Abstract
Alterations in working memory (WM) are common after traumatic brain injury (TBI). Frontal catecholaminergic systems, including the alpha-2 adrenergic system, modulate WM function and may be affected in TBI. We hypothesized that administration of an alpha-2 adrenergic agonist might improve WM after mild TBI (MTBI). Thirteen individuals with MTBI one month after injury and 14 healthy controls (HC) were challenged with guanfacine and placebo prior to administration of a verbal WM functional MRI task. Guanfacine was associated with improved WM performance in the MTBI but not the HC group. On guanfacine the MTBI group showed increased activation within a WM task-specific region of interest. Findings are consistent with the hypothesis that alterations in WM after MTBI may be improved with the alpha-2 agonist guanfacine.
Keywords: traumatic brain injury, alpha-2 adrenergic agonist, working memory, functional MRI, guanfacine
Introduction
Initial and persistent cognitive deficits are the most common complaints after traumatic brain injury (TBI) (Whyte et al. 2000) and are the major hindrance to return to baseline function (Cicerone et al. 2000). There are several predictable areas of cognitive impairment after TBI (Binder et al. 1997; Levin 1998; Levin et al. 1987; McMillan 1997), including working memory (WM), which is the ability to hold information in mind and to manipulate that material in light of incoming information. We previously used functional MRI (fMRI) to demonstrate that one month after mild TBI (MTBI) performance on a moderate difficulty WM task was associated with significantly greater cerebral activation (“compensatory activation”) and increased memory complaints despite WM task performance similar to that seen in controls (McAllister et al. 1999). We subsequently showed that MTBI patients were unable to further increase brain activation with a higher WM processing load (McAllister et al. 2001). We interpreted these results as evidence for abnormalities in the activation and allocation of WM processing resources after MTBI. In essence, patients were “working harder” to achieve the same results with a moderately difficult task, and thus had fewer additional processing resources to recruit during the more difficult task condition. Other groups have subsequently shown similar alterations in cerebral activation following MTBI (Chen et al. 2004; Perlstein et al. 2004).
Elucidation of the neural mechanisms underlying these observations might inform pharmacological strategies for treatment of WM complaints associated with TBI. A significant body of work suggests that catecholaminergic mechanisms play important roles in WM function, especially in the prefrontal cortex (PFC; see (Arnsten et al. 1998)), and there is evidence that TBI alters catecholamine tone and regulation after TBI (see McAllister et al 2004; Kobori et al. 2010 for reviews). Such abnormalities may play a role in alterations in activation and allocation of WM processing resources after MTBI. Although difficult to test this hypothesis directly, it is possible to use pharmacological challenges to probe whether individuals with MTBI differ in their response to catecholaminergic agents. Altered behavioral (cognitive) and neurophysiological (task-related cerebral activation) responses to a catecholamine agonist would provide indirect support for this hypothesis.
The catecholamine systems are complex, however, and different catecholamine agonists can have different effects on cognitive function. For example, in monkeys and rats localized prefrontal depletion of catecholamines (dopamine and norepinephrine) caused by infusion of 6-hydroxydopamine as well as global catecholamine depletion with agents such as reserpine lead to impaired performance on spatial WM tasks similar to that seen with ablation of neural tissue in the PFC (Brozoski et al. 1979; Cai et al. 1993). Normal aging in these animals results in lower levels of both dopamine (DA) and norepinephrine (NE) and is associated with declining performance on WM tasks (Arnsten 1998; Bartus et al. 1978; Luine et al. 1990), as is the use of alpha-2A (A2A) antagonists (Steere and Arnsten 1997; Tanila et al. 1996).
The role of the adrenergic system in WM varies as a function of receptor type, brain region, dose, and task difficulty. For example, in rodents and primates, blockade of the A2A system with infusion of A2A antagonists produces spatial WM impairment (Steere and Arnsten 1997; Tanila et al. 1996), whereas alpha-1 and beta adrenergic antagonists do not (Arnsten and Li, 2005). WM performance deficits brought about by normal aging, chemical depletion, or infusion of A2A antagonists can be reversed by A2A agonists (see Arnsten et al. 1988 for review; Arnsten and Goldman-Rakic 1985; Carlson et al. 1992; Franowicz and Arnsten 1998; Rama et al. 1996). However, A1A agonists can impair WM function, suggesting that different adrenergic receptors have opposing effects on cognitive function (Arnsten 1998; Birnbaum et al. 1999). In humans the A2A agonist guanfacine seems particularly effective at enhancing WM performance with a relatively low side effect profile (Arnsten et al. 1998; Arnsten 1998; Rama et al. 1996; Sirvio et al. 1991; Steere and Arnsten 1997). Jakala et al. (Jakala et al. 1999; Jakala et al. 1999) administered three different doses of clonidine (also an A2A agonist) or two doses of guanfacine to healthy controls using a double-blind, placebo-controlled design. Both low and high dose clonidine were associated with poorer performance on WM, and showed little effect on the other tasks. At the higher dose (29mcg/kg), guanfacine was associated with significant improvement on WM, the Tower of London task, and a verbal paired associates learning task. The researchers interpreted these results as consistent with guanfacine-enhanced frontal functioning in both spatial WM and planning. Taken together, these studies suggest that A2A effects may depend on individual characteristics of the agonist used and the dose, as well as the nature of the task tested. There are no studies of the ability of A2A agonists to enhance WM in individuals with TBI; however, there is evidence from other clinical populations that augmentation of the A2A system can improve aspects of WM and other cognitive functions (Arnsten et al. 1996; Hunt et al. 1995); see also Arnsten (1998) and McAllister and Arnsten (2008) for thorough discussion.
