Abstract
Patients with bipolar disorder and schizophrenia often show decision-making deficits in everyday circumstances. A failure to appropriately weigh immediate versus future consequences of choices may contribute to these deficits. We used the delay discounting task in individuals with bipolar disorder (BD) or schizophrenia (SZ) to investigate their temporal decision-making. Twenty-two individuals with BD, 21 individuals with SZ and 31 healthy individuals completed the delay discounting task along with neuropsychological measures of working memory and cognitive function. Both BD and SZ groups discounted delayed rewards more steeply than the healthy group even after controlling for current substance use, age, gender, and employment. Hierarchical multiple regression analyses showed that discounting rate was associated both with diagnostic group and working memory/intelligence composite scores. In each group, working memory or intelligence scores negatively correlated with discounting rate. The results suggest that 1) both BD and SZ groups value smaller, immediate rewards more than larger, delayed rewards compared to the healthy group and 2) working memory or intelligence is related to temporal decision-making in individuals with BD or SZ as well as in healthy individuals.
Keywords: temporal discounting, bipolar disorder, schizophrenia, working memory, intelligence
Bipolar Disorder (BD) and schizophrenia (SZ) are associated with impairments of decision-making and behavioral control in everyday circumstances. These deficits frequently suggest impaired reward processing and problems with self-control (Eichhorst et al., 2006; Nieuwenstein, Aleman, & de Haan, 2001). Laboratory tasks of decision-making have been utilized to better characterize the cognitive and motivational processes responsible for impaired decision-making. Such tasks typically involve cognitive and motivational processes including evaluation of experienced and expected outcomes and learning from the consequences of prior decisions.
Several studies demonstrated impaired decision-making performance in individuals with BD in different phases of the disorder. Individuals in the euthymic (Gorrindo et al., 2005) or depressed (Roiser et al., 2009) episode of BD showed difficulty maintaining the correct contingency after negative feedback (i.e., high “maintenance errors”) in the Probabilistic Reversal Learning (PRL) task. However, the high maintenance errors were observed only during the reversal phase, suggesting their deficits are specific to the difficulty adapting to changing environment. A recent study concluded that individuals in the depressed phase of BD showed equivalent performance to healthy individuals in the PRL task, but PRL performance measures used in the study were not specific to the reversal phase, which makes the comparison between studies difficult (Taylor Tavares et al., 2008). Previous studies using the Cambridge Gambling Task (CGT) suggest that individuals with BD may perform poorly on that task as well. Murphy et al. (2001) showed that individuals in a manic episode of BD preferred favorable outcomes less than healthy individuals (i.e., poorer “decision quality”), whereas decision quality among individuals in a depressed phase of BD was equivalent to that of healthy individuals. Meanwhile, individuals in both manic and depressed phases of BD showed suboptimal betting strategies compared to healthy individuals. Interestingly, severity of illness inversely correlated with decision quality only in individuals in a manic episode. Roiser and colleagues (Roiser, et al., 2009) recently tested unmedicated patients in a depressed episode of BD type II on the CGT and replicated the finding of Murphy and colleagues (i.e., intact decision quality and suboptimal betting strategies among individuals in a depressed episode of BD). In contrast, Rubinsztein and colleagues found that individuals with BD type I had poorer decision quality but equivalent betting strategies (Rubinsztein, Michael, Underwood, Tempest, & Sahakian, 2006). The Iowa Gambling Task (IGT) has been also widely used in individuals with BD, and ample evidence suggests that IGT performance is mood state dependent. While individuals in a manic episode of BD showed impaired performance on the IGT as indicated by fewer choices from good decks than healthy individuals (Clark, Iversen, & Goodwin, 2001), individuals in a euthymic or remitted phase of BD performed equally well as healthy individuals (Andreasen, 1989; Brzezinski, Abraham, Stone, Dean, & Bosron, 1994; S. P. Stone, Herbert, Chrisostomou, Vessey, & Horwood, 1993; Yechiam, Hayden, Bodkins, O'Donnell, & Hetrick, 2008). Finally in a two-choice prediction task, individuals with BD type I showed increased sensitivity to error rate, indicated by higher shifting rate in the BD group (Minassian, Paulus, & Perry, 2004).
Other decision-making studies demonstrated impaired performance in individuals with SZ. A meta-analysis of IGT studies with individuals with SZ showed that their task performance was usually impaired in that group (Sevy et al., 2007). Heerey and colleagues used the reward sensitivity task (Pizzagalli, Jahn, & O'Shea, 2005) and a modified CGT to examine whether decision-making impairment in SZ is due to their reduced reward sensitivity or their deficit in integrating cognitive and affective information for optimal choices. They found that reward sensitivity was intact in SZ, but the ability to weigh potential losses was poorer than in healthy individuals (Heerey, Bell-Warren, & Gold, 2008). Consistent with these findings, individuals with SZ also showed a reduced loss aversion when tested with buying and selling scenario paradigms (Tremeau et al., 2008). In the PRL task, similar to individuals with BD, individuals with SZ achieved significantly fewer reversals compared to healthy individuals, but again only during the reversal phase (G. Schweighofer & Pinz, 2006). Gold et al. (2008) reviewed recent decision-making studies in SZ and concluded that emotional processing and gradual habit learning systems might be intact in SZ. However, they also argued that SZ was associated with deficits in representing and integrating information about multiple options and in rapid reinforcement learning systems. Previous research also suggests that the negative symptoms in SZ play an important role in impaired reward processing. For example, behavioral performance and negative symptoms showed an inverse relationship in previous studies (Polgár et al., 2008; Waltz, Frank, Robinson, & Gold, 2007) when measured by the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1989). In addition, some functional magnetic resonance imaging (fMRI) studies showed that individuals with SZ showed reduced activation of the ventral striatum, a key region encoding expected reward, during reward anticipation and the reduction correlated with overall negative symptoms (Juckel et al., 2006) or apathy (Simon et al., 2010). Waltz et al (2006) also showed that negative symptoms correlated with activity in several brain regions during outcome anticipation and evaluation.
One limitation of some of the previous studies in the context of BD or SZ is that most decision-making tasks probe a multiplicity of processes, such as sensitivity to rewards and losses, error processing, learning, and sensitivity to varying event probabilities. Thus, it may be difficult to delineate the cognitive mechanisms responsible for specific decision-making deficits in individuals with BD or SZ. In this paper, we used the delay discounting task to assess the ability to decide between immediate versus future rewards, which has been little studied in BD and SZ.
The delay discounting task (Rachlin, Raineri, & Cross, 1991) is specifically designed to assess how individuals make tradeoffs between immediately-available small rewards versus large but delayed rewards. The task consists of a series of choices between a larger but delayed reward (e.g., $500 in a year) and a smaller but immediate reward (e.g., $100 now). From these choices, an individual’s discounting rate can be estimated, which measures the weight an individual gives to future consequences. Error processing and learning play a minimal role in performance because participants do not receive feedback. This task targets the processes of self-control and consideration for future consequences, which are necessary for adaptive decision-making. Furthermore, the ability to weigh immediate and future rewards is important performance in widely used tasks including the IGT and the PRL that involve learning. A previous study indeed observed a significant correlation between the IGT performance and discounting rate in individuals with cocaine dependence, suggesting temporal discounting is an overlapping construct in the IGT (Monterosso, Ehrman, Napier, O'Brien, & Childress, 2001). The use of the delay discounting task might isolate a specific parameter which influences decision-making deficits in individuals with BD or SZ.
