Abstract
Background
Microsatellite instability (MSI) is a marker of chemoresistance, but it is associated with improved survival when compared to microsatellite-stable (MSS) colon cancers. We hypothesized that MSI tumors over-express chemoresistance-associated genes and under-express DNA damage/repair genes. We used ultra high-throughput sequencing (UHTS) to assess the expression of representative genes in MSI and MSS colon cancer cell lines.
Methods
Solexa UHTS was used to examine gene expression in HCT116 (MSI) and HT29 (MSS) cells, and normal colonic mucosa (NCM). We compared expression of 40 genes involved in chemoresistance, DNA repair, DNA damage, and drug metabolism pathways.
Results
We observed gene expression differences between MSI and MSS cell lines in 8 out of 40 genes involved in mismatch repair (MMR), DNA repair, drug metabolism and chemoresistance. MMR gene expression was lower in MSI cells, which is consistent with the MSI phenotype, whereas DNA repair genes were highly expressed in these cells. Genes associated with chemoresistance and drug metabolism also had increased expression in MSI cells. No difference in expression of DNA damage genes was observed between MSI and MSS cell lines.
Conclusion
Using UHTS gene expression analysis, we identified differential expression of genes between MSI and MSS cell lines which may account for resistance to chemotherapy in MSI tumors. UHTS expression analysis has the potential to identify genome-wide predictors of response or resistance to chemotherapy.
Keywords: Microsatellite stable, microsatellite unstable, colon cancer, Solexa, gene expression, ultra-high throughput sequencing, DNA repair genes, DNA damage genes, 5-fluorouracil
Introduction
Approximately 15% of colorectal cancers are characterized with microsatellite instability (MSI) (1-11). These MSI colorectal tumors represent a distinct pathway of pathogenesis and genomic instability and are generally resistant to 5-fluorouracil (5-FU) based chemotherapy (6, 8-10, 12-15). This resistance has been attributed to deficient DNA mismatch repair (MMR) (8-10, 16). MSI tumors are typically located in the ascending colon with poor histological differentiation, mucin production, and lymphocytic infiltration (1, 6, 9, 12, 13). Although the clinical features of MSI tumors may be associated with a poor prognosis, these tumors are often associated with a better prognosis compared to microsatellite stable (MSS) tumors (2, 3, 6, 8, 9, 17). It is not known how deficiencies in the MMR pathway, which is a hallmark of MSI tumors, contribute to chemoresistance. Previous studies have focused on global patterns of gene expression rather than specific pathways that may contribute to treatment resistance, such as DNA repair pathways, DNA damage signaling, 5-FU drug metabolism, and chemoresistance genes (4, 5, 7).
Ultra high-throughput sequencing (UHTS) technology provides comprehensive transcriptome analysis, which can identify differences in gene expression by quantifying the exact number of gene transcripts present (18-27). Other methodologies, such as microarray assays only semi-quantitatively detect differences in gene expression levels (25, 26). Therefore, UHTS can accurately quantify levels of expression and characterize the expression of the entire genome, including genes of low abundance that cannot be quantified by microarray analysis.
Our objective was to identify differential gene expression in signaling pathways involved in DNA repair, DNA damage, 5-FU metabolism, and chemoresistance to identify potential genetic differences in chemotherapy-associated pathways between MSI and MSS colon cancer cells. We used the innovative Illumina Solexa expression assay which utilizes UHTS technology to compare RNA transcripts between MSI and MSS colon cancer cell lines to quantify gene expression levels and determine whether differences exist between MSI and MSS cells.
Methods
Cell lines and cultures
HCT116 and HT29 colon cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA), and normal colonic mucosa (NCM) cells were obtained from biopsy samples from normal colonic mucosa. All cell lines were grown in RPMI 1640 media and supplemented with 10% fetal bovine serum (FBS). Cell cultures were maintained at 37°C in an incubator with 5% CO2.
Colon cancer cells and exposure to 5-FU
Colon cancer cells (HCT116 and HT29) were seeded (3 × 103) in a 96-well plate. Cells were allowed to adhere overnight and then treated with 5-FU (1, 3, 5, and 10 μM). Experimental and untreated control cells were both maintained in growth medium supplemented with 10% FBS. Following exposure to 5-FU (24 and 48 h), colon cancer cells were lifted, counted, and assessed by the trypan blue exclusion method for cell viability. These assays were performed in triplicate.
