Abstract
Rationale
Cariprazine is a novel antipsychotic drug candidate that exhibits high selectivity and affinity to dopamine D3 and D2 receptors and moderate affinity to serotonin 5-HT1A receptors. Targeting receptors other than D2 may provide a therapeutic benefit for both positive and negative symptoms associated with schizophrenia. Positron emission tomography (PET) can be used as a tool in drug development to assess the in vivo distribution and pharmacological properties of a drug.
Objectives
The objective of this study was to determine dopamine D2/D3 and serotonin 5-HT1A receptor occupancy in monkey brain after the administration of cariprazine.
Methods
We examined three monkeys using the following PET radioligands: [11C]MNPA (an agonist at D2 and D3 receptors), [11C]raclopride (an antagonist at D2 and D3 receptors), and [11C]WAY-100635 (an antagonist at 5-HT1A receptors). During each experimental day, the first PET measurement was a baseline study, the second after a low dose of cariprazine, and the third after the administration of a high dose.
Results
We found that cariprazine occupied D2/D3 receptors in a dose-dependent and saturable manner, with the lowest dose occupying ~5% of receptors and the highest dose showing more than 90% occupancy. 5-HT1A receptor occupancy was considerably lower compared with D2/D3 occupancy at the same doses, with a maximal value of ~30% for the raphe nuclei.
Conclusions
We conclude that cariprazine binds preferentially to dopamine D2/D3 rather than to serotonin 5-HT1A receptors in monkey brain. These findings can be used to guide the selection of cariprazine dosing in humans.
Keywords: Cariprazine, Positron emission tomography (PET), Nonhuman primate brain, Dopamine D2, Dopamine D3, Serotonin 5-HT1A, Receptor occupancy
Introduction
Antipsychotic drugs have been shown to be beneficial for the treatment of schizophrenia. Antagonism at the dopamine D2 receptor is considered an essential component of their mechanism of action (Creese et al. 1976; Seeman et al. 1975). Antipsychotic drugs acting at other receptor targets, such as the D3 receptor, may provide a promising target due to the receptor’s anatomical localization and pharmacological properties (Sokoloff et al. 2006). Early in vitro studies have shown that the D3 receptor is concentrated in limbic areas (e.g., nucleus accumbens), whereas in vivo studies in primate and human brain using [11C]-(+)-PHNO have extended the earlier in vitro localization studies now showing high concentrations of the D3 receptor in areas such as globus pallidus <substantia nigra <ventral striatum <thalamus (Searle et al. 2010; Rabiner et al. 2009; Tziortzi et al. 2011). Thus, the receptor’s anatomical localization suggests that the receptors may be involved in modulating memory function, speech, and focused attention in schizophrenia (Sokoloff et al. 1990; Suzuki et al. 1998). Studies in mice lacking the dopamine D3 receptor aide in understanding the functional role of this receptor subtype, which may well be involved in the regulation of anxiety (Steiner et al. 1997), increased locomotor activity and rearing behavior (Accili and Fuchs 1996), and regulation of dopamine levels (Le Foll et al. 2005). In addition, a twofold elevation in D3 receptor expression has been found in postmortem tissue of schizophrenic patients (Gurevich et al. 1997). Thus, antipsychotic drugs acting selectively on the D3 receptor may provide an effective treatment for patients with schizophrenia.
Cariprazine is a novel antipsychotic drug candidate that exhibits high affinity for the D3 (Ki=0.085 nM) and D2 (Ki=0.49 nM) receptors, and moderate affinity for the 5-HT1A receptor (Ki=2.6 nM, Table 1; Kiss et al. 2010). Cariprazine was evaluated in multiple in vitro and in vivo functional assays and demonstrated antagonist–partial agonist properties at D2 and D3 receptors (Kiss et al. 2010). In addition, rodent models predicting antipsychotic-like activity such as conditioned avoidance response and inhibition of amphetamine-induced hypermotility confirmed that cariprazine does possess antipsychotic potential while devoid of cataleptogenic effect in comparison with reference antipsychotic drugs (Gyertyán et al. 2006). The affinity of cariprazine for the D3 receptor is significantly higher than that of marketed antipsychotic drugs and may provide additional therapeutic benefits compared with more D2-selective agents (Schotte et al. 1996; Millan et al. 1999).
Table 1.
In vitro receptor binding inhibition constants (Ki) of several compounds used in this study
| Compound | Receptor Ki (nM) | |||
|---|---|---|---|---|
| D2 | D3 | 5-HT1A | 5-HT2A | |
| Cariprazinea | 0.5 | 0.09 | 2.6 | 180 |
| Haloperidolb | 2.2 | 7.8 | 1500 | 200 |
| Clozapineb | 190 | 280 | 140 | 9.6 |
| Risperidoneb | 5.9 | 14 | 420 | 0.52 |
| Raclopridec | 1.1 | 1.4 | – | – |
| MNPAd | 0.09f | 1.02g | – | – |
| WAY-100635e | 79 | 67 | 0.24 | 1,100 |
Ki value is for the high-affinity state
Ki value was estimated for one affinity state (i.e., low)
Positron emission tomography (PET) can be used as a tool in drug development to assess the in vivo distribution and pharmacological properties of a drug (Lee and Farde 2006; Halldin et al. 2001; Wong et al. 2009; Hargreaves 2008). One approach is to measure the degree to which administration of a new drug candidate (such as cariprazine) competes with the specific binding of a characterized PET radioligand for the receptor target of interest (e.g., dopamine D2/D3 receptors). The effects of a new drug candidate are related to the percentage of receptor sites occupied by the drug and receptor occupancy reflected as the percent change in the PET outcome measure determined under the drug treatment condition compared with that under baseline measures. Another approach would be to extrapolate the in vitro determination of intrinsic activity using receptor binding assays to the in vivo situation of PET receptor occupancy studies (Lahti et al. 1992; Sibley and Creese 1983). Receptor occupancy studies utilizing a dual radioligand approach (i.e., radiolabeled agonist and radiolabeled antagonist) may provide a functional profile of the new drug candidate’s intrinsic activities. For example, if the candidate new drug has an antagonist profile, a comparison of the agonist/antagonist radioligand ratio of receptor occupancy would be ~1.0, whereas if the candidate new drug has an agonist profile, this ratio would result in a higher value. In the current study, we used PET imaging with an agonist and antagonist radioligand to determine the in vivo potency of cariprazine and assess its intrinsic activity as an agonist vs. as an antagonist.