Several factors could influence temporal discounting in SZ and BD, including cognitive capabilities, the personality trait of impulsivity, and current or past history of substance use disorders. Higher discounting rates, indicating greater preference for smaller, immediately-available rewards, are correlated with decreased general intelligence (IQ) (Shamosh & Gray, 2007) and working memory (WM) (Hinson, Jameson, & Whitney, 2003; Shamosh et al., 2008). For optimal decision-making on the task, participants need to integrate multiple features (e.g., reward amount and delays) of immediate and delayed rewards, which imposes WM demands (Hinson, et al., 2003). In addition, several studies have reported higher discounting rates among individuals who abuse or are dependent upon substances, including heroin and cocaine (Kirby & Petry, 2004), alcohol (Petry, 2001), and tobacco(Bickel, Odum, & Madden, 1999), compared to healthy individuals.
Impulsivity might also contribute to individual differences in temporal discounting rates in addition to cognitive or WM deficits, which might constrain the ability to conceptualize future outcomes. Both BD and SZ have been associated with increased impulsivity relative to healthy individuals. Mania, which is the defining phase of BD, is characterized by impulsive behavior, including impulsive spending, substance abuse, and risky sex (Swann, 2009). Marked impulsivity has been noted in individuals with BD during both the symptomatic (i.e., manic or depressed) and euthymic phases of the illness (Peluso et al., 2007). Although impulsivity or impulsive behavior is less well characterized in SZ, previous studies indicate that individuals with SZ have deficits in goal-directed behavior (Kerns, Nuechterlein, Braver, & Barch, 2008) and show apparent impulsivity at first episode (Friis, Sundet, Rund, Vaglum, & McGlashan, 2002).
Surprisingly, no study has employed the delay discounting task with individuals with BD, and to our knowledge only one study has evaluated discounting rate in a sample of individuals with SZ (Heerey, Robinson, McMahon, & Gold, 2007). Heerey et al. (2007) found higher discounting rates in individuals with SZ compared to healthy individuals. That is, individuals with SZ showed a greater preference for immediate rewards and their discounting rates were inversely correlated with verbal memory and marginally with WM. However, that study did not assess the possible relationship between discounting rates and comorbid substance abuse or dependence. It is important to account for substance use in BD and SZ because the lifetime prevalence of alcohol, nicotine, and recreational drug abuse or dependence in these populations is substantially higher than in the general population. For example, an Epidemiologic Catchment Area study found that 60.7% of individuals with type I BD and 47.0% of individuals with SZ met criteria for some form of substance abuse or dependence (Regier et al., 1990). Impulsivity, which could be a predisposing factor of steep delay discounting, might be higher in patients with substance abuse (Dervaux et al., 2001). Therefore, current or past substance use may be associated with impaired reward processing in individuals with BD or SZ.
The present study was designed to study discounting of rewards in psychiatric patients (BD and SZ) using the delay discounting task. Potential correlates including substance use, WM, IQ, parental education, employment, and impulsive personality traits were also measured. We predicted that individuals with BD or SZ would show higher discounting rates than healthy individuals (i.e. greater preference of smaller, immediate rewards). In addition, we predicted WM or IQ would be associated with the discounting rate (Shamosh, et al., 2008; Shamosh & Gray, 2007).
Method
Participants
Twenty-two individuals meeting Diagnostic and Statistical Manual IV (DSM-IV) (American Psychiatric Association. Task Force on DSM-IV, 1994) criteria for Type I bipolar disorder (BD), 21 individuals meeting DSM-IV criteria for schizophrenia or schizoaffective disorder (SZ), and 30 non-psychiatric healthy individuals participated in the present study. All patients received the Structured Clinical Interview for Axis I disorders (SCID-I) (Spitzer, Williams, Gibbon, & First, 1992) after initial screening for a likely diagnosis of BD or SZ. SCID interviews were conducted by a trained Bachelor’s level research assistant (J.K.F.) or doctoral level psychologist (A.B.). Inter-rater reliability coefficients (kappa) based on SCID interviews for paired raters at our site have ranged .91 and 1.0 (Ns > 10) for differentiation of SZ/schizoaffective disorder from BD (Brenner, Wilt, Lysaker, Koyfman, & O'Donnell, 2003). Final Diagnostic determinations were made by doctoral level psychologists (A.B., W.P.H., B.F.O.) on the basis of the SCID interview, medical records, clinician report, structured interviews which assessed current mood and psychotic symptoms in detail, and self-report questionnaires. The SCID-Non-Patient edition (SCID-NP) (Spitzer, Williams, & Gibbon, 1990) was used to rule out Axis I disorders in healthy individuals. Exclusion criteria for all individuals included head trauma with loss of consciousness for greater than five minutes, history of seizures or electroconvulsive therapy, neurological disorders, current drug abuse or drug dependence, and a positive urine drug screen. Additionally, healthy individuals were excluded from participation if they had a current or past diagnosis of an Axis I disorder (including substance or alcohol dependence), or a first-degree relative with BD or SZ. Since self-report of illicit substance use in psychiatric patients may be highly discrepant from objective measures (Hser, 1997; Kerns, et al., 2008; A. M. Stone, Greenstein, Gamble, & McLellan, 1993), urine screens were used for both clinical and healthy individuals prior to assessment to corroborate drug-free status.
The patient sample was recruited through outpatient and inpatient units at clinics and hospitals affiliated with the Indiana University School of Medicine (see Table 1 for characteristics). Healthy individuals were recruited through newspaper advertisements. All individuals received detailed information about the study protocol and gave written and oral informed consent. Participants were paid $10/hour. The protocol was approved by the Indiana University–Purdue University Indianapolis Human Subjects Review Committee.
Table 1.
Demographic and Clinical Data for the Bipolar Disorder (BD), Schizophrenia (SZ), and for the healthy groups.
