Abstract
Bisphenol A(BPA) is a ubiquitous high volume industrial chemical that is an estrogen and an environmental endocrine disrupting chemical. Bisphenol A is used extensively in the production of consumer goods, polycarbonate plastics, epoxy resins, coating used to line metallic food and beverage cans, and other products.There is great concern regarding the possible harmful effects from exposures that result from BPAleaching into foods and beverages from packaging or storage containers. The objective of this study was to independently assesswhether BPA contamination of water was occurring from different types of reusable drinking bottlesmarketed as alternatives to BPA-containing polycarbonate plastics. Using a sensitive and quantitative BPA-specific competitive enzyme-linked immunosorbent assaywe evaluated whether BPA migrated into water stored inpolycarbonateor copolyester plastic bottles, and different lined or unlined metallic reusable water bottles. At room temperature the concentration of BPA migrating from polycarbonate bottles ranged from 0.2–0.3 mg/L. Under identical conditions BPA migration from aluminium bottles lined with epoxy-based resins was variable depending on manufacturer ranging from 0.08 to 1.9 mg/L.Boiling water significantly increased migration of BPA from the epoxy lined bottles. No detectable BPA contamination was observed in water stored in bottles made from Tritan™ copolyester plastic, uncoated stainless steel, or aluminium lined with EcoCare™. The results from this study demonstrate that when used according to manufactures’ recommendations reusable water bottles constructed from “BPA-free” alternative materials are suitable for consumption of beverages free of BPA contamination.
Keywords: Bisphenol A, Copolyester, Epoxy resins, Endocrine disrupting chemicals, Polycarbonaste, Water bottles
1. Introduction
Bisphenol A (BPA) is an endocrine disrupting chemical (EDC) used extensively in the production of consumer products such as polycarbonate plastics, food cans, plastic packaging, dental sealants and water pipes. Analysis of BPA in urine samples showed that BPA is present in over 93% of the US population(Calafat et al., 2005). The mean urine BPA concentrations in various US populations are found to be in the range of 1–6 μg/L, or about 6–26 nM. Those levels are well within the dose range of animal studies demonstrating negative effects on the reproductive function resulting in decreased fecundity, alterations in embryonic development, and impacts on carcinogenesis (Richter et al., 2007). However other reproductive and chronic toxicological studies in some strains of rodents have failed to identify significant effects of BPA exposures (Howdeshell et al., 2008, Tyl et al., 2008).
Exposures to harmful xenobiotics that leach from packaging or storage containers into foods and beverages are a concern for many consumers, regulators and manufacturers. Much recent concern has focused on the potential adverse effects of BPA and its ability tonegatively impact human health through a variety of systems and modes of action.Bisphenol A has been shown to impact the actions of endogenous estrogenic steroid hormones (e.g. 17β-estradiol, esterone or estriol), but the full extents of its effects are yet to be fully understood. Bisphenol A is the monomer of polycarbonate plastic and is also used as a plasticizer, stabilizer and additive in many other plastic and non-plastic consumer products. As a result BPA has become ubiquitously used in the manufacturing of consumer goods, food and beverage containers, paper and in many other industrial applications. In some cases, migration of BPA from containers into water, drink solutions and food liquors has been well documented (e.g. Grumetto et al., 2008, Le et al., 2008,De Coensel et al., 2009,Maia et al., 2010). Our previous analysis showed that BPA migrates into water from polycarbonate drinking bottles, an effect that was stimulated by exposure of the plastic to increased temperatures(Le et al., 2008).