The aim of the present study was to determine whether cariprazine occupies dopamine D2/D3 and serotonin 5-HT1A receptors in monkey brain. We used both antagonist ([11C]raclopride) and agonist ([11C]MNPA) radioligands to measure D2/D3 receptor occupancy in striatum and [carbonyl-11C]WAY-100635 to measure 5-HT1A receptor occupancy in raphe nuclei and forebrain.
Materials and methods
Radioligand preparation
[11C]Raclopride, [11C]MNPA, and [carbonyl-11C]WAY-100635 were prepared as described previously (Langer et al. 1999; Finnema et al. 2005; Pike et al. 1995). The specific activity of [11C]raclopride, [11C]MNPA, and [carbonyl-11C]WAY-100635 at the time of injection was 10,103±2,163, 81,545±39,480, and 1,576±1,865 Ci/mmol, respectively. The injected mass of [11C]raclopride and [carbonyl-11C]WAY-100635 at the time of injection was 0.05±0.01, 0.006±0.002, and 1.2±0.9 µg, respectively. The radiochemical purity was ~99%.
PET imaging
A total of 15 PET experiments were performed in three cynomolgus monkeys (Macaca fascicularis) weighing 3–4 kg. The study was approved by the Animal Ethics Committee of the Swedish Animal Welfare Agency (Dnr 245/04 and 147/05) and was performed according to “Guidelines for Planning, Conducting and Documenting Experimental Research” (Dnr 4820/06-600) of Karolinska Institutet. Anesthesia was induced and maintained by repeated intramuscular injections of a mixture of ketamine hydrochloride (3.75 mg kg−1 h−1 Ketalar®, Pfizer) and xylazine hydrochloride (1.5 mg kg−1 h−1 Rompun® Vet., Bayer). The head was immobilized with a fixation device (Karlsson et al. 1993). Body temperature was maintained with a forced-air-heated air blanket (Bair Hugger model 505, Arizant Healthcare Inc., MN, USA) and monitored by a rectal thermometer (Precision Thermometer, Harvard Apparatus, MA, USA). Cardiac and respiratory rates were measured every 20 min.
After injection of either [11C]raclopride (53–55 MBq, n=6), [11C]MNPA (50–53 MBq, n=3), or [carbonyl-11C] WAY-100635 (52–58 MBq, n=6) in three monkeys, PET measurements were acquired for 93 min in 20 frames, with frames of 3 × 1 min, 4 × 3 min, and the remaining frames of 6 min. PET measurements were acquired in three-dimensional mode with the Siemens ECAT Exact HR 47 (Wienhard et al. 1994). Before radioligand injection, a 10-min transmission measurement for attenuation correction was collected using a 68Ge line source.
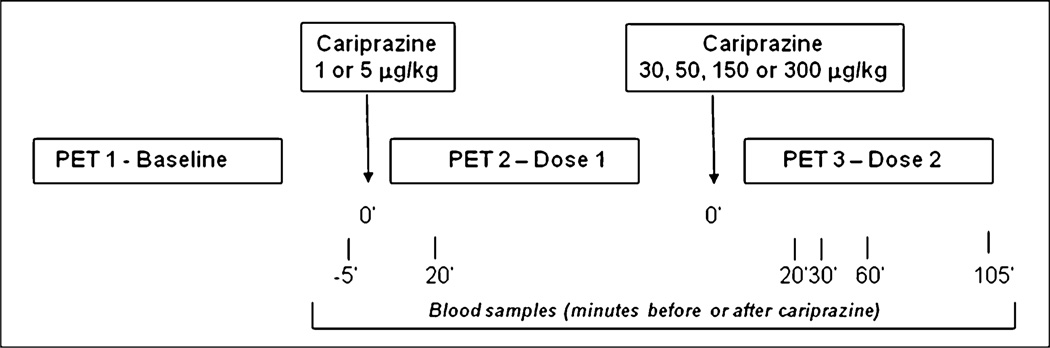
The monkeys participated in three consecutive PET measurements per experimental day. The first PET measurement was performed at baseline conditions, the second after a low dose (1–5 µg/kg) of cariprazine, and the third after the administration of a high dose (30–300 µg/kg). Cariprazine was injected intravenously over 30 s, approximately 15 min prior to radioligand injection. The study timeline for PET measurements, administration of study drug, and venous blood sampling is shown in Scheme 1. Gedeon Richter Plc. (Budapest, Hungary) provided cariprazine.
Scheme 1.