| Group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | BD (N=22) | SZ (N=21) | Healthy (N=30) | ||||||
| Mean | SD | Mean | SD | Mean | SD | F | dfw | p | |
| Age (years) | 39.9 | 9.4 | 40.3 | 11 | 37.0 | 11.9 | .73 | 70 | .487 |
| Years of Education a | 13.7 | 2.1 | 12.3 | 1.5 | 14.8 | 1.7 | 12.09 | 68 | <.001 |
| IQ a | 108.7 | 16.1 | 96.6 | 13.7 | 113.9 | 14.3 | 8.59 | 69 | <.001 |
| Father's education g | 3.3 | 1.4 | 3.8 | 1.6 | 3.7 | 1.4 | 1.17 | 2 | .557 |
| Mother's education g | 3.3 | 1.0 | 3.4 | 1.2 | 3.5 | .9 | .95 | 2 | .621 |
| Spatial Span Forward | 7.7 | 2.2 | 6.8 | 2.1 | 8.6 | 2.5 | 3.63 | 65 | .032 |
| Spatial Span Backward b | 7.3 | 2.7 | 6.2 | 2.5 | 8.0 | 1.8 | 3.43 | 65 | .038 |
| WM z-score b | .0 | .9 | −.4 | .9 | .3 | .8 | 4.48 | 65 | .015 |
| WM/IQ composite a | .0 | .9 | −.5 | .8 | .3 | .7 | 7.01 | 69 | .002 |
| BIS | |||||||||
| Attention c | 17.4 | 4.5 | 17.2 | 5.9 | 13.2 | 3.1 | 7.43 | 66 | .001 |
| Motor c | 24.2 | 5.2 | 23.7 | 4.1 | 20.4 | 3.1 | 6.28 | 66 | .003 |
| Non-planning c | 26.8 | 5.2 | 26.6 | 4.4 | 20.6 | 4.2 | 14.99 | 66 | <.001 |
| Trails A | 29.7 | 8.2 | 35.3 | 9.8 | 30.1 | 11.0 | 2.21 | 70 | .118 |
| Trails B d | 72.4 | 25.6 | 102.8 | 50 | 53.2 | 15.8 | 14.54 | 69 | <.001 |
| Executive Function (EF) d | 42.7 | 22.4 | 67.6 | 44 | 23.1 | 13.5 | 15.23 | 68 | <.001 |
| Digit Symbol e | 8.9 | 2.4 | 7.8 | 2.6 | 11.7 | 2.6 | 15.88 | 69 | <.001 |
| PANSS | NA | ||||||||
| Positive | 10.4 | 4.3 | 15.3 | 6.4 | 7.19 | 36 | .011 | ||
| Negative | 10.6 | 4.3 | 13.1 | 4.5 | 2.90 | 36 | .097 | ||
| General | 24.2 | 8.2 | 27.3 | 7 | 1.62 | 36 | .211 | ||
| YMRS | 8.3 | 8.5 | NA | ||||||
| MADRS | 10.8 | 8.9 | |||||||
| N | % | N | % | N | % | χ2 | df | p | |
| Gender | .32 | 2 | .852 | ||||||
| Male | 10 | 45.5 | 10 | 47.6 | 12 | 40.0 | |||
| Female | 12 | 54.5 | 11 | 52.4 | 18 | 60.0 | |||
| Employment | 35.0 | 15.0 | 92.0 | 27.83 | 2 | <.001 | |||
| Medication f | |||||||||
| Antipsychotics | 1.38 | 1 | .315 | ||||||
| Atypical (only) | 1 | 4.5 | 9 | 42.9 | 0 | 0 | |||
| Typical (only) | 12 | 54.5 | 3 | 14.3 | 0 | 0 | |||
| Atypical and typical | 1 | 4.5 | 4 | 19 | 0 | 0 | |||
| Lithium | 5 | 22.7 | 2 | 9.5 | 0 | 0 | 1.22 | 1 | .414 |
| Antidepressants | 7 | 31.8 | 4 | 19 | 0 | 0 | .38 | 1 | .491 |
| Anxiolytic | 7 | 31.8 | 3 | 14.3 | 0 | 0 | 1.63 | 1 | .284 |
| Anticonvulsant | 9 | 40.9 | 3 | 14.3 | 0 | 0 | 3.45 | 1 | .091 |
| Other psychoactive | 6 | 27.3 | 6 | 28.6 | 0 | 0 | .04 | 1 | 1.000 |
| Nev |
Past/ Curr |
Nev |
Past/ Curr |
Nev |
Past/ Curr |
||||
| Drug Abuse | 17 | 5/0 | 15 | 6/0 | 26 | 4/0 | |||
| Drug Dependence | 19 | 3/0 | 16 | 5/0 | 29 | 1/0 | |||
| Alcohol Abuse | 10 | 9/3 | 11 | 9/1 | 22 | 8/0 | |||
| Alcohol Dependence | 13 | 8/1 | 13 | 8/0 | 29 | 1/0 | |||
BD: Bipolar Disorder, SZ: Schizophrenia, WM: Working Memory, IQ: Intelligence, WM/IQ composite: Working Memory and Intelligence composite score, BIS: Barratt Impulsiveness Scale, PANSS: Positive and Negative Syndrome Scale, YMRS: Young Mania Rating Scale, MADRS: Montgomery-Asberg Depression Rating Scale. Nev: Never, Curr: Current
Healthy, BD > SZ
Healthy > SZ
BD, SZ > HC
SZ > BD > HC
Healthy > BD, SZ
Medication was compared between BD and SZ groups only.
The Kruskal-Wallis test was used instead of omnibus ANOVA. Mean values of ordinal variables are reported for simplicity. Values in the columns of F and dfw are in fact χ2 and df.
Clinical and Neuropsychological Assessment
Current symptom levels in the SZ group were assessed using the Positive and Negative Syndrome Scale (PANSS) (Kay, Fiszbein, & Opler, 1987). Current severity of depressive and manic symptoms in the BD group were assessed using the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery & Asberg, 1979) and the Young Mania Rating Scale (YMRS) (Young, Biggs, Ziegler, & Meyer, 1978), respectively. IQ for all individuals was estimated using the Wechsler Abbreviated Scale of Intelligence (WASI) (Nelson & Willison, 1982). WM was measured with Spatial Span Forward and Backward tests. Executive function (EF) was measured with Trails A and B tests (Reitan & Wolfson, 1993) using the index of the time difference between the two forms (RTTrail B–RTTrail A). The Barratt Impulsiveness Scale (BIS-11) (Patton & Stanford, 1995) was used to assess impulsive personality traits in all individuals. Parental education was coded using ordinal values; 1: grade school, 2: junior high, 3: high school, 4: some college, 5: bachelor’s degree, 6: master’s degree, and 7: doctoral degree.
Per exclusion criteria, no patient had a current diagnosis of drug abuse or dependence. For current alcohol, smoking, and drug use, ordinal values were used for correlation and regression analyses. Alcohol use per week was rated between 1 and 4 (1: none, 2: one or two drinks, 3: three to six beers, 4: six to twelve beers). Weekly amount of smoking was also rated between 1 and 5 (1: non-smoker, 2: less than one pack, 3: one to two packs, 4: two to three packs, 5: four or more packs) and drug use was rated between 1 and 4 (1: never taken, 2: very rarely, 3: about once a month, 4: about twice a month). Alternatively, those variables could be coded 1 for any current use and 0 for none. We found the alternative way of coding did not change any of findings reported in the Results section, thus we report findings with the ordinal values of alcohol, smoking and drug variables. History of drug and alcohol abuse/dependence diagnosed by SCID-I were also examined for their possible associations with the discounting rate.
Delay Discounting Task
The delay discounting task was completed on a computer. Participants were instructed to make choices about hypothetical scenarios involving money. Previous studies using the delay discounting task (e.g., Johnson & Bickel, 2002; Lagorio & Madden, 2005; Madden, Begotka, Raiff, & Kastern, 2003) showed that healthy individuals behaved similarly when offered real and hypothetical rewards, suggesting that hypothetical reward scenarios are valid for studying intertemporal choices. Participants were told that there were no correct or incorrect choices, and that they should choose whichever option they preferred using a mouse. The task began with a practice trial where the participant was offered an initial choice between $30 now and $60 in 8 months. Later, in critical trials, the participant made an initial choice between $400 now and $800 at 6 different delays: 2 weeks, 1 month, 6 months, 1 year, 3 years, and 10 years delay. Order of the critical trials (delays) was randomized for each participant. Choices were represented by two clickable rectangles on the screen. One rectangle showed the immediate amount ("$400 now") and the other showed the delayed amount (e.g., "$1000 in 8 months"). Order of the two rectangles on the screen was fixed within participants and counterbalanced across participants. A third clickable rectangle on the screen allowed participants to reset their choices within the current trial if they had made a mistake.
Each practice and critical trial consisted of 6 choices. The choices were designed to converge on the participant's indifference point for a particular delay by adjusting the immediate amount (Green & Myerson, 2004). For example, if the participant preferred $400 now rather than $800 in 1 year, then the next choice would be between $200 now and $800 in 1 year. If the participant then chose the $800 option, the next choice would be between $300 now and $800 in 1 year. The 6 choices per trial were used to estimate the value of the immediate amount at which the participant was indifferent between that immediate amount and the delayed amount. There was a 1 second pause between choices and a 3.5 second pause between trials.