DNA extraction and establishment of microsatellite status
Genomic DNA was extracted from colon cancer cell lines using the AllPrep DNA/RNA Mini Kit (Qiagen Inc., Chatsworth, CA). The microsatellite status of the cells was determined using five mononucleotide markers; BAT25, BAT26, NR21, NR24 and MONO27 (MSI Analysis System, Promega; Madison, WI). Each PCR reaction contained 7.85 μL nuclease free water, 1 μL GoldSTAR 10× Buffer, 1 μL 10× multiplex primer mix, and 0.15 μL AmpliTaq Gold DNA Polymerase. Lastly, 2μl of sample DNA was added for a total reaction volume of 12 μL.
Genotyping was performed with the ABI 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA) and data analysis was performed using GeneMapper software (Applied Biosystems). Tumors were classified as either microsatellite unstable (MSI) or microsatellite stable (MSS) as per standard definitions outlined by Boland et al. (1).
RNA isolation and Solexa sequencing
Total RNA was isolated from HCT116 and HT29 cell lines, and NCM cells using the QIAgen RNAeasy Mini Kit (Qiagen). RNA extracts were submitted for transcriptome analysis by Solexa sequencing (Illumina Inc., San Diego, CA). Genome-wide transcriptome analysis was performed by UHTS to quantitatively measure gene expression levels. Solexa sequencing was performed on HCT116 and HT29 cells, and NCM cells. A transcriptome library was compiled for all genes expressed in each cell line. We further assessed genes associated with DNA repair, DNA damage, drug metabolism, and chemoresistance.
Statistical analysis
Expression levels of genes associated with DNA repair, DNA damage, drug metabolism and chemoresistance in HCT116 and HT29 cells were compared to NCM cells. The fold-changes in gene expression in colon cancer cells compared to NCM cells were quantified as a log2 ratio (expression in cancer cells/expression in NCM cells). Subsequently, gene expression cut-offs were designated as high (> 2-fold increase) or low (< 0.5-fold increase) expression compared to NCM cells, as determined by prior publication (28). These cut-offs for evaluating gene expression have been shown to be appropriate for detecting statistically significant differences (P<0.05) in gene expression.
Results
Confirmation of MSS or MSI genotype
The genotype of each cell line was first determined. HCT116 cells were confirmed to have the MSI genotype by utilizing the panel of five markers, and HT29 was determined to be MSS. Two populations of NCM cells were also assessed for MSI, and confirmed to have the MSS genotype.
Colon cancer cells and resistance to 5-FU
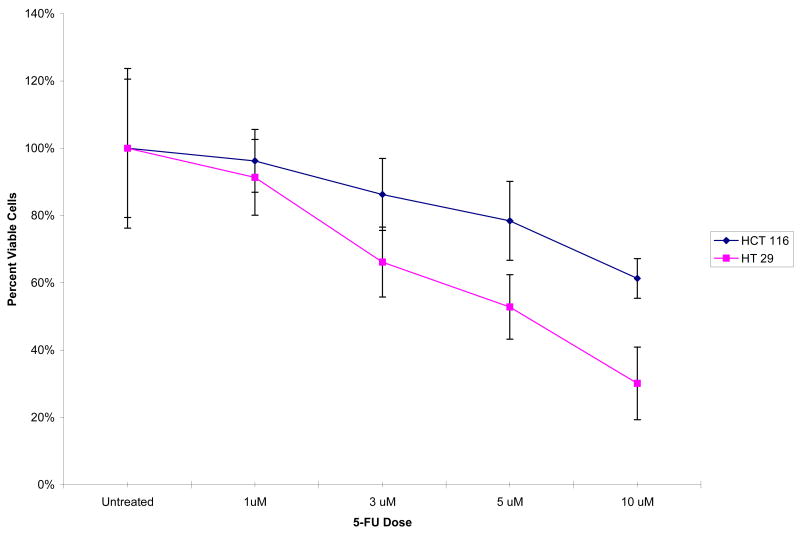
Cell viability for colon cancer cells (HCT116 and HT29) was assessed following exposure to 5-FU in vitro. After 24 h of exposure with 5-FU, we observed no difference in the number of viable cells between HCT116 and HT29 cell lines. After 48 h, there was significantly increased cell death in HT29 cells compared to HCT116 cells (70% versus 39%) (Figure 1). These results indicate that HCT116 colon cancer cells are more resistant to 5-FU treatment than HT29 colon cancer cells.
