979; Ligero et al., 1986, 1987; Lee and LaRue, 1992b). Nodule production can be stimulated by treatments of M. trunculata roots with AVG or Ag+, inhibitors of ethylene formation and perception, respectively (Peters and Crist-Estes, 1989; Ligero et al., 1991; Caba et al., 1998). These inhibitors partially restored nodulation in a subset of the low-nodulating pea mutants and in a unique Vicia sativa subsp. nigra symbiosis (Zaat et al., 1989; Fearn and LaRue, 1991; Guinel and LaRue, 1992). This indicates that some stage of nodule formation is oversensitive to ethylene inhibition in these plants. The hypernodulating phenotype of the “sickle” (skl) mutant of M. truncatula has been attributed to a mutation causing ethylene insensitivity (Penmetsa and Cook, 1997).
Despite the above results, ethylene may not play a significant role in nodule formation in all species. In studies with soybean, one of the most economically significant legume species, infection by B. japonicum caused an increase in ethylene production, but added exogenous ethylene did not inhibit nodulation and treatment with AVG did not increase nodule number (Lee and LaRue, 1992b; Hunter, 1993; Suganuma et al., 1995). The difference between results obtained with soybean and with other species may reflect substantive differences in the regulation of nodule formation in these plant species. Alternatively, the different findings with soybean may be attributable to the experimental methodologies used. Unpublished studies have been discussed in which ethylene inhibitors did increase nodule number in soybean (Caba et al., 1998).
We have isolated a number of soybean lines with decreased responsiveness to ethylene (Hoffman et al., 1999). These lines provide a tool for the analysis of many plant functions that are influenced by ethylene, including studies on root growth and development and on the control of symbiotic root nodule formation. A variety of mutant soybean lines were isolated that exhibit strong, intermediate, or weak ethylene insensitivity. Both recessive and incomplete-dominant mutant alleles were isolated (Hoffman et al., 1999). A similar range of mutant phenotypes has been observed in Arabidopsis, in which more than 100 ethylene-insensitive mutants have been isolated and the mutant genes have been grouped into more than eight genetic loci (Ecker, 1995). The ethylene-insensitive soybean lines are normal in growth and appearance under standard greenhouse conditions; differences in morphology are apparent only under particular environments (Hoffman et al., 1999; J.S. Schmidt, T.K. Hoffman, and A.F. Bent, unpublished data).
The present study used several soybean mutants to evaluate the role of ethylene in the control of nodule formation in this species. Soybean lines displaying an ethylene-insensitive phenotype were compared with near-isogenic parent lines in nodulation studies, with a particular focus on lines carrying the most strongly ethylene-insensitive mutation, etr1-1. Aspects of root growth and development other than symbiotic interaction were also characterized in wild-type and etr1-1 lines. Further nodulation experiments used Ag+ to inhibit ethylene signaling. In additional studies, previously available soybean mutants that form more or fewer nodules than the wild type were tested for their ethylene sensitivity. These resources were also used to examine the role of ethylene in nitrate-induced suppression of nodule formation. Our results indicate that significant differences may exist in the control of nodule formation by ethylene in different legume species, with ethylene playing a less-significant role in soybean.
MATERIALS AND METHODS
Plant Material
The isolation of soybean (Glycine max L. Merr.) lines that exhibit decreased responsiveness to ethylene has been described (Hoffman et al., 1999). Lines were derived from mutagenized populations of the var Hobbit 87 and var A90-312022. T119N54 was derived from Hobbit 87 after mutagenesis with nitrosomethyl urea, and has the genotype Hobbit 87 etr1-1/etr1-1. The term “wild type” is used to refer to lines such as Hobbit 87 that are the nonmutagenized parents of lines carrying induced mutations. Hypernodulating mutants used included nts382 and nts1116 (Bragg parent; Carroll et al., 1985a, 1985b), En6500 (Enrei parent; Akao and Kouchi, 1992), and NOD1-3, NOD2-4, and NOD3-7 (Williams parent; Gremaud and Harper, 1989). Additional soybean lines tested included Harosoy nonnodulating and nodulating isolines (Bernard, 1974) and the NN5 nonnodulating mutant derived from the normally nodulating Williams (Pracht et al., 1993).
Tests for Ethylene Insensitivity
To test directly for sensitivity to ethylene, the seedling triple-response assay of Bleecker et al. (1988) was used with slight modification. Seeds were planted in moist sand in light- and gas-tight boxes fabricated from sheets of polyvinyl chloride. Boxes were either aerated with humidified air carrying 20 μL L−1 (ppm) ethylene, or sealed and injected with small volumes of pure ethylene to achieve the specified air/ethylene mix. Boxes were typically opened for observation after 6 d, at which time germinated seedlings were scored for hypocotyl length, extent of curl of the hypocotyl hook, and/or radial swelling of the hypocotyl. Control experiments used the same boxes with no added ethylene.
ACC Sensitivity
For tests of sensitivity to ACC, plants were germinated in sterile, coarse vermiculite wetted with deionized water or aqueous solutions of ACC. After 2 d healthy seedlings were transferred to plastic growth pouches (Mega International, Minneapolis, MN) containing absorbent paper moistened with water or ACC solutions. Pouches were maintained in a controlled-environment chamber (25°C/18-h days, 22°C/6-h nights) in an upright, closely abutted position in a box with a lid designed to limit exposure of roots to light, and given water or ACC solution as needed. Lengths of main taproots were recorded at specified times after germination. In these and other experiments, mean, 95% confidence interval, and/or similarity of means at P < 0.05 (based in all cases on Student's t test) were calculated using the scientific graphing program SigmaPlot (version 4.14, Jandel Scientific, Corte Madera, CA).
