Abstract
Objective
Persons with elevated blood pressure show dampened emotional responses to affect-laden stimuli. We sought to further examine cardiovascular emotional dampening by examination of the relationship between resting hemodynamic measures and recognition of emotion in an African-American community-based sample.
Methods
Participants were 106 African American men and women (55 female; mean age 52.8 years), mainly low in socioeconomic status and part of the Healthy Aging in Nationally Diverse Longitudinal Samples (HANDLS-Pilot) Pilot Study. Participants evaluated emotional expressions in faces and in sentences using the Perception of Affect Test (PAT). Resting blood pressure, total peripheral resistance (TPR), cardiac output and heart rate were obtained continuously using a Portapres blood pressure monitor.
Results
Total PAT scores were inversely related to systolic (r = −.30) and diastolic (r = −.24) blood pressure, TPR (r = −.36) and age (r = − .31; p values < .01), and positively related to cardiac output (r = .27) and education (r = .38; p values <.01), and with mental state (r = .25) and body mass index (r = −.20; p values < .05). Accuracy of emotion recognition on the PAT tasks remained inversely related to TPR and blood pressure after adjustment for demographic variables, medication, mental state and body mass index.
Conclusions
Elevated blood pressure and TPR were associated with reduced perception of affect. TPR was the most consistent independent hemodynamic correlate of emotional dampening for the PAT scores. These results suggest potentially important links among CNS regulation of emotions, hemodynamic processes and hypertension development.
Keywords: Emotion regulation, blood pressure, hemodynamics, hypertension development, central nervous system, stress
The relationship between blood pressure control and central nervous system (CNS) function in the development of essential hypertension is complex and multidirectional. For example significant, sustained elevations in blood pressure (BP) can produce neuropsychological deficits in persons with established hypertension (1). Recently however, a growing body of evidence suggests that subtle changes in CNS function can accompany or possibly precede BP increases, even within the normotensive range (2–5). The nature of these changes in brain function and their possible role in developmental pathophysiology of essential hypertension remains to be fully characterized.
The well-established relationship between BP and pain sensitivity suggests a heretofore unappreciated mechanism of intimacy between brain function and BP control mechanisms. Reduced responsivity to painful stimuli has been well documented in hypertensive animals and humans (for a review see Ghione, 3). This inverse relationship between BP and pain sensitivity has been shown to extend throughout the normotensive range (2,6,7). Interestingly, this hypoalgesia has been observed in persons with a family history of hypertension before significant BP elevations have occurred (8,9), suggesting that the CNS changes may parallel or even precede significant blood pressure dysregulation. These findings suggest a previously uncharacterized interrelationship between cognitive appraisal of aversive stimuli and BP control.
Recent research suggests that BP-associated hypoalgesia may reflect a more generalized dampening of emotional responsivity. For example, several studies have shown significant relationships between BP and affective responses to pain (7,10,11). Moreover, this association between BP and affect dampening has been seen in emotionally laden contexts unrelated to acute or chronic pain. For example, Nyklicek and colleagues (12,13) found reduced subjective self report of stress in some hypertensives. Moreover, they noted positive correlations between pain sensitivity and negative appraisal of psychological stressors, further suggesting that BP associated hypoalgesia may reflect a more general effect on emotional responding.
These collective findings indicate that higher BP, even within the normotensive range, is associated with dampened response to emotionally-laden aversive stimuli. However, those studies did not systematically address a potential relationship between BP and positive affectivity. Therefore, we designed a study to assess BP and responses to emotionally positive as well as negative photographic scenes from the International Affective Series in normotensive, young adults (14). An emotional dampening hypothesis would postulate reduced affective response to both negatively and positively valenced stimuli. An alternative positivity bias hypothesis would postulate that persons with higher BP would show less negative appraisal of negatively valenced photos, and more positive appraisal of positively valenced photos (see Figure 1). Results showed that higher BP was associated with decreased intensity of emotional response to both positive and negative affective content, providing initial support for the cardiovascular emotional dampening hypothesis.
Figure 1.
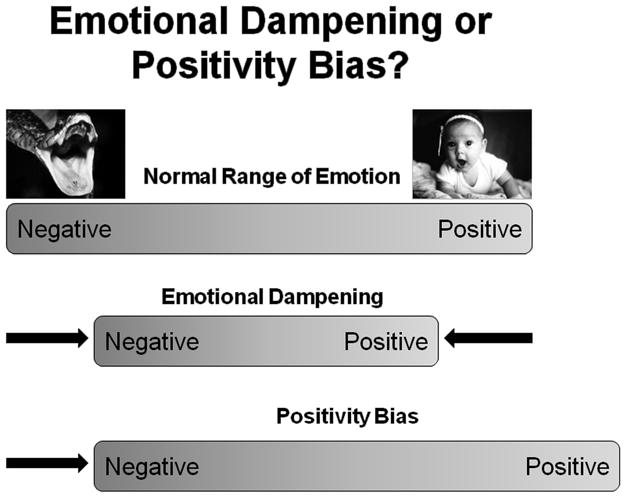
A comparison of the emotional dampening and positivity bias hypotheses. Cardiovascular emotional dampening indicates that persons with higher BP show reduced emotional response to stimuli with negative emotional valence as well as stimuli with positive emotional valence. Positivity bias indicates an overall positive shift where persons with higher BP show reduced emotional response to stimuli with negative emotional valence and increased emotional response to stimuli with positive emotional valence.
