Abstract
Previous studies have evaluated motor and extramotor cerebral cortical regions in patients with amyotrophic lateral sclerosis (ALS) using 1H MRS, but none have evaluated the thalamus or basal ganglia. The objective of this exploratory study was to evaluate the subclinical involvement of the basal ganglia and thalamus in patients with ALS using 1H MRS. Fourteen patients (52 ± 7 years) with sporadic definite ALS and 17 age-matched controls were studied using volumetric MRSI on a 3-T scanner. The concentration of the metabolites N-acetylaspartate (NAA), choline (Cho) and their ratio (NAA/Cho) were obtained bilaterally from the basal ganglia (lentiform nucleus, caudate) and thalamus. The maximum rates of finger and foot tap and lip and tongue movements were obtained to assess extrapyramidal and pyramidal tract function. In patients with ALS, relative to controls, the NAA concentration was significantly lower (p < 0.02) in the basal ganglia and thalamus, and the Cho concentration was higher (p < 0.01) in these structures, except in the caudate (p = 0.04). Correspondingly, the NAA/Cho ratio was significantly lower (p < 0.01) in these structures, except in the caudate (p = 0.03), in patients than in controls. There were mild to strong correlations (r = 0.4–0.7) between the metabolites of the basal ganglia and finger tap, foot tap and lip and tongue movement rates. In conclusion, decreased NAA in the basal ganglia and thalamus and increased Cho and decreased NAA/Cho in the lentiform nucleus and thalamus are indicative of neuronal loss or dysfunction and alterations in choline-containing membranes in these structures.
Keywords: amyotrophic lateral sclerosis, basal ganglia, MRS, extramotor, thalamus
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder characterized by variable loss of large pyramidal cells (upper motor neuron) in the motor cortex of the brain, brainstem motor nuclei (lower motor neuron) and anterior horn cells in the spinal cord (1). However, pathological (2-5), neuropsychological (6) and neuroimaging (7-10) studies have challenged the view that ALS is a disorder restricted to the motor system. Furthermore, results from several studies (2-6) have established dysfunction of the nonmotor cortex, especially the prefrontal and temporal lobe cortex, in patients with ALS.
Similar to the involvement of the extramotor system in the brain cortex, there is accumulating evidence from pathological (2,5) and neuroimaging (8-10) studies for widespread neuronal degeneration in other subcortical structures in patients with ALS. These include the basal ganglia, substantia nigra, thalamus, subthalamic nucleus, locus coeruleus, red nucleus, dentate nucleus, pontine tegmentum and cerebellum. In vivo studies using techniques, such as positron emission tomography (10), diffusion tensor imaging (8), functional MRI (8) and tensor-based morphometry (9), have confirmed the pathological findings of the involvement of several subcortical structures in ALS. However, metabolite abnormalities in these subcortical structures in patients with ALS have not been evaluated using 1H MRS.
In vivo 1H MRS techniques are used to quantify several brain metabolites noninvasively. These include N-acetylaspartate (NAA; a putative marker of viable neurons), choline (Cho; an indicator of membrane structural status and synthesis), and creatine and phosphocreatine (Cre; an indicator of cellular energetics). 1H MRS has been shown to be useful in patients with ALS for the early detection of neuronal loss/dysfunction in the motor cortex, corticospinal tract (7,11,12) and extramotor cortex (7), and for the assessment of longitudinal progression of the disease (12) and the effect of drug therapy (11). Previous MRS studies in patients with ALS (7,11,12) have evaluated metabolite alterations in the extramotor cortex, although none have assessed the subcortical structures, such as the basal ganglia and thalamus.
The objective of this exploratory study was to evaluate the subclinical involvement of the basal ganglia and thalamus in patients with ALS using MRS. The primary hypothesis of this study was that NAA would be decreased and Cho increased in the basal ganglia and thalamus in patients with ALS, relative to age-matched controls, and the secondary hypothesis was that these metabolite changes would correlate with the measures of extrapyramidal/pyramidal tract function and neurological disability.
METHODS
Subjects
Sixteen consecutive patients who met the revised El Escorial criteria (13) for sporadic definite ALS were recruited. Data from two of these patients were excluded from the analysis because of either poor quality of the data or incomplete data acquisition. The data from the remaining 14 patients (eight men, six women; age, 52 ± 7 years; range, 24–65 years; median, 57 years; 13 with limb onset and one with bulbar onset; symptom duration, 22 ± 10 months; median, 20 months; range, 9–42 months) and 17 age-matched controls (eight men, nine women; age, 51 ± 13 years; range, 39–63 years; median, 53 years) were analyzed. All patients and controls provided written informed consent approved by the Institutional Review Board.
