Abstract
Background
Biceps tendon pathology is common with rotator cuff tears. The mechanisms for biceps changes, and therefore its optimal treatment, are unknown. Our objective was to determine the effect of rotator cuff tears on regional biceps tendon pathology. We hypothesized that histological and compositional changes would appear before organizational changes, both would appear before mechanical changes, and changes would begin at the tendon’s insertion site.
Methods
Sixty-five Sprague-Dawley rats received either detachment of supraspinatus and infraspinatus tendons or sham surgery. Rats were sacrificed at 1, 4 or 8 weeks for regional measurements of histological, compositional, organizational (1, 4 and 8 weeks) or mechanical properties (4 and 8 weeks only).
Results
One week following tendon detachments, decreased organization and more rounded cell shape were found in the intra-articular space of the biceps tendon. Aggrecan expression was increased along the entire length of the tendon while all other compositional changes were at the tendon’s proximal insertion into bone only. With time, this disorganization and more rounded cell shape extended the length of the tendon. Organizational and cell shape changes also preceded detrimental mechanical changes, as decreased modulus in the intra-articular space was found after 8 weeks.
Conclusions
Results support a degenerative component to pathology in the biceps tendon. Additionally, changes resembling a tendon exposed to compressive loading occurring first in the intra-articular space indicate that the biceps tendon plays an increased role as a load bearing structure against the humeral head in the presence of rotator cuff tears.
Keywords: biceps tendon, rotator cuff, animal model, tendon injury, tendon pathology
Introduction
Biceps tendon pathology is commonly encountered, especially in the setting of rotator cuff disease.2,7 When pre-operative evaluation suggests that the long head of the biceps tendon is the source for pain, performing a biceps tenotomy or tenodesis has become standard of care. In cases where the biceps is noted to be flattened, widened and/or significantly frayed at the time of rotator cuff repair5, the decision to release the intraarticular portion of the biceps is straightforward.
In cases where treatment is aimed at rotator cuff repair, however, bicep tendon pathology may be incidentally identified. Without an understanding of the mechanism of how this pathology arose or the function of the intraarticular portion of the long head of the biceps, the decision to tenotomize or tenodese the biceps is made based only on anecdotal experience. After mechanisms of biceps tendon changes following rotator cuff tears are identified, clinicians will be better able to make an informed decision about its best treatment. For example, whether biceps changes will improve following rotator cuff repair or whether releasing the biceps tendon is the best option will be informed.
Before we can address whether bicep tendon pathology improves following a rotator cuff tear, we need a better understanding of the relationship between biceps tendon disease and rotator cuff tears. Little is also known about the effect of rotator cuff tendon tears on the regional properties of the biceps tendon over time, and therefore where these pathologic changes begin. Determining the origin of these changes would help to identify the mechanism responsible. For instance, changes including increased proteoglycan content and disorganized collagen first near the humeral head would indicate increased compressive loading1 while changes such as increased area first in the bicipital groove would indicate inflammation.7,10 This could result when sliding becomes difficult as the tendon hypertrophies. These questions are difficult to address in clinical studies due to the inability to harvest the entire biceps tendon during surgery. Additionally, in clinical studies it is difficult to evaluate the effect of time post-repair when the precise history, including the exact instance of tear initiation, is often unknown. It would also not be possible to obtain many specimens at time points soon after a rotator cuff tear occurs.
A previous study compared biceps tendons in the presence of rotator cuff tears to control tendons from uninjured animals in a rat model.8 The bony anatomy of the rat shoulder is very similar to the human and has previously been shown to be an appropriate model for studies of the rotator cuff.11 In addition, anatomy of the biceps tendon is also very similar in the rat and the human. In both the rat and the human, the long-head of the biceps tendon originates at the superior aspect of the glenoid (referred to here as the tendon’s proximal insertion into bone) and passes through the bicipital groove. This study evaluated only biomechanical changes and found increased area after 4 weeks and increased area and decreased modulus after 8 weeks in biceps tendons in the presence of rotator cuff tears compared to uninjured controls.8 Assays focusing on biological changes at these and earlier time points were not performed and the mechanism responsible for these changes remained unknown.
However, when evaluating earlier time points, it is possible that there may be effects of the surgical exposure itself which must be considered. Therefore, the objective of this study was to determine the histological, organizational, compositional and mechanical changes in the biceps tendon following a rotator cuff tear compared to a sham surgery at early, intermediate and late time points in order to investigate the relationship of cuff tears to biceps changes. Our hypotheses were that: 1) histological and compositional changes will appear before organizational changes, and both will appear before mechanical changes and 2) changes in all properties will begin at the tendon’s proximal insertion into bone and proceed along the length of the tendon at later time points.
Materials and Methods
Sixty-five male Sprague-Dawley rats (400–450g) were used in this IACUC approved study and separated into two treatment groups: supraspinatus+infraspinatus tendon detachments (n=34) and sham surgery (n=31). Animals were sacrificed at 1, 4 and 8 weeks post-surgery for histological analysis (n=4 or 5 per group and time point) and at 4 and 8 weeks post-surgery for mechanical testing (n=8–10 per group and time point).
In both groups, a unilateral surgery was performed on the animal’s left shoulder. Briefly, with the arm in external rotation, a 2 cm skin incision was made followed by blunt dissection down to the rotator cuff musculature. The rotator cuff was exposed and the tendons were visualized at their insertion on the humerus. The sham surgery ended here and the overlying muscle and skin were closed. In the supraspinatus+infraspinatus detachment group, the surgery continued with separation of the supraspinatus tendon from the other rotator cuff tendons and sharp detachment at its insertion on the greater tuberosity using a scalpel blade. The infraspinatus tendon was then detached in the same manner. Any remaining fibrocartilage at the insertion was left intact. Detached tendons were allowed to freely retract without attempt at repair, creating a gap ~4 mm from their proximal insertions into bone. The overlying muscle and skin were closed and the animals in both groups were allowed unrestricted cage activity.
