Abstract
Objectives
We sought to develop a clinical algorithm combining serum PSA with detection of TMPRSS2:ERG fusion and PCA3 in urine collected after digital rectal exam (post-DRE urine) to predict prostate cancer on subsequent biopsy.
Materials and Methods
Post-DRE urine was collected in 48 consecutive patients before prostate biopsy at two centers; qRT-PCR was used to detect PCA3 and TMPRSS2:ERG fusion transcript expression. Serum PSA was measured by clinical assay. The performance of TMPRSS2:ERG fusion, PCA3, and serum PSA as biomarkers predicting prostate cancer at biopsy was measured; a clinically practical algorithm combining serum PSA with TMPRSS2:ERG and PCA3 in post-DRE urine to predict prostate cancer was developed.
Results
Post-DRE urine sediment provided informative RNA in 45 patients; prostate cancer was present on subsequent biopsy in 15. TMPRSS2:ERG in post-DRE urine was associated with prostate cancer (OR = 12.02; p< 0.001). PCA3 had the highest sensitivity in predicting prostate cancer diagnosis (93%), whereas TMPRSS2:ERG had the highest specificity (87%). TMPRSS2:ERG had the greatest discriminatory value in predicting prostate cancer (AUC = 0.77 compared to 0.65 for PCA3 and 0.72 for serum PSA alone). Combining serum PSA, PCA3 and TMPRSS2:ERG in a multivariable algorithm optimized for clinical utility improved cancer prediction (AUC = 0.88; specificity = 90% at 80% sensitivity).
Conclusions
A clinical algorithm specifying biopsy for all patients with PSA ≥10ng/ml, while restricting biopsy among those with PSA <10ng/ml to only those with detectable PCA3 or TMPRSS2:ERG in post-DRE urine, performed better than the individual biomarkers alone in predicting prostate cancer.
Keywords: Screening, DRE, Biomarkers, Cancer Detection, Gene Fusion
Introduction
More than 20 years after introduction of PSA into clinical practice, substantial gains in prostate cancer mortality reduction have been realized, but concurrent limitations in the performance of PSA in selecting patients for prostate biopsy have persisted. These limitations have been further underscored by recently reported results from the European Randomized Study of Screening for Prostate Cancer (ERSPC) and Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO), that together indicate a continued need to improve upon serum PSA as our leading tool for prostate cancer early detection.1–6
The need for additional biomarkers that supplement PSA is reflected by the number of ongoing studies in this field. Amongst numerous experimental biomarkers for prostate cancer early detection, prostate cancer gene 3 (PCA3)7 and recurrent gene fusions involving TMPRSS2 and ETS family members (such as TMPRSS2:ERG)8 have shown promising results in preliminary studies and can be detected in post-DRE9–11 urine specimens that are suitable for possible use in clinical diagnostic strategies. One possible drawback of new molecular targets for early detection such as PCA3 or TMPRSS2:ERG fusion could be that they may only identify certain subgroups of cancer cases. In this context, one approach for optimizing performance of such biomarkers in predicting prostate cancer diagnosis is to develop models that combine different biomarkers to generate clinically useful, ‘multiplex’ algorithms for identifying candidates for prostate biopsy based on results of multiple biomarker assays combined with PSA test results.
In this study, we sought to verify the association of prostate cancer with detection in urine of TMPRSS2:ERG gene fusion, PCA3, and investigated how these biomarkers may be combined with serum PSA levels to develop clinically practical algorithms for predicting prostate cancer diagnosis.
Materials and Methods
Urine samples were collected after attentive digital rectal exam (DRE) from forty-eight men undergoing prostate biopsy at 2 clinical practice sites participating in the National Cancer Institute/Early Detection Research Network biopsy cohort. Institutional Review Board approval was obtained at the collaborating institutions and informed consent was obtained from these men. Demographic and pre-biopsy clinical parameters including age, race, history of smoking, height, weight, family history of prostate cancer, history of previous prostate biopsy, DRE findings, pre-biopsy PSA, and prostate volume as estimated by transrectal ultrasound scan (TRUS) were obtained using interviewer-administered questionnaires and abstracting data from patients’ medical records. Urine collection cups containing DNA/RNA preservative (Sierra Diagnostics LLC) were used to obtain urine from each subject after digital rectal examination was performed by the practicing urologist. Each patient subsequently underwent a prostate needle biopsy (PNB) by the urologist using the National Comprehensive Cancer Network (NCCN) guidelines.12 PNB results were reviewed and histology confirmed by institutional pathologists.