Clinical assessments
All patients with sporadic definite ALS underwent detailed physical and neurological examinations to assess for clinical evidence of extramotor involvement (extrapyramidal tract, sensory, cerebellar). The disease severity was evaluated using the ALS Functional Rating Scale-Revised [ALSFRS-R; range of scores, 0–48, with scores 0 and 48 indicating total disability and no disability (14), respectively] and percentage of predicted forced vital capacity (FVC). The maximum finger and foot tap rates (15,16) were obtained to evaluate the extrapyramidal and pyramidal tract function of these muscles, and are also components of the motor section of the Unified Parkinson’s Disease Rating Scale (17). Pyramidal tract function, in addition to the standard measures such as muscle stretch reflex and muscle tone, was quantified by counting the number of rapid foot taps, finger taps, lip movements with ‘pa-pa’ syllable repeat and tongue movements with ‘la-la’ syllable repeat in 10 s, and were converted to per second scores [rate (18,19)]. Furthermore, the rapid muscle movement rate at all these sites was repeated twice during the same session of the examination, after an interval of 1 min, to obtain the representative average rate for each site. The Mini-Mental State Examination [MMSE (20)] was administered to all patients and controls as a screening instrument for cognitive impairment.
For the remainder of this report, measures of pyramidal and extrapyramidal tract function constitute the number of rapid foot and finger taps, lip movements with ‘pa-pa’ syllable repeat and tongue movements with ‘la-la’ syllable repeat, and the measure of neurological disability constitutes the ALSFRS-R score.
MRSI protocol
All patients and controls underwent MR examination on a 3-T scanner (Siemens, Erlangen, Bavaria, Germany) with eight-channel phased-array detection. The MR protocol used for this study included MRI and whole-brain MRSI acquisitions. MRSI data were obtained using a volumetric spin-echo echo-planar sequence with the following parameters: TR/TE = 1710/70 ms; field of view, 280 × 280 × 180 mm3; phase encodes, 50 [read] × 50 [phase] × 18 [slice]; slab thickness, 135 mm; acquisition time, 26 min. The details of the data acquisition are provided in our earlier publication (21). An automated three-dimensional shimming procedure provided by the scanner manufacturer was used to optimize B0 field homogeneity over the volume of interest. This procedure generates three-dimensional phase maps from which the resonance frequency offset and the optimum shim currents for the adjustment of three linear and five second-order shim channels are calculated.
Data processing and analysis
MRSI data were processed using the fully automated MIDAS package (22). Briefly, the data processing steps included spatial smoothing and interpolation, signal normalization using the tissue water reference signal from the same voxel and intra-subject co-registration of T1-weighted MRI and MRSI for MRI-guided region of interest (ROI) metabolite data selection. The nominal MRSI voxel volume was approximately 0.3 mL (5.6 × 5.6 × 10 = 314 mm3). After applying Gaussian spatial smoothing in all three orthogonal directions and interpolation of processed metabolite images to 64 × 64 × 32 resolution, the volume of the voxel used for data analysis was increased to approximately 1 mL. For data analysis, the basal ganglia and thalamus structures were selected in T1-weighted MRI. To identify the basal ganglia substructures (caudate and lentiform nucleus) and thalamus, pixel intensity contrasts in MRI were used with isointense-to-gray matter for the caudate and putamen, and hypointense-to-gray matter for the globus pallidus and thalamus. Data from six ROIs (bilateral thalamus, caudate and lentiform nucleus; Fig. 1) were obtained to measure the relative concentrations of NAA, Cre and Cho (provided in institutional units) and the NAA/Cho ratio. For analysis, MRS data from the left and right side of the ROIs in both groups were used separately, and also summed to obtain average values. Similarly, the averages of the left- and right-side maximum rate of foot taps and finger taps were calculated for further data analysis. For correlation analysis, the bulbar muscle movement rates of ‘pa-pa’ and ‘la-la’ syllable repeats were combined.
Figure 1.
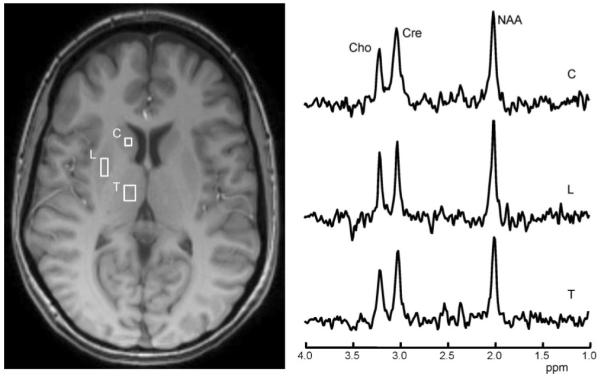
Spectra for various metabolites (Cho, choline; Cre, creatine; NAA, N-acetylaspartate), which were obtained from the regions of interest (ROIs) of the right caudate (C), lentiform nuclei (L) and thalamus (T) of a 37-year-old female patient with sporadic definite amyotrophic lateral sclerosis of 7 months’ symptom duration.