Animals were sacrificed for mechanical testing at 4 and 8 weeks following surgery. The scapula, long-head of the biceps tendon and associated muscle were removed and the tendons were fine dissected under a microscope. Five Verhoeff stain lines were then placed along the length of each tendon denoting the proximal insertion into bone (0–1.5mm), the portion of the tendon in the intra-articular space (1.5–3.5mm), and the portion in the bicipital groove (3.5–8.5mm). A fifth stain line was placed at 11.5mm to identify grip placement and these stain lines were used to determine the distribution of strain along the length of the tendon. These positions were determined using histology to identify the length of the proximal insertion into bone and gross dissections to determine the portion in the bicipital groove.8 Tendon geometry was measured in each tendon portion using a laser based system.3
The scapula was then embedded in a holding fixture using polymethylmethacrylate (PMMA) and inserted into a specially designed fixture. The proximal end of the tendon was then held at the fifth stain line (11.5mm) in a screw clamp lined with fine grit sandpaper. The specimen was then immersed in a 39°C PBS bath, preloaded to 0.1N, preconditioned for 10 cycles from 0.1N to 0.5N at a rate of 1%/sec, and held for 300sec. Immediately following, a stress relaxation experiment was performed by elongating the specimen to a strain of 4% at a rate of 5%/sec (0.575 mm/sec) followed by a 600sec relaxation period. Specimens were then returned to the initial preload displacement and held for 60 seconds and ramp to failure was then applied at a rate of 0.3%/sec. Using the applied stain lines, local tissue strain in each tendon portion was measured optically with a custom program (MATLAB). Elastic properties, such as stiffness and modulus, were calculated using linear regression from the visually determined linear region of the load-displacement and stress-strain curves, respectively. Peak and equilibrium load were determined from the stress relaxation test and percent relaxation was then calculated from these values.
Animals were sacrificed for histological analyses 1, 4 and 8 weeks following surgery. Cross-sectional area was also measured along the length of the tendon in the 1 week specimens. Each tendon was analyzed in 4 locations: the proximal insertion into bone (0–1.5mm), the intra-articular space (1.5–3.5mm), the proximal bicipital groove (3.5–6mm) and the distal bicipital groove (6–8.5mm). Sagittal sections (7µm) were collected serially and stained with hematoxylin and eosin. Quantitative polarized light microscopy was used to determine collagen fiber orientations on these H&E stained sections.4 The angular deviation (AD) of the collagen orientation, a measure of the fiber distribution spread, in each tendon location for each specimen was then calculated as used previously.14
H&E stained sections were also analyzed for changes in cell shape and cellularity. Changes in cell shape from the normal elongated phenotype to a more rounded cell shape may signify a more compressive loading environment compared to the normal tensile loading environment experienced by the biceps tendon. Increased cellularity may, for example, be due to degeneration or infiltration of inflammatory cells; however, cell type was not identified. Histological grading standards for cellularity and cell shape were produced by first organizing images for each tendon location from either least to most cellular or most elongated to more rounded cell shape. These images were then divided into four quadrants and the middle image from each quadrant was chosen as the representative image for that quadrant and assigned a grade of 0, 1, 2 or 3. Images were then assigned grades by 3 blinded graders who compared each image to these standards for each location. The median grade between the 3 blinded graders for each image was then assigned to that image.
Finally, the distribution of various extra-cellular matrix proteins was localized in the biceps tendon using immunohistochemistry. The same specimens were used as for histological and polarized light analyses and one section from each specimen was stained for collagens type I, II, III and XII as well as proteoglycans aggrecan, biglycan and decorin (Table I). The same 4 regions were analyzed for immunohistochemical staining as for polarized light analysis and histological grading. Images were taken at 100× and evaluated semi-quantitatively using a custom DAB intensity measurement program (MATLAB).12,15 Briefly, quartiles were produced for each tendon location using the total target DAB intensity ranges for each protein target, which were determined as the difference between the most and least stained specimens in each group. Each quartile was assigned a value of undetectable (0), low (1), moderate (2) or high (3) and each image was assigned one of these grades.
Table I.
Primary antibodies used for immunohistochemical staining.
| Protein Target |
Antibody | Host | Type | Enzyme pretreatment |
Dilution | Incubation Period (h) |
Source |
|---|---|---|---|---|---|---|---|
| Collagen I | AB755P | Rabbit | Polyclonal | Hyaluronidase | 1:200 | 16 | Chemicon (Temecula, CA) |
| Collagen II | II-116B3 | Mouse | Monoclonal | Hyaluronidase | 1:4 | 16 | DSHB (Iowa City, IA) |
| Collagen III | c7805 | Mouse | Monoclonal | Hyaluronidase | 1:50 | 38 | Sigma (St. Louis, MO) |
| Collagen XII | LB-1200 | Rabbit | Polyclonal | Hyaluronidase | 1:100 | 16 | Cosmo-bio (Tokyo, Japan) |
| Aggrecan | LF-113 | Rabbit | Polyclonal | Chondroritinase ABC | 1:200 | 16 | L. Fisher (Bethesda, MD) |
| Biglycan | LF-159 | Rabbit | Polyclonal | Chondroritinase ABC | 1:200 | 16 | L.Fisher |
| Deconin | JSCATE | Rabbit | Polyclonal | Chondroritinase AC | 1:300 | 38 | J. Sandy (Chicago, IL) |
Mechanical testing parameters and angular deviation were compared between groups at each time point using student’s t-tests and this data is presented as average +/− standard deviation. For histological and immunohistochemical analyses, median grades were compared for each tendon location between groups at each time point using a non- parametric Mann-Whitney test and therefore, this data is presented as median and interquartile ranges.