Figure 1. Cell viability after treatment with 5-FU for 48 hours.
Viable cells present in HCT116 and HT29 cells after treatment with 5-FU for 48 hours.
5-Fluorouracil Metabolism Genes
Clinically, MSI tumors do not respond to 5-FU based chemotherapy; as such, we examined genes involved in 5-FU metabolism in HCT116 and HT29 cells (Table 1). Of the genes involved in 5-FU metabolism, the uridine phosphorylase 1 (UPP1) gene showed increased expression in HCT116 cells and decreased expression in HT29 cells compared to NCM cells. In contrast, no differences in the expression levels of 5-FU metabolism genes, such as thymidylate synthetase (TYMS), dihydrofolate reductase (DHFR), dihydropyrimidine dehydrogenase (DPYD), thymidine phosphorylase (TYMP), and uridine monosphosphate synthetase (UMPS) were observed between HCT116 and HT29 cells when compared to NCM cells.
Table 1. Expression Levels of Genes Involved in Drug Metabolism of 5-Fluorouracil Chemotherapy.
| Gene | HCT 116 | HT 29 |
|---|---|---|
| DPYD | ↓ | ↓ |
| TYMP | ↓ | ↓ |
| TYMS | ↑ | ↑ |
| DHFR | ↑ | ↑ |
| UMPS | ↑ | ↑ |
| UPP1 | ↑ | ↓ |
↑= Expression level > 2× normal colonic mucosal cells; ↓ = Expression level < ½ normal colonic mucosal cells.
Mismatch Repair Genes
MSI tumors are characterized by their deficiency in DNA mismatch repair. We examined the expression of mismatch repair (MMR) genes: MLH 1 and 3, MSH 2, 3 and 6, and PMS2 (Table 2). The expression of MLH1 in HCT116 cells was down-regulated compared to HT29 cells, which is consistent with previous studies (29). The relative expression of the MSH3 gene was also decreased in HCT116 cells but was elevated in HT29 cells. Expression levels of MSH2, MSH6, and PMS2 were similarly increased in both HCT116 and HT 29 cells.
Table 2. Expression Levels of DNA Mismatch Repair Genes.
| Genes | HCT 116 | HT 29 |
|---|---|---|
| MLH1 | ↔ | ↑ |
| MLH3 | ↔ | ↔ |
| MSH2 | ↑ | ↑ |
| MSH3 | ↓ | ↑ |
| MSH6 | ↑ | ↑ |
| PMS2 | ↑ | ↑ |
↑= Expression level > 2× normal colonic mucosal cells; ↓ = Expression level < ½ normal colonic mucosal cells; ↔ = Expression level equal to normal colonic mucosal cells.
DNA Repair Genes
By definition, MSI tumors lack the ability to repair mismatches in DNA base-pairing, and thus alternative DNA repair pathways instead may contribute to treatment resistance. DNA repair genes were further analyzed in our study (Table 3). X-ray repair cross-complementing protein 1 (XRCC1) is a gene involved in base excision repair (BER) and functions to repair DNA single stranded breaks introduced by anti-metabolite based chemotherapies, such as 5-FU (30-33). The expression levels of XRCC1 were highly elevated in both HCT116 and HT29 cells compared to NCM cells. Other genes associated with BER, such as DNA ligase 3 (LIG3), also showed increased expression in HCT116 and HT29 cell lines (Table 3).