Statistics
For data analysis, Stat View II (Abacus Concepts, Inc., Berkley, CA, USA) was used. To evaluate differences between the measures of patients and controls, a two-tailed unpaired t-test was employed. A two-tailed paired t-test was used to evaluate differences in measures between the left- and right-side rapid movement rates of various muscles (see Clinical assessments section) and metabolite measures for the groups of controls and patients. Pearson’s product moment partial correlation was used to evaluate correlations between the metabolite measures and clinical measures, and the results are reported as correlation coefficients (r values). All data are expressed as means ± standard deviation. The median values are also provided for the ALSFRS-R score, percentage of predicted FVC, symptom duration of the disease and age of the patients and controls.
In this study, our primary hypothesis was that NAA would be decreased and Cho would be increased in the basal ganglia and thalamus in patients with ALS relative to age-matched controls. The secondary hypothesis was that these metabolite changes would correlate with measures of pyramidal/extrapyramidal tract function (timed movements of finger taps, foot taps and bulbar muscles) and the neurological disability measure (ALSFRS-R score). Consistent with the primary aim of evaluating abnormality in NAA and Cho in the basal ganglia and thalamus, the p value corrected for multiple comparisons (Bonferroni correction) was set to less than 0.025 to identify significant differences between the groups. The p value of significance for the secondary hypothesis was set to less than 0.05 and was not corrected for multiple comparisons.
RESULTS
Clinical features
Fourteen patients with definite sporadic ALS [13 limb onset, one bulbar onset (13)] had a variable degree of functional motor disability with a mean ALSFRS-R of 36.4 ± 6.7 (range, 26–46; median, 35) and mean FVC of 78.5 ± 20.3% (range, 40–102%; median, 76%). None of the patients had extramotor (extrapyramidal tract, sensory, cerebellar) abnormality on clinical examination, standard nerve conduction studies or neuropsychological evaluation with MMSE (ALS, 28.1 ± 1.5; range, 26–30; controls, 28.4 ± 1.1; range, 27–30; p = 0.5). Group comparisons between patients with ALS and controls showed significant abnormality of fine rapid movements in the muscles of the bulbar region (‘pa-pa’ syllable repeat rate of 3.6 ± 0.9 versus 4.4 ± 0.3, p = 0.03; ‘la-la’ syllable repeat rate of 3.5 ± 0.9 versus 4.5 ± 0.3, p = 0.02), upper limbs (right finger tap rate of 2.7 ± 0.9 versus 4.1 ± 0.6, p < 0.01; left finger tap rate of 2.4 ± 0.9 versus 4.1 ± 0.7, p < 0.01) and lower limbs (right foot tap rate of 2.4 ± 1.3 versus 3.5 ± 0.4, p = 0.04; left foot tap rate of 2.0 ± 1.2 versus 3.4 ± 0.5, p = 0.03).
MRS-observed metabolites
The mean and standard deviations of the metabolites and their ratios obtained at selected ROIs across both patient and control groups are shown in Table 1. No asymmetry of left versus right metabolites (absolute concentration in institutional units and ratio) was found in either controls or patients. Therefore, data from the left and right sides in each of the groups were averaged for use in all subsequent group comparisons. In patients with ALS, relative to controls, the NAA concentration was significantly lower in the basal ganglia and thalamus, and the Cho concentration was higher in these structures, except for the caudate (p = 0.04, Table 1). Correspondingly, the NAA/Cho ratio was significantly lower in these structures in patients than in controls, except for the caudate (p = 0.03, Table 1). The Cre concentration was similar in patients and controls in the lentiform nucleus (1720 ± 286 versus 1681 ± 198, p = 0.71), caudate nucleus (1779 ± 418 versus 1806 ± 346, p =0.89) and thalamus (1872 ± 192 versus 1829 ± 122, p = 0.53).
Table 1.