Results
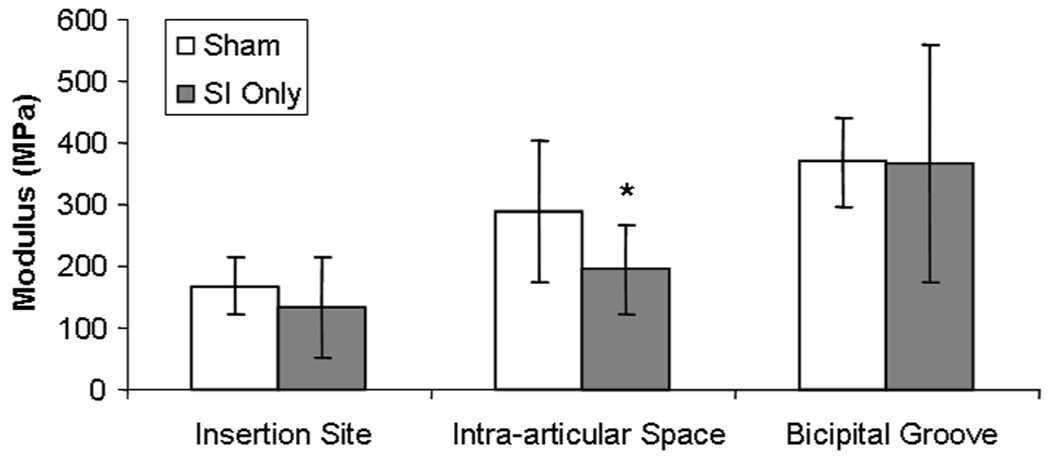
After 1 week, area was unchanged at the proximal insertion into bone, intra-articular space and bicipital groove in biceps tendons in the presence of a rotator cuff tear compared to sham (Table II). However, at 4 and 8 weeks area increased at all locations (Table II). Modulus was not different at any location 4 weeks post detachments and at 8 weeks modulus was decreased in the intra-articular space compared to sham (Figure 1). There were no differences in viscoelastic parameters at either time point.
Table II.
Area along the length of the biceps tendon at 1, 4 and 8 weeks post rotator cuff tendon detachments. Area was unchanged at 1 week but was increased with detachment along the entire tendon length at 4 and 8 weeks.
| Time post detachment (weeks) |
Group | Insertion Site Area (mm2) |
Intra-articular Space Area (mm2) |
Bicipital Groove Area (mm2) |
|---|---|---|---|---|
| 1 | Sham | 0.55±0.04 | 0.48±0.07 | 0.63±0.07 |
| Supra+Infra detach | 0.58±0.27 | 0.42±0.16 | 0.58±0.15 | |
| 4 | Sham | 0.83±0.18 | 0.71±0.15 | 0.66±0.18 |
| Supra+Infra detach | 1.09±0.21* | 0.84±0.19# | 0.85±0.20* | |
| 8 | Sham | 0.85±0.12 | 0.70±0.12 | 0.75±0.13 |
| Supra+Infra detach | 1.41±0.43* | 1.16±0.22* | 1.04±0.26* |
denotes p<0.05 compared to sham,
denotes p<0.1 compared to sham
Figure 1.
Modulus was decreased in the intra-articular space 8 weeks following detachment compared to sham. (* denotes p<0.05)
Angular deviation was increased in the intra-articular space at both 1 (Table III, Figure 2) and 4 weeks following rotator cuff tendon detachments (Table III, Figure 3). After 8 weeks, angular deviation was increased at all locations with cuff tears compared to sham surgery (Table III, Figure 4).
Table III.
Angular deviation was increased in the intra-articular space 1 and 4 weeks following detachment and was increased along the entire length of the tendon 8 weeks following detachment.