The details of methods used for RNA isolation from urine sediment and Transplex Whole Transcriptome Amplification (WTA) have been previously described.10 Quantitative RT-PCR was used to detect the expression of 2 prostate cancer biomarkers PCA3, and TMPRSS2:ERG and the control transcripts PSA and GAPDH from WTA-amplified cDNA using methods essentially as described.13 The primer sequences for GAPDH14 and PSA15 that serve as sample controls have been previously described; all samples were informative based on detectable levels of these controls. Threshold cycle (Ct) values for PCA3 and TMPRSS2:ERG for each sample were generated during the exponential phase of qPCR. TMPRSS2:ERG fusion status and PCA3 were each dichotomized as a binary variable to reflect positive or negative status as described.16
Demographic and pre-biopsy clinical parameters were compared between men diagnosed with prostate cancer vs. men without prostate cancer in their PNB using Kruskal-Wallis tests for continuous variables and Fisher’s exact tests for categorical variables. . Exact univariate logistic regression was used to examine the association between PSA, PCA3, and TMPRSS2:ERG gene fusion with the presence or absence of prostate cancer on PNB.
The performance of each biomarker, including serum PSA, as a screening test was evaluated and sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and the area under the receiver-operating characteristic curve AUC, with 95% bootstrap CI, were calculated. A multivariable logistic regression model predicting the presence of prostate cancer on biopsy was then developed using backward selection. The initial model contained serum PSA, PCA3, and TMPRSS2:ERG as potential predictor variables. The inclusion of serum PSA in the multivariable model was evaluated both as a continuous variable and as a categorical variable at PSA cut-offs of 2.5, 4, and 10 ng/mL. Calibration was evaluated by comparing average model risk and observed proportion with prostate cancer overall using Hosmer and Lemeshow’s goodness-of-fit chi-square test on deciles of risk and by calculating the AUC.
All analyses were done using SAS version 9.2 at 0.05 level of significance and two-sided p-values were reported.
Results
The demographic and pre-biopsy clinical parameters of 45 men from whom post-DRE urine samples were collected before prostate biopsy for these analyses and informative RNA was obtained are presented in Table 1. Fifteen (33%) men had a diagnosis of prostate cancer on biopsy of which 7 (47%) have a Gleason 7 or higher. Of the 30 (67%) men without prostate cancer on biopsy, 5 (17%) had either atypia or prostatic intraepithelial neoplasia (PIN) or both identified on histology.
Table 1.
Demographic and pre-biopsy clinical characteristics according to diagnosis on prostate needle biopsy
| Variable | No Prostate Cancer (n = 30) |
Prostate Cancer (n =15 ) |
p-value |
|---|---|---|---|
| Age (yrs) | 64 [56 – 70] | 65 [58 – 71] | 0.630 |
| BMI (kg/m2) | 27.1 [24.4 – 29.5] | 27.5 [24.4 – 30.9] | 0.718 |
| Non-Caucasian | 9 (30) | 2 (13) | 0.288 |
| Family History of Prostate cancer | 9(30) | 8 (53) | 0.193 |
| History of Smoking | 13(45) | 9 (64) | 0.332 |
| Abnormal DRE/suspicious of cancer | 6(20) | 4 (27) | 0.710 |
| PSA (ng/mL) | 4.2 [1.8 – 6.9] | 6.5 [3.8 – 22.30] | 0.019 |
| Prostate size by TRUS (cc) | 49 [33 – 65] | 36 [28 – 49] | 0.182 |
| PSA density (ng/mL/cc) | 0.09 [0.04 – 0.15] | 0.13 [0.10– 0.44] | 0.020 |
| History of previous prostate biopsy | 12 (40) | 9 (60) | 0.226 |
N (%) compared using Fisher’s exact tests; or median [inter-quartile range] compared using Kruskal-Wallis tests. All p-values of comparison of prostate cancer versus no cancer were greater than 0.05 except for PSA and PSA Density (here evaluated as continuous variables).