The mechanism by which A2A receptor stimulation results in improved WM function is not clear. A2A receptors are located both pre-synaptically on noradrenergic neuronal terminals, and post-synaptically on dendritic spines, as well as sites removed from traditional synapses. Presynaptically, A2A stimulation results in inhibition of NE release; post-synaptically A2A stimulation appears to result in increased signal to noise ratio, apparently the opposite of what is seen with alpha-1 receptor stimulation (Arnsten 1998). Adrenergic neuronal cell bodies arise in the brain stem within the locus coeruleus (LC) and project throughout most of the CNS. Thus LC activity is capable of altering a wide array of CNS functions. However, there are particularly strong reciprocal connections between PFC and LC, suggesting that adrenergic tone in one area can affect adrenergic tone in the other (Arnsten 1998). It is relevant that guanfacine is ten times weaker than clonidine in assays of pre-synaptic function, and this may in part explain why clonidine is more sedating than guanfacine (clonidine also likely has more potent effects on A2B receptors in the thalamus, which have powerful effects on arousal). However, the majority of A2A receptors in the brain are actually localized post-synaptic to NE neurons, and these are of particular relevance to WM.
The above literature suggests that the adrenergic system plays an important but complex role in the modulation of WM that is receptor, brain region, dose, and task specific. Enhancement of A2A system functioning can improve WM through stimulation of post-synaptic receptors in the PFC, which is a critical component of WM circuitry. However, depending on the A2A agonist used, there can be opposing effects. For example, agents that stimulate pre-synaptic A2A receptors in LC (e.g., clonidine) can result in reduced adrenergic tone in PFC and reduced arousal, both of which could impair WM function. Alternatively, excessive stimulation or conditions of stress (including perhaps arousal and stress associated with performance of difficult cognitive tasks), will impair WM and other cognitive functions (Arnsten and Li 2005). Guanfacine, because of its lower propensity for pre-synaptic A2A receptors (and hence less inhibition of LC firing) is ideally positioned to be of selective benefit to WM.
In this study we tested the hypothesis that problems in activation and allocation of WM resources in PFC after MTBI are related to dysregulation of prefrontal A2A post-synaptic receptors. We therefore hypothesized that stimulation of these receptors with a moderate dose of guanfacine would selectively improve WM performance and normalize task associated cerebral activation in WM circuitry relative to noninjured controls.
Methods
This was a prospective, placebo-controlled, double-blind, crossover study of consecutive patients with MTBI referred to Dartmouth-Hitchcock Medical Center (DHMC), a Level 1 Trauma Center.
Participants
Participants were diagnosed with MTBI by a Neuropsychiatrist (TM) using the American Congress of Rehabilitation Medicine criteria for mild brain injury (Kay et al. 1993). Exclusion criteria included a history of other neurologic disorders, significant systemic medical illness, or current DSM-IV Axis I diagnosis of psychiatric illness as assessed by the Structured Clinical Interview for DSM-IV (First et al. 1997). Healthy controls (HC) were recruited through community advertisements and were screened for neurologic, medical, or any past or current psychiatric illness. After complete description of the study to the participants, written informed consent was obtained. The study protocol and the informed consent document were approved by the Dartmouth College Committee for the Protection of Human Subjects.
Procedures
Participants were studied on two occasions approximately one week apart. The sequence of guanfacine and placebo administration was randomized and balanced across groups. The order of neuropsychological and fMRI task administration was also balanced, using alternate forms when available.
Study Protocol
Approximately two and a half hours after oral drug (2.0 mg guanfacine) or placebo ingestion, participants were positioned in the MRI scanner using laser alignment beams and a non-magnetic deformable foam head holder to stabilize head position. Stimuli for the cognitive tasks were programmed in Presentation (Neurobehavioral Systems, Inc., Albany, CA) and presented visually through an MRI compatible goggle system (Resonance Technology, Van Nuys, CA).
fMRI Scan Procedure
All scans were acquired using the same GE Horizon 1.5T LX scanner. A gradient echo, echo-planar sequence was used to provide whole brain coverage: TR=2500 ms, TE=40 ms, FOV=24 cm, NEX=1, 29 interleaved 5 mm thick sagittal slices with no skip, yielding a 64×64 matrix with 3.75 mm2 in-plane resolution. Initial volumes prior to spin saturation were discarded.