| Time post detachment (weeks) |
Group | Insertion Site Angular Deviation (°) |
Intra-articular Space Angular Deviation (°) |
Proximal Bicipital Groove Angular Deviation (°) |
Distal Bicipital Groove Angular Deviation (°) |
|---|---|---|---|---|---|
| 1 | Sham | 22.6±5.1 | 10.1±1.4 | 10.7±3.9 | 10.2±2.2 |
| Supra+Infra detach | 26.8±10.8 | 18.1±6.7* | 11.0±1.6 | 11.1±0.5 | |
| 4 | Sham | 21.9±6.4 | 9.8±2.4 | 8.9±2.6 | 10.6±3.7 |
| Supra+Infra detach | 25.9±10.0 | 13.0±1.7* | 10.2±1.0 | 9.3±2.0 | |
| 8 | Sham | 18.8±5.3 | 10.4±2.1 | 7.6±2.3 | 8.5±5.9 |
| Supra+Infra detach | 29.0±5.8* | 14.4±3.1* | 16.3±4.1* | 16.9±4.4* |
denotes p<0.05
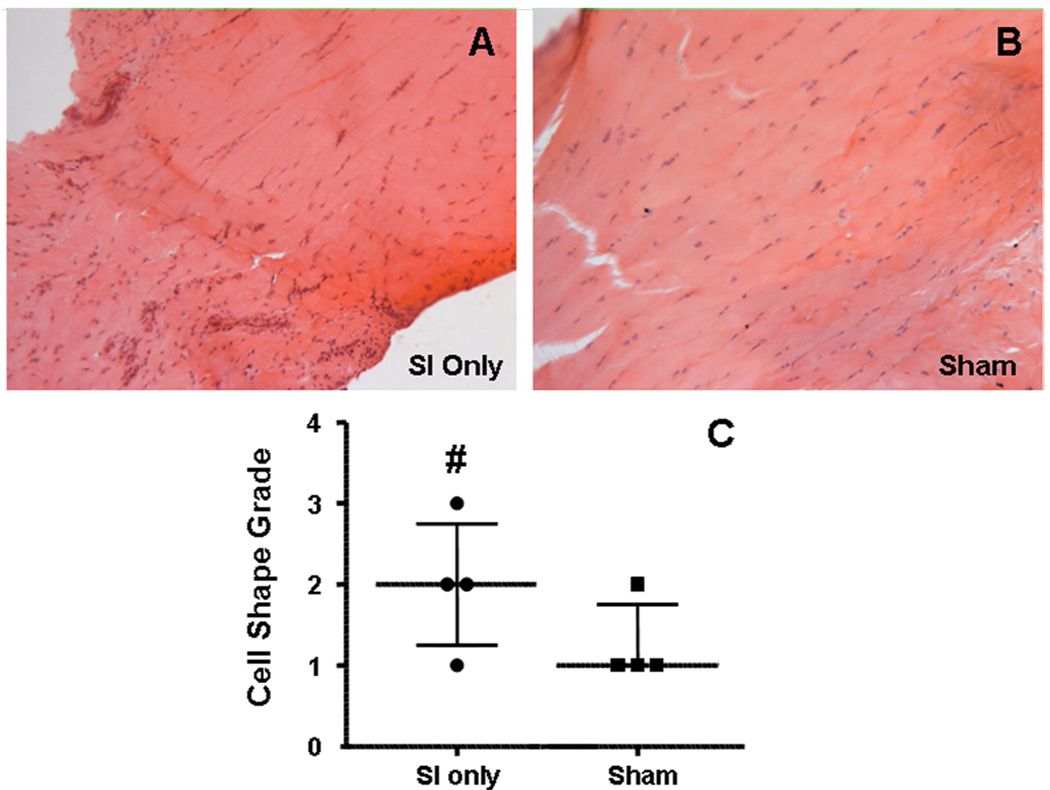
Figure 2.
After 1 week, a more rounded cell phenotype was seen in the intra-articular space with SI only (A) compared to sham (B). The median and inter-quartile ranges of the histological grading is seen in panel C. Also notice the more organized fibers in the sham image at this time point. (# denotes p<0.1)
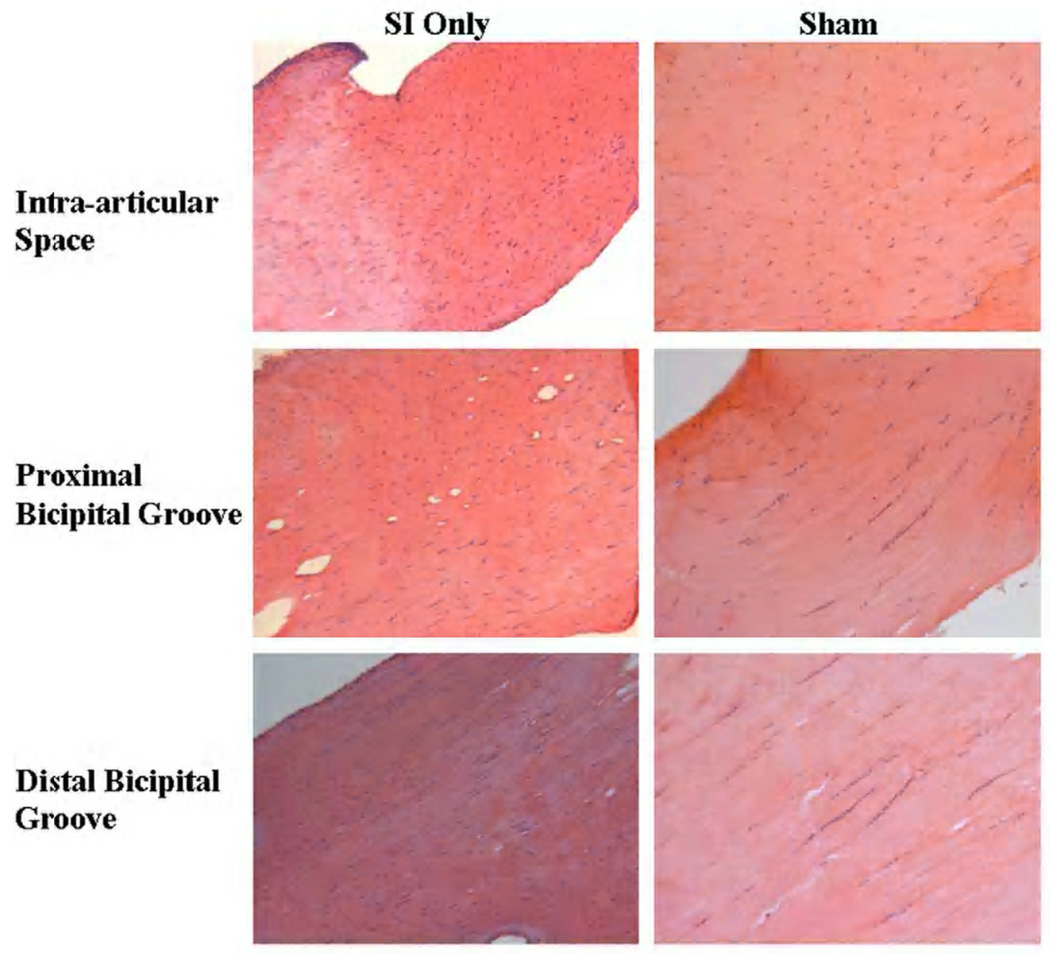
Figure 3.