Table 3. Expression Levels of DNA Repair Genes.
| Repair Pathway | Gene | HCT 116 | HT 29 |
|---|---|---|---|
| BER | XRCC1 | ↑ | ↑ |
| LIG3 | ↑ | ↑ | |
| NER | ERCC1 | ↑ | ↔ |
| ERCC2 | ↑ | ↑ | |
| ERCC3 | ↑ | ↑ | |
| ERCC4 | ↑ | ↔ | |
| ERCC5 | ↔ | ↔ | |
| ERCC6 | ↑ | ↔ | |
| XPA | ↑ | ↑ | |
| XPC | ↑ | ↑ | |
| HR / NHEJ | XRCC2 | ↑ | ↑ |
| XRCC3 | ↑ | ↑ | |
| XRCC4 | ↑ | ↑ | |
| XRCC6 | ↑ | ↑ | |
| ATM | ↑ | ↑ | |
| RAD51 | ↑ | ↑ | |
| BRCA1 | ↑ | ↑ |
Abbreviations: BER, Base excision repair; NER, Nucleotide excision repair; HR, Homologous recombination; NHEJ, Non-homologous end joining.
↑= Expression level > 2× normal colonic mucosal cells; ↓ = Expression level < ½ normal colonic mucosal cells; ↔ = Expression level equal to normal colonic mucosal cells.
Additional DNA repair pathways were also examined. Nucleotide excision repair (NER) is responsible for removing damaged or replaced nucleotides; (31-33) thus, genes associated with NER were examined (Table 3). Excision repair cross-complementing group (ERCC) 1, 4 and 6 showed increased expression in HCT116 cells compared to NCM cells. This contrasts levels observed in HT29 cells, which demonstrated similar expression levels to NCM cells. However, not all genes involved in NER showed differences in expression levels between HCT116 and HT29 cells. The pattern of expression levels of ERCC2, 3, 5, and xeroderma pigmentosum complementation group A and C (XPA and XPC) in HCT116 and HT29 cells were similar when each was compared to NCM cells.
Platinum-based chemotherapy, including agents such as oxaliplatin, and topoisomerase inhibitors, such as irinotecan, cause DNA double-stranded breaks that are repaired mainly by two pathways; homologous recombination (HR) and non-homologous end joining (NHEJ) (31-33). DNA repair genes involved in HR and NHEJ pathway, such as XRCC 2, 3, 4 and 6, were highly expressed in both HCT116 and HT29 cells compared to NCM cells (Table 3). DNA repair protein RAD51 (RAD51) and serine-protein kinase ATM (ATM) expression levels in HCT116 and HT29 were also elevated compared to NCM cells. Breast cancer 1 gene (BRCA1) which also participates in DNA damage signaling (34) showed increased expression levels in both HCT116 and HT29 cells.
Genes Associated with DNA Damage
The function of most chemotherapeutic regimens is to cause DNA damage which leads to cell death (32, 33). Seven genes involved in signaling DNA damage response showed elevated expression in both HCT116 and HT29 cells in comparison to NCM cells (Table 4). Serine/threonine-protein kinase ATR (ATR) is associated with a DNA damage response pathway that involves genes such as checkpoint homolog 1 (CHEK1) and retinoblastoma binding protein 8 (RBBP8). Two genes which encode proteins that interact with p53, tumor suppressor p53-binding protein 3 (TOPORS) and cell division cycle 25 homolog C (CDC25C), were also highly expressed in HCT116 and HT29 cells. In addition, expression of RAP1 interacting factor homolog 1 (RIF1) and structural maintenance of chromosomes 1A (SMC1A) genes, which encode proteins that interact with DNA, were increased in both cell lines compared to NCM cells. Although expression of genes responsible for DNA damage signaling was up-regulated in comparison to normal cells, no difference in expression was observed between MSI and MSS cell lines.
Table 4. Expression Levels of DNA Damage Signaling Genes.
| Gene | HCT 116 | HT 29 |
|---|---|---|
| RBBP8 | ↑ | ↑ |
| CDC25C | ↑ | ↑ |
| RIF1 | ↑ | ↑ |
| SMC1A | ↑ | ↑ |
| TOPORS | ↑ | ↑ |
| ATR | ↑ | ↑ |
| CHEK1 | ↑ | ↑ |
↑= Expression level > 2× normal colonic mucosal cells; ↓ = Expression level < ½ normal colonic mucosal cells.