WM Task
As in our previous studies (McAllister et al. 2006; McAllister et al. 1999; McAllister et al. 2001), a visually presented verbal “N-back” task was used to test WM. During scanning participants viewed a string of consonant letters (except L, W, and Y) presented at a rate of one every three seconds, in a four-condition, blocked design. Conditions were 0-, 1-, 2-, and 3-back. For each consonant seen, participants used a button press device (Photon Control Inc., Burnaby, BC) to signify whether the current letter was a match (i.e., was the same as the designated target or the letter presented 1, 2, or 3 back in the sequence, depending on the condition instructions) or was a non-match. Each task condition was presented in 27-second epochs preceded by three seconds of instruction (e.g., “the match is D” or “the match is one back”). The four experimental conditions were each presented three times in pseudo-random order for a total of 12 task blocks. Participants rehearsed a practice version of the task on a laptop prior to scanning to ensure comprehension of task demands.
Clinical Assessment
After the scan session, participants completed a neuropsychological test battery that assessed level of estimated general intellectual functioning (WRAT-3, Reading subtest (Wilkinson 1993); WAIS-III, Block Design subtest (The Psychological Corporation 1997)), verbal memory (California Verbal Learning Test (Delis et al. 1987) or California Verbal Learning Test-II (Delis et al. 2000)), psychomotor speed (WAIS-III, Digit Symbol-Coding subtest (The Psychological Corporation 1997)) and WM, executive, and attentional functioning (Trail Making Test, Parts A and B (Reitan and Wolfson 1993) or Delis-Kaplan Executive Function System (D-KEFS), Trail Making Test Conditions 2 and 4 (Delis et al. 2001)); D-KEFS Color-Word Interference Test (Delis et al. 2001); Controlled Oral Word Association Test (Lezak 1995; Spreen and Strauss 1998); Paced Auditory Serial Addition Test (PASAT) (Gronwall 1977); Gordon Continuous Performance Test (Gordon et al. 1986)).
Statistical Analysis
Cognitive Measures
WM in-scanner performance data and neuropsychological variables for both groups were compared using a repeated measures ANCOVA taking into account drug condition (guanfacine vs. placebo), diagnosis (MTBI vs. HC), age, education, and order of drug administration (drug/placebo vs. placebo/drug). For neuropsychological test data, raw or standard scores were used as indicated, with the exception of Trail Making. Because the version of the test changed during the protocol, raw scores for Trails A and B and D-KEFS conditions 2 and 4 were z-transformed using the HC group means in the placebo condition, with z-scores utilized for group comparisons. All models were fit using PROC MIXED in SAS V9, specifying a random subject effect for the repeated measures covariance structure.
fMRI Analyses
Spatial realignment using a six parameter model was performed on all raw scan data to remove any minor (subvoxel) motion-related signal change. All volumes for each subject were normalized into standardized Montreal Neurological Institute atlas space using SPM5 (Wellcome Department of Cognitive Neurology, University College, London). During spatial normalization all scans were resampled to 2 mm3 isotropic voxels. Spatial smoothing to a full width half maximum of 8 mm was performed prior to statistical analysis. fMRI analyses included statistical parametric mapping, on a voxel-by-voxel basis, using a general linear model approach (Friston et al. 1995) as implemented in SPM5. Smoothed normalized scans for all subjects were entered into the model, and contrast images comparing pairs of the WM processing load conditions (1-back > 0-back, 2-back > 0-back, 3-back > 0-back) were created for each subject. These contrast images were then used for the second level multi-subject/between-group random effects analyses. The random effects procedure performs a mixed model analysis to account for both random effects (scan) and fixed effects (task condition) (Holmes and Friston 1998).
Random effects analyses were conducted using ANCOVA to construct contrast maps of voxels in which brain activation differed between group and drug conditions (full factorial model in SPM5). Comparisons were conducted within an omnibus group- (two independent levels: MTBI, HC) by-drug (two non-independent levels: guanfacine, placebo) ANCOVA, covarying for age, education, and order of drug/placebo administration. The design matrix therefore included both conditions for both groups, accounting for the repeated measures nature of the drug factor (i.e., the matrix included four columns, one for each group on placebo and guanfacine). The critical significance threshold (Pcrit) was set to 0.01. Given the constraints of the sample size, only clusters of activated voxels with a whole-brain search cluster-level Puncorrected < 0.05 are discussed. For illustrative purposes, figures and tables also include results at a Pcrit of 0.05 where noted. Within the omnibus SPM5 design matrix between-group comparisons were conducted using weighted contrast vectors. For example, pair-wise comparisons of brain activation on guanfacine (MTBI vs. HC) were conducted by entering values of 1 and -1 in the appropriate columns in the matrix. In this manner determination of regions where HC showed greater activation than MTBI on guanfacine would be identified by entering 1 in the HC guanfacine column and -1 in the MTBI guanfacine column.
Results
Demographics
Thirteen subjects with MTBI and 14 HC completed the protocol (see Table 1). There were no significant group differences in WRAT-3 Reading scores, an estimate of baseline verbal intellectual ability. The groups did differ with respect to years of education, largely because several of the MTBI subjects were students at the time of study participation. Parental education did not differ between groups. MTBI participants were studied a mean of 37.2 days after injury (±17.6,) and had an average Glasgow Coma Scale score of 13.9 (±2.1). All participants were injured either by falls or in motor vehicle accidents, and none of the MTBI participants had focal injuries visible on computerized axial tomography (CT) scans done at the time of injury. The protocol was well tolerated by both groups.