Abbreviations: BMI=body mass index; PSA=prostate specific antigen; DRE=digital rectal exam; TRUS=trans-rectal ultrasound
In the univariate analysis of the pre-biopsy clinical parameters, men diagnosed with prostate cancer had significantly higher serum PSA level and increased PSA density as compared with men without prostate cancer (both p<0.05). The other demographic and pre-biopsy clinical variables were not significantly different between men found to have prostate cancer on biopsy and those not having cancer. The predicted classification of the presence or absence of prostate cancer on biopsy using serum PSA as a binary variable and the experimental urine biomarkers is as shown in Table 2. Fourteen (31%) men had positive TMPRSS2:ERG fusion status in urine while 33 (73%) men tested positive for urine PCA3. In univariate analysis of these urine biomarkers men diagnosed with prostate cancer were significantly more likely to have a positive test result for TMPRSS2:ERG with OR of 12.02 (95% CI 2.37–77.25, p=0.001) whereas positive test result for PCA3 (OR=7.81; 95% CI 0.93, 373.31, p=0.063) was not significant. Eight (18%) men had elevated serum PSA (cut-off of 10 ng/mL) and were more likely to have prostate cancer on biopsy as compared to men with PSA <10 ng/mL (OR=2.36; 95% CI 0.54, 12.54, p=0.011) but not at 2.5 or 4 ng/mL cut-offs. Although PCA3 had the highest sensitivity of 93% in predicting prostate cancer, TMPRSS2:ERG was more specific (specificity = 86.7%). TMPRSS2:ERG had the greatest discriminatory value with an AUC of 0.77 (95% CI 0.61, 0.90); the performance of serum binary PSA at a cut-off of 10 ng/mL (AUC = 0.67; 95% CI 0.53, 0.81) and PCA3 (AUC = 0.65; 95% CI 0.54, 0.76) were not as effective.
Table 2.
Performance of Urinary TMPRSS2:ERG, PCA3, and serum PSA in Predicting Prostate Cancer Diagnosis: Univariate Analyses
| Predictor | PNB Result | Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
AUC (95% CI) |
Univariate p value |
||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||||
| PSA (ng/mL) | ≥ 10 | 6 | 2 | 40. | 93 | 75 | 76 | 0.67 (0.53–0.81) | 0.011 |
| <10 | 9 | 28 | |||||||
| PCA3 | Positive | 14 | 19 | 93 | 37 | 42 | 92 | 0.65 (0.54–0.76) | 0.063 |
| Negative | 1 | 11 | |||||||
| TMPRSS2:ERG | Positive | 10 | 4 | 67 | 87 | 71 | 84 | 0.77 (0.61–0.90) | 0.001 |
| Negative | 5 | 26 | |||||||
Serum PSA at cut-offs of 2.5 (AUC=0.62; 95% CI 0.52, 0.72; sensitivity = 93%, specificity = 30%) and 4 ng/mL (AUC=0.60; 95% CI 0.50, 0.75; sensitivity = 73%, specificity = 47%) were not statistically significant predictors of prostate cancer (p>0.05) possibly due to sample size limitations.
Abbreviations: PNB=prostate needle biopsy; PSA=prostate specific antigen; PPV=positive predictive value; NPV=negative predictive value; AUC=area under curve
We next evaluated whether the performance of the individual tests in predicting prostate cancer diagnosis would be improved by combining serum PSA with urine TMPRSS2:ERG and PCA3 biomarkers. Multivariable logistic regression yielded a model wherein urine PCA3 and TMPRSS2:ERG were each significant (p values = 0.044 and 0.001 for PCA3 and TMPRSS2:ERG respectively; AUC = 0.82; 95 % CI 0.67, 0.96). Serum PSA (which was evaluated both as a continuous and a binary variable at cut-offs of 2.5, 4, and 10 ng/mL) was eliminated from the model at 0.05 level of significance, possibly due to sample size limitations. However, the AUC of this 2-variable model was not significantly better than that of serum PSA alone either as a continuous or a categorical variable at cut-off of 10 ng/mL (both p>0.05). Thus, recognizing the clinical utility of serum PSA and its significance in univariate analysis, we explored the effects of adding serum PSA back into the model (Figure 1). Although urine PCA3 became marginally insignificant (p=0.06), the performance of the multivariable model was optimized by including serum PSA at a cut-off of 10 ng/mL.