In response to consumer concern, manufacturers have been producing reusable metallic and non-polycarbonate plastic water bottles that are marketed as safe, “BPA-free”, alternatives to polycarbonate plastic bottles. Due to their durability and light weight, lined aluminium water bottles have become increasingly popular as reusable water bottles. The linings of some aluminium water bottles are composed of an epoxy resin. In some cases, the aluminium bottles with epoxy lining have been marketed as alternatives to polycarbonate and in some cases not to leach BPA. However,epoxy derivatives of BPA are the most common monomer substrates used to form the “epoxy resin” polymer. Depending on chemical reaction conditions associated with BPA derivatization and the cross-linking chemistry of polymerization, release 0f unmodified free-BPA contaminating the epoxy resins can occur. In 2008 it emerged thatthe purported non-BPA leaching lining used by the manufacture of high quality aluminium water bottlesSigg, was a BPA containing epoxy resin that could potentially contaminate water with free-BPA. Sigg is the manufacturer of the most popular aluminium water bottle and has responded by manufacturingtheir aluminium water bottles with their proprietaryEcoCare™ liningclaiming that this lining is completely BPA free. As a plastic alternative to their extremely popular polycarbonate water bottles, Nalgene now manufacture a presumed BPA-free plastic water bottle made of Eastman’s Tritan™ co-polyester polymer. There is however, wide spread consumer uncertainty related to the validity of marketing claims associated with “BPA-free” products, especially in light of the fact that few independent tests have confirmed the claim that BPA does not contaminate liquids stored in these water bottles. The purpose of this study was to independently determine whether BPA contamination of water was occurring in the different types of reusable drinking bottles.
2. Materials and Methods
2.1 Materials and Reagents
HPLC grade water (W5sk; Lot no. 095946) and methanol (A452sk; Lot no. 095586) was purchased from Fisher Scientific and used for all standardised washing and experimental aspects of the study. All reusable bottles were obtained from retail sources: the Nalgene, (Rochester, NY) 32 ounce loop-top polycarbonate bottles and Tritan™copolyesterbottles (Everyday™) were acquired from Campmor(Paramus NJ). One litre stainless steel bottles (#8203.80; Steel Works manufactured in China by Sigg, Zurich), aluminium epoxy resin lined bottles (Group A; #8127.90) and EcoCare™ lined bottles (#7533.30) were Swiss made by Sigg. The 800 ml New Balance branded aluminium epoxy resin lined bottles (Group B) were manufactured in China and purchased directly from the national discount retailer Target Corporation (Minneapolis, MN).
2.2 Bottle preparation and rinsing
As previously described all bottles and collection vials were washed and rinsed using a standardized protocol to ensure they were completely free of non-experimental contaminants (Le et al., 2008). Briefly, each bottle was rinsed with water generated from a Milli-Q Advantage A10 water system (resistivity < 18.2 MΩ and < 4 ppb total oxidizable organics). The interior of each bottle was scrubbed with a soft nylon bristle brush for approximately 30 seconds with half-strength solution of Alconox powdered precision cleaner (White Plains, NY).Bottles were then rinsed six times with BPA-free water, two times with HPLC grade water, and dried by inversion overnight in a dust-free laminar flow hood.
2.3 Experimental Design
Water bottles were divided into test groups based on their material or lining material: polycarbonate, stainless steel, aluminium copolyesterlined, aluminium epoxy resin lined (two different brands) and Tritan® co-polyester. The experimental design has been previously described (Le et al., 2008). Briefly, 100 ml of HPLC grade water was added to each bottle at day 0 and incubated for 5 days (120 h) at room temperature (22ºC). Three replicate experiments were performed for each bottle. Incubations were performed with rotation on a cell culture roller bottle system (Wheaton). The effects of hot water on BPA migration from the epoxy resin lined bottles was assessed by the addition of 100 ml HPLC grade water heated to 100ºC on day 0. Following transfer of boiling water, the bottles were incubated at room temperature with rotation for 24h during which time water samples cooled to ambient temperatures. All samples were collected by direct transfer into the washed 25 ml collection vials. All liquid transfers were performed using new autoclaved polypropylene pipette tips or graduated cylinders and beakers that were purchased new and washed as above.
2.4 ELISA assay of BPA concentration in water samples
A supersensitive ELISA (Ecologiena, Japan Environchemicals, Tokyo)previously validated to specifically detect BPA in samples of water was used. For water samples this ELISA shows an extremely high correlation with GC/MS/MS(http://www.abraxiskits.com/uploads/products/docfiles/81_PN590023info.pdf), findings that were confirmed independently (not shown). The ELISA was performed in accordance with a modified standard test kit protocol which provides a quantitative assay detection range of <0.05 ng/ml to 10 ng/ml (Le et al., 2008). For each assay a standard curve was generated and used to calculate the concentration of BPA present in each unknown sample (e.g. Fig. 1). In addition to known and experimental samples, control samples of HPLC water were included in each of the individual assays. For all control and experimental samples, triplicate measurements (n =3) were made at 450 nm with a microprocessor controlled SPECTRAFluor PLUS microplate reader (Tecan). Resulting data were analysed with Excel (Microsoft) and non-linear regression was performed with Prism version 5.03 (GraphPadSoftware Inc).