Metabolites and metabolite ratio in basal ganglia and thalamus for patients with amyotrophic lateral sclerosis (ALS) (n = 14) and control group (n = 17)
| Location | Group | NAA (mean ± SD)b | Cho (mean ±SD)b | NAA/Cho (mean ± SD) |
|---|---|---|---|---|
| Lentiform nucleus | ALS | 2097.07 ± 101.89 | 526.54 ± 34.76 | 4.00 ± 0.36 |
| Control | 2259.51 ± 166.19 | 456.35 ± 55.65 | 5.06 ± 0.66 | |
| p | 0.02a | <0.01a | <0.01a | |
| Caudate | ALS | 1904.84 ± 236.60 | 584.03 ± 170.53 | 3.14 ± 1.03 |
| Control | 2333.18 ± 313.44 | 414.51 ± 117.00 | 4.67 ± 1.29 | |
| p | 0.01a | 0.04 | 0.03 | |
| Thalamus | ALS | 2282.33 ± 189.08 | 605.56 ± 55.15 | 3.79 ± 0.15 |
| Control | 2650.69 ± 160.45 | 515.59 ± 50.76 | 5.19 ± 0.58 | |
| p | <0.01a | <0.01a | <0.01a |
Cho, choline; NAA, N-acetylaspartate; SD, standard deviation.
Significant p value, corrected for multiple comparisons, with cut-off set to less than 0.025.
Institutional unit.
Correlation between MRS measures and clinical measurements
The correlations between the metabolite and clinical measures are provided in Table 2. A significant positive correlation was found between the maximum finger tap rate and NAA/Cho in the lentiform nucleus (r = 0.7, p = 0.01; Fig. 2). In addition, there was a trend for a positive correlation for NAA in the caudate (r = 0.5, p = 0.05) and a trend for a negative correlation for Cho in the lentiform nucleus (r = −0.5, p = 0.05). There was no correlation between the finger tap rate and the remaining basal ganglia metabolite measurements (Table 2).
Table 2.
Correlation between metabolite and clinical measures
| Activity |
|||||||
|---|---|---|---|---|---|---|---|
| Finger tap rate |
Foot tap rate |
Bulbar muscle movement rateb |
|||||
| Location | r | p | r | p | r | p | |
| Lentiform nucleus | NAA | 0.5 | 0.08 | 0.6 | 0.02a | 0.4 | 0.20 |
| Cho | −0.5 | 0.05 | −0.4 | 0.11 | −0.5 | 0.08 | |
| NAA/Cho | 0.7 | 0.01a | 0.4 | 0.06 | 0.5 | <0.05a | |
| Caudate | NAA | 0.5 | 0.05 | 0.6 | 0.03a | 0.6 | 0.03a |
| Cho | −0.4 | 0.09 | −0.5 | 0.08 | −0.4 | 0.21 | |
| NAA/Cho | 0.4 | 0.11 | 0.6 | <0.05a | 0.6 | 0.03a | |
Cho, choline; NAA, N-acetylaspartate.
Significant p value, not corrected for multiple comparisons, with cut-off set to less than 0.05.
Bulbar muscle movement rate, maximum tongue and lip movement rate.
Figure 2.
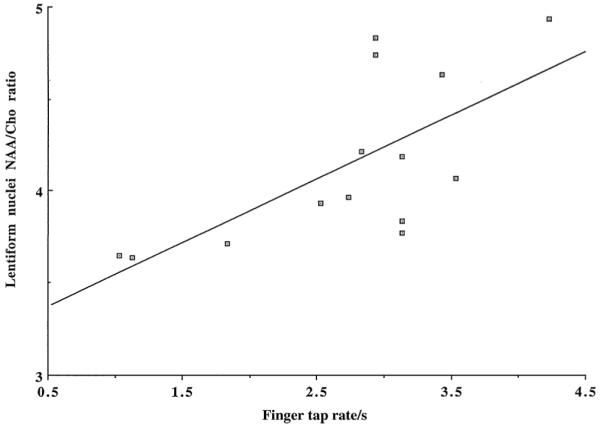
Scattered plot demonstrates a significant positive correlation (r = 0.7, p = 0.01) between the averages of the left- and right-hand maximum finger tap rates and the means of the left and right N-acetylaspartate/choline (NAA/Cho) ratios in the lentiform nucleus of 14 patients with sporadic definite amyotrophic lateral sclerosis.
Similarly, the maximum foot tap rate and metabolite measures of the basal ganglia of patients with ALS showed a positive correlation for NAA in the lentiform nucleus (r = 0.6, p = 0.02) and caudate (r = 0.6, p = 0.03), and for NAA/Cho in the caudate (r = 0.6, p < 0.05). There was a trend for a positive correlation for NAA/Cho in the lentiform nucleus (r = 0.4, p = 0.06), and a trend for a negative correlation for Cho in the caudate (r = −0.5, p = 0.08; Table 2).