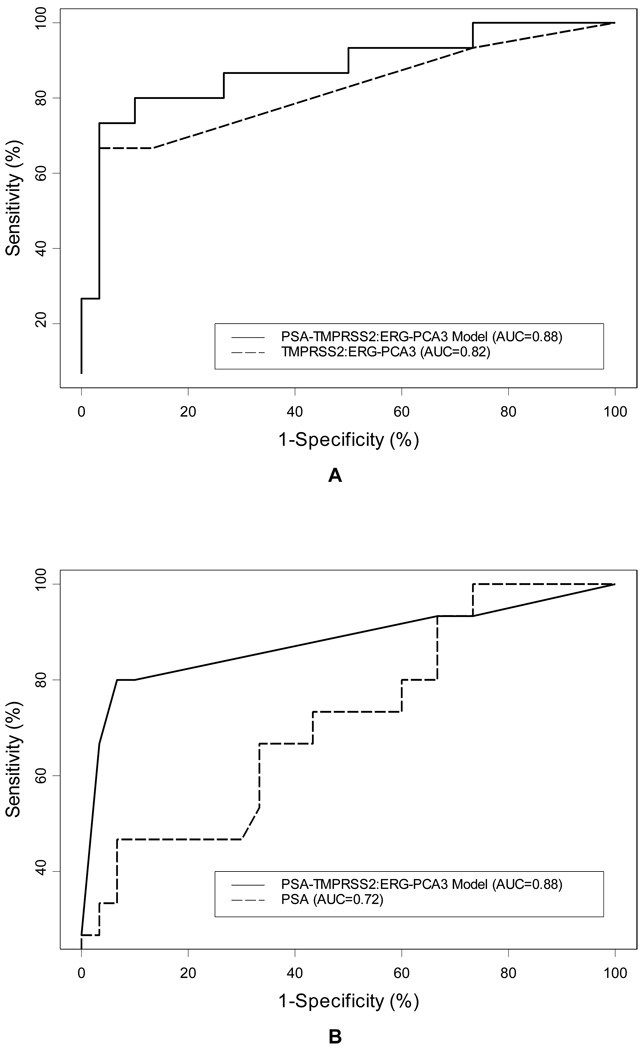
Figure 1.
Multivariable logistic regression resulted in a model combining urinary TMPRSS2:ERG and PCA3 (panel A, dashed, AUC=0.82). Allowing PSA to remain in the model either as a continuous variable (panel A solid line; AUC = 0.88) or categorical variable (Panel B solid-line; AUC=0.88) improved model performance as compared to PSA alone (panel B dashed line; AUC = 0.72).
A) The multiplex model combining PSA (continuous; β=0.165,SE=0.097,p-value=0.088), positive PCA3 (β=2.810, SE=1.449, p-value=0.053), and positive TMPRSS2:ERG fusion status (β=2.6067, SE=0.963 p-value= 0.007) outperforms PSA alone in predicting prostate cancer prior to biopsy. The multiplex model had a greater AUC than PSA alone (0.88 versus 0.72). B): Serum PSA as a categorical variable (cut-off = 10 ng/mL) in the multiplex model resulted in comparable model performance (Equal AUC of 0.88 for both multiplex models implementing serum PSA as continuous or categorical variable at a cut-off of 10 ng/mL). This represents a clinically more relevant and easier to use model (Table 3.)
The discriminatory ability of the multivariable model combining serum PSA (at cut-off of 10 ng/mL), urine PCA3 and TMPRSS2:ERG biomarkers, was better than any of the individual biomarkers alone [AUC = 0.88 (95% CI 0.75, 0.98) for the multivariable model vs., 0.62 for serum PSA at cut-off of 2.5 ng/mL , 0.60 for serum PSA at cut-off of 4 ng/mL , 0.67 for serum PSA at cut-off of 10 ng/mL, 0.65 for PCA3, and 0.77 for TMPRSS2:ERG; Table 2; Figure 1]. At a sensitivity of 80%, the specificity of PSA alone in this cohort ranged between 20 and 37% (corresponding to serum PSA values of 1.7 and 3.0 ng/mL respectively). At this level of sensitivity, the multivariable model using serum PSA at a cut-off of 10 ng/mL showed marked improvement in specificity to 90% (compared with 37% for serum PSA alone and 53% for the multivariable model using PSA as a continuous variable).
Table 3 shows the utility of this model in a clinically relevant format in making a decision to or not to send a patient for prostate biopsy. In a less restricted biopsy selection scenario at high sensitivity (93%), an individual with any of serum PSA ≥10 ng/mL or positive urine PCA3 or TMPRSS2:ERG test will be recommended to have a prostate biopsy. However, with the more restricted biopsy selection criteria at high sensitivity and specificity (80 and 90% respectively); at least two of the above conditions are required to make a recommendation for prostate biopsy.