Chemoresistance Genes
Differences in gene expression of chemoresistance-associated genes may explain the clinical behavior observed in MSI cells. HCT116 cells showed differential expression of two genes involved in resistance to chemotherapy (Table 5). ATP-binding cassette subfamily C2 (ABCC2) and hypoxia inducible factor 1 alpha (HIF-1A) showed greater than 2-fold increased expression in HCT116 cells compared to NCM cells. HT29 cells showed similar expression levels of ABCC2 and HIF-1A genes as NCM cells. Expression levels of ATP-binding cassette subfamily B1 (ABCB1) and carboxylesterase 1 (CES1) were decreased in both HCT116 and HT29 cells compared to NCM cells. These genes have also been implicated in resistance to chemotherapy (35-38).
Table 5. Expression Levels of Chemoresistance Genes.
| Gene | HCT 116 | HT 29 |
|---|---|---|
| ABCB1 | ↓ | ↓ |
| ABCC2 | ↑ | ↔ |
| CES2 | ↓ | ↓ |
| HIF1A | ↑ | ↔ |
↑= Expression level > 2× normal colonic mucosal cells; ↓ = Expression level < ½ normal colonic mucosal cells; ↔ = Expression level equal to normal colonic mucosal cells.
Discussion
Utilizing UHTS, we demonstrated that several pathway-specific genes are differentially expressed between two established colon cancer cell lines. Our observations show that differential expression of genes involved in DNA repair pathways and chemoresistance genes are consistent with the known clinical phenotype of MSI tumors (7, 10, 16, 33, 37, 39, 40). As such, UHTS is a powerful technique which can characterize tumors by their unique gene expression profile. Such comprehensive data can be utilized to further characterize candidate genes of treatment response or chemoresistance.
UHTS technology overcomes the limitations of microarrays, and genome-wide analyses of gene expression in tumor cells can now be performed to examine precise changes in levels of expression. UHTS directly quantifies the abundance of gene transcripts through next generation sequencing. This is advantageous as it avoids the high background noise resulting from non-specific probe hybridization present in microarray analysis, and allows for the sensitive detection of genes with low expression. Further uses of UHTS technology have been employed to identify unique transcription factor binding sites, detect areas of DNA methylation, and analyze the expression of small RNAs (20, 22, 24-26, 35). In our study, we utilized a novel technology to perform a focused examination of pathways and genes that may contribute to chemoresistance, in order to characterize the clinical phenotype observed in MSI tumors.
Two possible mechanisms can contribute to resistance to chemotherapy; direct or indirect. Direct pathways include mechanisms that process the chemotherapeutic agent. These typically involve drug metabolism pathways and mechanisms that influence drug delivery. Indirect pathways involve processes that respond to the effects of chemotherapeutic agents, such as DNA repair and DNA damage pathways. Both mechanisms may contribute to how MSI tumors fail to respond to 5-FU based chemotherapy. Here, we demonstrated a differential expression pattern for genes involved in 5-FU metabolism and chemoresistance between MSI and MSS tumors, including UPP1. Our results are inconsistent with prior studies which suggest a role for UPP1 in promoting the cytotoxic effects of 5-FU (41-44). Cao et al. demonstrated that the induction of UPP1 enhances the cytotoxic effects of the 5-FU pro-drug 5′-deoxy-5-fluorouridine (41). Therefore, the resistance to 5-FU observed with MSI tumors may in fact be unrelated to UPP1. We also observed that MSI tumors have increased expression of two genes, HIF-1A and ABCC2, which may influence the delivery of 5-FU to MSI tumors. Up-regulation of HIF-1A induces expression of drug efflux transporters, alters activity of DNA repair pathways, and promotes cell survival, which jointly counter the effects of chemotherapy (40). Recent studies also demonstrated the role of ABC transporters in multi-drug resistance (38). Currently, no clinical trials analyzing tumors with the MSI genotype have determined whether these tumors have increased levels of HIF-1A or ABCC2. However, we suspect that the expression of these genes may underlie, at least in part, the observed chemoresistance of MSI tumors.