The maximum bulbar muscle movement rate of syllable repeats and the metabolite measures in the basal ganglia of patients with ALS revealed positive correlations for NAA/Cho in the lentiform nucleus (r = 0.5, p <0 .05) and caudate (r = 0.6, p = 0.03), and NAA in the caudate (r = 0.6, p = 0.03; Table 2). The correlated ALSFRS-R score only with NAA/Cho in the caudate (r = 0.7, p = 0.02) among the metabolites in the basal ganglia.
DISCUSSION
The findings of this study in patients with ALS relative to controls were as follows: (i) the NAA concentration was significantly lower in the basal ganglia; (ii) the Cho concentration was higher in the basal ganglia except in the caudate (p = 0.04); (iii) the NAA/Cho ratio was significantly lower in the basal ganglia except in the caudate (p = 0.03; Table 1); and (iv) the concentration of NAA and the NAA/Cho ratio were significantly lower in the thalamus, and the Cho concentration was significantly higher in the thalamus. There were mild to strong correlations between the metabolite measures of the basal ganglia and the maximum movement rates subserved by the pyramidal/extrapyramidal systems (Table 2, Fig. 2).
The metabolite abnormalities observed in vivo in the basal ganglia and thalamus of patients with ALS support the results from previous in vitro pathological (2-5) and in vivo imaging (8-10) studies, suggesting subclinical dysfunction of these subcortical structures in ALS.
As NAA is present almost exclusively in neurons and synthesized in neuronal mitochondria, it is used as a biochemical marker of neuronal functional integrity. Reduced levels of in vivo NAA and NAA/Cre have been observed in various neurological disorders associated with neuronal loss or damage or dysfunction. This may result from either a decrease in the intracellular volume of the neurons per unit volume of the brain (caused by either neuronal loss or atrophy), or a decrease in the concentration of NAA within neurons as a result of metabolic dysfunction. Our findings of metabolic abnormalities (decreased NAA and NAA/Cho) in the basal ganglia and thalamus of patients with ALS suggest that there is degeneration or dysfunction of neurons in these subcortical structures, which is supported by the immunohistopathological studies of neuronal loss, astrocystic gliosis and ubiquitin-immunoreactive cytoplasmic inclusions in these structures in patients with ALS (4,5).
Cho is an indicator of the status of cell membrane synthesis, structural integrity and gliosis, and was found to be significantly higher in the basal ganglia and thalamus of patients with ALS, probably indicating gliosis secondary to neuronal loss. This increase in Cho concentration corroborates the reported histopathological findings of increased astrocytosis and glial proliferation in the basal ganglia and thalamus (2-5).
Basal ganglia dysfunction
Basal ganglia, through their afferent and efferent pathways, influence not only the primary motor cortex, but also the premotor and prefrontal cortices that are involved in language and cognitive functions (23,24). There was no difference in the scores of MMSE (20) between patients and controls; however, given the limitations of MMSE, it cannot be taken to indicate that there was no cognitive impairment in the patients.
Similar to our observations of subclinical dysfunction of the basal ganglia in patients with ALS, investigations using in vivo imaging techniques [positron emission tomography (25,26) and single photon emission computed tomography (27)] have shown abnormal presynaptic and postsynaptic striatal dopaminergic function in these patients. The intensity of reduction of postsynaptic striatal D2-receptor binding in some of the patients with ALS was similar to that seen in patients with multisystem atrophy (28). Degeneration in the dopaminergic neurons in the basal ganglia and midbrain has also been observed in a transgenic mouse model of familial ALS (29). Neurodegeneration beyond motor neurons in the cortex, brainstem and spinal cord is particularly evident in patients with ALS who survive for prolonged periods on a respirator (3). It is uncertain whether this widespread neurodegeneration, observed beyond the motor neurons in the cortex at an advanced stage of the disease, represents primary (30,31) or secondary (10) degeneration. Moreover, this uncertainty about the extent of contribution from either of these two degenerative processes may account for the variability in the degree of dysfunction of subcomponents of the basal ganglia and thalamus in patients with ALS observed in this study and others (2-5,8-10,25-28). Further studies are required to resolve this issue.