Root Morphology
For root growth/branching experiments, soybean lines were planted in square pots (9 × 9 × 9 cm) in sand, 1:1:1 sand:soil:perlite mixture, or coarse vermiculite (as indicated), watered with tap water, and grown in a controlled-environment chamber set to 25°C/18-h days and 22°C/6-h nights. After 14 d plants were removed from pots by inverting, and soil was removed from root systems by gentle immersion in a large volume of standing water, followed by a final rinse in water. The length of the main taproot and the number of lateral root sections were then determined for each plant, and root dry weights were determined after at least 2 d of drying at elevated temperature. Root-hair-density experiments were performed on plants that had been grown from seed in square pots filled with sand (as described above) or in 11-cm-deep sand in a closed ethylene triple-response test box with no added ethylene. Root hairs were examined on fresh roots in water mounts using a stereomicroscope (model SZH10, Olympus) with bright-field illumination and no staining. Roots were maintained in distilled water or on very moist paper towels and were examined within minutes after removal from sand. For statistical analysis individual lateral or main roots were rated on a scale of 1 to 4 based on observed root-hair density, and differences were analyzed using the nonparametric Wilcoxon two-sample test.
Nodulation Tests
Nodulation tests were performed as described by Gremaud and Harper (1989). Soybean seeds were inoculated with a commercial strain of Bradyrhizobium japonicum (Urbana Laboratories, St. Joseph, MO) and then planted in a gravel bench that was periodically subirrigated with nitrogen-free nutrient solution (Gremaud and Harper, 1989). After 14 d plants were gently uprooted and shaken to remove gravel, and the number of nodules present on the root system of each plant was recorded.
For tests of ACC sensitivity of nodulation, plants were germinated and grown as for the ACC-sensitivity tests (above). One day after transplantation to pouches, 20 μL of a suspension of B. japonicum strain USDA110 (approximately 1 × 108 colony-forming units/mL grown in liquid yeast-mannitol broth medium) was applied to the root system of each seedling. At 14 d after inoculation the number of nodules present on each root system was recorded.
Inhibition of nodulation by Ag+ was studied by a modification of the method of Caba et al. (1998). Surface-sterilized seeds were germinated in sterile, coarse vermiculite wetted with sterile, deionized water. After 2 d germinated seedlings were transferred to 25- × 150-mm glass tubes containing coarse vermiculite and two strips of filter paper to promote even wetting. Tubes were covered with foil to block light entry. Vermiculite in tubes was wetted with nutrient solution containing defined concentrations of nitrate (Rigaud and Puppo, 1975). Eight days after transplantation to tubes, seedlings were watered with 2 mL of the same nutrient solution plus added Ag+. Ag+ solutions were made from fresh stocks of a 1:4 silver nitrate:sodium thiosulfate mixture (Veen, 1983) that was added to the appropriate defined nitrate nutrient solution to achieve the designated final concentrations of nitrate and Ag+. Two hours later, root systems were inoculated with 1 mL of B. japonicum strain USDA110 (approximately 1 × 108 colony-forming units/mL). Nodule numbers were recorded 14 d after inoculation.
RESULTS
Ethylene Sensitivity of Soybean Mutants
Several soybean lines with decreased responsiveness to ethylene have been isolated in our laboratory (Hoffman et al., 1999). The assay for ethylene sensitivity that was used to initially identify these mutants was also used in the present studies. This assay tests germinating seedlings for the ethylene “triple response” under etiolating conditions (Bleecker et al., 1988). When wild-type seedlings are germinated in the dark in air, the hypocotyl becomes excessively elongated. When wild-type seedlings are germinated in the dark in the presence of small amounts of ethylene (1–20 μL L−1), the hypocotyl remains very short and exhibits radial swelling and the seedling develops exaggerated curvature of the hypocotyl hook (Bleecker et al., 1988; Ecker, 1995). We observed previously that the different ethylene-insensitive soybean mutants displayed varying degrees of ethylene insensitivity in this triple-response assay, as is summarized in the top portion of Table I (Hoffman et al., 1999). Many subsequent studies used soybean line T119N54 because it is strongly ethylene insensitive, developing a wild-type etiolated phenotype despite the presence of ethylene. The mutant etr1-1 allele in line T119N54 exhibits incomplete dominance with respect to the wild type, which (by analogy with known Arabidopsis ethylene-insensitive mutants) indicates that this mutation may disrupt the gene for an ethylene receptor (Schaller and Bleecker, 1995).
Table I.