Cell shape and cellularity were increased in the intra-articular space and proximal and distal bicipital groove 4 weeks following detachments. Also note the more disorganized collagen in the intra-articular space in the SI only group at this time point.
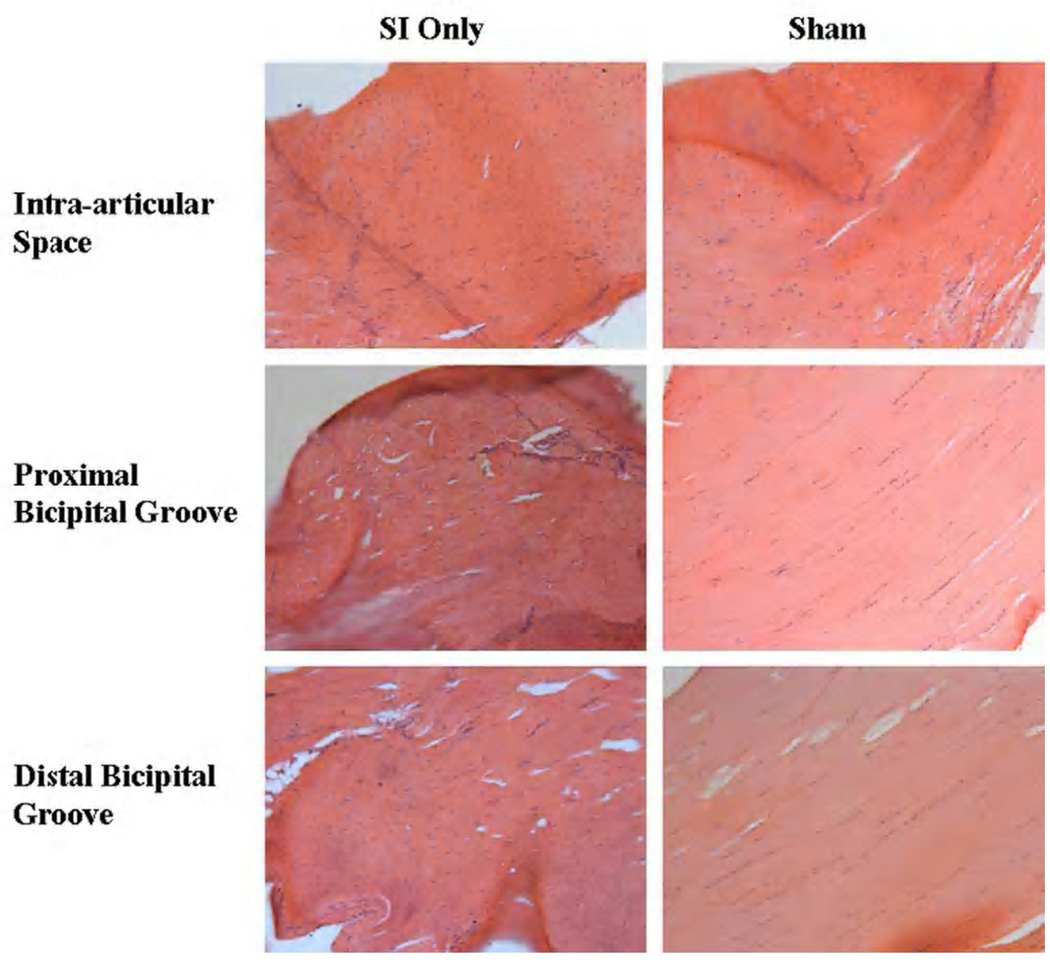
Figure 4.
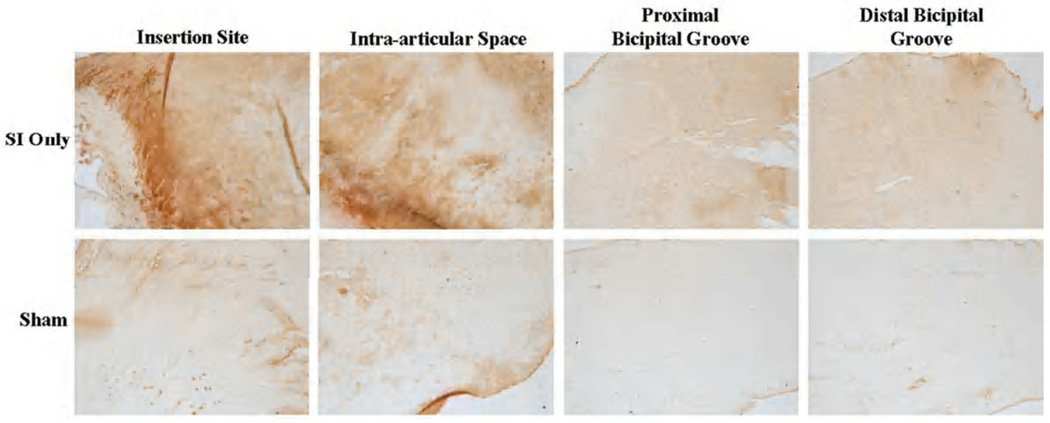
Cell shape and cellularity were still increased after 8 weeks in the intra-articular space and proximal and distal bicipital groove compared to sham. Also note the more disorganized collagen at all locations in the SI only images.