Coupled with the pain sensitivity data, these findings suggest that persons with elevated BP or other hypertension risk factors may also have impaired recognition of emotional meaning in, for example, facial expressions, written communication and possibly other important modalities of emotion expression. If this is the case, then persons at risk for hypertension may show significant impairment in recognition and response to emotionally-relevant communication by others. This emotional dampening may produce potentially adverse affects on social relationships, with increased psychosocial distress and possibly additional BP dysregulation.
The present study seeks to further examine emotional dampening by examination of: 1) its effects on recognition of emotion in faces and written narratives, 2) its generalizability to an older high risk population, and 3) its underlying hemodynamic correlates. We used the Perception of Affect Test (PAT; 15) to determine if elevated resting cardiovascular levels were related to reduced recognition of emotionally salient stimuli in an African-American community-based sample (HANDLS-Pilot). We hypothesize that higher BP will be related to decreased accuracy in recognition of emotion in verbal (sentences) and nonverbal (faces) content. In addition, we examined the role of total peripheral resistance and cardiac output in the relationship between BP control and emotional dampening. Finally, the influence of resting cardiovascular levels on perception of affect was examined in a sample of mostly low SES African American adults with an average age of 52.6 years.
Methods
Participants were part of the Healthy Aging in Nationally Diverse Longitudinal Samples Pilot (HANDLS-Pilot) Study. Demographic characteristics for the HANDLS-Pilot sample are shown in Table 1 (N=106, 52% female, age = 52.6 ± 14.6 years). Most participants were of low socioeconomic status (SES) based on national norms, and living in neighborhoods with high rates of polydrug substance use, violent crime, and sexually transmitted diseases. The data were collected in a custom built mobile laboratory or in a community room in an apartment building, which were located in an urban, predominantly African-American Baltimore neighborhood. Although all participants reported at least some African-American background, the only inclusion criterion was age eighteen or older. Participants received $40 upon completion of the HANDLS-Pilot study.
Table 1.
Means, Standard Deviations, and Ranges of Demographic, Average Cardiovascular Levels and PAT Accuracy Scores
| Variable | Total | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | M | SD | Range | |
| Age | 52.6 | 14.6 | 21–92 | 55.1 | 15.6 | 21–92 | 49.8 | 13.1 | 26–83 |
| Education | 11.9 | 3.3 | 3–18 | 11.3 | 3.3 | 3–17 | 12.5 | 3.2 | 3–18 |
| MMSE | 26.4 | 3.0 | 15–30 | 26.4 | 2.4 | 20–30 | 26.4 | 3.5 | 15–30 |
| BMI | 27.7 | 7.6 | 16–52 | 29.9 | 7.8 | 16–52 | 25.2* | 6.6 | 16–49 |
| Average CV | |||||||||
| Levels | |||||||||
| SBP | 143.9 | 27.4 | 101–254 | 147.4 | 28.7 | 104–230 | 140.1 | 25.7 | 101–254 |
| DBP | 84.4 | 12.0 | 63–124 | 82.0 | 10.1 | 63–103 | 87.1* | 13.3 | 63–124 |
| TPR | 1.5 | 0.8 | 0.7–5.8 | 1.4 | 0.6 | 0.7–3.1 | 1.7* | 1.0 | 0.7–5.8 |
| CO | 5.1 | 1.7 | 1.9–10.8 | 5.4 | 1.8 | 2.2–10.8 | 4.8 | 1.6 | 1.9–8.8 |
| HR | 76.0 | 12.7 | 47–107 | 76.9 | 12.2 | 46–107 | 75.0 | 13.2 | 49–104 |
| PAT scores | |||||||||
| Total | 74.3 | 13.5 | 37–99 | 74.1 | 13.0 | 37–99 | 74.4 | 14.3 | 43–93 |
| Faces | 74.3 | 13.7 | 40–100 | 74.3 | 13.5 | 40–100 | 74.4 | 14.0 | 41–91 |
| Sentences | 74.2 | 16.6 | 23–100 | 74.0 | 16.0 | 23–97 | 74.4 | 17.3 | 34–100 |
| Negative | 70.3 | 14.9 | 30–100 | 70.6 | 15.4 | 30–100 | 70.0 | 14.5 | 38–93 |
| Positive | 81.6 | 15.0 | 35–100 | 80.8 | 13.5 | 40–100 | 82.5 | 16.5 | 35–100 |
p < .05 for gender difference
Protocol
Written informed consent was received prior to the experimental session. The participant sat in a chair directly across from the experimenter while the experimenter provided an overview of the study. The experimenter recorded the participant’s age and gender. The experimenter then connected the participant to a recording device that collected beat-to-beat readings of BP and heart rate (HR) while the participant sat and rested for five minutes. The participant then completed the sentences and faces sub-tasks of the Perception of Affect test. An additional five minute rest period ensued after task completion. Approximate dates of data collection were from September, 2000 to September, 2002. All procedures were approved by the Medstar/Harbor Hospital institutional review board.
Sentences sub-task
The experimenter gave the participant blank forms for the sentences sub-task as well as instructions on how to complete items. The experimenter asked the participant to read (or listen to experimenter read out loud) 35 sentences and match the perceived emotional response of the underlined character in each sentence to one of the following response options: happy, sad, fear, anger, disgust, surprise and neutral emotion. Five sets of sentences represented each of the seven emotions. For example, one anger item from the PAT sentences is: “Being sure that his players did nothing wrong, a coach demands an explanation from the referee about the penalty call.”