Statistical Analysis
We considered both the hyperbolic and exponential models of delay discounting, both of which have a single free parameter. The hyperbolic model (Mazur, 1987) has the form: V = A / (1+kD), where a reward amount A after a delay D is discounted to a subjective value V in a participant with a discounting rate k. In the exponential function, V = Ae−kD. Previous studies suggest that intertemporal choice behavior of human and animals are well described by the hyperbolic model (Green & Myerson, 2004). However, a recent study showed that healthy individuals’ data can be better explained by the exponential rather than the hyperbolic model with temporal constraints (N. Schweighofer et al., 2006). It is possible that the exponential model may better fit the data of BD and SZ, so we compared the models first across all individuals, and then in each group separately. We used root mean square error (RMSE) as a model-fit index, with lower RMSE values indicating a better model fit. Paired t-tests were used to examine the differences between two models. The discounting rate (k) of each model was estimated for each individual participant by searching for the value of k that maximized R2 (minimizing the least square errors) for all indifferent points in 6 delays. Pearson correlation (r, two-tailed) and hierarchical multiple linear regression analyses were used to explore possible relationships between discounting rates and demographic, clinical and neuropsychological variables including age, years of education, parental education, employment, estimated IQ, alcohol use, drug use, smoking, medication, WM capacity, impulsive traits (3 BIS factor scores–Attentional, Motor, and Non-planning impulsivity), manic/depressive symptoms in the BD group (YMRS, MADRS), and positive/negative symptoms (PANSS) in the SZ group. Correlation analyses were conducted within each group separately while hierarchical multiple linear regression analyses were conducted with all individuals across all three groups unless otherwise specified.
We computed WM scores and WM/IQ composite scores in the following way. For each individual, separate z-scores were computed for Spatial Span forward and Spatial Span backward, and the means of these two scores were used as the WM z-scores. Means of WM z-scores and IQ z-scores were used as WM/IQ composite scores. We used WM/IQ composite scores for correlation and hierarchical multiple linear regression analyses because WM is a strongly related construct to IQ (Conway, Kane, & Engle, 2003) and they appear to share similar brain networks (Gray & Thompson, 2004). WM and IQ are also strongly correlated (in our dataset, r = .810, .691, and .436 in the BD, SZ, and healthy groups, respectively; p < .01 for BD and SZ groups, p < .02 for the healthy group), so using them separately in regression analyses would result in a collinearity problem. Weighted mean values were used when an individual had missing data in any of three measures (Spatial Span forward, Spatial Span backward, and IQ).
When comparing the results across three groups, omnibus ANOVA was first used and then independent t-tests were conducted to compare each pair of groups (two-tailed). Kruskal-Wallis and Mann-Whitney U tests were used to compare ordinal variables across groups. Statistical analyses were considered significant if significance (p value) was below .05 (two-tailed). Some participants did not complete all of the items on the questionnaires, and these questionnaire scores were pro-rated. Ln(k) (natural log) instead of k was used for all analyses to approximately normalize its distribution across participants. When reporting effect sizes, we used Cohen’s d when comparing a pair of groups, Cohen’s f2 when doing hierarchical multiple regression analyses, and correlation coefficients for correlational analyses. By convention, effect sizes are regarded small, medium, and large, respectively, when Cohen’s d is about .2, .5, and .8, Cohen’s f2 is about .02, .15, .35, or a correlation coefficient ranges from .1 to .23, .24 to.36, and >.37 (Cohen, 1988).
Results
Clinical Measures
The groups differed significantly on years of education and IQ measures (see Table 1 for summaries and statistical information). Healthy individuals and individuals with BD had more years of education and higher IQ scores than did individuals with SZ, but healthy individuals did not differ from individuals with BD. Neither parental education nor maternal education was significantly different across groups. BIS subscale scores differed significantly across groups. In pair-wise comparisons, both the BD and SZ groups scored higher than the healthy group on all three BIS subscales, suggestive of elevated impulsivity by self-report. As seen in Table 1, no individual was diagnosed as having current drug abuse or dependence, only three BD (13.6%) and one SZ patient (4.8%) was diagnosed as having current alcohol abuse, and only one BD (4.5%) was diagnosed as having current alcohol dependence.
Delay Discounting Choice Behavior
The model comparison results indicated that the hyperbolic model had a better fit than the exponential model, which is consistent with most of the existing literature (Green & Myerson, 2004). Across all participants in the three groups (N = 73), means (and the standard deviations (SDs)) of RMSEs for the hyperbolic and the exponential models were 95.6(60.5) and 108.9(69.8), respectively. The difference was highly significant (t(72) = 5.09, p = 2.75E-06). When two models were compared in each group, the hyperbolic model still performed better than the exponential one (in BD: 91.1(51.7) vs. 104.3(68.1), t(21) = 2.20, p < .04; in SZ: 112.7(70.0) vs. 135.0(72.8), t(20) = 5.94, p < 8.3E-06; in healthy: 87.1(59.2) vs. 93.9(65.9), t(29) = 2.03, p = .05). Furthermore, estimated discounting rates were highly correlated across two models (r = .982), which suggests that our findings would not be affected by the choice of temporal discounting. It was also noted that the means of RMSE were higher in the clinical groups than the healthy group. However, the group difference was not significant in any of the possible pairs (tested by independent t-tests, all p values were greater than .162 when the hyperbolic model was used). We also tested whether these results would be affected by outliers by removing participants whose RMSE was in the upper 5% (N = 5) and re-conducting all the analyses. The mean (and SD) RMSE of 5 outliers was 223.5(24.7) and we found removing outliers did not affect any of the findings reported in this work. With the choice of the hyperbolic model, both BD and SZ groups had higher (steeper) discounting rates than the healthy group with very large effect sizes (healthy vs.BD: t(50) = 4.14, p < .001, Cohen’s d = 1.15; healthy vs. SZ: t(49) = 4.27, p < .001, Cohen’s d = 1.20). However, the discounting rates of the BD and SZ groups were not significantly different (t(41) = .26, p = .794). Note that the higher a participant’s ln(k), the more temporally impulsive he/she is. The means (and standard deviations) of ln(k) for the BD, SZ, and healthy groups were −2.49(2.0), −2.33(2.1), and −4.68(1.8), respectively.