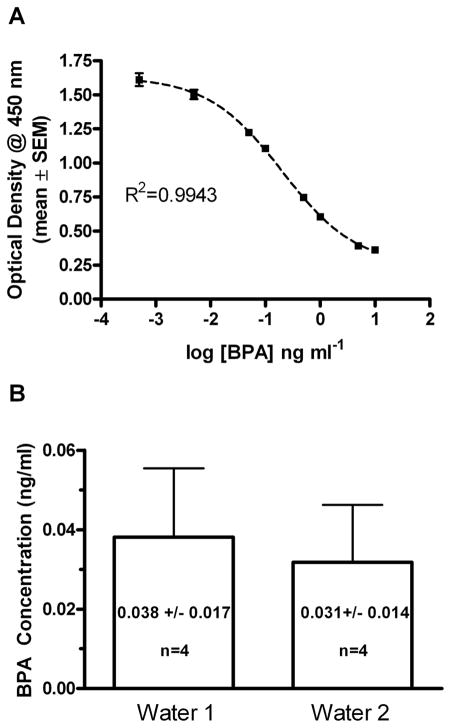
Figure 1. Bisphenol A (BPA) ELISA standard curve and analysis of control water samples.
(A) The standard curve resulting from a non-linear sigmoidal curve fit of the BPA standard from a representative experiment is shown graphically. Data points are mean optical density values ± SEM at each known concentration.(B) The concentrations of BPA-like immunoreactivity present in samples of HPLC water used in this study were estimated using the BPA ELISA assay. For each experiment the concentration of BPA was determined in each of 4 samples. The mean values ± SD are indicated and in each experiment concentrations found were below the established detection limit of 0.05 ng ml−1 for the assay. The mean calculated BPA concentration for each experimental determination is indicated in each bar.
2.5 Statistical Analysis
All presented results are representative of at least 3 replicate experiments or quantitative determinations. As appropriate for the experimental design, data are reported as the mean value ± SD or SEM with statistical analysis conducted using an unpaired t test, or one-way analysis of variance (ANOVA) with post-test comparison between treatment groups using Tuckey-Kramer multiple comparison test. A minimal level of statistical significance for differences in values was considered to be p<0.05. The levels of statistical significance are indicated withasterisks. Data were analyzed with Excel 2007 (Microsoft) and GraphPad Prism® version 5.04 (GraphPad Software Inc).
3. Results
Previous and current results confirm that the BPA-specific ELISA approach used here was highly sensitive and specific for detection of BPA in samples of water (Le et al., 2008). Shown in Fig. 1 is a representative example of the standard curve that was generated for each assay performed in this study. Two separate control experiments were performed that demonstrated the lack of BPA in HPLC grade water used as a dilutant for these experiments. In two different experiments the mean estimated concentration of BPA was below the assay’s theoretical detection limitof 0.05 ng/ml (Fig.1B).As was observed previouslyin other polycarbonate bottles increased levels of BPA were detected in water samples that were incubated in each of 4 different polycarbonate bottles (Table 1, Fig 2A). Over the incubation period a mean sample concentration of 0.234 ng/ml (S.D. ± 0.064) was observed. Those results confirmed that significant amounts of BPA can leach into liquids from new polycarbonate bottles at room temperature.
Table 1.