Ethylene sensitivity of parental and mutant soybean lines
| Soybean Line | Phenotype | Genotype | Ethylene Response: Hypocotyl Lengtha |
|---|---|---|---|
| cm | |||
| Hobbit 87 | Wild type | Parent | 2.1 ± 0.3 |
| T119N54 | Ethylene insensitive | etr1-1 | 12.8 ± 1.1* |
| T124N38B | Intermediate/partial ethylene insensitive | ? | 8.9 ± 0.7* |
| A90-312022 | Wild type | Parent | 2.1 ± 0.4 |
| T15N23 | Weak ethylene insensitive | etr2-1 | 3.4 ± 0.6* |
| T58N5 | Weak ethylene insensitive | etr3? | 2.9 ± 0.3* |
| Bragg | Wild type | Parent | 1.7 ± 0.4 |
| nts382 | Hypernodulating | ? | 1.6 ± 0.3 |
| nts1116 | Hypernodulating | ? | 1.7 ± 0.4 |
| Enrei | Wild type | Parent | 2.0 ± 0.2 |
| En6500 | Hypernodulating | rj7 | 2.2 ± 0.5 |
| Harosoy | Wild type | Parent | 1.7 ± 0.4 |
| Harosoy NN | Nonnodulating | rj1 | 1.6 ± 0.5 |
| Williams 82 | Wild type | Parent | 1.3 ± 0.2 |
| NOD1-3 | Hypernodulating | rj7 | 1.5 ± 0.3 |
| NOD2-4 | Hypernodulating | rj7 | 1.4 ± 0.2 |
| NOD3-7 | Hypernodulating | rj7 | 1.6 ± 0.2 |
| NN5 | Nonnodulating | rj5, rj6 | 1.4 ± 0.2 |
Etiolation response of seedlings germinated for 6 d in the dark in 20 μL L−1 ethylene; soybean seedlings germinated in the same assay system but in air typically form hypocotyls that are 12 to 15 cm in length (Hoffman, et al., 1999). Values marked with an asterisk are significantly different from those of the parental line according to the Student's t test (P < 0.05).
Values are means ± sd.
In view of previous reports linking ethylene to the suppression of nodule formation, we examined the ethylene sensitivity of previously isolated soybean mutants that exhibit altered nodulation in response to B. japonicum. These tests focused on hypernodulating mutants that form an aberrantly high number of nodules (nts382 and nts1116 [Bragg], En6500 [Enrei], and NOD1-3, NOD2-4, and NOD3-7 [Williams]). Nonnodulating mutants that form few or no nodules were also tested (Harosoy NN and Williams NN5). Table I shows the triple-response phenotype of these mutant lines. In all tests the altered-nodulation soybean mutants resembled wild-type plants in their ethylene sensitivity.
Ethylene Insensitivity in Roots of the etr1-1 Mutant
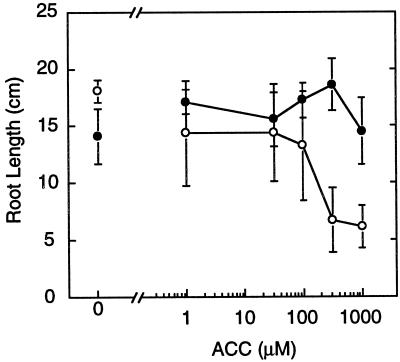
Further tests specifically assayed the ethylene sensitivity of etr1-1 roots. Exogenously applied ACC mimics applied ethylene, because ACC is the immediate precursor of ethylene and is converted to ethylene by constitutive ACC oxidases (Ecker, 1995). We grew soybean plants with their roots exposed to ACC. As expected (Penmetsa and Cook, 1997), dose-response experiments revealed a decrease in root elongation in wild-type plants grown in ACC concentrations ranging from 100 μm to 1 mm (Fig. 1). However, the ethylene-insensitive etr1-1 line displayed little or no decrease in root elongation, indicating that this mutant exhibits ethylene insensitivity in the root (Fig. 1).
Figure 1.
Ethylene insensitivity of etr1-1 roots. Seedlings were germinated and grown in the presence of ACC at the designated concentrations. Lengths of tap roots were measured 7 d after germination. Data are presented as means ± a 95% confidence interval. •, etr1-1 (T119N54); ○, wild type (Hobbit 87).
Ethylene has also been reported to stimulate root-hair development (Jackson, 1991; Abeles et al., 1992). Ten-day-old seedlings of Hobbit 87 and the etr1-1 mutant were shifted to an atmosphere of 20 μL L−1 ethylene for 16 h, after which time roots were examined either immediately or 8 h after plants were shifted back to air. In Hobbit 87 profuse root-hair development was apparent to within 1 to 2 mm of the tip of actively growing lateral roots. The etr1-1 line did not exhibit this ethylene response, and instead had few or no root hairs within 3 to 4 mm of the tips of lateral roots. Preliminary estimates of average epidermal cell length in the region 2 to 4 mm from the tip of these roots revealed no differences between Hobbit 87 and the etr1-1 line. No obvious differences in root-hair density or location were observed between Hobbit 87 and the etr1-1 mutant in samples from 10-d-old plants grown in air, and no differences in root-hair density were apparent in basal (older) regions of roots that had received the 16-h ethylene exposure.
Nodulation Phenotype of Ethylene-Insensitive Mutants
In light of the postulated regulatory role of ethylene in the limitation of nodule numbers, quantitative studies of nodule formation were performed with the ethylene-insensitive soybean mutants. No significant difference in nodule number was detected between the ethylene-insensitive mutants and their near-isogenic parents (Fig. 2). Similar results were obtained in additional gravel-bench experiments with 12 other less fully characterized ethylene-insensitive soybean lines (data not shown), as well as in pouch and test-tube nodulation tests (discussed below). The hypernodulating NOD1-3 soybean mutant was tested for comparison, and this line consistently formed approximately three times as many nodules per plant as its wild-type parent (e.g. Fig. 2).
Figure 2.
Nodule formation on soybean mutants. Seeds inoculated with B. japonicum were grown in a subirrigated gravel bench and the number of nodules present on root systems of individual plants was recorded 14 d after inoculation. Data are presented as means ± a 95% confidence interval. Genotypes and phenotypes of soybean lines are listed in Table I.