Table 1.
Sample Characteristics
| HC [Mean±SD or N(%)] N=14 | MTBI [Mean±SD or N(%)] N=13 | p-value | |
|---|---|---|---|
| Age (years) | 32.0 ± 8.5 | 27.2 ± 11.7 | 0.23 |
| Education (years) | 16.5 ± 2.1 | 13.6 ± 2.8 | 0.005 |
| WRAT-3 Reading SS | 106 ± 4.7 | 107 ± 9.3 | 0.69 |
| Mother's Education (years) | 14.2 ± 2.5 | 14.1 ± 2.8 | 0.90 |
| Father's Education (years) | 14.6 ± 2.9 | 14.7 ± 3.0 | 0.97 |
| Male Gender | 6 (42.9%) | 7 (53.8%) | 0.57 |
| Placebo first | 8 (57.1%) | 8 (61.5%) | 0.82 |
WRAT-3 = Wide Range Achievement Test, 3rd edition; SS = Standard Score
Cognitive Performance
There was a main effect of drug order in our initial modeling of cognitive outcomes (better performance on the second assessment), probably because the protocol called for repeated cognitive assessments over a short interval and both test performance and imaging results may be vulnerable to the influence of practice and exposure effects. Even when alternate forms of a task are given, there can be improvement in performance related to improved strategy/efficiency in task approach. Thus we included order (drug or placebo first) as a covariate in subsequent analyses. Although the two groups did not differ significantly with respect to age, the literature supports a relationship between age and performance across a variety of cognitive measures, including the age range spanned by our participants. In addition, there is evidence that age affects task-related blood oxygen level dependent (BOLD) response. Thus subsequent analyses reported used age, education and order of drug administration as covariates.
N-Back Performance
The MTBI and HC groups showed similar performance on the N-back task. Both groups showed the expected WM load effect on performance, regardless of drug condition (see Table 2), with percentage of correct responses (adjusted for guessing) decreasing with increasing WM load requirements. The HC group generally did worse on guanfacine relative to placebo, although this was not statistically significant. The MTBI group showed a statistically significant improvement on the moderate WM load (2-back) condition on guanfacine relative to placebo (p=0.018), and there was a significant drug-by-diagnosis interaction (p=0.007) for the 2-back as well as a trend for the mean back (p=0.07), with the MTBI group demonstrating improved performance on guanfacine relative to placebo and the HC group showing a decline in performance on the drug.
Table 2.
N-Back Performance
| HC (N= 14) | MTBI (N= 13) | HC vs. MTBI p-values | Overall p-values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N-Back Processing Load | Placebo Mean(SE) | Guanfacine Mean(SE) | P-value | Placebo Mean(SE) | Guanfacine Mean(SE) | P-value | Placebo | Drug | Diagnosis | Drug | Interaction |
| Corrected 1-Back | 86.7(4.1) | 90.0(4.4) | 0.53 | 84.7(4.7) | 89.2(4.1) | 0.43 | 0.97 | 0.92 | 0.97 | 0.32 | 0.88 |
| Corrected 2-Back | 79.9(6.0) | 70.7(6.6) | 0.13 | 76.9(6.0) | 88.8(3.1) | 0.018 | 0.28 | 0.18 | 0.88 | 0.46 | 0.007 |
| Corrected 3-Back | 62.3(6.4) | 55.9(6.4) | 0.36 | 73.4(5.5) | 68.5(7.1) | 0.48 | 0.30 | 0.24 | 0.20 | 0.26 | 0.89 |
| Mean-Back | 76.3(4.0) | 72.2(3.8) | 0.19 | 78.3(4.2) | 82.2(4.1) | 0.19 | 0.99 | 0.22 | 0.51 | 0.97 | 0.07 |
Out of Scanner Cognitive Tasks
Overall, the MTBI group and the HC group did not differ on out of scanner cognitive test performance, with the exception of the fastest condition of the PASAT (PASAT D), where HC demonstrated better performance than the MTBI group. There was a variable effect of guanfacine on performance in a small number of out of scanner cognitive tests, with a positive main effect of drug on the D-KEFS Color-Word Interference Test Word Reading Trial (p=0.007), but a negative main effect on CPT simple reaction time number correct (p=0.036); and CPT vigilance reaction time (p=0.009); as well as Trails 2/A (p=0.02).