Faces sub-task
Next, the experimenter showed the participant 35 photographs of faces with varying expressions and asked the participant to indicate which emotion (of the seven options above) was the best match for the expression in the face. Five sets of faces stimuli represented each of the seven emotions.
The sentences and faces sub-tasks were counter-balanced and have been normed in previous research with community-based aging populations (16). This task has proven to be reliable and sensitive to multiple measures of emotion awareness, anxiety, defensiveness and coping style (15,16). After the protocol the experimenter debriefed the participant.
Cardiovascular Assessment
A PORTApres beat-to-beat BP monitor was used to collect cardiovascular measures. The PORTApres (17) is an ambulatory monitor that utilizes the arterial clamp method (Penaz Method) to collect continuous BP and HR waveforms that are analyzed offline using a Modelflow (18) technique and BEATSCOPE 1.1 software (19) to produce reliable estimates of stroke volume, total peripheral resistance (TPR), and cardiac output (CO). This software uses the Wesseling Algorithm, which computes aortic flow waveform from arterial pressure signal. Aortic diameter can be different based on age and gender parameters. Thus, the Modelflow technique uses age and gender group norms to estimate diameter and stroke volume. In turn, measures of HR and stroke volume allow for computation of CO (L/min) and TPR (mmHg·s/mL). In humans, this technique compares well with CO and stroke volume measured by radial artery catheterization, and CO as determined by Doppler ultrasound (20,21). For example, the overall CO root-mean-squared normalized error was 15.3% with respect to the direct arterial measures and 15.1% with respect to Doppler ultrasound measures.
The experimenter placed a finger BP cuff on the middle phalanx of two fingers of the non-dominant hand and then attached a height correction transducer from the front end unit of the PORTApres to the upper arm of the participant’s non-dominant arm at heart level. Average cardiovascular parameters were calculated across all values obtained at rest.
Analysis of PAT
Various scores representing the correct identification of emotional stimuli (higher scores mean better recognition) were computed as follows. Total PAT score was calculated as the proportion of correct responses to total responses (across faces and sentences). Total Faces score was calculated as the percentage of correct responses for the faces task. Total Sentences score was calculated as the percentage of correct responses for the sentences task. Proportion scores for each task were calculated if participants had more than 29 responses. Nine participants had four or more missing values and were excluded from analyses. Individual emotion scores were calculated as the proportion of correct responses to total responses across and within tasks (for each emotion). Scores for each emotion were prorated if participants had fewer than five, and participant data were not used if less than three responses were obtained. No participants had more than one missing value for any emotion-task category. Cronbach’s alpha for each subtask and for the total PAT score were: Faces = 0.72 (N = 99), Sentences = 0.78 (N = 93), and total = 0.83 (N =87).
Prior research suggests that the valence of emotional stimuli (positive or negative) may provide valuable information about emotion recognition (22,23). Therefore, proportion scores were computed for negative and positive stimuli across faces and sentences tasks.
Measures of education and cognitive function
Educational attainment was measured as the total number of years of formal education. Thus, scores could range from one to 20 years. The mean educational attainment (see Table 1) was consistent with the demographic characteristics of the surrounding neighborhood. Men had marginally higher educational attainment than women [t(104) = -1.79; p = .07].
Total scores on the Mini-Mental state exam (MMSE; 24) were analyzed to determine if cognitive capacity confounds PAT performance, especially the sentences. As the sample is primarily low on educational attainment it could be argued that poor PAT performance could in large part be a function of poor cognitive skills associated with inadequate educational attainment. The Mini-mental has been used as an indicator of cognitive aging in African American and White aging populations (25). In the present study, there was no gender difference in mean MMSE score (see Table 1).
Measures of health risk: Hypertension and body mass index
Prevalence of hypertension and diabetes are highly correlated, and at least some of their pathophysiologies and treatments are known to affect CNS function (26–28), so a dummy variable (yes or no) was created to assess hypertension/diabetes medication usage (including ace-inhibitors, beta-blockers, calcium channel blockers, diuretics, hypoglycemics or other related medications). In the current sample, 44 persons (42%) were coded as positive for medication usage.
Numerous epidemiological studies have found BMI, to be a risk factor for poor cardiovascular health outcomes (28). We obtained measures of height and weight in inches and pounds from each subject, then converted these values into meters and kilograms to compute BMI scores (BMI = kg / m2). The mean BMI score for the total sample and by gender is shown in Table 1. Women had significantly higher mean BMI score than men.
Plan for Statistical Analysis
Mean scores for TPR, CO, systolic and diastolic BP (SBP and DBP), and HR were computed for the rest periods. Analyses were performed with SPSS-PC software and an alpha level of 0.05 was adopted for all statistical tests. Statistical analyses included correlational tests for demographic variables, MMSE, resting cardiovascular levels and the various PAT scores. We conducted a series of hierarchical multiple regression tests with independent variables entered in the following steps: 1) gender, age, education and BMI, 2) medication usage, 3) MMSE score, and 4) the specific average resting cardiovascular levels, individually, with the selected PAT score as the dependent measure for each test. Thus, we tested the role of resting cardiovascular levels on perception of affect after adjustment first for relevant sociodemographic factors, then for medication status, and then for cognitive functioning.