Imaging study timeline. Top boxes reflect the intravenous doses of cariprazine administered ~15 min prior to PET measurement 2 or PET measurement 3. The boxes below reflect the series of PET measurements per study day. The information below the series of PET measurements refers to timing in minutes of the blood samples for estimation of the plasma concentration of cariprazine, desmethyl-, and didesmethyl-cariprazine
Image analysis and calculation of outcome measures
To calculate dopamine and serotonin receptor occupancy, the outcome measure was quantified with two methods, the transient equilibrium method and the two-parameter multilinear reference tissue model (MRTM2). The first outcome measure was the transient equilibrium method (Farde et al. 1992; Andree et al. 1998; Nyberg et al. 1996; Ito et al. 1998). Briefly, the transient equilibrium method calculates the ratio of bound/free as defined as the concentration in tissue of interest (CT(t)) minus the concentration in the reference region (CREF(t)) (bound) and the total radioactivity in a reference region (CREF(t)) (free) with negligible density of D2/D3 and 5-HT1A receptors. The radioactivity in the cerebellum was used as an approximate value for free and non-specifically bound radioligand concentration, bearing in mind the negligible density of D2/D3 (Hall et al. 1994, 1996) and 5-HT1A receptors in the cerebellum (Hall et al. 1997). The ratio bound/free obtained at the peak of specific binding time–activity curve is assumed to represent transient equilibrium (Farde et al. 1989). The second outcome measure we used is the two-parameter multilinear reference tissue model (Ichise et al. 2003) which fits the time–activity curves of the striatum and the cerebellum from 1 min to the end of scan (93 min).
The regions of interest were identified from images created by the two-parameter multilinear reference tissue model (Ichise et al. 2003). This model generates two parametric images from each scanning session: One shows binding potential (BPND) and another shows blood flow relative to the reference region. Anatomical regions of interest were manually defined on the fused image for left and right striatum and cerebellum for dopamine D2/D3 radioligands, temporal and frontal cortex (defined as forebrain), raphé nuclei, and cerebellum for the 5HT1A radioligand. The MRTM2 model requires a priori estimation of a reference region clearance rate (k2′) which was estimated using the three-parameter MRTM from the target and reference region of interest activities. All parametric imaging was performed in PMOD (Mikolajczyk et al. 1998) installed on a PC workstation. Brain uptake was expressed as a standardized uptake value (%SUV) which normalizes for injected activity and body weight.
To calculate D2/D3 and 5-HT1A receptor occupancy, the striatum, forebrain, and raphe nuclei were used as the regions of interest, respectively. Receptor occupancy was defined as the percentage change in the PET outcome measures quantified with two methods, the transient equilibrium method and the two-parameter multilinear reference tissue model. The effects of cariprazine are related to the percentage of receptor sites occupied by the drug which is reflected by the reduced receptor availability of the PET radioligands.
Receptor occupancy was operationally defined as:
Cariprazine, desmethyl-, and didesmethyl-cariprazine concentration in plasma