MSI tumors have deficient MMR pathways. It is not yet clear as to how decreased MMR function results in 5-FU resistance. We demonstrated differential expression of alternate DNA pathways, particularly the NER pathway. The NER DNA repair pathway may play a larger role than other DNA repair pathways such as NHEJ and HR in reversing the damage caused by 5-FU. We showed that HCT116 cells have increased expression of genes involved in NER when compared to NCM cells. DNA damage signaling can mediate repair pathways, inhibit cell cycle progression, and signal apoptosis. No distinction between MSI and MSS cell lines could be found from the differential expression of these DNA damage signaling genes, suggesting that these genes may not contribute to the chemoresistance observed in MSI tumors.
Microsatellite instability is one of the earliest described molecular alterations in colorectal cancer (3, 12, 13). We showed that HCT116 cells, which harbor the MSI genotype, have differential expression of genes associated with DNA repair pathways, chemoresistance genes, and 5-FU metabolism. These studies demonstrate the utility of UHTS for detecting pharmacogenetic differences in cancer cells. This technology provides a powerful approach to better understand genes involved in chemotherapeutic response and resistance and could be used to investigate other MSI and MSS cell lines to identify additional genes and signaling pathways related to 5-FU-based therapies. Ultimately, this technology may lead to optimized and individualized therapy for patients with rectal cancer.
Acknowledgments
The authors thank Nicola Solomon, Ph.D., for assistance in writing and editing the manuscript. This study was supported by the National Institutes of Health (NIH), National Cancer Institute (NCI) R01 Grant CA090559 (JGA). ClinicalTrials.org Identifier: NCT00335816.
Footnotes
Meeting Presentation: Presented at the 6th Annual Academic Surgical Congress, February 2nd, 2011, Huntington Beach, California.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, R-B MA, Fodde R, Ranzani GN, Srivastava S. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 2.Choi SW, Lee KJ, Bae YA, Min KO, Kwon MS, Kim KM, Rhyu MG. Genetic classification of colorectal cancer based on chromosomal loss and microsatellite instability predicts survival. Clin Cancer Res. 2002;8:2311–2322. [PubMed] [Google Scholar]
- 3.de la Chapelle A, Hampel H. Clinical relevance of microsatellite instability in colorectal cancer. J Clin Oncol. 2010;28:3380–3387. doi: 10.1200/JCO.2009.27.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunican DS, McWilliam P, Tighe O, Parle McDermott A, Croke DT. Gene expression differences between the microsatellite instability (MIN) and chromosomal instability (CIN) phenotypes in colorectal cancer revealed by high-density cDNA array hybridization. Oncogene. 2002;21:3253–3257. doi: 10.1038/sj.onc.1205431. [DOI] [PubMed] [Google Scholar]
- 5.Duval A, Hamelin R. Genetic instability in human mismatch repair deficient cancers. Ann Genet. 2002;45:71–75. doi: 10.1016/s0003-3995(02)01115-2. [DOI] [PubMed] [Google Scholar]
- 6.Hemminki A, Mecklin JP, Järvinen H, et al. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology. 2000;119:921–928. doi: 10.1053/gast.2000.18161. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Nam SW, Rhee H, Li LS, Kang HJ, Koh KH, Kim NK, Song J, Liu ET, Kim H. Different gene expression profiles between microsatellite instability-high and microsatellite stable colorectal carcinomas. Oncogene. 2004;23:6218–6225. doi: 10.1038/sj.onc.1207853. [DOI] [PubMed] [Google Scholar]
- 8.Ng K, Schrag D. Microsatellite instability and adjuvant fluorouracil chemotherapy: A mismatch? J Clin Oncol. 2010;28:3207–3210. doi: 10.1200/JCO.2010.28.9314. [DOI] [PubMed] [Google Scholar]