fMRI
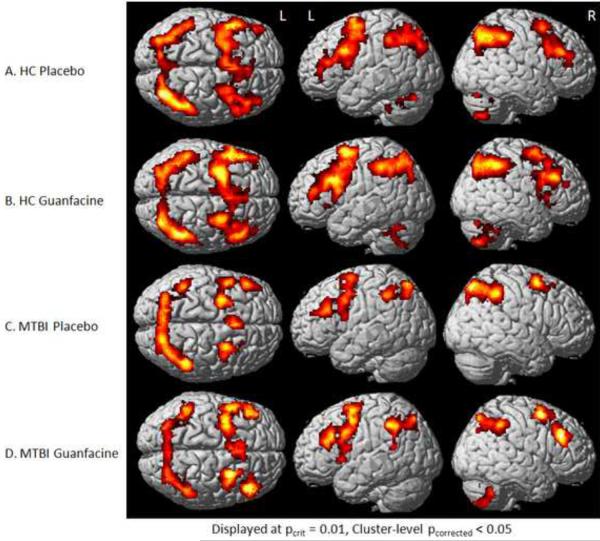
Both diagnostic groups showed the expected WM activation pattern on both drugs (Figure 1), including bilateral frontal, parietal, and cerebellar circuitry. This pattern of activation is consistent with previous results with the N-back task (McAllister et al. 1999; McAllister et al. 2001). As noted above, the two groups showed a significant diagnosis-by-drug interaction in task performance at the moderate (2-back) WM load. All WM conditions were examined, but similar to the findings for task performance, cerebral activation differences were most marked for the 2-back condition and are displayed in Figures 1 and 2, and Table 3. Visual inspection of these maps indicates a prominent increase in right frontal activation in the MTBI group on guanfacine relative to placebo (Figure 1C compared to 1D). Within-group statistical comparisons are shown in Figure 2. Brain regions with significant activation in both groups on both drugs are displayed in red, while drug contrasts of interest are overlaid in blue (e.g., regions where HC showed greater activation on guanfacine than placebo are shown in blue in Figure 2A). Figure 2 again demonstrates increased right frontal activation on guanfacine relative to placebo in the MTBI group. In contrast, the HC group shows increased activation outside typical WM circuitry on guanfacine relative to placebo. This pattern is similar to that of the MTBI group on placebo, where greater activation is also noted outside WM circuitry. This pattern was also evident on the 3-back condition (not shown).
Figure 1.
Group Activation Patterns during 2-Back in Response to Pharmacological Challenge
Activation maps (SPM5) for each group (MTBI and HC) under each drug condition (placebo or guanfacine). Note increased right frontal activation in the MTBI group on guanfacine when compared to placebo (1C and 1D).
Figure 2.
Within-Group Differences in Brain Activation during 2-Back in Response to Pharmacological Challenge
The combined SPM5 activation map for the 2-back > 0-back contrast for both groups (MTBI and HC) in both drug conditions (placebo and guanfacine) is displayed in red. Note the bilateral frontal, parietal, and cerebellar network typically activated during the N-back task. Group contrasts of interest (discussed further in text) are overlaid in blue, using the MRIcroGL software package.
Table 3.
Regions of Significant Activation Differences for 2-back > 0-back Contrast as a Function of Diagnosis and Drug Condition
| Pcrit < .01 | Pcrit < .05 | |||||||
|---|---|---|---|---|---|---|---|---|
| MNI coordinates (x y z) | Cluster extent (k) | Cluster-level Pcorrected | Cluster-level Puncorrected | Cluster extent (k) | Cluster-level Pcorrected | Cluster-level Puncorrected | T | Region description (for cluster peak) |
|
HC Placebo > Guanfacine
| ||||||||
| −44 24 −20 | 134 | .965 | .039 | 1440 | .143 | .001 | 3.77 | Left superior temporal gyrus |
| 34 −66 46 | 177 | .822 | .020 | 612 | .952 | .021 | 3.54 | Right superior parietal lobule |
| 12 −50 62 | 1244 | .254 | .002 | 3.50 | Right precuneus | |||
| 14 12 0 | 632 | .939 | .019 | 3.29 | Right caudate | |||
| −24 −64 −8 | 609 | .954 | .021 | 3.26 | Left fusiform gyrus | |||
|
| ||||||||
|
HC Guanfacine > Placebo
| ||||||||
| 52 −14 60 | 150 | .926 | .030 | 475 | .996 | .038 | 3.60 | Right postcentral gyrus |
| −26 −44 70 | 160 | .892 | .026 | 3.43 | Left postcentral gyrus | |||
| −40 −28 50 | 1135 | .345 | .003 | 3.64 | Left postcentral gyrus (same cluster as previous line) | |||
| 42 −44 −14 | 421 | .999 | .049 | 4.05 | Right fusiform gyrus | |||
| −42 −52 6 | 1788 | .052 | .000 | 3.77 | Left superior temporal gyrus | |||
| 46 18 −32 | 419 | .999 | .049 | 3.27 | Right superior temporal gyrus | |||
| 28 −16 50 | 946 | .562 | .006 | 3.08 | Right precentral gyrus | |||
|
| ||||||||
|
MTBI Placebo > Guanfacine
| ||||||||
| 52 −58 4 | 287 | .324 | .005 | 810 | .747 | .009 | 4.46 | Right superior temporal gyrus |
| 56 10 −6 | 172 | .844 | .022 | 931 | .582 | .006 | 4.22 | Right superior temporal gyrus |
| −8 −78 0 | 424 | .076 | .001 | 4731 | .000 | .000 | 3.82 | Left lingual gyrus |
| 8 −64 6 | 185 | .785 | .018 | 3.74 | Right cuneus (same cluster as previous line) | |||
| −32 −80 −14 | 179 | .813 | .020 | 3.73 | Left inferior occipital gyrus (same cluster as previous line) | |||
| −42 −50 −34 | 131 | .970 | .041 | 3.49 | Left cerebellum (same cluster as previous line) | |||
| 18 −58 −14 | 130 | .972 | .042 | 3.28 | Right cerebellum (same cluster as previous line) | |||
| 22 −76 44 | 514 | .991 | .032 | 2.85 | Right precuneus | |||
| 4 40 −16 | 442 | .998 | .044 | 2.82 | Right medial frontal gyrus | |||
|
| ||||||||
|
MTBI Guanfacine > Placebo
| ||||||||
| −48 −50 36 | 471 | .997 | .039 | 3.87 | Left inferior parietal lobule | |||
| 42 −6 30 | 1372 | .175 | .001 | 3.62 | Right precentral gyrus | |||