Results
Table 1 shows descriptive statistics for PAT Total, PAT-Sentences, and PAT-Faces, PAT Negative, and PAT Positive scores. The results indicate that the participants in the current study have lower PAT scores than the predominately White sample in Lane et al. (15). However, the minimum PAT scores are higher for participants in the HANDLS-Pilot study. While there were no significant gender differences for any PAT score, supplemental analyses suggest that older men had the most trouble correctly identifying emotional expressions, especially negative emotions.
Zero order correlations showed that PAT scores were inversely related to systolic and diastolic BP, TPR and age, and positively related to cardiac output, education, mental state and body mass index (BMI, see Table 2). In particular, higher SBP, DBP and TPR were significantly correlated with lower scores on PAT Total, PAT Faces, PAT Positive and PAT Negative. Lower CO was linked with lower PAT scores. HR was not significantly correlated with PAT scores.
Table 2.
Zero-order Correlations (2-tailed) for Average Cardiovascular Levels, Demographic Variables, and Mini-mental State Exam (MMSE) with PAT Accuracy Scores
| Systolic BP | Diastolic BP | TPR | Cardiac Output | Heart Rate | Age | Education | MMSE | BMI | |
|---|---|---|---|---|---|---|---|---|---|
| PAT Total | −0.30* | −0.24* | −0.36* | 0.27* | 0.15 | −0.31* | 0.38* | 0.25+ | 0.20+ |
| PAT Faces | −0.31* | −0.19+ | −0.33* | 0.25* | 0.18 | −0.38* | 0.27* | 0.15 | 0.18 |
| PAT Sentences | −0.23* | −0.22+ | −0.31* | 0.23* | 0.10 | −0.19 | 0.39* | 0.28* | 0.17 |
| PAT Negative | −0.24* | −0.20+ | −0.35* | 0.29* | 0.13 | −0.22+ | 0.32* | 0.27* | 0.27* |
| PAT Positive | −0.33* | −0.24* | −0.29* | 0.18 | 0.16 | −0.38* | 0.32* | 0.15 | 0.07 |
TPR = Total Peripheral Resistance
p < .05
p < .01
Zero-order correlations for demographics and MMSE with PAT accuracy scores (see Table 2) showed that increasing age was associated with lower PAT Total and for Faces, Positive and Negative subtest scores. Higher education levels were significantly associated with higher PAT Total and all subtests. Lower MMSE scores were correlated with lower PAT Total, Sentences, and Negative scores. BMI was positively correlated with PAT Total and PAT Negative scores.
PAT Total
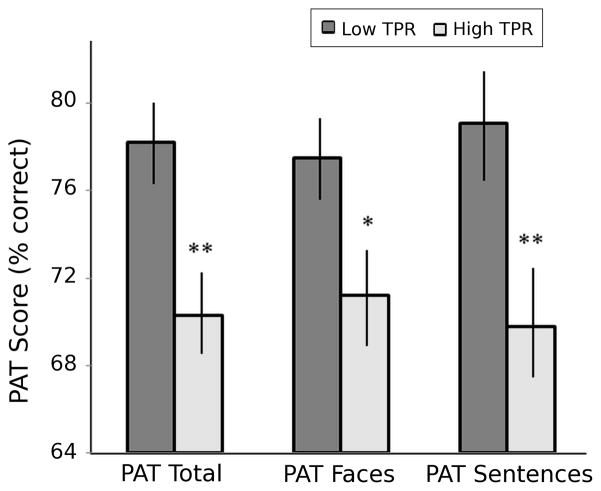
Table 3 shows results for the hierarchical multiple regression models for each cardiovascular variable [Steps 4(a)–4(e)] and PAT Total score. Higher TPR was significantly related to lower PAT Total scores (p=.033), with nonsignificant trends for DBP (p=.065) and SBP (p=.094). Higher education and BMI, and lower age were significantly related to higher PAT Total scores. There was a nonsignificant (p=.055) trend for higher MMSE scores to be associated with higher PAT Total scores. Figure 2 shows the average (+/− standard error) PAT Total scores for high and low TPR groups based on a TPR median split. Differences between groups were significant (t(104)=3.155, p=.002).
Table 3.
Regression of Demographics, medication usage (Rx), Mini-mental Score and average cardiovascular levels on PAT Total Score
| R-square | R-square change | Sig. F Change | beta | t (beta) | Significance | |
|---|---|---|---|---|---|---|
| Step 1: | ||||||
| Gender | .215 | .215 | .000 | −.020 | −.203 | .839 |
| Age | −.269 | −2.720 | .008 | |||
| Education | .255 | 2.580 | .011 | |||
| BMI | .222 | 2.268 | .026 | |||
|
| ||||||
| Step 2: | ||||||
| Rx | .216 | .001 | .711 | .041 | .371 | .711 |
|
| ||||||
| Step 3: | ||||||
| MMSE | .248 | .032 | .055 | .181 | 1.944 | .055 |
|
| ||||||
| Step 4(a): | ||||||
| Systolic BP | .271 | .023 | .094 | −.181 | −1.693 | .094 |
|
| ||||||
| Step 4(b): | ||||||
| Diastolic BP | .276 | .028 | .065 | −.175 | −1.871 | .065 |
|
| ||||||
| Step 4(c): | ||||||
| TPR | .286 | .038 | .033 | −.234 | −2.170 | .033 |
|
| ||||||
| Step 4(d): | ||||||
| CO | .255 | .007 | .376 | .112 | .890 | .376 |
|
| ||||||
| Step 4(e): | ||||||
| HR | .259 | .011 | .252 | .114 | 1.152 | .252 |
Figure 2.