Concentration and apparent rates of Bisphenol A migrating into water from bottles
| ID Number | Material | Brand | Conc. (ng ml−1) | Migration (ng hr−1) |
|---|---|---|---|---|
| 1 | Polycarbonate | Nalgene | 0.306 ± 0.060 | 0.240 ± 0.033 |
| 2 | 0.178 ± 0.028 | 0.154 ± 0.021 | ||
| 3 | 0.199 ± 0.024 | 0.170 ± 0.017 | ||
| 4 | 0.251 ± 0.066 | 0.216 ± 0.044 | ||
| 1 | Tritan™ | Nalgene | 0.007 ± 0.005 | 0.007 ±0.003 |
| 2 | 0.008 ± 0.002 | 0.005 ±0.001 | ||
| 3 | 0.008 ± 0.003 | 0.006 ±0.002 | ||
| 1 | Stainless steel | SteelWorks | 0.026 ± 0.023 | 0.014 ± 0.007 |
| 2 | Sigg | 0.056 ± 0.018 | 0.043 ± 0.012 | |
| 3 | 0.010 ± 0.004 | 0.009 ± 0.003 | ||
| 1 | Al EcoCare™ | Sigg | 0.006 ± 0.003 | 0.005 ± 0.001 |
| 2 | 0.028 ± 0.008 | 0.021 ± 0.002 | ||
| 3 | 0.016 ± 0.001 | 0.014 ± 0.005 | ||
| 4 | 0.016 ± 0.001 | 0.013 ± 0.004 | ||
| 1A | Al/Epoxy | Sigg | 0.140 ± 0.014 | 0.120 ± 0.005 |
| 2A | 0.131 ± 0.019 | 0.151 ± 0.005 | ||
| 3A | 0.091 ± 0.004 | 0.075 ± 0.001 | ||
| 4A | 0.081 ± 0.015 | 0.065 ± 0.011 | ||
| 5A | 0.104 ± 0.022 | 0.094 ± 0.006 | ||
| 6A | 0.059 ± 0.019 | 0.044 ± 0.009 | ||
| 1B | Al/Epoxy | New Balance | 1.902 ± 0.522 | 1.710 ± 0.311 |
| 2B | 0.767 ± 0.058 | 0.658 ± 0.016 | ||
| 3B | 0.931 ± 0.211 | 0.840 ± 0.080 | ||
| 4B | 1.305 ± 0.979 | 1.072 ± 0.072 |
Values are shown ± standard deviation; n = 3; Al: aluminum; conc: concentration
Figure 2. Comparison of bisphenol A (BPA) migration into water from reusable drinking bottles.
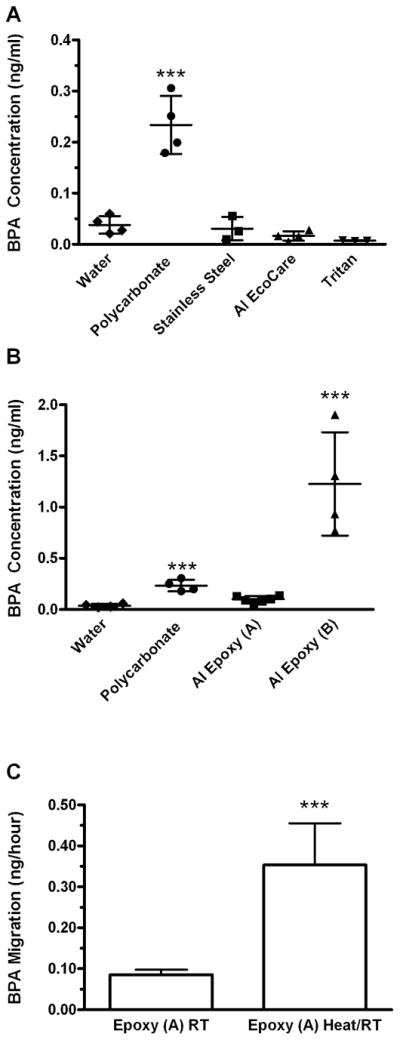
The comparative analysis of BPA concentration values calculated following room temperature incubation for 120 hr in new polycarbonate, stainless steel, EcoCare™ lined or Tritanwater bottles are show. (B) The analysis of the BPA concentrationcalculated following room temperature incubation for 120 hr in new polycarbonate, and two different epoxy lined aluminium water bottlesare show. In both panels the mean values are indicated and graphically represented with a horizontal line. Error bars represent the standard deviation. *** indicates that values are significantly different (p< 0.001) from the negative control water samples as assessed with a one-way ANOVA and also different from the 0.05 ng/ml detection limit as calculated with a one sample t-test using a theoretical mean of 0.05. (C) Comparison of the calculated rates of migration of BPA from epoxy lined bottle group that were incubated with room temperature water (RT) or incubated at room temperature with water that was added to the bottleheated to boiling (Heat/RT). Apparent mean migration rates are shown in ng/hour (± SD)*** indicates that values are significantly different (p< 0.001) from each other.