| 26 −36 58 | 496 | .994 | .034 | 3.37 | Right postcentral gyrus | |||
| −4 20 16 | 860 | .679 | .008 | 3.31 | Left anterior cingulate gyrus | |||
| −8 −34 68 | 1362 | .180 | .001 | 3.21 | Left paracentral lobule | |||
| 54 32 30 | 439 | .999 | .045 | 2.79 | Right middle frontal gyrus | |||
Discussion
These results indicate that individuals with MTBI have altered cognitive and cerebral activation patterns in response to the A2A receptor agonist guanfacine relative to controls, consistent with the main study hypothesis. Of particular interest is that the observed effect was largely specific to WM task performance. On guanfacine relative to placebo, the MTBI group showed increased activation in WM circuitry, particularly in the right prefrontal area, in and around the middle frontal gyrus. This change in activation was associated with improved performance on the 2-back WM task. The HC group showed no increase in activation in WM circuitry on guanfacine relative to placebo, but did show increased activation in regions not typically involved in WM and in particular N-back performance. This pattern was associated with generally poorer task performance. Although guanfacine is generally less sedating than other alpha-2 agonists such as clonidine, it may be that the decrements observed on some timed tests (CPT measures and Trails 2/A) may be related to decreased arousal associated with the drug, perhaps through pre-synaptic effects on noradrenergic neurons in the LC.
The observation that increased N-back performance was associated with increased activation in PFC, particularly in regions involved with WM processing, is of interest. The A2A system is complex and A2A agonist effects are regionally specific. At the level of central adrenergic tone, stimulation of pre-synaptic A2A receptors on noradrenergic neurons in the LC can reduce activity of these neurons. However, the majority of A2A receptors are found post-synaptic to noradrenergic neurons (Arnsten and Li 2005), particularly on the dendritic spines of pyramidal neurons in the superficial layers of the PFC, where they are positioned to play an important role in the cortical-cortical networks that subserve WM (Arnsten and Li 2005; McAllister and Arnsten 2008). Here the A2A receptors are co-localized with hyperpolarisation-activated cyclic nucleotide-gated (HCN) cation channels (Barth et al. 2008; Ramos et al. 2006). Stimulation of the A2A receptors (with subsequent reduction in production of cyclic AMP) “closes” the HCN channels, making them less leaky and increasing pyramidal cell excitability (Ramos et al. 2006). This in turn enhances local connectivity and is associated with reduced distractibility and enhanced WM (Wang et al. 2007). Additional work has suggested that TBI is associated with a hypercatecholaminergic state, at least in part related to increased tyrosine hydroxylase expression and activity with subsequent increased catecholamine synthesis (Kobori and Dash 2006). Thus it has been suggested that A2A agonists such as guanfacine might enhance WM pre-synaptically by reducing overall catecholamine release at the level of the LC, while also working post-synaptically through enhancing cortical to cortical networks in the PFC (McAllister and Arnsten 2008). In an animal model, guanfacine was found to increase regional cerebral blood flow in monkeys performing a WM task (Avery et al. 2000). More recently Clerkin et al. (2009) found that guanfacine (1.0 mg) significantly reduced reaction time in a warning signal paradigm, and this was associated with increased activation in the dorsolateral PFC as well as related frontal subcortical circuitry. Our results suggest enhanced WM associated with guanfacine in the MTBI group as well as increased PFC activation. Although we did not find improved performance on timed tests, this could be related to the higher guanfacine dose used in our study.
The pattern of greater activation outside typical WM circuitry in the MTBI group on placebo and the HC group on guanfacine accompanied by poorer task performance also merits comment. As shown in Figure 2A and 2D, activation in precentral and superior and middle temporal gyri was apparent in these contrasts. These regions are typically deactivated during WM processing, with prior research in healthy subjects and other clinical populations relating poorer WM task performance to a failure to deactivate such regions (in addition to failure to activate typical WM circuitry) (Anticevic et al. 2010; Crossley et al. 2009; Nejad et al. 2011; Tomasi et al. 2006). The current finding of relatively poorer task performance accompanied by greater activation (or failure to deactivate) outside of expected WM circuitry may offer further evidence that successful WM performance relies on region-specific alterations in brain activation, which can be differentially disrupted by pharmacological challenge in clinical populations relative to healthy controls.