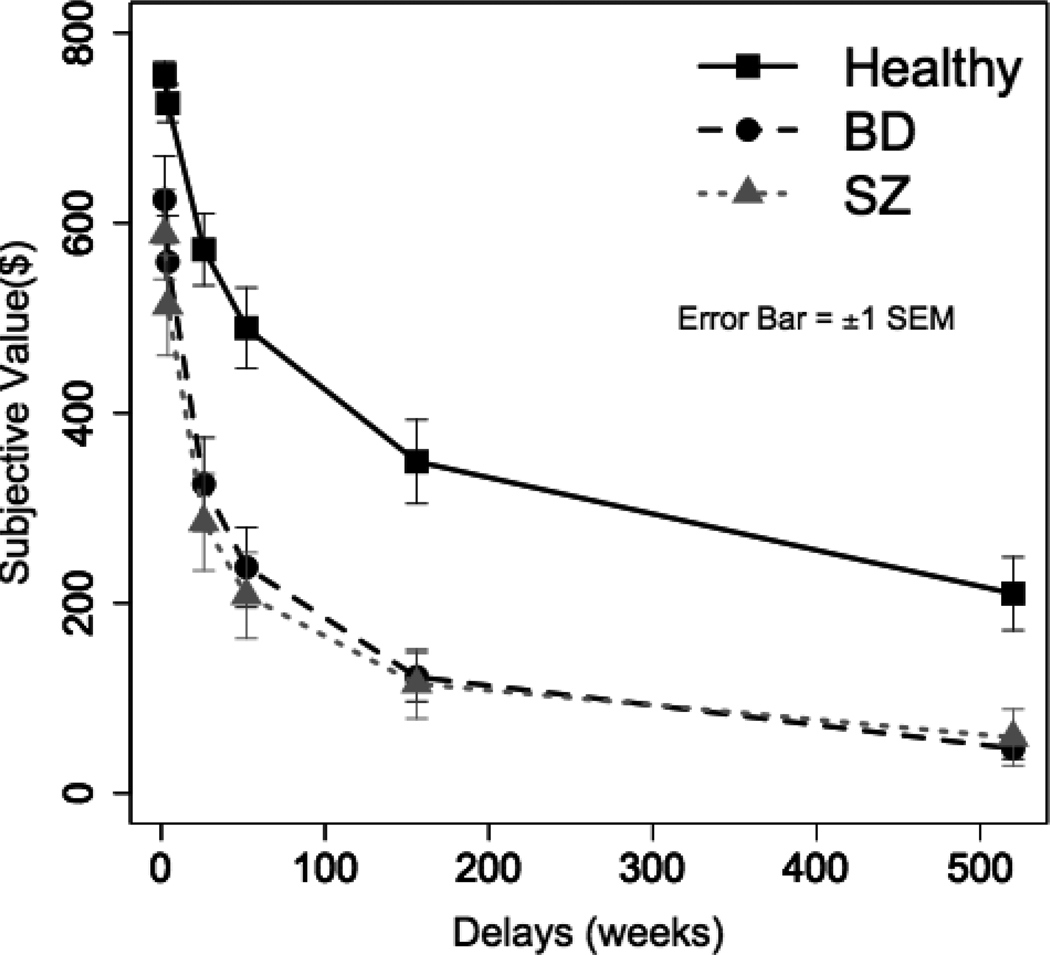
Figure 1 shows how the value of a delayed reward was discounted as a function of the delay to its receipt for each group. It clearly illustrates that BD and SZ groups discounted the delayed rewards more steeply compared to the healthy group. When tested with independent t-tests, delayed rewards at each delay were significantly more discounted in BD and SZ groups compared to the healthy group (p < .05 at every delay). Again, the BD and SZ groups’ subjective values did not differ significantly (p > .16 at every delay).
Figure 1.
Mean subjective values of delayed rewards as a function of the delay for each group. Each participant’s subjective values were computed based on his/her discount rate (k) and then averaged within each group. BD: Bipolar Disorder, SZ: Schizophrenia. Error bars represent ±1 SEM.
Relationship of Intellectual Function and Group Membership on the Discounting Rate
It is possible that the higher discounting rate in BD or SZ group was due to impaired WM and IQ, or current substance or alcohol use. Hierarchical multiple linear regression analyses were used to determine whether BD or SZ membership predicted discounting rates beyond WM/IQ composite scores, substance use, and employment. WM/IQ composite scores, employment (1 for employed and 0 for unemployed), and current drug use measures (alcohol, smoking, and substance use) were entered in the first step as predictors in the multiple linear regression analysis (5 predictors in the first step). Then, BD membership (1 for BD patients and 0 otherwise) and SZ membership (1 for SZ patients and 0 otherwise) were entered in the second step. The dependent variable was the discounting rate, ln(k). The change in R2 at the second step shows how much of the variance in the discounting rate is shared with psychiatric memberships.
The results summarized in Table 2 showed that 34.6% of variance in the discounting rate was accounted for by WM/IQ composite, employment, and drug use measures, R = .588, F(5,58) = 6.138, p < .001 and the WM/IQ composite score was the only significant predictor of ln(k). In the second model, BD membership and SZ membership accounted for additional 8.7% of variance in the discounting rate, R = .659, F(2,56) = 4.830, p = .018. BD and SZ memberships as well as the WM/IQ composite score were all significant predictors. BD and SZ memberships had greater than medium effect sizes (Cohen’s f2 > .015) even after controlling for other variables. All substance use measures and employment variable were nonsignificant in the second step (p > .247). In sum, the results indicate that WM/IQ composite alone does not explain the steep discounting in the BD and SZ groups.
Table 2.
Results of Hierarchical Multiple Linear Regression Analysis
| Model | Independent Variable | Standardized Coefficients (β) |
t | Sig. | Cohen's f2 |
|---|---|---|---|---|---|
| 1 | WM/IQ composite | −.405** | −3.51 | .001 | .196 |
| Employment | −.234† | −1.78 | .081 | .058 | |
| Smoking Use | .084 | .68 | .500 | .007 | |
| Drug Use | .170 | 1.57 | .121 | .030 | |
| Alcohol Use | .112 | 1.02 | .313 | .013 | |
| 2 | WM/IQ composite | −.371** | −3.25 | .002 | .160 |
| Employment | −.012 | −.08 | .936 | .000 | |
| Smoking Use | .044 | .37 | .710 | .002 | |
| Drug Use | .092 | .86 | .393 | .009 | |
| Alcohol Use | .122 | 1.17 | .247 | .015 | |
| BD membership | .397** | 2.87 | .006 | .187 | |
| SZ membership | .374* | 2.34 | .023 | .163 | |
Note. R2 = .346 for Step 1 (p < .001): R2 = .087 for Step 2 (p = .018). BD: Bipolar Disorder, SZ: Schizophrenia, WM: Working Memory, IQ: intelligence, WM/IQ composite: Working Memory and Intelligence composite score.
p < .10
p < .05
p < .01
To further examine the relationship among the discounting rate, correlation coefficients between discounting rate and WM, IQ, WM/IQ composite scores, WM scores, and IQ scores were calculated. The results in Table 3 revealed that WM/IQ composite scores significantly correlated with discounting rate in the BD group (r = −.573, p = .01) and at a trend level in SZ and healthy groups (r = −.391, p = .08 for SZ; r = −.362, p = .053 for healthy,). While WM scores significantly correlated with discounting rate in all three groups, IQ scores correlated with discounting rate only in the BD group (r = −.642, p < .001).
Table 3.
Pearson correlations between the Discounting Rate and WM/IQ measures
| Correlation coefficient with Ln(k) | BD | SZ | Healthy |
|---|---|---|---|
| WM/IQ composite score | −.573** | −.391† | −.362† |
| WM z-score | −.535* | −.484* | −.383* |
| IQ z-score | −.642** | −.110 | .186 |
Note.BD: Bipolar Disorder, SZ: Schizophrenia, WM: Working Memory, IQ: intelligence, WM/IQ composite: Working Memory and Intelligence composite score.
p < .10
p < .05
p < .01
In summary, the results show that both BD and SZ groups have steeper discounting rates than the healthy group, and the difference persists even after accounting for substance use and other demographic and neuropsychological factors. Correlation analyses and hierarchical multiple regression analyses demonstrate that BD and SZ membership contributes significantly to steeper discounting rates even after accounting for WM/IQ composite scores, and WM or IQ is significantly associated with discounting rate in all three groups.