In contrast to the polycarbonate bottles, no BPA wasobserved to migrate from EcoCare™ lined aluminium, stainless steel, or Tritan™ plastic water bottles over the 120 hr incubation period. In each case BPA levels were well below established detection limits of the assay (Table 1; Fig, 2B). Interestingly, one of the two types of epoxy-lined aluminium bottles (group A, 8127.90; Sigg) leached low, but detectable amounts of BPA that reached an average concentration of 0.101 ng/ml ± 0.o30 and ranged from 0.14 ng/ml to 0.o6 ng/ml for individual bottles (Table 1). Although the surface area of the bottles in group B (800 ml volume bottles) was significantly less, the amount of BPA leaching from group B epoxy-lined aluminium bottles (mean of 1.23 ng/ml ± S.D. 0.50) was more than 12 times greater than the group A bottles average (Table 1; Fig 2C.). To determine whether exposure to increased liquid temperatures impacted the low levels of BPA leaching from the epoxy resin lining from the group A bottles (8127.90; Sigg), the leaching of BPA for bottles that were incubated with room temperature water or heated water were compared. As above leaching from unheated bottles was low (0.086 ng/hr S.D. ± 0.017). In contrast, epoxy resin lined bottles that were initially exposed to boiling water and allowed to cool during the incubation period the migration of BPA into water was increased significantly by more than 4 fold to 0.354 ng/hr ± S.D. 0.101 (Fig. 2C).
4. Discussion
Previous study has shown that BPA migrated into water from polycarbonate drinking bottles and that amounts of BPA were proportional to incubation time(Le et al., 2008). The main goal of the study was to determine whether or not the commercially available materials used for reusable water bottles were indeed free of BPA that could migrate into water stored in these vessels. Because BPA is widely used in the manufacture of many consumer products, especially plastics where it is used as a plasticiser or a stabilizing coating for many forms of plastics and other materials, it is difficult to know based solely on the nature of the polymer whether the materials are truly free of BPA. Because of the proprietary nature of many of the alternative polymers or linings, and the lack of rigorous and independent confirmation of BPA-free claims, there is a level of scepticism surrounding manufacture’s marketing claims that their products are “safe” or “BPA-free”. Eastman’s polycarbonate alternative Tritan™ is a copolyester polymer that is being used to manufacture “BPA-free” water bottles. In agreement with manufactures statements, our analysis found that BPA was not detected in water incubated in Nalgen branded water bottles made of Tritan. As anticipated, the uncoated stainless steel bottles similarly did not leach BPA in to water.
During the 120 hour incubation period used in the current study the amounts of BPA (mean = 0.23 ng/ml; range 0.18 to 0.31 ng/ml; n=4) migrating into water from the positive control polycarbonate bottles was comparable, but lower (p=0.0444) than the levels reported previously by Le and co-workers(2008) over the same incubation period (mean = 0.52 ng/ml; range 0.28 to 0.68 ng/ml; n=3). This finding may be due to a relative decrease in sensitivity of the assay. Quality control studies suggest that differences can result due to cumulative effects of small variations in experimental conditions or the ELISA assay itself (e.g. variations in goodness of standard curve fits orreagent/antibody characteristics). However the observed difference in migration could be related in unknown ways to the age of the bottles – the bottles used here were acquired at the same time as those used by Le et al (2008), and stored in the dark unused for more than 2 years under climate controlled laboratory conditions (22°C).
The Swiss supplier Sigg manufactures aluminium bottles that have a BPA-free liner (EcoCare™) with a proprietary formula. There was some concern in 2008 that water bottles manufactured by Sigg were made with an epoxy-based liner that theoretically could leach BPA; however, the manufacturer purportedly claimed that extensive testing had revealed that the lining did not leach BPA. Since August of 2008 all Sigg bottles were manufactured using the EcoCare™ liner, which is guaranteed to contain no BPA. Consistent with that claim, BPA was undetectable in water samples following incubation in EcoCare™ lined Sigg bottles. Testing of the BPA leaching properties of Sigg bottles that were manufactured prior to August 2008 revealed that low, but detectable levels of BPA were migrating into water incubated in these older epoxy resin lined bottles. Upon adding heated water to those bottles, the migration of BPA was increased. Clearly BPA migrates from these linings, and while not absent, the amounts of BPA migrating into the water is considered low and unlikely to constitute a major source of BPA for the consumer if the manufactures guidelines of consuming liquids relatively rapidly and not storing heated liquids in the bottles are followed.