Venous blood samples (1–2 mL) were obtained from the femoral vein at baseline before drug administration (−5 min), at 20 min after the administration of the low dose (PET 2), and at 20, 30, 60, and 105 min after the injection of the highest dose of the compound. Samples were collected into spray-dried EDTA tubes and centrifuged. The plasma samples were stored frozen at or below −70°C prior to the analysis.
The plasma samples were analyzed for cariprazine and its metabolites, desmethyl- and didesmethyl-cariprazine, by a selective and sensitive LC-MS/MS method. Liquid–liquid extraction was used to isolate the compounds from the biological matrix. The extracts were subjected to reversed-phase HPLC with MS/MS detection. The mass spectrometer was equipped with a Turbo IonSpray interface and operated in positive ion multiple reaction monitoring mode. Deuterated derivatives were used as internal standards. For all the three analyses, the lower limit of quantification of the method was 0.5 ng/mL using 100 µL of plasma, and the calibration curve ranged from 0.5 to 250 ng/mL.
Results
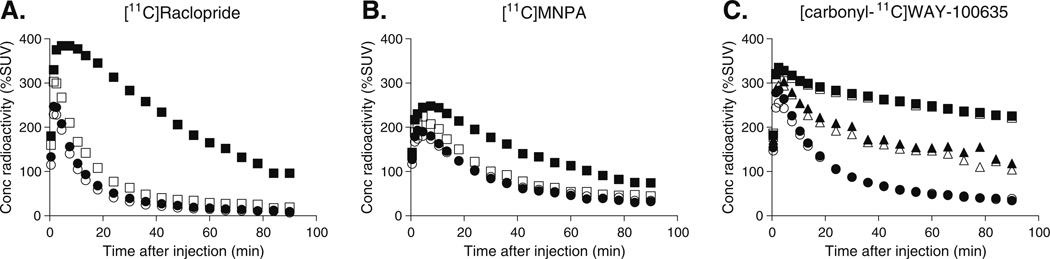
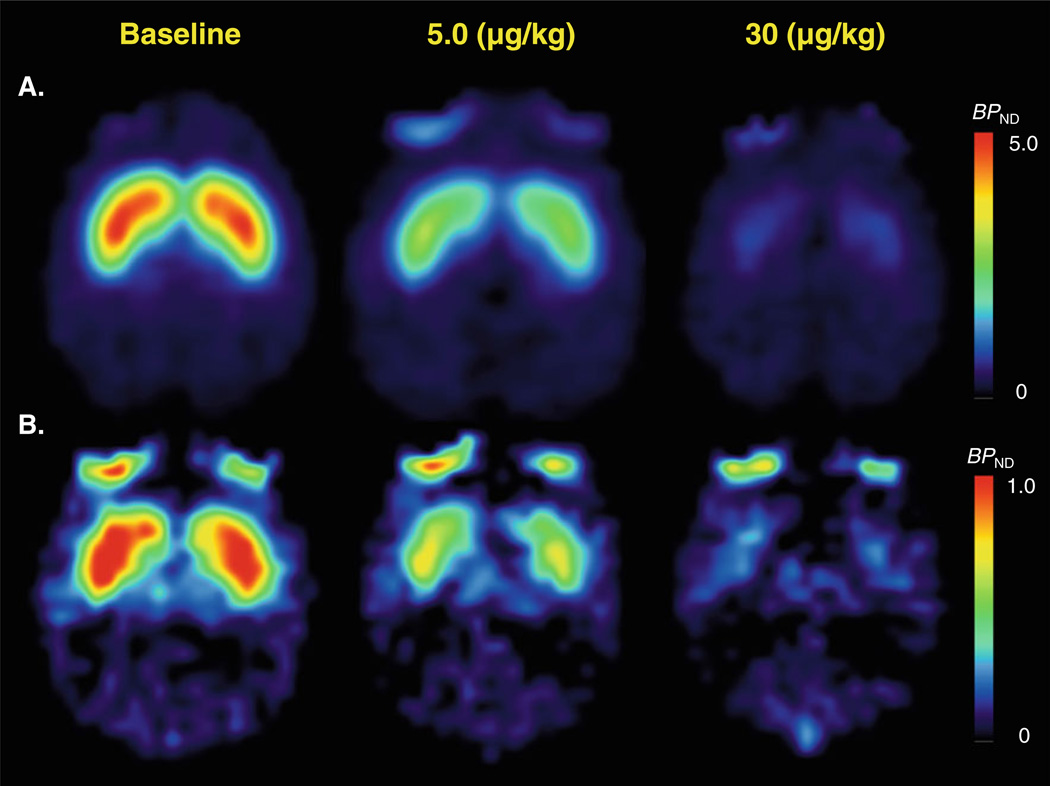
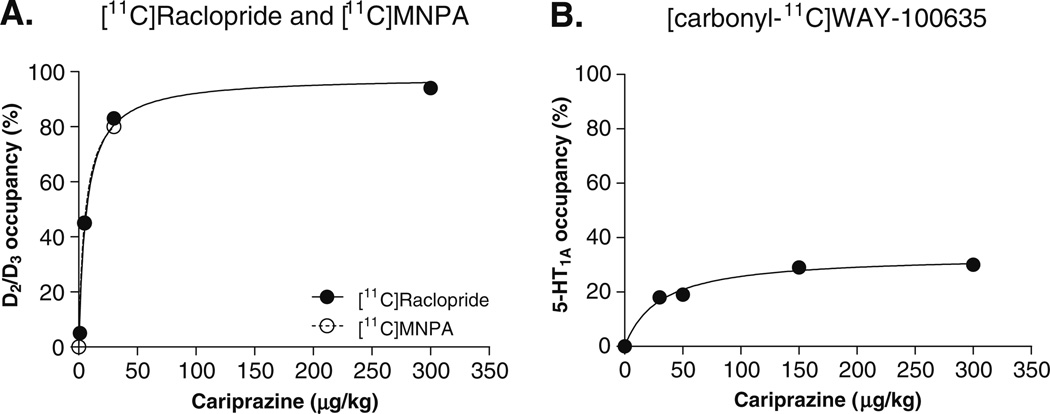
After the injection of [11C]raclopride or [11C]MNPA, uptake of radioactivity was highest in the striatum, with lower levels in the cerebellum (Fig. 1a, b). Administration of cariprazine (30 µg/kg) reduced the striatal uptake of both radioligands to the level of nonspecific binding compared with baseline PET measurements (Fig. 1a, b). Cariprazine had negligible effect on the time–activity curves in the cerebellum. At doses of 5.0 and 30 µg/kg, cariprazine caused a dose-dependent dopamine D2/D3 receptor occupancy of ~45% and ~80% for both antagonist [11C] raclopride and agonist radioligand [11C]MNPA (Figs. 2 and 3). Receptor occupancy of dopamine D2/D3 receptors calculated using the transient equilibrium and the MRTM2 methods ranged from 5% at the lowest dose (1.0 µg/kg) to 94% at the highest dose (300 µg/kg; Fig. 3a and Table 2). A comparison of antagonist/agonist radioligand ratio of receptor occupancy showed a ratio of ~1.0, suggesting that occupancy of D2/D3 receptors by cariprazine equally displaced the agonist and antagonist radioligand.
Fig. 1.
Time–activity curves for regional brain radioactivity concentration (%SUV) after injection of [11C]raclopride (a), [11C]MNPA (b), and [carbonyl-11C]WAY-100635 (c) at baseline conditions (closed symbols) and after intravenous administration of cariprazine at 30 µg/ kg (open symbols). Squares and circles (a, b) represent radioactivity concentrations in the striatum and cerebellum, respectively. Square, triangle, and circle (c) represent radioactivity concentrations in the forebrain, raphe, and cerebellum, respectively
Fig. 2.
Parametric images of [11C]raclopride (a) and [11C]MNPA (b) binding potential (BPND) estimated by the MRTM2 at baseline and after 5.0 and 30 µg/kg of cariprazine
Fig. 3.
A hyperbolic curve fit of dopamine D2/D3 (a) ([11C]raclopride (filled circle) and [11C]MNPA (empty circle)) and (b) serotonin 5-HT1A ([carbonyl-11C]WAY-100635 (filled circle)) receptor occupancy plotted as a function of cariprazine dose (µg/kg). Receptor occupancy reflects the percent change in BPND estimated by MRTM2 under the post-dose drug treatment condition compared with baseline measurements
Table 2.