Changes in cell shape and cellularity were seen with histological grading. At 1 week, a more rounded cell shape was seen in the intra-articular space with rotator cuff detachments compared to sham (Figure 2). Four weeks following detachments, cellularity was increased and a more rounded cell phenotype was found along the entire length of the tendon with rotator cuff tears compared to sham (Figure 3). Cellularity continued to be increased with cuff detachments at all tendon locations at 8 weeks compared to sham. Further, a more rounded cell shape was seen in the intra-articular space as well as proximal and bicipital grooves in the presence of a rotator cuff tear compared to sham surgery (Figure 4). Unfortunately, staining for cell type was not included in this study.
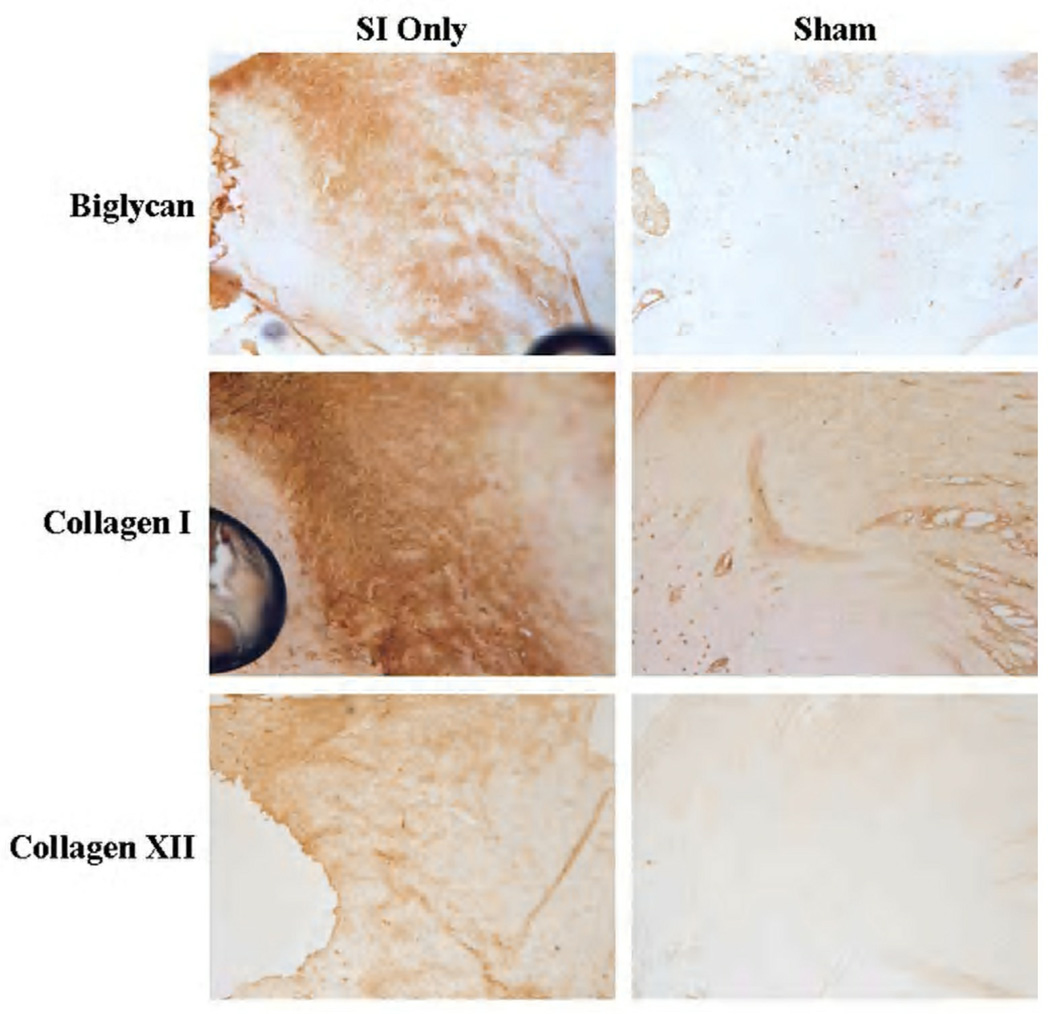
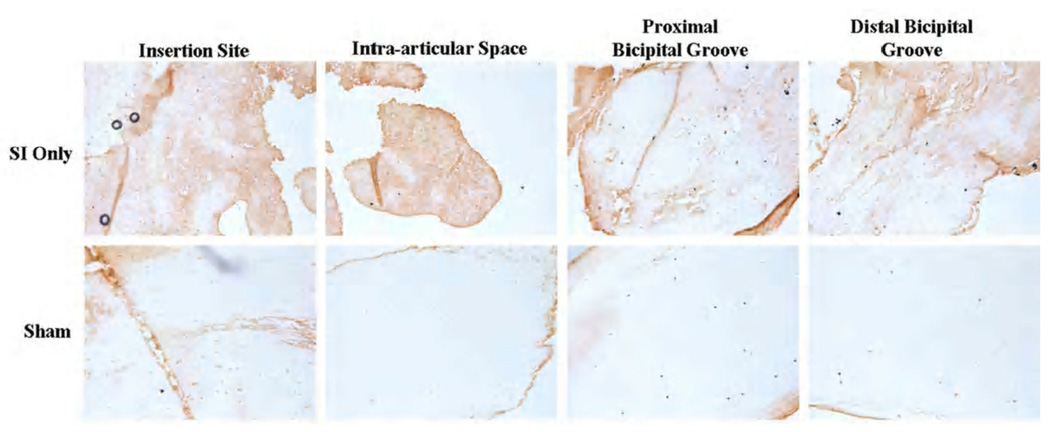
Changes in protein expression were found with immunohistochemical staining. At 1 week, aggrecan was increased along the entire tendon length in the presence of a rotator cuff tear compared to sham (Figure 5). Also at 1 week, biglycan and collagens I and XII were increased compared to sham at the proximal insertion into bone (Figure 6). Four weeks following detachments, aggrecan was increased in the proximal bicipital groove compared to the sham surgery. Biglycan was increased in the intra-articular space and decorin was increased in the intra-articular space and proximal and distal grooves with rotator cuff detachment compared to sham. Collagens III and XII were increased in the presence of a cuff tear at the proximal insertion into bone. Finally, 8 weeks post detachments, there were trends for increases in aggrecan in the distal bicipital groove and in biglycan (Figure 7) along the entire tendon length compared to sham. Decorin was increased with cuff detachments compared to sham at the proximal insertion into bone and in the intra-articular space. Collagen I was increased along the entire tendon length and Collagen XII was increased at the proximal insertion into bone with rotator cuff tears compared to sham surgery.