Relationship Between Discounting Rates and Clinical Variables
Exploratory analyses evaluated whether discounting rate was related to a variety of other variables, including medication (typical and atypical antipsychotic, lithium, antidepressant, anxiolytic, anticonvulsant), past drug abuse/dependence, current and past alcohol abuse/dependence, impulsivity (BIS 3 subscales), EF scores, speed of information processing (Digit Symbol test), parental (father and mother) education, and BD/SZ symptoms (PANSS Positive & Negative & General, YMRS, and MADRS). In the BD group, ln(k) did not correlate with any of the variables including BIS subscales (p > .21), PANSS scales (p > .34), BD symptoms (p > .26), EF (p < .08), and Digit Symbol test (p < .10). In the SZ group, ln(k) correlated only with BIS non-planning impulsivity (r = .52, p < .03), but not with any of other variables including other BIS subscales (p < .17) or SZ symptoms (p > .21).
Discussion
This study was designed to examine the delay discounting of rewards in individuals with BD or SZ. We predicted that individuals with BD or SZ would show greater preference for immediate, smaller rewards over larger, delayed rewards relative to healthy individuals. The key findings of the present study supported our prediction: both BD and SZ groups had higher discounting rates than the healthy group. Compared to healthy individuals, individuals with BD or SZ showed a stronger preference for smaller immediate rewards over larger later rewards. An examination of the contributing factors to such choice behavior revealed that the higher discounting rates exhibited by the BD and SZ groups were not significantly accounted for by their current substance use, medication, employment, and other demographic variables. However, WM/IQ composite scores were strongly associated with discounting rates in both psychiatric groups as well as in the healthy group.
The results of this study are consistent with a previous study employing the delay discounting task in SZ (Heerey, et al., 2007). Heerey and colleagues reported that individuals with SZ showed steeper discounting rates than the healthy individuals, and that the discounting rate inversely correlated with memory tests but not with antipsychotic medication status. In contrast to previous studies of individuals with substance dependence but without comorbid psychotic disorder or BD, discounting rate was not related to current substance or alcohol use. This was likely due to the exclusion of participants currently diagnosed with illicit drug abuse or dependence and the use of a urine screen to establish drug free status prior to testing. Only five of 43 individuals with BD or SZ had a current diagnosis of alcohol abuse (N=4) or dependence (N=1). Although some individuals, especially those in the BD and SZ groups, had a past history of substance abuse or dependence, previous studies suggest overall past users have discounting rates comparable to never-users of illicit substances (see Reynolds, 2006, for a review). Future studies which include clinical groups who are comorbid for current substance dependence and SZ or BD could clarify whether these factors have additive effects on discounting rate.
Although no study has used the delay discounting task with individuals with BD, a recent study (Strakowski et al., 2009) used a conceptually similar task, the delayed reward task (DRT). In the DRT, participants need to wait for a longer time to obtain a larger monetary reward instead of immediate small reward. Unlike the delay discounting task, however, the delay period is in the time scale of seconds, information about delay periods is not explicitly provided to participants, and participants receive feedback on each trial. In addition, only the percent of impulsive responses and reaction times are often measured. In the DRT, individuals with BD showed a greater preference for immediate but smaller rewards (more impulsive choices) than healthy individuals, which is in agreement with our findings. However, further research is needed to examine whether the tasks measure similar constructs (Bornovalova, Lejuez, Daughters, Zachary Rosenthal, & Lynch, 2005).
The previous literature assessing healthy individuals suggests that delay discounting inversely correlates with WM (Shamosh, et al., 2008) and IQ (Shamosh & Gray, 2007), and our findings are in general agreement with those findings. The present data extended the association between WM/IQ composite and discounting rate by showing that higher delay discounting in psychiatric groups (BD and SZ) were also related to their WM or IQ capacities. Specifically, WM scores were inversely correlated with discounting rate in all three groups. WM is important for maintaining and integrating cognitive and affective information, and also for focusing on relevant information during goal-directed behavior (D'Esposito, 2007). Previous studies have also shown that WM ability inversely correlated with optimal choice behavior in individuals with SZ (Heerey, et al., 2008; Heerey, et al., 2007). Although several studies report reduced IQ (Bearden, Hoffman, & Cannon, 2001) and WM capacity (Hsiao et al., 2009) in individuals with BD, few studies have examined their associations with reward processing and decision-making in the group.
A significant association between discounting rate and impulsivity, as measured by the BIS questionnaire, was only found for the SZ group for non-planning impulsivity. Our findings of nonsignificant correlations are consistent with previous clinical studies showing only modest or nonsignificant associations between BIS scores and cognitive task performance in BD (Strakowski, et al., 2009) and SZ (Enticott, Ogloff, & Bradshaw, 2008). To date, findings suggest that impulsivity measured by questionnaires and by cognitive tasks reflect different characteristics (Swann, Anderson, Dougherty, & Moeller, 2001; Swann, Pazzaglia, Nicholls, Dougherty, & Moeller, 2003), and as Strakowski et al. (2009) argued, self-report measures alone may not adequately characterize cognitive components of impulsivity.
A common hypothesis for decision-making deficits in individuals with BD is that they might have deficits in reward processing and more specifically, an exaggerated response to negative feedback (Roiser, et al., 2009). However, this model would not account for steeper discounting rate in BD because the discounting task does not involve feedback. Reward processing involves multiple systems such as valuation, action selection, outcome evaluation, and reward learning (Rangel, Camerer, & Montague, 2008). In this study, we showed that individuals with BD or SZ have alterations in a specific valuation system: temporal discounting of delayed rewards. Previously, steeper temporal discounting has been regarded as central to substance use problems. The present findings suggest that such alterations may extend to mood disorders and psychotic disorders. The underlying mechanisms, however, may differ between clinical disorders. Delay discounting rates could, for example, be affected by a failure to inhibit responses to immediate rewards, an inability to conceptualize the future, inadequate search of a problem space, an overriding need for present resources, or apathy and avolition.
An important issue in evaluating these findings is whether the discounting rate alterations in SZ and BD represent a specific or differential deficit, or simply reflect a generalized cognitive deficit in these clinical groups. Comparison of the delayed discounting task to conventional accuracy based tests of performance poses several challenges. First, the discounting rate is not a “deficit,” and responses to individual items are not “correct” or “incorrect.” Consequently, comparing or matching performance with other tasks which are based on accuracy of performance, such as Wechsler subtests or psychophysical tests (as in O’Donnell et al., 2002) is not feasible. Second, matching on true score variance (as suggested by Chapman & Chapman, 2001) poses similar computational issues regarding derivation of reliability on the basis of item scores. For discounting tasks, the model fit as measured by RMSE is used to assess performance of the discounting model rather than reliability. Finally, true score variance as a measure of discriminating power may not be a sensitive measure of discriminating power. A recent stimulation study by Kang and Macdonald (2010) showed that true score variance accounted for only about 10% of variance of discriminating power, suggesting that true score variance is not an adequate measure to predict differential deficits.
As an alternative statistical approach, a hierarchical multiple linear regression analysis was used to examine whether higher discounting rate in the BD or SZ group remained significant after entry of WM/IQ composite scores and other factors. The hierarchical multiple linear regression analysis (Table 2) showed that BD and SZ memberships were significantly associated with higher discounting rate even after accounting for the contribution of WM/IQ composite scores. In addition, individuals with BD showed equivalent IQ and WM scores compared to healthy individuals, but individuals with BD still had significantly higher discounting rates than healthy individuals, again arguing against the discounting rate being solely a function of intellectual impairment between groups. Future studies would benefit from experimentally manipulating the WM load or general intellectual capacity (e.g., Hinson, et al., 2003) to demonstrate that alteration in temporal discounting among individuals with BD or SZ is a specific deficit.