Dopamine and serotonin receptor occupancy after intravenous administration of increasing doses of cariprazine. Receptor occupancy was measured by the percent change in BPND estimated with the two-parameter multilinear reference tissue model (MRTM2) and the transient equilibrium method
| Receptor occupancy (%) | |||||||
|---|---|---|---|---|---|---|---|
| Cariprazine (µg/kg) | |||||||
| Radioligand | BPND | 1 | 5 | 30 | 50 | 150 | 300 |
| [11C]Raclopride | MRTM2 | 5 | 45 | 83 | –‡ | – | 94 |
| Transient equilibrium | 8 | 49 | 87 | – | - | 97 | |
| [11C]MNPA | MRTM2 | – | 45 | 80 | – | – | – |
| Transient equilibrium | – | 49 | 85 | – | – | – | |
| [11C]WAY-100635 | MRTM2 | – | – | 18 | 19 | 29 | 30 |
| Transient equilibrium | – | – | 11 | 12 | 27 | 24 | |
Not determined
Following injection of [carbonyl-11C]WAY-100635, uptake of radioactivity was highest in the forebrain and raphe nuclei with lower levels in the cerebellum (Fig. 1c). Intravenous administration of 30 µg/kg of cariprazine slightly reduced radioactivity in the raphe nuclei compared with baseline and had no effect in the forebrain. 5-HT1A receptor occupancy could be described by a hyperbolic function, and the maximal reduction of [carbonyl-11C]WAY-100635 binding was ~30% for the raphe nuclei (Fig. 3b).
The plasma concentrations of cariprazine increased with dose, with values ranging from <1.0 ng/mL at the lower doses (1–5 µg/kg) to 3.11–34.1 ng/mL at the higher doses (30–300 µg/kg). The concentration of desmethyl- or didesmethyl-cariprazine at all doses of cariprazine studied was <5 ng/mL.
Discussion
In this study, we have shown that administration of cariprazine at increasing doses resulted in a dose-dependent and saturable reduction in the specific binding of [11C]raclopride (an antagonist at D2 and D3 receptors) and [11C]MNPA (an agonist at D2 and D3 receptors). On the other hand, administration of cariprazine slightly reduced the specific binding of [carbonyl-11C]WAY-100635 (an antagonist at 5-HT1A receptors). For example, administration of cariprazine at 30 µg/kg induced higher striatal D2/D3 receptor occupancy of ~87% compared with only ~20% for 5-HT1A receptor in raphe nuclei. These in vivo receptor occupancy studies confirmed the in vitro measurements showing that cariprazine has a much lower affinity for 5-HT1A than D2/D3 receptors.
The pharmacological properties of cariprazine using multiple in vitro and in vivo functional assays demonstrated antagonist–partial agonist properties at D2 and D3 receptors (Kiss et al. 2010). To explore the in vivo pharmacological properties of cariprazine, we compared receptor occupancy of D2/D3 receptors using a dual radioligand approach (i.e., using a radiolabeled agonist and a radiolabeled antagonist). At tracer doses, the agonist radioligand is thought to bind almost exclusively to the high-affinity state of the receptor, whereas the antagonist radioligand binds with equal affinity to both high- and low-affinity states. It has been suggested that the preference of a drug for the high-affinity state (reflected as an agonist/antagonist ratio of drug inhibition of >1) may relate to the intrinsic activity to the receptor in in vitro studies (Lahti et al. 1992). Thus, an agonist/antagonist radioligand receptor occupancy ratio may provide an in vivo estimation of intrinsic activity. The agonist/antagonist receptor occupancy ratio for cariprazine was ~1.0, potentially suggesting antagonistic properties. However, in a similar PET study, we showed comparable D2/D3 receptor occupancy for the agonist apomorphine when comparing apomorphine’s occupancy of a radiolabeled agonist and a radiolabeled antagonist (Finnema et al. 2009). Thus, we therefore cannot exclude the (partial) agonistic properties of cariprazine.
The lack of subtype selectivity of the dopamine receptor PET radioligands used in this study for D2 vs. D3 receptors makes it difficult to assess the relative occupancy at these receptor subtypes. After the injection of [11C]raclopride or [11C]MNPA, uptake of radioactivity is high in D2 receptor-rich regions, such as the dorsal striatum, and lower in D3 receptor-rich regions, such as the ventral striatum (Gurevich and Joyce 1999). The striatal regions of interest in this study account for the radioligand binding in the dorsal striatum, which would primarily reflect receptor occupancy at the D2 receptor. Cariprazine has been shown to have in vitro greater affinity for the D3 receptor (Ki=0.09 nM) compared with the D2 receptor (Ki=0.5 nM; Table 1). Since cariprazine has higher in vitro affinity to the D3 receptor, estimating the in vivo selectivity to the D3 over the D2 receptor may be possible utilizing PET radioligands such as [11C]-(+)-PHNO (a preferential affinity for D3 over D2 receptors). Imaging studies utilizing PET radioligands which are selective to the D3 receptor (i.e., [11C]-(+)-PHNO) combined with a mixed receptor subtype radio-ligand such as [11C]raclopride may aide in understanding the in vivo selectivity of receptor occupancy for the D3 over D2 receptors by comparing the receptor occupancy in the dorsal striatum (e.g., caudate plus putamen) vs. the globus pallidus in the same subject (Graff-Guerrero et al. 2009). Thus, future studies including the D3 receptor preferring radioligands such as [11C]-(+)-PHNO would help determine the occupancy of D3 receptors by cariprazine (Tziortzi et al. 2011; Searle et al. 2010).