Table 3.
Clinical application of the multivariable model in selecting patients for biopsy
| PSA (ng/mL) |
PCA3 | T2-ERG | Probability of PCa at Biopsy (95% CI) |
Biopsy selection scenarios | |
|---|---|---|---|---|---|
| Less restricted* | More restricted* | ||||
| <10 | Negative | Negative | 1.3 (0.1 – 20.9) | No Biopsy | No Biopsy |
| <10 | Negative | Positive | 16.8 (1.5 – 72.9) | Biopsy | No Biopsy |
| <10 | Positive | Negative | 17.0 (6.2 – 38.7) | Biopsy | No Biopsy |
| <10 | Positive | Positive | 76.0 (38.8 – 94.0) | Biopsy | Biopsy |
| ≥ 10** | Negative | Negative | 7.3** | Biopsy | No Biopsy |
| ≥ 10 | Negative | Positive | 54.8 (7.0 – 95.1) | Biopsy | Biopsy |
| ≥ 10 | Positive | Negative | 55.1 (14.9 – 89.6) | Biopsy | Biopsy |
| ≥ 10 | Positive | Positive | 95.0 (64.8 – 99.5) | Biopsy | Biopsy |
Probability ≥ 1.4% for less restricted biopsy selection scenario and ≥ 23.0% for more restricted biopsy selection scenario with sensitivity/specificity of 93.3/26.7% and 80/90% respectively.
In this cohort all patients with PSA ≥ 10 had either TMPRSS2:ERG, PCA3, or both detectable in their urine
Comment
The performance of serum PSA as a biomarker for prostate cancer early detection is undermined by several limitations, including substantial false positive and false negative tests results because PSA is produced in benign as well as malignant prostate tissue. Hence, there is a need to identify biomarkers that are more specific for prostate cancer and that may thereby improve prospects for prostate cancer screening and early detection.5
Recent advancements in high throughput technologies have provided a large inventory of candidate biomarkers that might prove to be more selective and potentially more useful than individual biomarker when combined in a multiplex model.17 Multi-gene tests have for example been established for breast cancer to predict recurrence after tamoxifene treatment.18 Tomlins et al8, 19 identified the recurrent gene fusion of the androgen regulated gene TMPRSS2 to members of the ETS family of transcription factors (ERG, ETV1 or ETV4) in majority of prostate cancers. TMPRSS2:ERG is by far the most common subtype of ETS fusions accounting for approximately 85% of all ETS fusion-positive samples. In PSA-screened prostatectomy cohorts from North America, Europe and Asia, the TMPRSS2:ERG fusion has been reported to occur in approximately 50% of prostate cancers.20 The presence of TMPRSS2:ERG fusion in prostate cancers seems to predict a more aggressive natural course of the disease and associated increase in prostate cancer-related deaths has been reported.21, 22 Thus, patients with TMPRSS2:ERG fusion might possibly benefit from curative therapy. Moreover, the fusion transcripts can not only be detected in specimens from prostatectomy, but recent studies have shown that TMPRSS2:ERG fusion can reliably be detected in the urine of patients collected after DRE by PCR9, 10 which has the advantage of being a convenient, non-invasive procedure. Briefly, urine specimen is collected after DRE, a process comparable to that used for commercial urine PCA3 test. The urine sample is transported on ice and can be stored at 4°C for up to 4 hours before centrifugation to separate sediment and supernatant, with the cellular sediment then stored and transported at −80°C until further processing using RT-PCR assay. Although laboratory processing has been further simplified for forthcoming studies with the commercial assay, the costs of these molecular assays may be substantial. Despite this limitation, the assay may help some men avoid the anxiety and discomfort associated with undergoing prostate biopsy.
In this study, we detected TMPRSS2:ERG fusion in post-DRE urine sediment of 10/15 (67%) of prostate cancer positive cases whereas 26/30 patients without cancer were TMPRSS2:ERG fusion negative, resulting in a specificity of 87%. Two other studies investigated TMPRSS2:ERG fusion in urine and reported similarly high specificities of 93% and 73%, respectively; and one study evaluated TMPRSS2:ERG fusion in expressed prostatic secretions reporting a specificity of 80%.23 However, prior studies have not evaluated the utility of combining serum PSA test results with noninvasive detection of TMPRSS2:ERG and PCA3 in developing models to predict prostate cancer diagnosis in a clinically relevant context, as we explored in this study.