Higher discounting rates in SZ could be interpreted as deficits in delay of gratification, but it is possible that their higher discounting rate might be due to apathy (i.e., a loss of motivation) or cognitive “short-sightedness”. Fellows and Farah (2005) suggested that poor decisions regarding future events (“future thinking”) might have two aspects: temporal discounting of distant rewards and future time perspective. Future time perspective refers to the actual chronological time being considered when thinking about one’s own life events (e.g., career plan, buying a house, etc.). In SZ, apathy or working memory deficits may limit the ability to conceptualize future events. Future time perspective may be vulnerable to dysregulation of frontal lobe systems. Fellows and Farah (2005) examined the two aspects of decision-making in patients with dorsolateral prefrontal cortex (DLPFC) and ventromedial prefrontal cortex (VMPFC) damage, and showed that patients with VMPFC lesion, but not those with DLPFC lesion, had significantly shorter future time extension than the control group. They also showed that their apathy scale score, but not BIS scores, significantly correlated with future time perspective. However, it is notable that diminished time perspective was apparent in patients with ventromedial frontal lobe lesions, but temporal discounting was unaffected in both lesion groups. Thus, deficits in future time perspective and temporal discounting may be dissociated in clinical populations, and should be assessed independently.
While we did not include a self-report measure of a construct similar to apathy or avolition, we did examine the correlation between the PANSS observer rating of Negative Symptoms and discounting rate, which was not significant (r = −.177, p = .457). Simon et al. (2010) found that while apathy scores inversely correlated with the ventral striatum activation during reward anticipation, PANSS negative symptoms did not, which might suggest that apathy is more specifically related to reward anticipatory process than overall negative symptoms (Simon, et al., 2010). The behavioral study by Polgár et al. (2008) also had a similar finding: behavioral performance correlated significantly and inversely with SANS scores, but not with PANSS negative symptoms. The present data provide evidence of increased self-report impulsivity in SZ and increased discounting rate. The relationship of these findings to experimentally derived measures of future time perspective, apathy or avolition would be an important issue to address in future research.
Limitations and Future Directions
There are several limitations to this study. First, the sample size was relatively small, and therefore was only able to detect moderate to large effect sizes associated with diagnosis, WM, and IQ. The study may have lacked sufficient power to detect small effects of other factors, such as the current mood state, history of dependence or current recreational illicit substance use, on discounting rates. Second, we did not assess participants’ income levels, which may have inverse relationships with the discounting rate (Green, Myerson, Lichtman, Rosen, & Fry, 1996; Hausman, 1979; Kirby et al., 2002; Pender, 1996). The heterogeneous medications used to treat BD and SZ might also interact in complex ways with discounting rates. Future studies which incorporated delay discounting as an outcome measures for pharmacological or psychosocial interventions could be highly informative. For example, a recent study showed that cognitive remediation training not only improved WM performance in SZ patients, but also increased their prefrontal cortical function (Haut, Lim, & MacDonald, 2010). Interestingly, a recent study demonstrated that WM training decreased discounting rate of individuals with stimulant dependence (Bickel, Yi, Landes, Hill, & Baxter, 2011). Also, studies of relatives of individuals with BD or SZ could indicate whether increased discounting rate is associated with genetic and environmental risk factors for these illnesses.
In summary, we found greater delay discounting of rewards in individuals with BD or SZ than healthy individuals that were unrelated to current or past substance use, but related to WM and IQ. Our findings suggest that the increased rate of temporal discounting among individuals with BD or SZ may significantly contribute to their decision-making deficits in their lives and its implications in current theories of decision-making deficits in BD were discussed. Future studies may examine how alterations in temporal discounting are related to other aspects of decision-making and everyday planning. Treatment targeting awareness of long-term outcomes of decisions and WM/IQ composite improvement might enable patients to make more adaptive decisions for their future and improve their quality of life.
Acknowledgments
This work was supported byte National Institutes of Mental Health (R01 MH62150, 1 R21 MH091774-01, and CTSA UL1RR025761-01 to BFO, and R01 MH074983 to WPH).The authors thank Yong Wook Shin for his helpful comments on an earlier draft of this manuscript.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn
The authors declare that they have no competing financial interests.
References
- American Psychiatric Association. Task Force on DSM-IV. DSM-IV: diagnostic and statistical manual of mental disorders. 1994. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. British Journal of Psychiatry. 1989;(7):49–58. [PubMed] [Google Scholar]
- Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disorders. 2001;3(3):106. doi: 10.1034/j.1399-5618.2001.030302.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Lejuez CW, Daughters SB, Zachary Rosenthal M, Lynch TR. Impulsivity as a common process across borderline personality and substance use disorders. Clinical Psychology Review. 2005;25(6):790–812. doi: 10.1016/j.cpr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Brenner C, Wilt M, Lysaker P, Koyfman A, O'Donnell B. Psychometrically Matched Visual-Processing Tasks in Schizophrenia Spectrum Disorders. Journal of Abnormal Psychology. 2003;112(1):28–37. [PubMed] [Google Scholar]
- Brzezinski MR, Abraham TL, Stone CL, Dean RA, Bosron WF. Purification and characterization of a human liver cocaine carboxylesterase that catalyzes the production of benzoylecgonine and the formation of cocaethylene from alcohol and cocaine. Biochemical Pharmacology. 1994;48(9):1747–1755. doi: 10.1016/0006-2952(94)90461-8. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Commentary on two articles concerning generalized and specific cognitive deficits. Journal of Abnormal Psychology. 2001;110(1):31–39. doi: 10.1037//0021-843x.110.1.31. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. A neuropsychological investigation of prefrontal cortex involvement in acute mania. American Journal of Psychiatry. 2001;158(10):1605–1611. doi: 10.1176/appi.ajp.158.10.1605. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum; 1988. [Google Scholar]
- Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences. 2003;7(12):547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- D'Esposito M. From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1481):761. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervaux A, Bayle F, Laqueille X, Bourdel M, Le Borgne M, Olie J, et al. Is substance abuse in schizophrenia related to impulsivity, sensation seeking, or anhedonia? American Journal of Psychiatry. 2001;158(3):492. doi: 10.1176/appi.ajp.158.3.492. [DOI] [PubMed] [Google Scholar]
- Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107(3):885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- Enticott P, Ogloff J, Bradshaw J. Response inhibition and impulsivity in schizophrenia. Psychiatry Research. 2008;157(1–3):251–254. doi: 10.1016/j.psychres.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Dissociable elements of human foresight: a role for the ventromedial frontal lobes in framing the future, but not in discounting future rewards. Neuropsychologia. 2005;43(8):1214–1221. doi: 10.1016/j.neuropsychologia.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Friis S, Sundet K, Rund BR, Vaglum P, McGlashan TH. Neurocognitive dimensions characterising patients with first-episode psychosis. The British Journal of Psychiatry. 2002;43:s85–s90. doi: 10.1192/bjp.181.43.s85. [DOI] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophrenia Bulletin. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. American Journal of Psychiatry. 2005;162(10):1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nature Reviews Neuroscience. 2004;5(6):471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin. 2004;130(5):769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal discounting in choice between delayed rewards: the role of age and income. Psychology and Aging. 1996;11(1):79–84. doi: 10.1037//0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- Hausman J. Individual discount rates and the purchase and utilization of energy-using durables. The Bell Journal of Economics. 1979;10(1):33–54. [Google Scholar]