The finding of the relatively low maximal 5-HT1A receptor occupancy suggests that cariprazine could have limited pharmacological actions via this target. These PET data demonstrated an ED50 value of 5 µg/kg at the D2/D3 receptors and a five times greater dose induced only ~18% occupancy at the 5-HT1A receptor. These receptor occupancy values may be underestimated since [carbonyl-11C] WAY-100635 is an antagonist and binds with equal affinity to the high- and low-affinity states of the receptor, as described earlier. If cariprazine acts as a partial agonist, it may bind preferentially to a subpopulation of these receptors that are in the high-affinity state. However, the 5-HT1A full agonist 8-OH-DPAT and partial agonist buspir-one have been shown to displace all of the antagonist radioligand [carbonyl-11C]WAY-100635 binding in monkey brain (Mathis et al. 1994; Farde et al. 1997). The primary CNS actions of cariprazine are therefore related to the D3 and D2 receptors, with minimal 5-HT1A-related effects at therapeutic dose levels.
Several methodological considerations should be taken into account when interpreting the results of the present PET study. (1) The dual radioligand approach using the agonist and antagonist D2/D3 receptor radioligand used a small sample size, and the reliability of this approach has to be confirmed in larger samples. (2) The small ROI for the raphe nuclei may have caused noisy time–activity curves, and the resolution of PET restricts our ability to quantify accurately the BPND in this area. (3) Autoradiographic studies with [carbonyl-11C]WAY-100635 have found the cerebellum almost devoid of 5-HT1A receptors (Hall et al. 1997), with the exception of the vermis that contains a moderate density of sites. As described in detail by Parsey et al. (2005), we excluded the vermis from the cerebellar region of interest since this method allows for a more accurate estimation of the reference region. (4) Ketamine and xylazine were used to induce and maintain anesthesia and may have affected radioligand binding. Ketamine administered at greater doses has been found to reduce [11C]raclopride binding in monkey striatum (Tsukada et al. 2000), whereas several studies in humans at lower doses have found no change in [11C]raclopride striatal binding (Aalto et al. 2002; Kegeles et al. 2002). In addition, ketamine can inhibit serotonin uptake and increase the levels of serotonin in the rat brain (Martin et al. 1982). Thus, ketamine may have some effect on radioligand binding in these PET studies.
We applied two methods to calculate the binding potential and to quantify receptor occupancy. The first approach estimated the binding potential by using the transient equilibrium method that has been applied extensively to both pre-clinical monkey and human clinical PET studies (Farde et al. 1988, 2000). The second method to calculate binding potential was estimated with MRTM2; this model incorporates two strategies to improve parameter estimation at the voxel noise level: (1) The number of parameters are reduced from three to two by fixing k2′ to a value estimated from the selected regions of interest and (2) rapid linear least squares estimation algorithm allows faster computation (Ichise et al. 2003). In addition, MRTM is identical to SRTM reference tissue model when MRTM t* is set near zero. However, the clear advantage of MRTM2 is that this reference tissue model can be used for radioligands with two tissue compartmental model kinetics (Ichise et al. 2003). The D2/D3 receptor BPND and receptor occupancy values were similar between the two quantitative methods applied in these studies (Table 2), demonstrating that the methods are comparable. By using two different methods, we allow comparison with a large body of previous work that used the transient equilibrium method.
In summary, we have shown that cariprazine occupies dopamine D2/D3 receptors in a dose-dependent and saturable manner and that cariprazine displays lower occupancy of serotonin 5-HT1A receptors. Cariprazine had similar occupancy measured with both an agonist ([11C]MNPA) and an antagonist ([11C]raclopride) radioligand, potentially suggesting antagonist properties. These findings can be used to guide the selection of cariprazine dosing in humans.
Acknowledgments
We thank the members of the Karolinska PET group for their assistance. This research was supported in part by the Intramural Program of the National Institute of Mental Health, Bethesda, Maryland, USA.
Contributor Information
Nicholas Seneca, Department of Clinical Neuroscience, Psychiatry Section, Karolinska Institutet, Stockholm 171 76, Sweden; Molecular Imaging Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA.
Sjoerd J. Finnema, Department of Clinical Neuroscience, Psychiatry Section, Karolinska Institutet, Stockholm 171 76, Sweden
István Laszlovszky, Gedeon Richter Ltd., Budapest 1103, Hungary.
Béla Kiss, Gedeon Richter Ltd., Budapest 1103, Hungary.
Attila Horváth, Gedeon Richter Ltd., Budapest 1103, Hungary.
Gabriella Pásztor, Gedeon Richter Ltd., Budapest 1103, Hungary.
Margó Kapás, Gedeon Richter Ltd., Budapest 1103, Hungary.
István Gyertyán, Gedeon Richter Ltd., Budapest 1103, Hungary.
Sándor Farkas, Gedeon Richter Ltd., Budapest 1103, Hungary.
Robert B. Innis, Molecular Imaging Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA
Christer Halldin, Department of Clinical Neuroscience, Psychiatry Section, Karolinska Institutet, Stockholm 171 76, Sweden.
Balás Gulyás, Email: balazs.gulyas@ki.se, Department of Clinical Neuroscience, Psychiatry Section, Karolinska Institutet, Stockholm 171 76, Sweden.