Average percent correct (+/− standard error) Perception of affect (PAT) scores in persons with low versus high resting total peripheral resistance (TPR). * p<.025, ** p<.005 in high versus low TPR groups.
PAT Negative
Regression results for PAT Negative are shown in Table 4. Higher TPR was significantly associated with lower PAT Negative (p=.029). Higher Education, BMI and MMSE scores were significantly related to higher PAT Negative performance. There was a nonsignificant (p=.061) trend for older Age to be associated with lower performance on the PAT Negative subtest.
Table 4.
Regression of Demographics, medication usage (Rx), Mini-mental Score and average cardiovascular levels on PAT Negative Score
| R-square | R-square change | Sig. F Change | beta | t (beta) | Significance | |
|---|---|---|---|---|---|---|
| Step 1: | ||||||
| Gender | .186 | .186 | .001 | −.001 | −.006 | .995 |
| Age | −.191 | −1.896 | .061 | |||
| Education | .227 | 2.257 | .026 | |||
| BMI | .289 | 2.900 | .005 | |||
|
| ||||||
| Step 2: | ||||||
| Rx | .187 | .001 | .775 | .032 | .287 | .775 |
|
| ||||||
| Step 3: | ||||||
| MMSE | .230 | .043 | .027 | .213 | 2.251 | .027 |
|
| ||||||
| Step 4(a): | ||||||
| Systolic BP | .244 | .013 | .215 | −.136 | −1.248 | .215 |
|
| ||||||
| Step 4(b): | ||||||
| Diastolic BP | .249 | .019 | .137 | −.143 | −1.500 | .137 |
|
| ||||||
| Step 4(c): | ||||||
| TPR | .271 | .041 | .029 | −.242 | −2.229 | .029 |
|
| ||||||
| Step 4(d): | ||||||
| CO | .243 | .013 | .218 | .157 | 1.242 | .218 |
|
| ||||||
| Step 4(e): | ||||||
| HR | .240 | .009 | .302 | .104 | 1.037 | .302 |
PAT Positive
Regression results for PAT Positive are shown in Table 5. Higher systolic (p=.025) and diastolic BPs (p=.033) were significantly associated with lower PAT Positive scores. Older age was significantly related to lower performance on the PAT Positive subtest. There was a nonsignificant (p=.087) trend for higher Education to be associated with higher PAT Positive scores.
Table 5.
Regression of Demographics, medication usage (Rx), Mini-mental Score and average cardiovascular levels on PAT Positive Score
| R-square | R-square change | Sig. F Change | beta | t (beta) | Significance | |
|---|---|---|---|---|---|---|
| Step 1: | ||||||
| Gender | .188 | .188 | .001 | −.024 | −.240 | .811 |
| Age | −.346 | −3.436 | .001 | |||
| Education | .174 | 1.731 | .087 | |||
| BMI | .110 | 1.107 | .271 | |||
|
| ||||||
| Step 2: | ||||||
| Rx | .189 | .001 | .709 | .042 | .374 | .709 |
|
| ||||||
| Step 3: | ||||||
| MMSE | .196 | .007 | .377 | .086 | .887 | .377 |
|
| ||||||
| Step 4(a): | ||||||
| Systolic BP | .241 | .044 | .025 | −.249 | −2.284 | .025 |
|
| ||||||
| Step 4(b): | ||||||
| Diastolic BP | .237 | .040 | .033 | −.209 | −2.172 | .033 |
|
| ||||||
| Step 4(c): | ||||||
| TPR | .212 | .016 | .179 | −.153 | −1.354 | .179 |
|
| ||||||
| Step 4(d): | ||||||
| CO | .196 | .000 | .954 | .007 | .057 | .954 |
|
| ||||||
| Step 4(e): | ||||||
| HR | .208 | .011 | .260 | .116 | 1.134 | .260 |
PAT Sentences
The hierarchical multiple regression models for PAT Sentences showed higher TPR was significantly (p=.032) related to lower PAT Sentences scores with a nonsignificant trend for DBP (p=.095). Both higher Education and MMSE were significantly associated with higher PAT Sentences performance. There was a nonsignificant (p=.097) trend for higher BMI to be associated with higher PAT Sentences scores. Figure 2 shows the average (+/− standard error) PAT Sentences scores for high and low TPR groups based on a TPR median split. Differences between groups were significant (t(104)=2.995, p=.003).
PAT Faces
The hierarchical multiple regression models for PAT Faces indicate that PAT Faces scores were not significantly associated with any of the cardiovascular variables, although both younger Age and higher BMI was associated with higher PAT Faces performance. Figure 2 shows the average (+/− standard error) PAT Faces scores for high and low TPR groups based on a TPR median split. Differences between groups were significant (t(104)=2.437, p=.017).
Discussion
The present results show a significant relationship between resting cardiovascular function and recognition of affective content in pictures of faces and written narratives. Persons with high resting BP and TPR show significantly reduced responses to both positively and negatively valenced faces and sentences. While recognition accuracy in the current population is also related to age, education, and mental status, the relationship between cardiovascular function and emotional dampening appears independent of medication, mental status, education, BMI, age and gender. This reduced ability to detect and/or respond to emotional cues is consistent with the relationships between BP levels and responses to pain (6,7) affective images (14) and self-reported stress (13). Paradoxically, dampened subjective reports of emotion in persons with elevated BP may be accompanied by increased autonomic and circulatory reactivity to stress (29). Thus, there appears to be a complex intimacy between emotional dampening and cardiovascular dysregulation. This psychophysiological link between cardiovascular control and regulation of emotion remains to be fully understood. Moreover, the potential clinical significance of cardiovascular emotional dampening in the etiology of essential hypertension urges further investigation.