Effect of Ag+ on Nodule Formation
Ag+ is an inhibitor that blocks binding of ethylene to ethylene receptors (Matoo and Suttle, 1991; Abeles et al., 1992). If ethylene stimulates plant processes that limit nodule formation, then disruption of ethylene perception by Ag+ should cause an increase in nodule formation. This effect has been reported in pea and alfalfa (Fearn and LaRue, 1991; Guinel and LaRue, 1992; Caba et al., 1998). We examined the effect of Ag+ on nodule formation in soybean. Figure 3 demonstrates that no significant increase in nodule formation was observed in wild-type plants treated with Ag+ (open circles). Nodulation of the etr1-1 mutant also was not responsive to Ag+ (open squares). In a repeat of the experiment reported in Figure 3, rather than increasing slightly, the average number of nodules on wild-type plants decreased as Ag+ concentration went from 0 to 1 to 10 μm, going from 28 to 26 to 22. In both experiments the differences in nodule number between the Ag+ treatments were not statistically significant either for wild-type plants or for the etr1-1 line (Fig. 3 and data not shown). Although the average number of nodules on etr1-1 roots was lower than that on wild-type roots in both Ag+ experiments, the difference was statistically significant at only one of three Ag+ levels in each experiment. No difference in nodule number between mutant and wild type was observed in other experiments (e.g. Fig. 2).
Figure 3.
Nodule formation in the presence of Ag+ and/or nitrate. Seedlings were grown in the presence of B. japonicum at low (0.5 mm) or moderately high (8.0 mm) concentrations of nitrate. Some plants also received Ag+ (as silver thiosulfate) at the designated concentrations. The number of nodules present on root systems of individual plants was recorded 14 d after inoculation. Data are presented as means ± a 95% confidence interval. ○, Wild-type (Hobbit 87) plants grown at low nitrate; □, etr1-1 (T119N54) plants grown at low nitrate; •, wild-type plants grown at high nitrate; and ▪, etr1-1 plants grown at high nitrate.
Inhibition by Nitrate
When legumes such as soybean are grown in the presence of soil nitrogen, root-nodule formation is more strongly inhibited as the nitrogen concentration increases (Harper, 1987; Streeter, 1988). Further experiments reported in Figure 3 revealed that Ag+ did not increase soybean nodulation when plants were grown at nitrate levels that limit nodule numbers. In addition, the ethylene-insensitive etr1-1 line nodulated at a rate similar to the wild type when both lines were grown at the higher nitrogen level (Fig. 3). Similar data were obtained in repeat experiments (not shown). These results indicate that the limitation of nodule formation by the host in response to nitrogen availability can occur independent of ethylene.
ACC and Nodulation
To further test for an effect of ethylene on nodule formation, nodulation tests were performed in the presence of varying concentrations of ACC. As reported above, ACC treatment inhibited elongation of Hobbit 87 roots but not the roots of the ethylene-insensitive etr1-1 mutant (Fig. 1). ACC treatment also caused a decrease in the number of nodules formed on Hobbit 87 roots (Fig. 4A). Other experiments in this study indicated that the control of nodule number is independent of ethylene signaling, making this effect of ACC on nodulation contrary to what might have been predicted. However, the effect of ACC on nodule number may be largely attributable to the stunted growth of Hobbit 87 roots. In the presence of ACC, the formation of root nodules in Hobbit 87 was restricted to a region close to the uppermost crown of branching secondary roots (Fig. 4B), as might be expected in plants for which the length of the entire root system was significantly decreased. ACC treatment of roots of the etr1-1 line caused only a slight, statistically insignificant decline in average nodule number, providing further evidence that these roots are highly insensitive to ethylene (Fig. 4A). The previously observed similarity of nodule number on etr1-1 and wild-type Hobbit 87 roots in the absence of added ACC (Figs. 2 and 3) was once again demonstrated in these tests (Fig. 4A, [ACC] = 0).
Figure 4.
Nodule formation in the presence of ACC. Seedlings grown in the presence or absence of ACC were inoculated with B. japonicum strain USDA110 3 d after germination. The number of nodules present on root systems of individual plants was recorded 14 d after inoculation (A), and the distance from the uppermost lateral root to the lowest nodule was noted (B). Data are presented as means ± a 95% confidence interval. ○, Wild type (Hobbit 87); •, etr1-1 (T119N54).
Other Root Phenotypes of the etr1-1 Mutant
In addition to the effects on nodulation, other reported effects of ethylene on root growth and development include reduction of root biomass and root elongation, altered lateral branching, and promotion of root-hair formation (Jackson, 1991; Abeles et al., 1992; Lee and LaRue, 1992b; Masucci and Schiefelbein, 1994; Tanimoto et al., 1995; Heidstra et al., 1997; and refs. therein). However, even more so than with control of nodule numbers, the reported results can be conflicting depending on the species and assay system used (Jackson, 1991). As reported above and in Figure 1, we observed that ACC inhibited root elongation and ethylene stimulated root-hair formation in nonmutagenized soybean, and found that these responses to ACC were greatly diminished or absent in the ethylene-insensitive etr1-1 line. Hobbit 87 and etr1-1 plants were also examined for other differences in root development. In the ethylene triple-response test Hobbit 87 and other nonmutagenized soybean seedlings were similar to other plant species in exhibiting a dramatic stunting of root development. Mutant etr1-1 plants did not show this response, developing similar root biomass when grown in 20 μL L−1 ethylene or in air (e.g. 0.013 versus 0.011 g mean dry weight, respectively).
In visual comparisons of Hobbit 87 and etr1-1 plants grown “normally” in soil mix, sand, or vermiculite in controlled-environment chambers in air with no added ethylene, the etr1-1 plants often developed a more extensive root system. Although the mean number of lateral roots present was higher on the etr1-1 mutant than on the parental Hobbit 87 line in all tests, statistically significant differences were observed in only two of five tests (Table II and data not shown). The length of the main taproot of air-grown plants was similar between the two lines (Table II and data not shown). The overall root biomass was also similar between the two lines in three of five experiments (Table II and data not shown).