Several limitations should be considered in the interpretation of these findings. We did not include MTBI participants with significant medical and psychiatric disorders; therefore these results may not generalize to all individuals with MTBI. This MTBI group had very mild injuries and the results may not apply to those with moderate and severe injuries. It is also important to point out that the regions of increased activity evident on guanfacine do not necessarily indicate the exact locations of the adrenergic effect. Although BOLD fMRI activation in these areas indicates local increased cerebral blood flow and metabolic activity, guanfacine administration could indirectly modulate this activity through striatal or other interconnected subcortical or cortical sites. This is a relatively small sample size and thus should be considered a preliminary finding. However, the fact that we are able to detect a specific effect on the hypothesized cognitive domain (WM) and that this effect is associated with increased activation in WM circuitry, even in this sample size, is of interest and suggests that psychopharmacological probes in association with fMRI may be a promising way to test hypotheses about the neural mechanisms of cognitive complaints and deficits after MTBI.
These results are consistent with the hypothesis that MTBI is associated with subtle dysregulation of frontal alpha-2 adrenergic systems in the first 4–6 weeks after injury, and that augmentation strategies with alpha-2 agonists might improve WM function. However, the majority of A2A receptors in the brain are actually localized post-synaptic to NE neurons, particularly in the dendritic spines of prefrontal neurons critical to cortical-cortical networks involved in WM (Arnsten and Li 2005; Ramos et al. 2006) Further studies are needed to clarify the effects that different dosing strategies, severity of injury, and the injury to treatment interval may all have on outcome.
Acknowledgments
This research was supported in part by NIDRR Grants H133G70031 & H133000136 and NIH Grant R01 NS40472-01. The authors would like to thank Brian Greenlee and Kaloyan Tanev, Neuropsychiatry Fellows, James Ford and Heather Pixley, Department of Psychiatry, and Robert Ferranti, Alice Davison, Shreve Soule and Robert Shaffer, Department of Radiology, for their assistance on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Location of Work: Section of Neuropsychiatry and Brain Imaging Laboratory, Departments of Psychiatry, Radiology, Community and Family Medicine (Biostatistics), Emergency Medicine, Dartmouth Medical School, Lebanon, NH
References
- Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. NeuroImage. 2010;49(3):2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A, Li B-M. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biological Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. Journal of Neuroscience. 1988;8(11):4287–98. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230(4731):1273–6. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Steere JC, Jentsch DJ, Li BM. Noradrenergic influences on prefrontal cortical cognitive function: opposing actions at postjunctional alpha 1 versus alpha 2-adrenergic receptors. Advances in Pharmacology (New York) 1998;42:764–7. doi: 10.1016/s1054-3589(08)60859-5. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Catecholamine modulation of prefrontal cortical cognitive function. Trends in Cognitive Sciences. 1998;2(11):436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Steere JC, Hunt RD. The contribution of alpha-2 noradrenergic mechanisms to prefrontal cortical cognitive function: potential significance to Attention Deficit Hyperactivity Disorder. Archives of General Psychiatry. 1996;53:448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23(3):240–9. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Barth A, Vizi E, Zelles T, Lendvai B. Alpha2-adrenergic receptors modify dendritic spike generation via HCN channels in the prefrontal cortex. J Neurophysiol. 2008;99:394–401. doi: 10.1152/jn.00943.2007. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Fleming D, Johnson HR. Aging in the rhesus monkey: debilitation effects on short-term memory. Journal of Gerontology. 1978;33:858–871. doi: 10.1093/geronj/33.6.858. [DOI] [PubMed] [Google Scholar]
- Binder LM, Rohling ML, Larrabee J. A review of mild head trauma. Part I: Meta-analytic review of neuropsychological studies. Journal of Clinical and Experimental Neuropsychology. 1997;19(3):421–431. doi: 10.1080/01688639708403870. [DOI] [PubMed] [Google Scholar]
- Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biological Psychiatry. 1999;46(9):1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Brozoski T, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–931. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Cai JX, Xu L, Hu X. Reserpine impairs spatial working-memory performance in monkeys: Reversal by the alpha-2 adrenergic agonist clonidine. Brain Research. 1993;614:191–196. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]
- Carlson S, Tanila H, Rama P, Mecke E, Pertovaara A. Effects of medetomidine, an alpha-2 adrenoceptor agonist and atipamezole, an alpha-2 antagonist, on spatial memory performance in adult and aged rats. Behav Neural Biol. 1992;58(2):113–9. doi: 10.1016/0163-1047(92)90327-z. [DOI] [PubMed] [Google Scholar]