References
- Aalto S, Hirvonen J, Kajander J, Scheinin H, Nagren K, Vilkman H, Gustafsson L, Syvalahti E, Hietala J. Ketamine does not decrease striatal dopamine D2 receptor binding in man. Psychopharmacology. 2002;164:401–406. doi: 10.1007/s00213-002-1236-6. [DOI] [PubMed] [Google Scholar]
- Accili D, Fuchs S. A new look at dopamine D3 receptors. Mol Psychiatry. 1996;1:93–94. [PubMed] [Google Scholar]
- Andree B, Nyberg S, Ito H, Ginovart N, Brunner F, Jaquet F, Halldin C, Farde L. Positron emission tomographic analysis of dose-dependent MDL 100,907 binding to 5-HT2A receptors in the human brain. J Clin Psychopharmacol. 1998;18:317–323. doi: 10.1097/00004714-199808000-00012. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of anti-schizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Farde L, Wiesel FA, Halldin C, Sedvall G. Central D2 dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry. 1988;45:71–76. doi: 10.1001/archpsyc.1988.01800250087012. [DOI] [PubMed] [Google Scholar]
- Farde L, Wiesel FA, Nordstrom AL, Sedvall G. D1 and D2 dopamine receptor occupancy during treatment with conventional and atypical neuroleptics. Psychopharmacology. 1989;99 Suppl:S28–S31. doi: 10.1007/BF00442555. [DOI] [PubMed] [Google Scholar]
- Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- Farde L, Ginovart N, Ito H, Lundkvist C, Pike VW, McCarron JA, Halldin C. PET-characterization of [carbonyl-11C]WAY-100635 binding to 5-HT1A receptors in the primate brain. Psychopharmacology. 1997;133:196–202. doi: 10.1007/s002130050391. [DOI] [PubMed] [Google Scholar]
- Farde L, Andree B, Ginovart N, Halldin C, Thorberg S. PET-determination of robalzotan (NAD-299) induced 5-HT1A receptor occupancy in the monkey brain. Neuropsychopharmacology. 2000;22(4):422–429. doi: 10.1016/S0893-133X(99)00125-6. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Seneca N, Farde L, Shchukin E, Sovago J, Gulyas B, Wikstrom HV, Innis RB, Neumeyer JL, Halldin C. A preliminary PET evaluation of the new dopamine D2 receptor agonist [11C]MNPA in cynomolgus monkey. Nucl Med Biol. 2005;32:353–360. doi: 10.1016/j.nucmedbio.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Halldin C, Bang-Andersen B, Gulyás B, Bundgaard C, Wikström HV, Farde L. Dopamine D2/D3 receptor occupancy of apomorphine in the nonhuman primate brain—a comparative PET study with [11C]raclopride and [11C]MNPA. Synapse. 2009;63:378–389. doi: 10.1002/syn.20615. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mamo D, Shammi CM, Mizrahi R, Marcon H, Barsoum P, Rusjan P, Houle S, Wilson AA, Kapur S. The effect of antipsychotics on the high-affinity state of D2 and D3 receptors: a positron emission tomography study with [11C]-(+)-PHNO. Arch Gen Psychiatry. 2009 Jun;66(6):606–615. doi: 10.1001/archgenpsychiatry.2009.43. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Bordelon Y, Shapiro RM, Arnold SE, Gur RE, Joyce JN. Mesolimbic dopamine D3 receptors and use of antipsychotics in patients with schizophrenia A postmortem study. Arch Gen Psychiatry. 1997;54:225–232. doi: 10.1001/archpsyc.1997.01830150047009. [DOI] [PubMed] [Google Scholar]
- Gyertyán I, Kiss B, Sághy K, Laszy J, Szabó Gy K, Ágai-Csongor É, Gy D, Tihanyi K, Zs S. RGH-188, an atypical antipsychotic with dopamine D3/D2 antagonist/partial agonist properties: behavioral characterization. Int J Neuropsychopharm. 2006;9 suppl 1:S222. [Google Scholar]
- Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1 and D2 dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11:245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- Hall H, Halldin C, Dijkstra D, Wikstrom H, Wise LD, Pugsley TA, Sokoloff P, Pauli S, Farde L, Sedvall G. Autoradiographic localisation of D3 dopamine receptors in the human brain using the selective D3 dopamine receptor agonist (+)-[3H]PD 128907. Psychopharmacology. 1996;128:240–247. doi: 10.1007/s002130050131. [DOI] [PubMed] [Google Scholar]
- Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, Fletcher A, Cliffe IA, Barf T, Wikstrom H, Sedvall G. Autoradiographic localization of 5-HT1A receptors in the postmortem human brain using [3H]WAY-100635 and [11C]WAY-100635. Brain Res. 1997;745:96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- Halldin C, Gulyas B, Farde L. PET studies with carbon-11 radioligands in neuropsychopharmacological drug development. Curr Pharm Des. 2001;7:1907–1929. doi: 10.2174/1381612013396871. [DOI] [PubMed] [Google Scholar]
- Hargreaves RJ. The role of molecular imaging in drug discovery and development. Clin Pharmacol Ther. 2008;83:349–353. doi: 10.1038/sj.clpt.6100467. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Ito H, Hietala J, Blomqvist G, Halldin C, Farde L. Comparison of the transient equilibrium and continuous infusion method for quantitative PET analysis of [11C]raclopride binding. J Cereb Blood Flow Metab. 1998;18(9):941–950. doi: 10.1097/00004647-199809000-00003. [DOI] [PubMed] [Google Scholar]
- Johansson L, Sohn D, Thorberg SO, Jackson DM, Kelder D, Larsson LG, Rényi L, Ross SB, Wallsten C, Eriksson H, Hu PS, Jerning E, Mohell N, Westlind-Danielsson A. The pharmacological characterization of a novel selective 5-HT1A receptor antagonist, NAD-299. J Pharmacol Exp Ther. 1997;283:216–225. [PubMed] [Google Scholar]
- Karlsson P, Farde L, Halldin C, Swahn CG, Sedvall G, Foged C, Hansen KT, Skrumsager B. PET examination of [11C]NNC 687 and [11C]NNC 756 as new radioligands for the D1 dopamine receptor. Psychopharmacology. 1993;113:149–156. doi: 10.1007/BF02245691. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Martinez D, Kochan LD, Hwang DR, Huang Y, Mawlawi O, Suckow RF, Van Heertum RL, Laruelle M. NMDA antagonist effects on striatal dopamine release: positron emission tomography studies in humans. Synapse. 2002;43:19–29. doi: 10.1002/syn.10010. [DOI] [PubMed] [Google Scholar]