Table II.
Symbiosis-independent root phenotypes of parental and mutant soybean lines
| Soybean Line | Lateral Roots | Root Length | Root Dry Wt |
|---|---|---|---|
| no. | cm | g | |
| Test 1 | |||
| Hobbit 87 | 61.2 | 13.3 | 0.124 |
| etr1-1 | 72.3* | 14.3 | 0.208* |
| Test 2 | |||
| Hobbit 87 | 76.1 | 16.0 | 0.210 |
| etr1-1 | 91.9* | 17.2 | 0.187* |
| Test 3 | |||
| Hobbit 87 | 53.7 | – | 0.145 |
| etr1-1 | 61.0 | – | 0.130 |
Data for each of three phenotypes are presented as sample means. Values marked with an asterisk indicate a significant difference between etr1-1 and Hobbit 87 within a given test according to the Student's t test (P < 0.05).
A striking “push-up” phenotype was often observed when seeds of the etr1-1 line were planted near the surface of shallow sand and germinated in humid air under etiolating conditions (i.e. in zero ethylene controls for the ethylene triple-response test). Under these conditions the tips of etr1-1 root systems often remained in one location instead of growing laterally when they reached the bottom of the sand bed, so root elongation caused the etr1-1 root systems to push up into the humid airspace and push the entire upright or lodged seedling ahead and across the sand surface. Lateral roots emerging from these aboveground root systems also tended to grow down to, but not through, the sand, and elongation of lateral roots caused the primary root to be propped up well above the sand surface. Relative frequencies of this phenomenon in a representative experiment are reported in Table III.
Table III.
Occurrence of seedling push-up phenotype in different planting configurations
| Soybean Line | Planted <1 cm Deep in 12 cm of Sand | Planted 2 cm Deep in 3 cm of Sand | Planted <1 cm Deep in 3 cm of Sand |
|---|---|---|---|
| Hobbit 87 | 0 /19 | 0 /18 | 9 /19 |
| etr1-1 | 0 /20 | 8 /20 | 18 /18 |
Data are presented as the number of seedlings with the root system growing in the airspace above the sand surface divided by the total number of seedlings.
The push-up phenotype was observed in Hobbit 87 seedlings only when they were planted right at the surface of shallow (3 cm deep) sand (Table III). The push-up effect apparently occurred when the nascent root system did not adequately anchor the young seedling into the adjacent sand matrix. This prompted an examination of root-hair density in very young seedlings. When roots from seedlings grown in moist sand were examined 54 h after imbibition, there was overlap in the root-hair densities observed among multiple Hobbit 87 and etr1-1 plants, but a clear overall tendency toward less-profuse root-hair formation was observed in the etr1-1 plants (P = 0.01 for the Wilcoxon two-sample test).
DISCUSSION
Leguminous plants are genetically programmed to form root nodules in symbiosis with Rhizobium or Bradyrhizobium bacteria, but they are also programmed to limit the number of infection sites that develop into root nodules. An apparent discrepancy exists between data implicating ethylene signaling in the limitation of nodule numbers in alfalfa, pea, and Vicia sativa subsp. nigra and data providing no evidence for the involvement of ethylene in this process in soybean (see the introduction). We recently isolated soybean mutants that display decreased ethylene sensitivity, and in the present study we used these mutants to explore the role of ethylene in the control of nodule formation. No significant differences in the number of nodules formed were observed between mutant and wild-type soybean. In addition, previously isolated soybean mutants defective in the down-regulation of nodule number were found in the present study to display wild-type ethylene sensitivity. In a third set of experiments root systems were treated with Ag+, an inhibitor of ethylene perception, and in these studies as well no effect on nodulation was observed.
Our data are consistent with those of Hunter (1993) and Lee and LaRue (1992b), who showed that enhanced ethylene levels do not decrease nodule number in soybean, and Suganuma et al. (1995), who found that inhibition of ethylene production by AVG does not increase nodule number in soybean. Although neither the etr1-1 mutation nor Ag+ treatment caused an increase in nodule number, ACC did have an inhibitory effect on nodule number in wild-type plants. However, in light of the overall shortening of the root system of these plants, the observed effect of ACC on nodulation of wild-type soybean is of questionable significance.
It is interesting to compare our results with those of Xie et al. (1996). They screened 161 soybean cultivars and identified lines with exceptionally high or low ethylene responsiveness, as measured by leaf senescence and chitinase-induction assays. Their most highly ethylene-sensitive line formed a reasonably normal number of nodules in standard (air) assays. However, when grown in a split-level Leonard jar assembly in the presence of added ethylene, this line formed nodules only on roots in the upper chamber. This demonstrates that some residual effect of ethylene on soybean nodulation exists that can be discerned in highly ethylene-sensitive lines treated with high concentrations of ethylene. Their most ethylene-insensitive line nodulated normally in the presence or absence of added ethylene. Quantitative comparisons with the nodulation rates for lines with normal ethylene sensitivity were not attempted, presumably because of the extensive genetic diversity of the lines used in that study (Xie et al., 1996).