- 9.Ribic C, Sargent DJ, Moore M, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shephard LE, Tu D, Redston M, Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, Ribic C, Grothey A, Moore M, Zaniboni A, Seitz J, Sinicrope F, Gallinger S. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-The stable evidence. Nat Rev Clin Oncol. 2010;7:153–162. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 13.Shibata D, Peinado MA, Ionov Y, Malkhosyan S, Perucho M. Genomic instability in repeated sequences is an early somatic event in colorectal tumorigenesis that persists after transformation. Nat Genet. 1994;6:273–281. doi: 10.1038/ng0394-273. [DOI] [PubMed] [Google Scholar]
- 14.Gustavsson B, Kaiser C, Carlsson G, Wettergren Y, Odin E, Lindskog EB, Niyikiza C, Ma D. Molecular determinants of efficacy for 5-FU-based treatments in advanced colorectal cancer: mRNA expression for 18 chemotherapy-related genes. Int J Cancer. 2009;124:1220–1226. doi: 10.1002/ijc.23852. [DOI] [PubMed] [Google Scholar]
- 15.Kim ST, Lee J, Park SH, Park JO, Lim HY, Kang WK, Kim JY, Kim YH, Chang DK, Rhee P, Kim DS, Yun H, Cho YB, Kim HC, Yun SH, Lee WY, Chun H, Park YS. Clinical impact of microsatellite instability in colon cancer following adjuvant FOLFOX therapy. Cancer Chemother Pharmacol. 2010;66:659–667. doi: 10.1007/s00280-009-1206-3. [DOI] [PubMed] [Google Scholar]
- 16.Tajima A, Hess MT, Cabrera BL, Kolodner RD, Carethers JM. The mismatch repair complex hMutS-alpha recognizes 5-fluorouracil-modified DNA: Implications for chemosensitivity and resistance. Gastroenterology. 2004;127:1678–1684. doi: 10.1053/j.gastro.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 18.Agaton C, Unneberg P, Sievertzon M, Holmberg A, Ehn M, Larsson M, Odeberg J, Uhlen M, Lundeberg J. Gene expression analysis by signature pyrosequencing. Gene. 2002;289:31–39. doi: 10.1016/s0378-1119(02)00548-6. [DOI] [PubMed] [Google Scholar]
- 19.Armour CD, Castle JC, Chen R, Babak T, Loerch P, Jackson S, Shah JK, Dey J, Rohl CA, Johnson JM, Raymond CK. Digital transcriptome profiling using selective hexamer priming for cDNA synthesis. Nat Methods. 2009;6:647–649. doi: 10.1038/nmeth.1360. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh Y, Yeo HC, Yeo XY, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung W, Clarke ND, Wei C, Ng H. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Chiang DY, Getz G, Jaffe DB, O'Kelly MJT, Zhao X, Carter SL, Russ C, Nusbaum C, Meyerson M, Lander ES. High-resolution mapping of copy-number alterations with massively parallel sequencing. Nat Methods. 2009;6:99–103. doi: 10.1038/nmeth.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox S, Filichkin S, Mockler TC. Applications of ultra-high-throughput sequencing. Methods Mol Biol. 2009;553:79–108. doi: 10.1007/978-1-60327-563-7_5. [DOI] [PubMed] [Google Scholar]
- 23.Marioni JC, Mason CE, Mane SM. RNA-seq: An assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008;18:1509–1517. doi: 10.1101/gr.079558.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 25.Hoen PA, Ariyurek Y, Thygesen HH, Vreugdenhil E, Vossen RH, de Menezes RX, Boer JM, van Ommen GJ, den Dunnen JT. Deep sequencing-based expression analysis shows major advances in robustness, resolution and inter-lab portability over five microarray platforms. Nucleic Acids Res. 2008;36:e141. doi: 10.1093/nar/gkn705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wall PK, Leebens-Mack J, Chanderbali AS, Barakat A, Wolcott E, Liang H, Landherr L, Tomsho LP, Hu Y, Carlson JE, Ma H, Schuster SC, Soltis DE, Soltis PS, Altman N, dePamphilis CW. Comparison of next generation sequencing technologies for transcriptome characterization. BMC Genomics. 2009;10:347. doi: 10.1186/1471-2164-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou D, Rao MS, Walker R, Khrebtukova I, Haudenschild CD, Miura T, Decola S, Vermaas E, Moon K, Vasicek TJ. Massively parallel signature sequencing. Methods Mol Biol. 2006;331:285–311. doi: 10.1385/1-59745-046-4:285. [DOI] [PubMed] [Google Scholar]