Figure 5.
Aggrecan staining was increased along the entire tendon length 1 week following rotator cuff detachments compared to sham.
Figure 6.
After 1 week, biglycan, collagen I and collagen XII staining was increased at the tendon’s proximal insertion into bone compared to sham.
Figure 7.
Biglycan staining was increased along the entire tendon length 8 weeks following detachment compared to sham surgery.
Discussion
This study defines the pathologic changes in the long head of the biceps tendons resulting from a rotator cuff tear. Changes in both composition and organization were found at the earliest time point investigated in this study. Results support the hypothesis that organizational changes precede changes in mechanics, but do not support our hypothesis that compositional changes would precede organizational change. More disorganized tissue was seen in the intra-articular space at 1 and 4 weeks followed by a decrease in modulus in the intra-articular space 8 weeks following detachment. Increased cellularity was not seen until the 4 and 8 week time points, at which increases were seen along the entire tendon length. However, it is possible that cellularity was increased after 1 week with both the sham and rotator cuff detachment surgeries, which decreased in the sham animals but not in the presence of detachments.
It is also interesting to note that changes in histological grading are consistent with changes seen in organization, with more rounded cells present in disorganized tissue and more elongated cells with increased organization. After 1 week, a more disorganized tendon was seen in the intra-articular space. Also at this time point, there was a trend toward a more rounded cell phenotype at this location. After 4 weeks, it seems that cell shape changes precede organizational changes as again the tendon was only disorganized in the intra-articular space but a more rounded cell shape was seen along the entire tendon length. Finally, after 8 weeks decreased organization and a more rounded cell phenotype were present along the entire tendon length. Unfortunately, the cell types present were not identified in this study. Therefore, it is unclear if the more rounded cells found are new cells infiltrating the tendon or cells that were already present. This more rounded cell shape and decreased organization are characteristics indicative of tendons more adapted to compressive, rather than purely tensile, loading. Following a sham surgery, biceps tendons resemble those adapted to purely tensile loading. With the most significant superior stabilizers of the humeral head (supraspinatus and infraspinatus) detached, we speculate, as supported by our results indicating tendons more adapted to a compressive loading environment, that the biceps tendon is required to play a more significant role in humeral head depression and stabilization. This theory is supported by the increase in aggrecan (a proteoglycan found most often in cartilage) at 1 week post detachments. Biglycan, an ECM protein associated with injury, was also increased at the tendon’s proximal insertion into bone after 1 week. Increased biglycan expression progressed along the length of the tendon with time as increased expression was seen at 4 weeks in the intra-articular space and at 8 weeks along the entire tendon length. This finding supports the theory there may be a degenerative component to the tendon response following rotator cuff detachments, rather than a purely inflammatory process as has been speculated previously.7 Collagen I, the most prevalent collagen seen in tendons, was increased at the tendon’s proximal insertion into bone at 1 week and along the entire tendon length at 8 weeks, perhaps indicating that the tendon is trying to return its properties to normal by producing more collagen adapted to tensile loading. While mechanical properties were not returned to normal by this time point8, many tendon properties did improve at 16 weeks post detachment9, which may be due to the increased collagen production found here at 8 weeks.
Immunohistochemical results support our hypothesis that changes begin at the tendon’s proximal insertion into bone as, other than aggrecan, all changes at 1 week were only at that location. However, histological grading and organizational results indicate changes may begin in the intra-articular space. The lack of changes in organization seen at the tendon’s proximal insertion into bone may be due simply to the fact that a disorganized collagen matrix already exists at this location and further disorganization may be difficult to detect. Following sham surgery, the organization is the same in the intra-articular space and proximal and distal bicipital grooves whereas following rotator cuff detachments the intra-articular space is more disorganized than the more distal locations, perhaps indicating it is experiencing types of loading more like that seen at the tendon’s proximal insertion into bone.
While not all changes occur first at the tendon’s proximal insertion into bone, it was consistently shown that changes appear in the intra-articular portion of the tendon before the extra-articular portion. These results are supported by recent work demonstrating the intra- and extra-articular portions of the biceps tendon to be markedly different when in the presence of rotator cuff tears.6 However, that study did not compare to uninjured tendons and therefore there may be some differences in the intra- and extra-articular portions that are inherent to the tendon and not a result of rotator cuff tears. In this study, it was possible to compare to a sham surgery and therefore any changes seen are the direct result of the rotator cuff tear.