We were also interested in using our ethylene-insensitive soybean lines to explore the down-regulation of nodule formation in response to nitrate. Alfalfa plants produce additional ethylene upon inoculation with Rhizobium meliloti or upon growth in elevated nitrate, and inhibition of ethylene biosynthesis (using AVG) or perception (using Ag+) significantly increased nodule formation in the presence of inhibitory levels of nitrate (Ligero et al., 1986, 1987, 1991; Caba et al., 1998). Many soybean mutants that display a hypernodulation phenotype also display degrees of nitrate-insensitive nodulation; these and other data indicate at least partial overlap in the control of these processes (Gresshoff, 1993). In the present study, neither the strongly ethylene-insensitive etr1-1 mutation nor inhibition of ethylene perception with Ag+ caused a significant loss of the ability to inhibit nodule formation in response to high nitrate. This, again, is in contrast to the findings of previous studies with other legume species, although Lee and LaRue (1992a) also failed to overcome the inhibitory effect of nitrate with Ag+ application in pea.
Ethylene-insensitive soybean mutants, and the strongly insensitive etr1-1 mutant in particular, provide a tool to examine the effects of ethylene on many phenotypes beyond those involved in symbiosis. When etr1-1 seedlings are grown in ethylene they resemble wild-type or etr1-1 seedlings grown in air. Using an assay for induction of leaf chlorosis by ethylene, we have also observed ethylene insensitivity in mature leaves of the etr1-1 line (T.K. Hoffman, unpublished data). When the ethylene sensitivity of roots was examined in the present study, the parental line Hobbit 87 showed the expected inhibition of root elongation by ACC, but elongation of the roots of etr1-1 plants was not inhibited. Stimulation of root-hair formation by ethylene was also disrupted in etr1-1 plants. This demonstrated that ethylene insensitivity is expressed in the roots of the etr1-1 mutant.
Although not the primary focus of the present work, we did conduct preliminary studies on other root phenotypes. When plants were grown in air with no added ethylene, the etr1-1 mutation caused only subtle changes in the overall architecture of the root system. Visual inspection indicated that there was slightly more branching of the lateral root system in the etr1-1 plants, but quantitative studies revealed significant differences in only two of five experiments. The density and position of root-hair formation on lateral roots was very similar between etr1-1 and Hobbit 87 when the two lines were grown in air. However, our observation of the push-up phenotype of shallow-planted seedlings prompted an inspection of root-hair density on primary roots in recently germinated seedlings, and we did observe a reduction in root-hair numbers on etr1-1 roots at that stage of development.
The push-up phenotype occurred when the forces generated by root elongation against the solid bottoms of our plant containers were greater than the root-anchoring forces that usually keep root systems underground. We hypothesize that the push-up phenotype was caused by inadequate attachment to the soil matrix, which was a more frequent occurrence in etr1-1 seedlings because of the lower density of root hairs on these lines at a time before the formation of lateral roots. However, as with ethylene-insensitive mutants of Arabidopsis (Ecker, 1995), most aspects of root development that we examined were quite normal in the soybean etr1-1 mutant. Apparently, ethylene-independent mechanisms predominate or can substitute for ethylene signaling in fostering most stages of root development in unstressed plants. Our ethylene-insensitive soybean lines are available to the community and may be of use in other laboratory or field studies of plant growth, development, and productivity.
The difference in the effects of ethylene on nodulation in soybean as opposed to other leguminous plants is intriguing and is at present unexplained. As observed with the ripening of climacteric as opposed to nonclimacteric fruit (Matoo and Suttle, 1991; Abeles et al., 1992), ethylene apparently plays a significant role in the control of root-nodule formation only in some plant species. It is possible that the mechanistic basis of this difference is simply quantitative, with very similar regulatory pathways present in the different legume species, but with the ethylene pathway exerting a less-predominant influence in soybean.
The possibility must also be considered that, despite our findings, ethylene signaling is significant in the regulation of nodule numbers in soybean. Ethylene-sensing pathways relevant to nodulation may not have been completely blocked in the etr1-1 soybean line used in this study. In Arabidopsis and tomato a small number of ETR1 gene homologs are present in the genome and some of these genes encode functional ethylene receptors that are active in particular tissues or at certain stages of plant growth (Hua and Meyerowitz, 1998; Hua et al., 1998; Lashbrook et al., 1998; Sakai et al., 1998). Mutant etr1 alleles typically have a dominant negative effect on ethylene signaling, but the soybean etr1-1 allele exhibited only incomplete dominance in heterozygous plants (Hoffman et al., 1999). Homozygous etr1-1/etr1-1 plants with a strong ethylene-insensitive phenotype were used in the present study, but it remains possible that some ethylene signaling remained effective in specific tissues. However, we specifically observed ethylene-insensitivity traits in roots of soybean etr1-1/etr1-1 plants, and our experiments that used Ag+ were in agreement with the mutant studies. Other studies have also indicated that ethylene signaling is of minor importance in the regulation of nodule numbers in soybean (Lee and LaRue, 1992b; Hunter, 1993; Suganuma et al., 1995).
The different effects of ethylene on nodulation in different species may be related to the clear dichotomy that exists in nodule development between soybean and the other species for which this topic of ethylene and nodulation has been addressed. Plants of Medicago, Vicia, Pisum, Trifolium, and other well-studied genera form indeterminate nodules in which a nodule meristem gives rise to the bulk of the nodule tissues (Hirsch and LaRue, 1997). Initial cell divisions at the earliest stages of nodule development occur inside the pericycle in the inner cortex of the root, typically opposite a protoxylem pole. In contrast, soybean, as well as other Glycine, Lotus, Vigna, and Phaseolus hosts, form determinate nodules through cell divisions that are not localized to a discrete nodule meristem. The first cell divisions in soybean nodule development occur among cells in the outer cortex of the root, just below the epidermis (Hirsch and LaRue, 1997).