- Kiss B, Horváth A, Némethy Z, Schmidt E, Laszlovszky I, Bugovics G, Fazekas K, Hornok K, Orosz S, Gyertyán I, Agai-Csongor E, Domány G, Tihanyi K, Adham N, Szombathelyi Z. Cariprazine (RGH-188), a dopamine D3 receptor-preferring, D3/D2 dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333:328–340. doi: 10.1124/jpet.109.160432. [DOI] [PubMed] [Google Scholar]
- Lahti RA, Figur LM, Piercey MF, Ruppel PL, Evans DL. Intrinsic activity determinations at the dopamine D2 guanine nucleotide-binding protein-coupled receptor: utilization of receptor state binding affinities. Mol Pharmacol. 1992;42:432–438. [PubMed] [Google Scholar]
- Langer O, Halldin C, Dolle F, Swahn CG, Olsson H, Karlsson P, Hall H, Sandell J, Lundkvist C, Vaufrey F, Loc'h C, Crouzel C, Maziere B, Farde L. Carbon-11 epidepride: a suitable radioligand for PET investigation of striatal and extrastriatal dopamine D2 receptors. Nucl Med Biol. 1999;26:509–518. doi: 10.1016/s0969-8051(99)00005-0. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Lee CM, Farde L. Using positron emission tomography to facilitate CNS drug development. Trends Pharmacol Sci. 2006;27:310–316. doi: 10.1016/j.tips.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Martin LL, Bouchal RL, Smith DJ. Ketamine inhibits serotonin uptake in vivo. Neuropharmacology. 1982;21:113–118. doi: 10.1016/0028-3908(82)90149-6. [DOI] [PubMed] [Google Scholar]
- Mathis CA, Simpson NR, Mahmood K, Kinahan PE, Mintun MA. [11C]WAY 100635: a radioligand for imaging 5-HT1A receptors with positron emission tomography. Life Sci. 1994;55 doi: 10.1016/0024-3205(94)00324-6. PL403-7. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk K, Szabatin M, Rudnicki P, Grodzki M, Burger C. A JAVA environment for medical image data analysis: initial application for brain PET quantitation. Med Inform. 1998;23:207–214. doi: 10.3109/14639239809001400. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Gobert A, Joly F, Bervoets K, Rivet J, Newman-Tancredi A, Audinot V, Maurel S. Contrasting mechanisms of action and sensitivity to antipsychotics of phencyclidine versus amphetamine: importance of nucleus accumbens 5-HT2A sites for PCP-induced locomotion in the rat. Eur J Neurosci. 1999;11:4419–4432. doi: 10.1046/j.1460-9568.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Nakashima Y, Nordström AL, Halldin C, Farde L. Positron emission tomography of in vivo binding characteristics of atypical antipsychotic drugs. Review of D2 and 5-HT2 receptor occupancy studies and clinical response. Br J Psychiatry. (Suppl. 29) 1996;168:40–44. [PubMed] [Google Scholar]
- Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- Pike VW, McCarron JA, Lammerstma AA, Hume SP, Poole K, Grasby PM, Malizia A, Cliffe IA, Fletcher A, Bench CJ. First delineation of 5-HT1A receptors in human brain with PET and [11C]WAY-100635. Eur J Pharmacol. 1995;283:R1–R3. doi: 10.1016/0014-2999(95)00438-q. [DOI] [PubMed] [Google Scholar]
- Rabiner E, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, Diwan M, Wilson AA, McCormick P, Gentile G, Gunn RN, Laruelle MA. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: studies in non-human primates and transgenic mice. Synapse Sep. 2009;63(9):782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, Mugnaini M, Griffante C, Wilson AA, Merlo-Pich E, Houle S, Gunn R, Rabiner EA, Laruelle M. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry. 2010;68:392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Seeman P, Chau-Wong M, Tedesco J, Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci USA. 1975;72:4376–4380. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, Creese I. Regulation of ligand binding to pituitary D2 dopaminergic receptors: effects of divalent cations and functional group modification. J Biol Chem. 1983;258:4957–4965. [PubMed] [Google Scholar]
- Skinbjerg M, Namkung Y, Halldin C, Innis RB, Sibley DR. Pharmacological characterization of 2-methoxy-N-propylnorapomorphine's interactions with D2 and D3 dopamine receptors. Synapse. 2009;63:462–475. doi: 10.1002/syn.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor D3 as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Steiner H, Fuchs S, Accili D. D3 dopamine receptor-deficient mouse: evidence for reduced anxiety. Physiol Behav. 1997;63:137–141. doi: 10.1016/s0031-9384(97)00430-7. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res. 1998;779:58–74. doi: 10.1016/s0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Harada N, Nishiyama S, Ohba H, Sato K, Fukumoto D, Kakiuchi T. Ketamine decreased striatal [11C]raclopride binding with no alterations in static dopamine concentrations in the striatal extracellular fluid in the monkey brain: multiparametric PET studies combined with microdialysis analysis. Synapse. 2000;37:95–103. doi: 10.1002/1098-2396(200008)37:2<95::AID-SYN3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Wienhard K, Dahlbom M, Eriksson L, Michel C, Bruckbauer T, Pietrzyk U, Heiss WD. The ECAT EXACT HR: performance of a new high resolution positron scanner. J Comput Assist Tomogr. 1994;18:110–118. [PubMed] [Google Scholar]
- Wong DF, Tauscher J, Grunder G. The role of imaging in proof of concept for CNS drug discovery and development. Neuropsychopharmacology. 2009;34:187–203. doi: 10.1038/npp.2008.166. [DOI] [PubMed] [Google Scholar]