Although our results indicate that all the components of the basal ganglia are affected in patients with ALS, the lentiform nucleus is slightly more affected than the head of the caudate nucleus (Table 1). The reasons for these discrepancies are not clear. Possible reasons include technical issues and the evolutionary origin (32) of these structures. The head of the caudate nucleus is adjacent to the inferior frontal lobe region, where residual susceptibility-induced magnetic field distortions commonly impact the magnetic field. This technical limitation may give rise to greater variation in linewidth between subjects, leading to more strongly scattered data, as noted by the higher standard deviation in the caudate compared with the lentiform nucleus or thalamus in both groups (Table 1). Phylogenetically, the caudate nucleus is derived from telencephalic structures and contains different cell types from the lentiform nucleus (32). The globus pallidus, similar to that of the thalamus, is also derived from the diencephalon (32). It is possible that neurons in the globus pallidus and thalamus are more susceptible than caudate neurons to disease processes. However, on the basis of the results of autopsy (2-5,33,34), neuroimaging (8-10,25-28) and the transgenic mouse model of ALS (29,30), the involvement of these subcortical structures in ALS is very variable.
Correlation between MRS metabolite metrics and motor function
The division of the motor system into pyramidal and extrapyramidal systems, a simple dichotomy, is not satisfactory, as several other brain structures, such as the red nucleus, motor nuclei of the brainstem and cerebellum, are known to mediate voluntary movements (32,35). The extrapyramidal and pyramidal systems are extensively interconnected and functionally cooperate in the control of movement (32,35). The impairment of fine rapid movements of various muscles may occur in the dysfunction of either system (15,16,32,35).
Measures of the extrapyramidal tract/pyramidal tract correlated more strongly with the basal ganglia metabolites (Table 2) than with the ALSFRS-R score, which correlated only with NAA/ Cho in the caudate. The reasons for these discrepancies are unclear. Although the ALSFRS-R score represents disability related to both the lower motor neuron and upper motor neuron, the extent of contribution from each component is very variable based on the results of clinicopathological and cliniconeurophysiological correlation studies in ALS (2,36-38).
Limitations
The sample size used in this exploratory study was small. MMSE was used to evaluate cognitive function, which is less sensitive in detecting subtle cognitive abnormality. Metabolite relaxation times (T1 and T2) were not measured in this study because of their lengthy measurement time, and so the observed metabolite concentration differences between the groups could not be ascribed specifically to changes in metabolite concentrations.
CONCLUSION
This study showed the presence of altered metabolite levels in the basal ganglia and thalamus in patients with ALS, indicating that these extramotor noncortical anatomical structures are also affected in the disease. The use of clinical tools sensitive and specific for the evaluation of extrapyramidal tract dysfunction may help to elicit subtle signs related to the abnormality of the basal ganglia in patients with ALS. Additional studies are necessary to evaluate whether the degree and extent of involvement of subcortical structures are similar to those of the extramotor cortex in patients with ALS, and whether the alterations in these extramotor structures are of primary or secondary origin.
Acknowledgements
This study was supported by the Stanley Glaser Foundation and National Institutes of Health Grant #R01 NS 060874 (to VG). The data processing software used was developed under RO1 EB000822. Kris Arheart kindly provided guidance for data analysis. Thanks are extended to Professor Walter G. Bradley for critical review of the manuscript, and Regina Menendez-Choy for help in the preparation of the manuscript.
Abbreviations used
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
ALS Functional Rating Scale-Revised
- Cho
choline
- Cre
creatine and phosphocreatine
- FVC
forced vital capacity
- MMSE
Mini-Mental State Examination
- NAA
N-acetylaspartate
- ROI
region of interest
- SPECT
single photon emission computed tomography
Footnotes
Presented as an Abstract in part at the 62nd American Academy of Neurology Meeting, 14th April 2010, Toronto, ON, Canada.