- Haut KM, Lim KO, MacDonald A., 3rd Prefrontal cortical changes following cognitive training in patients with chronic schizophrenia: effects of practice, generalization, and specificity. Neuropsychopharmacology. 2010;35(9):1850–1859. doi: 10.1038/npp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biological Psychiatry. 2008;64(1):62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cognitive Neuropsychiatry. 2007;12(3):213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29(2):298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Hser YI. Self-reported drug use: results of selected empirical investigations of validity. NIDA Research Monographs. 1997;167:320–343. [PubMed] [Google Scholar]
- Hsiao Y-L, Wu Y-S, Wu JY-W, Hsu M-H, Chen H-C, Lee S-Y, et al. Neuropsychological functions in patients with bipolar I and bipolar II disorder. Bipolar Disorders. 2009;11(5):547–554. doi: 10.1111/j.1399-5618.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- Johnson M, Bickel W. Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior. 2002;77(2):129. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kang S, MacDonald A., III Limitations of True Score Variance to Measure Discriminating Power: Psychometric Simulation Study. Journal of Abnormal Psychology. 2010;119(2):300–306. doi: 10.1037/a0018400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S, Fiszbein A, Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biological Psychiatry. 2008;64(1):26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Godoy R, Reyes-García V, Byron E, Apaza L, Leonard W, et al. Correlates of delay-discount rates: Evidence from Tsimane'Amerindians of the Bolivian rain forest. Journal of Economic Psychology. 2002;23(3):291–316. [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99(4):461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Lagorio C, Madden G. Delay discounting of real and hypothetical rewards III: Steady-state assessments, forced-choice trials, and all real rewards. Behavioural Processes. 2005;69(2):173–187. doi: 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Experimental and Clinical Psychopharmacology. 2003;11(2):139–145. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- Mazur J. An adjusting procedure for studying delayed reinforcement. Quantitative Analyses of Behavior. 1987;5:55–73. [Google Scholar]
- Minassian A, Paulus MP, Perry W. Increased sensitivity to error during decision-making in bipolar disorder patients with acute mania. Journal of Affective Disorder. 2004;82:203–208. doi: 10.1016/j.jad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ehrman R, Napier KL, O'Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001;96(12):1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Montgomery S, Asberg M. A new depression scale designed to be sensitive to change. The British Journal of Psychiatry. 1979;134(4):382. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES, et al. Decision-making cognition in mania and depression. Psychological Medicine. 2001;31(4):679–693. doi: 10.1017/s0033291701003804. [DOI] [PubMed] [Google Scholar]
- Nelson H, Willison J. National adult reading test (NART): Test manual. Nfer-Nelson Windsor; 1982. [Google Scholar]
- Nieuwenstein M, Aleman A, de Haan E. Relationship between symptom dimensions and neurocognitive functioning in schizophrenia: a meta-analysis of WCST and CPT studies. Journal of Psychiatric Research. 2001;35(2):119–125. doi: 10.1016/s0022-3956(01)00014-0. [DOI] [PubMed] [Google Scholar]
- O’Donnell B, Potts G, Nestor P, Stylianopoulos K, Shenton M, McCarley R. Spatial frequency discrimination in schizophrenia. Journal of Abnormal Psychology. 2002;111(4):620. doi: 10.1037//0021-843x.111.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J, Stanford M. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peluso MA, Hatch JP, Glahn DC, Monkul ES, Sanches M, Najt P, et al. Trait impulsivity in patients with mood disorders. Journal of Affective Disorders. 2007;100(1–3):227–231. doi: 10.1016/j.jad.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Pender J. Discount rates and credit markets: Theory and evidence from rural India. Journal of Development Economics. 1996;50(2):257–296. [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154(3):243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biological Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgár P, Farkas M, Nagy O, Kelemen O, Réthelyi J, Bitter I, et al. How to find the way out from four rooms? The learning of "chaining" associations may shed light on the neuropsychology of the deficit syndrome of schizophrenia. Schizophrenia Research. 2008;99(1):200–207. doi: 10.1016/j.schres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Papaxanthis C, Petit JL, Schweighofer N, Stucchi N. Kinematic features of movement tunes perception and action coupling. Behavioural Brain Research. 2006;169(1):75–82. doi: 10.1016/j.bbr.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. Journal of the Experimental Analysis of Behavior. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience. 2008;9(7):545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behavioural Pharmacology. 2006;17(8):651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Cannon DM, Gandhi SK, Taylor Tavares J, Erickson K, Wood S, et al. Hot and cold cognition in unmedicated depressed subjects with bipolar disorder. Bipolar Disorders. 2009;11(2):178–189. doi: 10.1111/j.1399-5618.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein JS, Michael A, Underwood BR, Tempest M, Sahakian BJ. Impaired cognition and decision-making in bipolar depression but no 'affective bias' evident. Psychological Medicine. 2006;36(5):629–639. doi: 10.1017/S0033291705006689. [DOI] [PubMed] [Google Scholar]
- Schweighofer G, Pinz A. Robust pose estimation from a planar target. IEEE Transactions on Pattern Analysis and Machine Intelligence. 2006;28(12):2024–2030. doi: 10.1109/TPAMI.2006.252. [DOI] [PubMed] [Google Scholar]
- Schweighofer N, Shishida K, Han CE, Okamoto Y, Tanaka SC, Yamawaki S, et al. Humans can adopt optimal discounting strategy under real-time constraints. PLoS Computational Biology. 2006;2(11):e152. doi: 10.1371/journal.pcbi.0020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, et al. Iowa Gambling Task in schizophrenia: A review and new data in patients with schizophrenia and co-occuring cannabis use disorders. Schizophrenia Research. 2007;92:74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway AR, et al. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychological Science. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, Gray JR. Delay discounting and intelligence: A meta-analysis. Intelligence. 2007 [Google Scholar]
- Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, et al. Neural correlates of reward processing in schizophrenia--relationship to apathy and depression. Schizophrenia Research. 2010;118(1–3):154–161. doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M. First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Archives of General Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stone AM, Greenstein RA, Gamble G, McLellan AT. Cocaine use by schizophrenic outpatients who receive depot neuroleptic medication. Hospital and Community Psychiatry. 1993;44(2):176–177. doi: 10.1176/ps.44.2.176. [DOI] [PubMed] [Google Scholar]
- Stone SP, Herbert P, Chrisostomou J, Vessey C, Horwood C. The assessment of disability in patients on an acute medical ward for elderly people. Disability and Rehabilitation. 1993;15(1):35–37. doi: 10.3109/09638289309165867. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Fleck DE, DelBello MP, Adler CM, Shear PK, McElroy SL, et al. Characterizing impulsivity in mania. Bipolar Disorders. 2009;11(1):41–51. doi: 10.1111/j.1399-5618.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC. Impulsivity in mania. Current Psychiatry Reports. 2009;11(6):481–487. doi: 10.1007/s11920-009-0073-2. [DOI] [PubMed] [Google Scholar]
- Swann AC, Anderson JC, Dougherty DM, Moeller FG. Measurement of inter-episode impulsivity in bipolar disorder. Psychiatry Research. 2001;101(2):195–197. doi: 10.1016/s0165-1781(00)00249-3. [DOI] [PubMed] [Google Scholar]
- Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. Impulsivity and phase of illness in bipolar disorder. Journal of Affective Disorder. 2003;73(1–2):105–111. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42(3):1118–1126. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremeau F, Brady M, Saccente E, Moreno A, Epstein H, Citrome L, et al. Loss aversion in schizophrenia. Schizophrenia research. 2008;103(1–3):121–128. doi: 10.1016/j.schres.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biological Psychiatry. 2007;62:756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E, Hayden EP, Bodkins M, O'Donnell BF, Hetrick WP. Decision making in bipolar disorder: a cognitive modeling approach. Psychiatry Research. 2008;161(2):142–152. doi: 10.1016/j.psychres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133(5):429. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]