We also found that of those who had negative prostate biopsy result in our cohort, 4/30 (13%) had a positive TMPRSS2:ERG fusion status. The finding of TMPRSS2:ERG fusion positive cases in patients with negative biopsy is a rare event: for example, no Fluorescent In-Situ Hybridization (FISH)-based assay has reported ETS gene rearrangements in normal prostate glands, proliferative inflammatory atrophy (PIA), or benign prostatic hyperplasia (BPH).24, 25 So this raises the question of false negative prostate biopsies (a limitation of PNB which is widely recognized) and highlights the possible utility of TMPRSS2:ERG fusion status in identifying a subgroup of patients that need to be followed up more closely for possible repeat biopsy, despite their negative prostate biopsy result.
Another urine biomarker that we evaluated in this study was Prostate Cancer Gene 3 (PCA3), a non-coding RNA that is highly over-expressed in prostate cancer.26 The urine PCA3 test has been shown in some studies to be superior to serum PSA testing in predicting positive prostate biopsies.11, 27, 28 The sensitivity of urine PCA3 in predicting prostate cancer has been reported to be as high as 62%.9 In our cohort, we observed an even higher sensitivity of 93%. Combining PCA3 and TMPRSS2:ERG detection from post-DRE urines has been evaluated in two prior studies: Hessels et al.9 reported an increase in sensitivity from 62% (PCA3 only) to 73% when combined with TMPRSS2:ERG. Laxman et al16 evaluated the performance of a multivariate model combining TMPRSS2:ERG fusion, PCA3, and other urine biomarkers in early detection of prostate cancer and reported an improved AUC of 0.756 with the model compared with 0.662 for PCA3 alone.
Our findings corroborate the utility of combining detection of PCA3 and TMPRSS2:ERG in post-DRE urine, and extend this paradigm to include consideration of concurrent serum PSA test results in a simple clinical algorithm that would include consideration of all 3 test results as components of clinical decisions regarding prostate biopsy. We found an improved discriminatory ability over either of these biomarkers alone when serum PSA, urine PCA3 and TMPRSS2:ERG fusion status were combined in a multivariable model (AUC = 0.72 for PSA, 0.65 for PCA3, 0.77 for TMPRSS2:ERG vs. 0.88 for the multivariable model; Figure 1). The multiplex model is simple and clinically relevant as illustrated in Table 3 where in a less restricted biopsy selection scenario, patients with serum PSA ≥10 ng/mL or any of positive urine PCA3 or TMPRSS2:ERG test will be recommended to have a prostate biopsy. On the other hand, in a more restricted screening scenario with high sensitivity and specificity of 80 and 90% respectively, only patients with at least two of serum PSA ≥10 ng/mL, positive urine PCA3 and TMPRSS2:ERG will be recommended for prostate biopsy. Unnecessary biopsies would thus have been avoided. For example in the latter scenario, 30/45 (67%) of prostate biopsies would have been avoided at the risk of a false negative rate (FNR) of 3/15 (20 %). Hence combining serum PSA, urine PCA3 and TMPRSS2: ERG showed marked improvement over serum PSA alone which at the 80% level of sensitivity, the specificity ranges between 20 and 37%.
There are, however, several limitations to this study. Notable among these is the small number of patients. Hence, this represents only a pilot in evaluating the feasibility and performance of combining PCA3, TMPRSS2:ERG and serum PSA in a multiplex model that provides a basis for selecting patients for biopsy. Validation of the urine TMPRSS2:ERG fusion assay and the multiplex model’s performance and feasibility in a larger cohort are thus warranted. In addition, we used a quantitative RT-PCR assay to measure PCA3 in urine sediment, and evaluation of this model with the commercially available TMA assay of whole urine would help inform broader clinical applicability.28 Furthermore, a larger validation study would help determine whether a multiplex model combining PCA3, TMPRSS2:ERG and serum PSA can better predict aggressive versus indolent prostate cancer than any of these biomarkers alone.
Conclusions
Early detection of prostate cancer is crucial to providing an effective therapy to patients. These new biomarkers can potentially fulfill this task more effectively than serum PSA alone, when used in combination. We have shown in this pilot study, that TMPRSS2:ERG gene fusion and PCA3, especially in combination with serum PSA, are very attractive targets with respect to early detection of prostate cancer. Both markers can be detected in patients’ urine after attentive digital rectal examination which could be the basis for a non-invasive, easy-to-use clinical test.