REFERENCES
- 1.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 2.Brownell B, Oppenheimer DR, Hughes JT. The central nervous system in motor neuron disease. J. Neurol. Neurosurg. Psychiatry. 1970;33:338–357. doi: 10.1136/jnnp.33.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato S, Oda M, Hayashi H. Neuropathology in amyotrophic lateral sclerosis patients on respirators: uniformity and diversity in 13 cases. Neuropathology. 1993;13:229–236. [Google Scholar]
- 4.Piao YS, Wakabayashi K, Kakita A, Yamada M, Hayashi S, Morita T, Ikuta F, Oyanagi K, Takahashi H. Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol. 2003;12:10–22. doi: 10.1111/j.1750-3639.2003.tb00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geser F, Brandmeir N, Linda K, Kwong L, Martinez-Lage M, Elman L, McCluskey L, Xie S, Lee V, Trojanowski J. Evidence for multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch. Neurol. 2008;65:636–641. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- 6.Ringholz G, Appel S, Bradshaw M, Cooke N, Mosnik D, Schultz PE. Prevalence and pattern of cognitive impairment in sporadic amyotrophic lateral sclerosis. Neurology. 2005;65:586–590. doi: 10.1212/01.wnl.0000172911.39167.b6. [DOI] [PubMed] [Google Scholar]
- 7.Rule R, Suhy J, Schuff N, Gelinas D, Miller RG, Weiner MW. Reduced NAA in motor and non-motor regions in amyotrophic lateral sclerosis: across-sectional and longitudinal study. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2004;5:141–149. doi: 10.1080/14660820410017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Chen Q, Yu B, Xue K, Luo C, Gong Q, He C, Zhou D, He L, Yao D. Structural and functional changes mapped in the brain of amyotrophic lateral sclerosis patients with/without dysphagia: pilot study. Amyotrophic Lateral Sclerosis. 2009;10:280–287. doi: 10.3109/17482960902893342. [DOI] [PubMed] [Google Scholar]
- 9.Agosta F, Goron-Tempini M, Pagani E, Sala S, Caputo D, Perini M, Bartolomei I, Fruguglietti M, Filippi M. Longitudinal assessment of grey matter contraction in amyotrophic lateral sclerosis: a tensor based morphometry study. Amyotrophic Lateral Sclerosis. 2009;10:168–174. doi: 10.1080/17482960802603841. [DOI] [PubMed] [Google Scholar]
- 10.Turner MR, Cagnin A, Turkheimer FE, Miller C, Shaw CE, Brooks DJ, Leigh PN, Banati RB. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C]-PK11195 positron emission tomography study. Neurobiol. Dis. 2004;15:601–609. doi: 10.1016/j.nbd.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Kalra S, Cashman NR, Genge A, Arnold DL. Recovery of N-acetylaspartate in corticomotor neurons of patients with ALS after riluzole therapy. NeuroReport. 1998;9:1757–1761. doi: 10.1097/00001756-199806010-00016. [DOI] [PubMed] [Google Scholar]
- 12.Pohl C, Block W, Karitzky J, Traber F, Schmidt S, Grothe C, Lamerichs R, Schild H, Klockgether T. Proton magnetic resonance spectroscopy of the motor cortex in 70 patients with amyotrophic lateral sclerosis. Arch. Neurol. 2001;58:729–735. doi: 10.1001/archneur.58.5.729. [DOI] [PubMed] [Google Scholar]
- 13.Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on motor neuron disease. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 14.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J. Neurol. Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 15.Agostino R, Berardelli A, Curra A, Accornero N, Manfrendi M. Clinical impairment of sequential finger movements in Parkinson’s disease. Mov. Disord. 1998;13:418–421. doi: 10.1002/mds.870130308. [DOI] [PubMed] [Google Scholar]
- 16.Norlinah IM, Bhatia KP, Ostergaard K, Howard R, Arabia G, Quinn N. Primary lateral sclerosis mimicking atypical parkinsonism. Mov. Disord. 2007;22:2057–2062. doi: 10.1002/mds.21645. [DOI] [PubMed] [Google Scholar]
- 17.Goetz CG, Tilley BC, Shaftman SR, Stebbins G, Fahn S, Martinez-Martin P, Poewe W, Sampaino C, Stern M, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang A, Lees A, Leurgans S, LeWitt P, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi J, van Hilten J, LaPelle N, UPDRS Revision Task Force Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 18.Mitsumoto H, Ulung A, Pullman S, Gooch C, Chan S, Tang M, Mao X, Hays A, Floyd A, Battista V, Montes J, Hayes S, Dashanaw S, Kaufmann P, Gordon P, Hirsch J, Levin B, Rowland LP, Shungu D. Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology. 2007;68:1402–1410. doi: 10.1212/01.wnl.0000260065.57832.87. [DOI] [PubMed] [Google Scholar]