Cardiovascular Emotional Dampening and Stress
The precise causal pathways between cardiovascular function and emotional dampening have not yet been fully clarified, but are likely complex and multidirectional. For example, emotional dampening could directly result from circulatory function via visceral afferent processes or, in advanced clinical hypertension, CNS microcirculatory pathology. Alternatively, emotional dampening could be a marker for CNS changes that contribute directly to BP elevation. Additionally, the phenomenon of cardiovascular emotional dampening could reveal parallel and perhaps progressive changes in both CNS function and autonomic control of circulation. Separately or in combination with the above, emotional dampening could also increase psychological stress, thus further increasing blood pressure. For example, emotional dampening could increase psychosocial distress through emotionally inappropriate interactions with others, including family, friends, co-workers and supervisors. Thus emotional dampening could contribute directly to chronic stress levels, further exacerbating potentially pathogenic cardiovascular responses.
Reduced recognition of emotional content could be associated with increased psychosocial distress in several ways. For example, effective stress management relies on sensitive and accurate recognition of emotional, threatening, and/or stressful situations in order to appraise and respond appropriately in complex environments. Social relations, including social support networks, are bolstered by empathy, emotional bonding, and trust. Impaired recognition of emotions in verbal and nonverbal communication could be associated with reduced quality of social relationships, social isolation, and emotional distancing from family, friends and work associates. Therefore impaired recognition of emotional cues is likely more than a simple marker of the parallelism between cardiovascular and CNS function. It is also a mechanism that could increase psychosocial distress, producing additional neuroendocrine and autonomic disturbance of cardiovascular function. Moreover, the dampening of emotionally positive experiences may interfere with the restorative functions of leisure, hobbies, and positive social relationships. Thus the potential threats of emotional dampening to normal psychological, social and physiological functioning merit extensive future study to innumerate the correlates, causes, consequences and possible therapeutic interventions.
Regulation of Emotion and Blood Pressure
The present study supports and extends the emotional dampening hypothesis in several significant ways. First, this study extends the relationship between cardiovascular function and assessment of emotionally laden stimuli to older, predominantly low SES African Americans at elevated risk for cardiovascular disease. Second, cardiovascular emotional dampening has been linked to reduced accuracy in recognition of emotional expression in narrative texts and faces. Third, after adjustment for potentially confounding variables, emotional dampening remains associated with increased blood pressure and total peripheral resistance.
Emotional dampening in a population of older African American men and women at elevated risk for cardiovascular disease is important for several reasons. Hypoalgesia has been observed in hypertensive humans and animals (3). The relationship between BP and pain sensitivity is well-documented throughout the normotensive range, and has been observed in persons with a family history of hypertension (7,8), including newborn infants (30,31). To our knowledge, this is the first evidence of emotional dampening in older, mostly lower SES African Americans. Resting cardiovascular parameters were significantly related to PAT score after correction for demographics (i.e. age, education, gender), medications and mental status. Cardiovascular emotional dampening appears conceptually similar to alexithymia (15,16), but emerging evidence suggests that it is an independent phenomenon. For example, a recent preliminary study of healthy young adults from our lab shows that blood pressure significantly related to PAT scores (p=.001) after adjustment for both age and alexithymia (32). This suggests that the cardiovascular emotional dampening observed in the HANDLS sample may be independent of alexithymia. The present findings point to a basic CNS mechanism linking regulation of emotion and cardiovascular function, independent of age, education, gender, medications and mental status.
Neurocirculatory Implications
The precise mechanisms linking BP and/or hypertension risk and emotional dampening are not yet fully clarified. Although there is significant evidence linking both sinoaortic and cardiopulmonary baroreflex mechanisms to pain sensitivity (33–35), a recent study suggested that emotional dampening may not be mediated via baroreflexes in persons with parental hypertension (36). A series of studies has implicated endogenous opioid peptides in BP associated hypoalgesia; the body of evidence suggests involvement of both opioid and nonopioid mechanisms (6,7). While most of the original studies on pain sensitivity emphasized the relationship with either BP or familial risk for hypertension, the present study is one of the first to examine indices of total peripheral resistance. PAT scores were most consistently related to TPR after correction for demographics, medications, mental status, suggesting that the primary, independent cardiovascular phenomenon may reflect vascular processes, at least in the current population. These processes could include both active vasoconstriction, but may also reflect vascular compliance and other structural changes as well. Prior studies have found that dampening is strongly tied to chronic resting BP levels. This suggests that the common CNS and circulatory mechanisms may be embodied in regulation of the set point of BP that determines the chronic level of BP rather than acute BP changes around that set point. While certain antihypertensive medications have been shown to normalize pain sensitivity in hypertensives, these effects are not necessarily linked directly with BP lowering (37). This supports the notion that the common mechanism may be most closely linked to the chronic baroreflex set point rather than acute baroreceptor sensitivity. Regardless of the precise mechanism, the intimate relationship between emotional dampening and BP control mechanisms may help better characterize the role of the CNS in autonomic and circulatory function during the early stages of development of essential hypertension.