Acknowledgments
Funding: NCI-EDRN CEVC U01 CA113913
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: SAT, AC and MAR are listed as inventors in a patent related to diagnostic use of TMPRSS2:ERG
References
- 1.Andriole GL, Levin DL, Crawford ED, et al. Prostate Cancer Screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial: findings from the initial screening round of a randomized trial. J Natl Cancer Inst. 2005;97:433. doi: 10.1093/jnci/dji065. [DOI] [PubMed] [Google Scholar]
- 2.Crawford ED, DeAntoni EP, Etzioni R, et al. Serum prostate-specific antigen and digital rectal examination for early detection of prostate cancer in a national community-based program. The Prostate Cancer Education Council. Urology. 1996;47:863. doi: 10.1016/s0090-4295(96)00061-1. [DOI] [PubMed] [Google Scholar]
- 3.Hugosson J, Aus G, Lilja H, et al. Results of a randomized, population-based study of biennial screening using serum prostate-specific antigen measurement to detect prostate carcinoma. Cancer. 2004;100:1397. doi: 10.1002/cncr.20126. [DOI] [PubMed] [Google Scholar]
- 4.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. Jama. 2004;291:2713. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 5.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 6.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Kok JB, Verhaegh GW, Roelofs RW, et al. DD3(PCA3), a very sensitive and specific marker to detect prostate tumors. Cancer Res. 2002;62:2695. [PubMed] [Google Scholar]
- 8.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 9.Hessels D, Smit FP, Verhaegh GW, et al. Detection of TMPRSS2-ERG fusion transcripts and prostate cancer antigen 3 in urinary sediments may improve diagnosis of prostate cancer. Clin Cancer Res. 2007;13:5103. doi: 10.1158/1078-0432.CCR-07-0700. [DOI] [PubMed] [Google Scholar]
- 10.Laxman B, Tomlins SA, Mehra R, et al. Noninvasive detection of TMPRSS2:ERG fusion transcripts in the urine of men with prostate cancer. Neoplasia. 2006;8:885. doi: 10.1593/neo.06625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Gils MP, Hessels D, van Hooij O, et al. The time-resolved fluorescence-based PCA3 test on urinary sediments after digital rectal examination; a Dutch multicenter validation of the diagnostic performance. Clin Cancer Res. 2007;13:939. doi: 10.1158/1078-0432.CCR-06-2679. [DOI] [PubMed] [Google Scholar]
- 12.Kawachi MH, Bahnson RR, Barry M, et al. Prostate cancer early detection. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2007;5:714. [PubMed] [Google Scholar]
- 13.Tomlins SA, Mehra R, Rhodes DR, et al. Whole transcriptome amplification for gene expression profiling and development of molecular archives. Neoplasia. 2006;8:153. doi: 10.1593/neo.05754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Specht K, Richter T, Muller U, et al. Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol. 2001;158:419. doi: 10.1016/S0002-9440(10)63985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laxman B, Morris DS, Yu J, et al. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res. 2008;68:645. doi: 10.1158/0008-5472.CAN-07-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 18.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 19.Tomlins SA, Laxman B, Varambally S, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demichelis F, Fall K, Perner S, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 22.Attard G, Clark J, Ambroisine L, et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27:253. doi: 10.1038/sj.onc.1210640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark JP, Munson KW, Gu JW, et al. Performance of a single assay for both type III and type VI TMPRSS2:ERG fusions in noninvasive prediction of prostate biopsy outcome. Clin Chem. 2008;54:2007. doi: 10.1373/clinchem.2008.108845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perner S, Mosquera JM, Demichelis F, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- 25.Tomlins SA, Bjartell A, Chinnaiyan AM, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol. 2009;56:275. doi: 10.1016/j.eururo.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 26.Bussemakers MJ, van Bokhoven A, Verhaegh GW, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59:5975. [PubMed] [Google Scholar]
- 27.Fradet Y, Saad F, Aprikian A, et al. uPM3, a new molecular urine test for the detection of prostate cancer. Urology. 2004;64:311. doi: 10.1016/j.urology.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 28.Marks LS, Fradet Y, Deras IL, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology. 2007;69:532. doi: 10.1016/j.urology.2006.12.014. [DOI] [PubMed] [Google Scholar]