One of the most interesting results was the comparison of the epoxy lined bottles from a discount retailer with those from Sigg. The discount epoxy lined bottles, released much higher levels of BPA even without first being exposed to high temperature liquid. The difference in the amount of BPA migrating from the discount aluminium epoxy lined bottles and those manufactured by Sigg was 12 fold. In fact, the concentrations of BPA in water exposed to those bottles was more than 5 times the concentration of water samples from polycarbonate bottles which have a significantly larger internal surface area.Along with higher temperatures increasing the BPA migration from polycarbonate, these studies indicate that elevated temperatures greatly increase the liberation of BPA from epoxy-based liners; these studies have confirmed that there areunknown variables related to the manufacture of the epoxy liners that determines how much BPA migration occurs. Those variables are unknown, however the observations reported in this study is that under normal, and especially after exposure to extreme condition (e.g. temperatures above manufacture recommendations) BPA can leach from epoxy resins regardless of perceived quality. However, commercially available “BPA free” alternatives in the form of plastic reusable drinking bottles and lined or unlined metallic drinking bottles were identified. No detectable BPA was observed to migrate from EcoCare™ lined aluminium, stainless steel, or Tritan™plastic water bottles. Therefore, based on the presented results it appears that reusable water bottles constructed of those alternative materials and when used according to manufacturer’s recommendations, can be considered suitable for storage and consumption of beverages free of container mediated BPA contamination.
Conclusions
As shown previously by our results and those of many others, BPA migrates into water stored inpolycarbonate plastic, especially when heated to high temperatures. Relatively high levels of BPA arefound to be released into drinking water from reusable aluminium bottles withan epoxy-based liner. The amount of BPA released from the epoxy coating was greatly increased by heating. No detectable BPA was observed in water to migrate from unlined stainless steel, EcoCare™ copolyester lined aluminium, or copolyester Tritan™plastic water bottles. To avoid exposures to BPA unlined stainless steel, copolyester lined aluminium or copolyester plastic drinking bottles should be used.
BPA migration from different reusable water bottles was assessed
BPA migrated into water from aluminum bottles lined with epoxy-based resins
BPA contamination was not observed from bottles made from copolyester plastic
Stainless steel, or copolyester lined aluminum lined bottles did not release BPA
Acknowledgments
These studies were supported by NIH/NIEHS grant, RC2-ES-018765; R01-ES015145, University of Cincinnati Centre for Environmental Genetics (P30-ES06096) and T32 ES016646-02. The authors are grateful to Steve Casimiro (http://www.theadventurelife.org) for his interest enthusiastic encouragement topursuing this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Coensel N, David F, Sandra P. Study on the migration of bisphenol-A from baby bottles by stir bar sorptive extraction-thermal desorption-capillary GC-MS. Journal of Separation Science. 2009;32:3829–3836. doi: 10.1002/jssc.200900349. [DOI] [PubMed] [Google Scholar]
- 3.Grumetto L, Montesano D, Seccia S, Albrizio S, Barbato F. Determination of Bisphenol A and Bisphenol B Residues in Canned Peeled Tomatoes by Reversed-Phase Liquid Chromatography. Journal of Agricultural and Food Chemistry. 2008;56:10633–10637. doi: 10.1021/jf802297z. [DOI] [PubMed] [Google Scholar]
- 4.Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, vomSaal FS, Welshons WV. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. EnvironHealthPerspect. 2003;111:1180–1187. doi: 10.1289/ehp.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE., Jr Gestational and Lactational Exposure to Ethinyl Estradiol, but not Bisphenol A, Decreases Androgen-Dependent Reproductive Organ Weights and Epididymal Sperm Abundance in the Male Long Evans Hooded Rat. ToxicolSci. 2008;102:371–382. doi: 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- 6.Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicology Letters. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maia J, Cruz JM, Sendón R, Bustos J, Cirugeda ME, Sanchez JJ, Paseiro P. Effect of amines in the release of bisphenol A from polycarbonate baby bottles. Food Research International. 2010;43:1283–1288. [Google Scholar]
- 8.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive Toxicology. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, Seely JC, Dimond SS, Van Miller JP, Shiotsuka RN, Beyer D, Hentges SG, Waechter JM. Two-Generation Reproductive Toxicity Study of Dietary Bisphenol A in CD-1 (Swiss) Mice. Toxicological Sciences. 2008;104:362–384. doi: 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]