HANDLS Sample Characteristics
The present study was designed to focus on a neighborhood sample that was primarily older African American and lower SES. Therefore, our sample has characteristics that reflect that mission. For example, women in this sample have a significantly higher BMI than men (see Table I). Moreover, the wide range of MMSE scores in this sample suggests caution in interpretation of results. Our diverse HANDLS sample included individuals up to 92 years of age, and educational attainment as low as 3 years. This is a demographic range where normative MMSE screening guidelines are not well established. However, in a subsidiary analysis, exclusion of total MMSE scores below 24 did not substantially alter our findings. In light of these results, and adjustment for MMSE scores in the full sample models, the overall findings are not likely distorted by persons with possible dementia. Most importantly, our emotional dampening findings are supported despite the wide range of age, education, BMI and MMSE scores in the current HANDLS sample.
Conclusions
Emotional dampening is related to levels of resting blood pressure, total peripheral resistance and other indices of hemodynamic function in a sample of predominantly middle aged, lower SES African Americans who are at significantly elevated risk for cardiovascular disease. The present findings provide support for a cardiovascular emotional dampening hypothesis and are consistent with the neurovisceral integration model (38). The role of emotional dampening in the developmental etiology of hypertension requires further study, but the present results suggest potentially important links among CNS regulation of emotions, neural control of circulation and hypertension development.
Acknowledgments
The Healthy Aging in Neighborhoods across the Life Span study is supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. Support was also provided by the National Center on Minority Health and Health Disparities, NIH.
Glossary
- BP
blood pressure
- CNS
central nervous system
- PAT
perception of affect test
- HANDLS-Pilot
Healthy Aging in Nationally Diverse Longitudinal Samples Pilot Study
- SES
socioeconomic status
- CV
cardiovascular
- HR
heart rate
- TPR
total peripheral resistance
- CO
cardiac output
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- MMSE
mini mental status total score
Contributor Information
James A. McCubbin, Department of Psychology, Clemson University
Marcellus M. Merritt, Department of Psychology, University of Wisconsin-Milwaukee
John J. Sollers, III, Department of Psychological Medicine, University of Auckland
Michele K. Evans, Laboratory of Immunology, National Institute on Aging
Alan B. Zonderman, Laboratory of Behavioral Neuroscience, National Institute on Aging
Richard D. Lane, Department of Psychiatry, University of Arizona
Julian F. Thayer, Department of Psychology, Ohio State University
References
- 1.Waldstein SR, Brown JRP, Maier KJ, Katzel LI. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Ann Behav Med. 2005;29:174–180. doi: 10.1207/s15324796abm2903_3. [DOI] [PubMed] [Google Scholar]
- 2.Bruehl S, Carlson CR, McCubbin JA. The relationship between pain sensitivity and blood pressure in normotensives. Pain. 1992;48:463–467. doi: 10.1016/0304-3959(92)90099-W. [DOI] [PubMed] [Google Scholar]
- 3.Ghione S. Hypertension-associated hypalgesia. Evidence in experimental animals and humans, pathophysiological mechanisms, and potential clinical consequences. Hypertens. 1996;28:494–504. doi: 10.1161/01.hyp.28.3.494. [DOI] [PubMed] [Google Scholar]
- 4.France CR. Decreased pain perception and risk for hypertension: considering a common physiological mechanism. Psychophysiol. 1999;36:683–92. [PubMed] [Google Scholar]
- 5.Jennings JR, Zanstra Y. Is the brain the essential in hypertension? Neuroimage. 2009;47:914–21. doi: 10.1016/j.neuroimage.2009.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCubbin JA, Bruehl S. Do endogenous opioids mediate the relationship between blood pressure and pain sensitivity in normotensives? Pain. 1994;57:63–67. doi: 10.1016/0304-3959(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 7.McCubbin JA, Helfer SG, Switzer FS, Galloway C, Griffith WV. Opioid analgesia in persons at risk for hypertension. Psychosom Med. 2006;68:116–120. doi: 10.1097/01.psy.0000195742.24850.79. [DOI] [PubMed] [Google Scholar]
- 8.al’Absi M, Buchanan T, Lovallo WR. Pain perception and cardiovascular responses in men with positive parental history for hypertension. Psychophysiol. 1996;33:655–61. doi: 10.1111/j.1469-8986.1996.tb02361.x. [DOI] [PubMed] [Google Scholar]
- 9.France C, Ditto B, Adler P. Pain sensitivity in offspring of hypertensives at rest and during baroreflex stimulation. J Behav Med. 1991;14:513–25. doi: 10.1007/BF00845108. [DOI] [PubMed] [Google Scholar]
- 10.Fillingim RB, Maixner W, Bunting S, Silva S. Resting blood pressure and thermal pain responses among females: effects on pain unpleasantness but not pain intensity. Int J Psychophysiol. 1998;30:313–8. doi: 10.1016/s0167-8760(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 11.Duschek S, Dietel A, Schandry R, del Paso GA. Increased sensitivity to heat pain in chronic low blood pressure. Eur J Pain. 2009;13:28–34. doi: 10.1016/j.ejpain.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Nyklícek I, Vingerhoets AJ, Van Heck GL. Hypertension and objective and self-reported stressor exposure: a review. J Psychosom Res. 1996;40:585–601. doi: 10.1016/0022-3999(95)00647-8. [DOI] [PubMed] [Google Scholar]