In alfalfa and other indeterminate nodulators, infection events that do not lead to nodulation are typically arrested in the epidermis, with no activation of cell division in the subtending inner cortical cells. In contrast, nonproductive infections in soybean are associated with activation and subsequent early arrest of cell divisions in the root outer cortex (Caetano-Anolles and Gresshoff, 1990; Caetano-Anolles et al., 1991). It is possible that only the former process is subject to significant regulation by ethylene. It must be added, however, that the correlation between ethylene regulation and determinate versus indeterminate nodulation may not be absolute. An early study reported that ethylene could inhibit nodule formation in excised roots of bean, a determinate nodulator (Grobbelaar et al., 1971). However, several additional differences exist in the molecular and cellular details of plant-rhizobium interactions between different host and bacterial species (Sprent, 1989; Spaink, 1995; Hirsch and LaRue, 1997). Even within a single species, control of nodule number is likely to involve multiple mechanisms. For example, not all Medicago hypernodulation mutants exhibit ethylene insensitivity. These differences allow ample opportunity for divergence in the mechanisms that regulate nodule formation in different legume species.
Using a new experimental route, our results reinforce the previously observed contrast between the effect of ethylene on nodulation in soybean as opposed to other frequently studied legumes. Ethylene apparently plays a less significant role in regulating nodule development in soybean.
ACKNOWLEDGMENT
We thank Nicole Lavaggi for her assistance with many experiments.
Abbreviation:
- AVG
aminoethoxyvinylglycine
Footnotes
This research was funded by a grant to A.F.B. from the Illinois Soybean Program Operating Board.
LITERATURE CITED
- Abeles GB, Morgan PW, Saltveit ME. Ethylene in Plant Biology. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Akao S, Kouchi H. A supernodulating mutant isolated from soybean cultivar Enrei. Soil Sci Plant Nutr. 1992;38:183–187. [Google Scholar]
- Bernard RL. Notice of release of Clark and Harosoy isolines. Soybean Genet Newsl. 1974;1:57–75. [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Caba JM, Recalde L, Ligero F. Nitrate-induced ethylene biosynthesis and the control of nodulation in alfalfa. Plant Cell Environ. 1998;21:87–93. [Google Scholar]
- Caetano-Anolles G, Gresshoff PM. Early induction of feedback regulatory responses governing nodulation in soybean. Plant Sci. 1990;71:69–81. [Google Scholar]
- Caetano-Anolles G, Gresshoff PM. Plant genetic control of nodulation. Annu Rev Microbiol. 1991;45:345–382. doi: 10.1146/annurev.mi.45.100191.002021. [DOI] [PubMed] [Google Scholar]
- Caetano-Anolles G, Paparozzi ET, Gresshoff PM. Mature nodules and root tips control nodulation in soybean. J Plant Physiol. 1991;137:389–396. [Google Scholar]
- Carroll BJ, McNeil DL, Gresshoff PM. Isolation and properties of soybean (Glycine max) mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci USA. 1985a;82:4164–4166. doi: 10.1073/pnas.82.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, McNeil DL, Gresshoff PM. A supernodulation and nitrate tolerant symbiotic (nts) soybean mutant. Plant Physiol. 1985b;78:34–40. doi: 10.1104/pp.78.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan DS, Norton C. The effect of Ethrel on nodulation in Pisum sativum. Plant Soil. 1972;36:53–57. [Google Scholar]
- Ecker JR. The ethylene signal transduction pathway in plants. Science. 1995;268:667–674. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- Fearn JC, LaRue TA. Ethylene inhibitors restore nodulation to sym5 mutants of Pisum sativum L. cv Sparkle. Plant Physiol. 1991;96:239–244. doi: 10.1104/pp.96.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlass G, Smith KA. Effects of ethylene on root extension and nodulation of pea (Pisum sativum L.) and white clover (Trifolium repens L.) Plant Soil. 1979;51:387–395. [Google Scholar]
- Gremaud MF, Harper JE. Selection and initial characterization of partially nitrate tolerant nodulation mutants of soybean. Plant Physiol. 1989;89:169–173. doi: 10.1104/pp.89.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresshoff PM. Molecular genetic analysis of nodulation genes in soybean. Plant Breed Rev. 1993;11:275–318. [Google Scholar]
- Grobbelaar N, Clarke B, Hough M (1971) The nodulation and nitrogen fixation of isolated roots of Phaseolus vulgaris L. III. The effect of carbon dioxide and ethylene. Plant Soil Spec Vol 215–221
- Guinel FC, LaRue TA. Ethylene inhibitors partly restore nodulation to pea mutant E107 (brz) Plant Physiol. 1992;99:515–518. doi: 10.1104/pp.99.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JE (1987) Nitrogen metabolism. In JR Wilcox, ed, Soybeans: Improvement, Production and Uses, Ed 2. American Society of Agronomy, Madison, WI, pp 497–533
- Heidstra R, Yang WC, Yalcin Y, Peck S, Emons A, van Kammen A, Bisseling T. Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development. 1997;124:1781–1787. doi: 10.1242/dev.124.9.1781. [DOI] [PubMed] [Google Scholar]
- Hirsch AM, LaRue TA. Is the legume nodule a modified root or stem or an organ sui generis? Crit Rev Plant Sci. 1997;16:361–392. [Google Scholar]