- 28.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article 3. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos N, Nicolaides NC, Wei YF, Ruben SM, Carter KC, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM, Adams MD, Venter JC, Hamilton SR, Peterson GM, Watson P, Lynch HT, Peltomaki P, Mecklin J, de-la-Chapelle A, Kinzler KW, Vogelstein B. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994;263:1559–1560. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- 30.David SS, O'Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoeijmakers JHJ. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 32.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miquel C, Jacob S, Grandjouan S, Aime A, Viguier J, Sabourin J, Sarasin A, Duval A, Praz F. Frequent alteration of DNA damage signaling and repair pathways in human colorectal cancers with microsatellite instability. Oncogene. 2007;26:5919–5926. doi: 10.1038/sj.onc.1210419. [DOI] [PubMed] [Google Scholar]
- 34.Scully R, Ganesan S, Vlasakova K, Chen J, Socolovsky M, Livingston DM. Genetic analysis of BRCA1 function in a defined tumor cell line. Mol Cells. 1999;4:1093–1099. doi: 10.1016/s1097-2765(00)80238-5. [DOI] [PubMed] [Google Scholar]
- 35.Halapi E, Hakonarson H. Advances in the development of genetic markers for the diagnosis of disease and drug response. Expert Rev Mol Diagn. 2002;2:411–421. doi: 10.1586/14737159.2.5.411. [DOI] [PubMed] [Google Scholar]
- 36.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 37.Pare L, Marcuello E, Altes A, del Rio E, Sedano L, Salazar J, Cortes A, Barnadas A, Baiget M. Pharmacogenetic prediction of clinical outcome in advanced colorectal cancer patients receiving oxaliplatin/5-fluorouracil as first-line chemotherapy. Br J Cancer. 2008;99:1050–1055. doi: 10.1038/sj.bjc.6604671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theile D, Grebhardt S, Haefeli WE, Weiss J. Involvement of drug transporters in the synergistic action of FOLFOX combination chemotherapy. Biochem Pharmacol. 2009;78:1366–1373. doi: 10.1016/j.bcp.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Popat S, Wort R, Houlston RS. Inter-relationship between microsatellite instability, thymidylate synthase expression, and p53 status in colorectal cancer: Implications for chemoresistance. BMC Cancer. 2006;6:150. doi: 10.1186/1471-2407-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravizza R, Molteni R, Gariboldi MB, Marras E, Perletti G, Monti E. Effect of HIF-1 modulation on the response of two-and three-dimensional cultures of human colon cancer cells to 5-fluorouracil. Eur J Cancer. 2009;45:890–898. doi: 10.1016/j.ejca.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Cao D, Pizzorno G. Uridine phosphorylase: An important enzyme in pyrimidine metabolism and fluoropyrimidine activation. Drugs Today (Barc) 2004;40:431–443. doi: 10.1358/dot.2004.40.5.850491. [DOI] [PubMed] [Google Scholar]
- 42.Im Y, Shin HK, Kim H, Jeong S, Kim S, Kim Y, Lee DH, Jeon S, Lee H, Choi J. Enhanced cytotoxicity of 5-FU by bFGF through up-regulation of uridine phosphorylase 1. Mol Cells. 2009;28:119–124. doi: 10.1007/s10059-009-0116-x. [DOI] [PubMed] [Google Scholar]
- 43.Liu M, Cao D, Russell R, Handschumacher RE, Pizzorno G. Expression, characterization, and detection of human uridine phosphorylase and identification of variant uridine phosphorylitic activity in selected human tumors. Cancer Res. 1998;58:5418–5424. [PubMed] [Google Scholar]
- 44.Wan L, Cao D, Zeng J, Yan R, Pizzorno G. Modulation of uridine phosphorylase gene expression by tumor necrosis factor-alpha enhances the antiproliferative activity of the capecitabine intermediate 5′-deoxy-5-fluorouridine in breast cancer cells. Mol Pharmacol. 2006;69:1389–1395. doi: 10.1124/mol.105.018515. [DOI] [PubMed] [Google Scholar]