- 13.Nyklícek I, Vingerhoets AJ, Van Heck GL. Hypertension and appraisal of physical and psychological stressors. J Psychosom Res. 2001;50:237–44. doi: 10.1016/s0022-3999(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 14.Pury CLS, McCubbin JA, Helfer SG, Galloway C, McMullen LJ. Elevated resting blood pressure and dampened emotional response. Psychosom Med. 2004;66:583–587. doi: 10.1097/01.psy.0000130490.57706.88. [DOI] [PubMed] [Google Scholar]
- 15.Lane RD, Sechrest L, Riedel R, Weldon V, Kaszniak AW, Schwartz GE. Impaired verbal and nonverbal emotion recognition in alexithymia. Psychosom Med. 1996;58:203–10. doi: 10.1097/00006842-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Lane RD, Sechrest L, Riedel R, Shapiro DE, Kaszniak AW. Pervasive emotion recognition deficit common to alexithymia and the repressive coping style. Psychosom Med. 2000;62:492–501. doi: 10.1097/00006842-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 17.TNO-TPD Biomedical Instrumentation. Portapres Model-2 User’s Guide. Amsterdam, The Netherlands: 1999. [Google Scholar]
- 18.Wesseling KH, Jansen JRC, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Applied Physiol. 1993;74:2566–73. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 19.TNO-TPD Biomedical Instrumentation. Beatscope 1.0 User’s Guide. Amsterdam, The Netherlands: 1999b. [Google Scholar]
- 20.Lu Z, Mukkamala R. Continuous cardiac output monitoring in humans by invasive and noninvasive peripheral blood pressure waveform analysis. J Appl Physiol. 2006;101:598–608. doi: 10.1152/japplphysiol.01488.2005. [DOI] [PubMed] [Google Scholar]
- 21.Voogel AJ, Van Montfrans GA. Reproducibility of twenty-four-hour finger arterial blood pressure, variability and system. J Hypertens. 1997;15:1761–5. doi: 10.1097/00004872-199715120-00086. [DOI] [PubMed] [Google Scholar]
- 22.Crews WD, Harrison DW. Sex differences and cerebral asymmetry in facial affect perception as a function of depressed mood. Psychobiol. 1994;22:112–16. doi: 10.2466/pms.1994.79.3f.1667. [DOI] [PubMed] [Google Scholar]
- 23.Thayer JF, Johnsen BH. Sex differences in judgment of facial affect: A multivariate analysis of recognition errors. Scand J Psychol. 2000;41:243–6. doi: 10.1111/1467-9450.00193. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Shadlen MF, Larson EB, Gibbons L, McCormick WC, Teri L. Alzheimer’s disease symptom severity in blacks and whites. J Amer Geriatric Soc. 1999;47:482–6. doi: 10.1111/j.1532-5415.1999.tb07244.x. [DOI] [PubMed] [Google Scholar]
- 26.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 2000;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 27.Lovallo WR, Gerin W. Psychophysiological reactivity: mechanisms and pathways to cardiovascular disease. Psychosom Med. 2003;65:36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- 28.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Internal Med. 2001;161:1581–6. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 29.McCubbin JA, Surwit RS, Williams RB. Endogenous opiate peptides, stress reactivity, and risk for hypertension. Hypertension. 1985;7:808–811. doi: 10.1161/01.hyp.7.5.808. [DOI] [PubMed] [Google Scholar]
- 30.France CR, Taddio A, Shah VS, Pagé MG, Katz J. Maternal family history of hypertension attenuates neonatal pain response. Pain. 2009;142:189–93. doi: 10.1016/j.pain.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 31.McCubbin JA. Prenatal maternal stress hormones, risk for hypertension, and the neonatal pain response: Comment on France et al. “Maternal family history of hypertension attenuates neonatal pain response”. Pain. 2009;142:173–4. doi: 10.1016/j.pain.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCubbin JA, Loveless JP, Hall GA, Robinson G, Moore D. Cardiovascular emotional dampening is independent of alexithymia. Psychosom Med. 2011;73:A115–116. [Google Scholar]
- 33.Elbert T, Rockstroh B, Lutzenberger W, Kessler M, Pietrowsky R. Baroreceptor stimulation alters pain sensation depending on tonic blood pressure. Psychophysiol. 1988;25:25–9. doi: 10.1111/j.1469-8986.1988.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 34.Mini A, Rau H, Montoya P, Palomba D, Birbaumer N. Baroreceptor cortical effects, emotions and pain. Intl J Psychophysiol. 1995;19:67–77. doi: 10.1016/0167-8760(94)00084-r. [DOI] [PubMed] [Google Scholar]
- 35.Ditto B, Lewkowski MD, Rainville P, Duncan GH. Effects of cardiopulmonary baroreceptor activation on pain may be moderated by risk for hypertension. Biol Psychol. 2009;82:195–197. doi: 10.1016/j.biopsycho.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson DZ, France CR. Attenuation of positive and negative affect in men and women at increased risk for hypertension: a function of endogenous barostimulation? Psychophysiol. 2009;46:114–21. doi: 10.1111/j.1469-8986.2008.00757.x. [DOI] [PubMed] [Google Scholar]
- 37.Guasti L, Zanotta D, Diolisi A, Garganico D, Simoni C, Gaudio G, Grandi AM, Venco A. Changes in pain perception during treatment with angiotensin converting enzyme-inhibitors and angiotensin II type 1 receptor blockade. Hypertens. 2002;20:485–91. doi: 10.1097/00004872-200203000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affective Disorders. 2000;61:201–16. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]