- Hoffman T, Schmidt JS, Zheng X, Bent AF. Isolation of ethylene-insensitive soybean mutants that are altered in pathogen susceptibility and gene-for-gene disease resistance. Plant Physiol. 1999;119:935–949. doi: 10.1104/pp.119.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QHG, Bleecker AB, Ecker JR, Meyerowitz EM. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter WJ. Ethylene production by root nodules and effect of ethylene on nodulation in Glycine max. Appl Environ Microbiol. 1993;59:1947–1950. doi: 10.1128/aem.59.6.1947-1950.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB. Ethylene in root growth and development. In: Matoo AK, Suttle JC, editors. The Plant Hormone Ethylene. Boca Raton, FL: CRC Press; 1991. pp. 159–181. [Google Scholar]
- Kneen BE, LaRue TA. Nodulation resistant mutant of Pisum sativum. J Hered. 1984;75:238–240. [Google Scholar]
- Kneen BE, Weeden NF, LaRue TA. Non-nodulating mutants of Pisum sativum (L.) cv. Sparkle. J Hered. 1994;85:129–133. [Google Scholar]
- Lashbrook CC, Tieman DM, Klee HJ. Differential regulation of the tomato ETR gene family throughout plant development. Plant J. 1998;15:243–252. doi: 10.1046/j.1365-313x.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- Lee KH, LaRue TA. Ethylene as a possible mediator of light- and nitrate-induced inhibition of nodulation of Pisum sativum L. Sparkle. Plant Physiol. 1992a;100:1334–1338. doi: 10.1104/pp.100.3.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, LaRue TA. Exogenous ethylene inhibits nodulation of Pisum sativum L. cv Sparkle. Plant Physiol. 1992b;100:1759–1763. doi: 10.1104/pp.100.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligero F, Caba JM, Lluch C, Olivares J. Nitrate inhibition of nodulation can be overcome by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 1991;97:1221–1225. doi: 10.1104/pp.97.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligero F, Lluch C, Olivares J. Evolution of ethylene from roots of Medicago sativa plants inoculated with Rhizobium meliloti. J Plant Physiol. 1986;125:361–365. [Google Scholar]
- Ligero F, Lluch C, Olivares J. Evolution of ethylene from roots and nodulation rate of alfalfa (Medicago sativa L.) plants inoculated with Rhizobium meliloti as affected by the presence of nitrate. J Plant Physiol. 1987;129:461–467. [Google Scholar]
- Masucci JD, Schiefelbein JW. The rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol. 1994;106:1335–1346. doi: 10.1104/pp.106.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoo AK, Suttle JC. The Plant Hormone Ethylene. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Penmetsa RV, Cook DR. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science. 1997;275:527–530. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]
- Peters NK, Crist-Estes DK. Nodule formation is stimulated by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 1989;91:690–693. doi: 10.1104/pp.91.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M, Bauer WD. A rapid regulatory response governing nodulation in soybean. Plant Physiol. 1983;73:286–290. doi: 10.1104/pp.73.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pracht JE, Nickell CD, Harper JE. Genes controlling nodulation in soybean: Rj5 and Rj6. Crop Sci. 1993;33:711–713. [Google Scholar]
- Rigaud J, Puppo A. Indole-3-acetic acid catabolism by soybean bacteroids. J Gen Microbiol. 1975;88:223–228. [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:5812–5817. doi: 10.1073/pnas.95.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB. Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science. 1995;270:1809–1811. doi: 10.1126/science.270.5243.1809. [DOI] [PubMed] [Google Scholar]
- Shantharam S, Mattoo AK. Enhancing biological nitrogen fixation: an appraisal of current and alternative technologies for N input into plants. Plant Soil. 1997;194:205–216. [Google Scholar]
- Spaink HP. The molecular basis of infection and nodulation by rhizobia: the ins and outs of sympathogenesis. Annu Rev Phytopathol. 1995;33:345–368. doi: 10.1146/annurev.py.33.090195.002021. [DOI] [PubMed] [Google Scholar]
- Spaink HP. Ethylene as a regulator of Rhizobium infection. Trends Plant Sci. 1997;2:203–204. [Google Scholar]
- Sprent JI. Tansley review no. 15: Which steps are essential for the formation of functional legume nodules? New Phytol. 1989;111:129–153. doi: 10.1111/j.1469-8137.1989.tb00675.x. [DOI] [PubMed] [Google Scholar]
- Streeter J. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit Rev Plant Sci. 1988;7:1–23. [Google Scholar]
- Suganuma N, Yamauchi H, Yamamoto K. Enhanced production of ethylene by soybean roots after inoculation with Bradyrhizobium japonicum. Plant Sci. 1995;111:163–168. [Google Scholar]
- Tanimoto M, Roberts K, Dolan L. Ethylene is a positive regulator of root-hair development in Arabidopsis thaliana. Plant J. 1995;8:943–948. doi: 10.1046/j.1365-313x.1995.8060943.x. [DOI] [PubMed] [Google Scholar]
- Veen H. Silver thiosulphate: an experimental tool in plant science. Sci Hortic. 1983;20:211–214. [Google Scholar]
- Xie Z-P, Staehelin C, Wiemken A, Boller T. Ethylene responsiveness of soybean cultivars characterized by leaf senescence, chitinase induction and nodulation. J Plant Physiol. 1996;149:690–694. [Google Scholar]
- Zaat SAJ, Van Brussel AAN, Tak T, Lugtenberg BJJ, Kijne JW. The ethylene-inhibitor aminoethoxyvinylglycine restores normal nodulation by Rhizobium leguminosarum biovar. viciae on Vicia sativa subsp. nigra by suppressing the thick and short roots' phenotype. Planta. 1989;177:141–150. doi: 10.1007/BF00392802. [DOI] [PubMed] [Google Scholar]