This study is not without limitation. The rotator cuff tendon tears in this study were made acutely. However, biceps tendon pathology occurs over time without surgical injury to the tendon itself in this model. It is also possible that additional biological changes may be taking place that were not detected with the immunohistochemical staining performed in this study. A group of targets were selected that, according to previous studies12,13,15, would be expected to show differences across both time and the length of the tendon and have been extensively used for rotator cuff tendons in the rat model. Additionally, staining to identify cell types present was not performed in this study and in the future could help determine the source of increased cellularity found here. Future work with this model may include additional biological assays such as PCR or staining for targets involved in collagen turnover or to determine cell types present. Additionally, the effect of altered loading scenarios in addition to rotator cuff detachments on all properties measured here will be investigated.
Conclusion
In summary, alterations in the balance of forces following a rotator cuff tear are detrimental to surrounding tissues. A rotator cuff tear alone is enough to elicit changes in the long head of the biceps tendon. It was shown here that organizational and compositional changes precede changes in area, which in turn precede changes in mechanical properties. In addition, it was shown that organizational and mechanical property changes begin in the intra-articular space while most immunohistochemical changes began at the tendon’s proximal insertion into bone. These results illustrate that changes in the biceps tendon occur gradually over time and indicate a response to the presence of rotator cuff tears that may have a degenerative component and is not purely inflammatory in nature. Finally, changes in tendon properties indicating a more compressive loading environment occurring first in the intra-articular space indicate the biceps tendon may play an increased role as a humeral head depressor in the presence of rotator cuff tears.
Acknowledgments
Funding provided by: NIH AR051000 and NIH AR050950 IRB approval: N/A (Animal study approval provided by University of Pennsylvania Institutional Animal Care and Use Committee (IACUC) protocol #802540)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No disclosures
References
- 1.Berenson MC, Blevins FT, Plaas AH, Vogel KG. Proteoglycans of human rotator cuff tendons. J Orthop Res. 1996;14:518–525. doi: 10.1002/jor.1100140404. DOI: none (prior to 2002), PMID: 8764859. [DOI] [PubMed] [Google Scholar]
- 2.Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58:1189–1193. doi: 10.1097/01.ta.0000170052.84544.34. No DOI, PMID: 15995469. [DOI] [PubMed] [Google Scholar]
- 3.Favata M. Scarless healing in the fetus: Implications and strategies for postnatal tendon repair. Philadelphia: University of Pennsylvania; 2006. p. 216. No DOI. [Google Scholar]
- 4.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739–749. doi: 10.1016/j.jbiomech.2003.09.019. DOI: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Itoi E, Hsu HC, Carmichael SW, Morrey BF, An KN. Morphology of the torn rotator cuff. J Anat. 1995;186(Pt 2):429–434. DOI: none (prior to 2002), PMID: 7649844. [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph M, Maresh CM, McCarthy MB, Kraemer WJ, Ledgard F, Arciero CL, et al. Histological and molecular analysis of the biceps tendon long head post-tenotomy. J Orthop Res. 2009 doi: 10.1002/jor.20868. DOI: 10.1002/jor.20868. [DOI] [PubMed] [Google Scholar]
- 7.Murthi AM, Vosburgh CL, Neviaser TJ. The incidence of pathologic changes of the long head of the biceps tendon. J Shoulder Elbow Surg. 2000;9:382–385. doi: 10.1067/mse.2000.108386. DOI: none, PMID: 11075320. [DOI] [PubMed] [Google Scholar]
- 8.Peltz CD, Perry SM, Getz CL, Soslowsky LJ. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J Orthop Res. 2009;27:416–420. doi: 10.1002/jor.20770. DOI: 10.1002/jor.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peltz CD, Hsu JE, Zgonis MH, Trasolini NA, Glaser DL, Soslowsky LJ. Biceps tendon properties worsen initially but improve over time following rotator cuff tears in a rat model. J Orthop Res. 2011 doi: 10.1002/jor.21325. DOI: 10.10021/jor.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Refior HJ, Sowa D. Long tendon of the biceps brachii: sites of predilection for degenerative lesions. J Shoulder Elbow Surg. 1995;4:436–440. doi: 10.1016/s1058-2746(05)80035-7. DOI: none (prior to 2002), PMID: 8665288. [DOI] [PubMed] [Google Scholar]
- 11.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. DOI: none (prior to 2002), PMID: 8933461. [DOI] [PubMed] [Google Scholar]
- 12.Wurgler-Hauri CC, Dourte LM, Baradet TC, Williams GR, Soslowsky LJ. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg. 2007;16:S198–S203. doi: 10.1016/j.jse.2007.04.003. DOI: 10.1016/j.jse.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokota A, Gimbel JA, Williams GR, Soslowsky LJ. Supraspinatus tendon composition remains altered long after tendon detachment. J Shoulder Elbow Surg. 2005;14S:72–78. doi: 10.1016/j.jse.2004.09.021. DOI: 10.1016/j.jse.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Zar JH. Biostatistical Analysis. Princeton: Prentice Hall; 1984. [Google Scholar]
- 15.Zgonis M, Wurgler-Hauri CC, Perry SM, Soslowsky LJ. Transactions of the Orthopaedic Research Society. Edited, 854. San Diego: 2007. The effect of rest after overuse on extracellular matrix proteins in rat supraspinatus tendon: An immunohistochemical analysis; p. 854. [Google Scholar]