- 19.Kasarskis EJ, Scarlata D, Hill R, Fuller C, Stambler N, Cedarbaum JM, BDNF Phase, III, and CNTF treatment study (ACTS) groups A retrospective study of percutaneous endoscopic gastrostomy in ALS patients during the BDNF and CNTF trials. J. Neurol. Sci. 1999;169:118–125. doi: 10.1016/s0022-510x(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein S, McHugh PR. Mini-mental state: a practical method for the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Govind V, Gold S, Kaliannan K, Saigal G, Falcone S, Arheart K, Harris L, Jagid J, Maudsley A. Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. J. Neurotrauma. 2010;27:1–14. doi: 10.1089/neu.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maudsley AA, Darkazanli A, Alger JR, Hall LO, Schuff N, Studholme C, Yu Y, Ebel A, Frew A, Goldgof D, Gu Y, Pagare R, Rousseau F, Sivasankaran K, Soher BJ, Weber P, Young K, Zhu X. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed. 2006;19:492–503. doi: 10.1002/nbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch. Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 24.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H, Snow BJ, Bhatt MH, Peppard R, Eisen A, Calne DB. Evidence for a dopaminergic deficit in sporadic amyotrophic lateral sclerosis on positron emission scanning. Lancet. 1993;342:1016–1018. doi: 10.1016/0140-6736(93)92878-w. [DOI] [PubMed] [Google Scholar]
- 26.Przedborsk S, Dhawan V, Donaldson DM, Murphy PL, McKenna-Yasek D, Mandel FS, Brown RH, Jr, Eidelberg D. Nigrostriatal dopaminergic function in familial amyotrophic lateral sclerosis patients with and without copper/zinc superoxide dismutase mutations. Neurology. 1996;47:1546–1551. doi: 10.1212/wnl.47.6.1546. [DOI] [PubMed] [Google Scholar]
- 27.Borasio GD, Linke R, Schwarz J, Schlamp V, Abel A, Mozley P, Tatsch K. Dopaminergic deficit in amyotrophic lateral sclerosis assessed with [I-123] IPT single photon emission computed tomography. J. Neurol. Neurosurg. Psychiatry. 1998;65:263–265. doi: 10.1136/jnnp.65.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogels OJ, Veltman J, Oyen WJ, Horstink MW. Decreased striatal dopamine D2 receptor binding in amyotrophic lateral sclerosis (ALS) and multisystem atrophy (MSA): D2 receptor down-regulation versus striatal cell degeneration. J. Neurol. Sci. 2000;180:62–65. doi: 10.1016/s0022-510x(00)00429-9. [DOI] [PubMed] [Google Scholar]
- 29.Kostic V, Gurney ME, Deng H, Siddique T, Epstein CJ, Przedborski S. Midbrain dopaminergic neuronal degeneration in a transgenic mouse model of familial amyotrophic lateral sclerosis. Ann. Neurol. 1997;41:497–504. doi: 10.1002/ana.410410413. [DOI] [PubMed] [Google Scholar]
- 30.Rathke-Hartlieb S, Schmidt VC, Jokusch H, Schmitt-John T, Bartsch JW. Spatiotemporal progression of neurodegeneration and glia activation in the wobbler neuropathy of the mouse. NeuroReport. 1999;10:3411–3416. doi: 10.1097/00001756-199911080-00028. [DOI] [PubMed] [Google Scholar]
- 31.Kiernan JA, Hudson AJ. Frontal lobe atrophy in motor neuron diseases. Brain. 1994;117:747–757. doi: 10.1093/brain/117.4.747. [DOI] [PubMed] [Google Scholar]
- 32.Cote L, Ciutcher M. Motor functions of the basal ganglia and diseases of transmitter metabolism. In: Kandle ER, Schwartz JH, editors. Principles of Neural Science. 2nd edn. Elsevier Science Publishing; New York: 1985. pp. 523–535. [Google Scholar]
- 33.Smith MC. Nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 1960;23:269–282. doi: 10.1136/jnnp.23.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orrell RW, King A, Hilton D, Campbell M, Lane R, de Belleroch Familial amyotrophic lateral sclerosis with point mutation of SOD-1: intrafamilial heterogeneity of disease duration associated with neurofibrillary tangles. J. Neurol. Neurosurg. Psychiatry. 1995;59:266–270. doi: 10.1136/jnnp.59.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidoff RA. The pyramidal tract. Neurology. 1990;40:332–339. doi: 10.1212/wnl.40.2.332. [DOI] [PubMed] [Google Scholar]
- 36.Ince PG, Evans J, Knopp M, Forster G, Hamdalla H, Wharton SB, Shaw PJ. Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology. 2003;60:1252–1258. doi: 10.1212/01.wnl.0000058901.75728.4e. [DOI] [PubMed] [Google Scholar]
- 37.Kaufmann P, Pullman S, Shungu D, Chan S, Hays A, Floyd A, Battista V, Montes J, Hayes S, Del Bene M, Dover M, Vukic M, Rowland LP, Mitsumoto H. Objective tests for upper motor neuron involvement in amyotrophic lateral sclerosis (ALS) Neurology. 2004;62:1753–1757. doi: 10.1212/01.wnl.0000125182.17874.59. [DOI] [PubMed] [Google Scholar]
- 38.Kent-Braun J, Walker CH, Weiner M, Miller RG. Functional significance of upper and lower motor neuron impairment in amyotrophic lateral sclerosis. Muscle Nerve. 1998;21:762–768. doi: 10.1002/(sici)1097-4598(199806)21:6<762::aid-mus8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]