- Chen K, Reiman EM, Alexander GE, Bandy D, Renaut R, Crum WR, Fox NC, Rossor MN. An automated algorithm for the computation of brain volume change from sequential MRIs using an iterative principal component analysis and its evaluation for the assessment of whole-brain atrophy rates in patients with probable Alzheimer's disease. Neuroimage. 2004;22(1):134–43. doi: 10.1016/j.neuroimage.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Cicerone K, Dahlberg C, Kalmar K, Langenbahn DM, Malec J, Bergquist TF, Felicetti T, Giacino JT, Harley JP, Harrington DE, Herzog J, Kneipp S, Laatsch L, Morse PA. Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Archives of Physical Medicine and Rehabilitation. 2000;81:1596–1615. doi: 10.1053/apmr.2000.19240. [DOI] [PubMed] [Google Scholar]
- Clerkin S, Schulz K, Halperin J, Newcorn J, Ivanov I, Tang C, Fan J. Guanfacine Potentiates the Activation of Prefrontal Cortex Evoked by Warning Signals. Biol Psychiatry. 2009;66:307–312. doi: 10.1016/j.biopsych.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Fusar-Poli P, Broome MR, Matthiasson PJ, L.C., Bramon EV, L., Williams SCR, McGuire PK. Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Human Brain Mapping. 2009;30(12):4129–4137. doi: 10.1002/hbm.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult version. The Psychological Corporation; New York: 1987. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Second Edition: Adult Version Manual. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Delis DC, Kaplan E, Kramer JF. Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press; Washington, D.C.: 1997. [Google Scholar]
- Franowicz JS, Arnsten AF. The alpha-2a noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology. 1998;136(1):8–14. doi: 10.1007/s002130050533. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gordon M, McClure FD, Aylward GP. Gordon Diagnostic System: Instruction Manual and Interpretive Guide. Gordon Systems, Inc.; DeWitt, NY: 1986. [Google Scholar]
- Gronwall D. Paced Auditory Serial Addition Task: A measure of recovery from concussion. Perceptual Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Holmes A, Friston K. Generalisability, random effects and population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- Hunt RD, Arnsten AFT, Asbell MD. An open trial of guanfacine in the treatment of Attention Deficit Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- Jakala P, Riekkinen M, Sirvio J, Koivisto E, Kejonen K, Vanhanen M, Riekkinen P., Jr. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20(5):460–70. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Jakala P, Sirvio J, Riekkinen M, Koivisto E, Kejonen K, Vanhanen M, Riekkinen P., Jr. Guanfacine and clonidine, alpha 2-agonists, improve paired associates learning, but not delayed matching to sample, in humans. Neuropsychopharmacology. 1999;20(2):119–30. doi: 10.1016/S0893-133X(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Kay T, Harrington DE, Adams R, Anderson T, Berrol S, Cicerone K, Dahlberg C, Gerber D, Goka R, Harley P, Hilt J, Horn L, Lehmkuhl D, Malec J. Definition of Mild Traumatic Brain Injury. Journal of Head Trauma Rehabilitation. 1993;8(3):86–87. [Google Scholar]
- Kobori N, Dash PK. Reversal of brain injury-induced prefrontal glutamic acid decarboxylase expression and working memory deficits by D1 receptor antagonism. Journal of Neuroscience. 2006;26(16):4236–46. doi: 10.1523/JNEUROSCI.4687-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori N, Hu B, Dash PK. Altered adrenergic receptor signaling following brain injury contribures to dysfunction of the prefrontal cortex. 2010 doi: 10.1016/j.neuroscience.2010.10.048. Neuroscience electronically published http://dx.doi.org/10.1016/j.neurosceince.2010.10.048. [DOI] [PMC free article] [PubMed]
- Levin HS. Cognitive function outcomes after traumatic brain injury. Current Opinion in Neurology. 1998;11:643–646. doi: 10.1097/00019052-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Levin HS, Mattis S, Ruff RM, Eisenberg HM, Marshall LF, Tabaddor K, High WM, Jr., Frankowski RF. Neurobehavioral outcome following minor head injury: A three-center study. Journal of Neurosurgery. 1987;66(2):234–243. doi: 10.3171/jns.1987.66.2.0234. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. Oxford University Press; New York: 1995. [Google Scholar]
- Luine V, Bowling D, Hearns M. Spatial memory deficits in aged rats: Contributions of monoaminergic systems. Brain Researsh. 1990;537:271–278. doi: 10.1016/0006-8993(90)90368-l. [DOI] [PubMed] [Google Scholar]
- McAllister T, Arnsten A. Pharmacologic Approaches to Cognitive Rehabilitation. In: Stuss D, Winocur G, Robertson I, editors. Cognitive Neurorehabilitation. Cambridge University Press; Boston: 2008. pp. 298–320. [Google Scholar]
- McAllister TW, Flashman LA, McDonald BC, Saykin AJ, McAllister TW, Flashman LA, McDonald BC, Saykin AJ. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. Journal of Neurotrauma. 2006;23(10):1450–67. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Flashman LA, Sparling MB, Saykin AJ. Working memory deficits after mild traumatic brain injury: Catecholaminergic mechanisms and prospects for catecholaminergic treatment-a review. Brain Injury. 2004;18(4):331–350. doi: 10.1080/02699050310001617370. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian A, Weaver J, Yanofsky N. Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14(5):1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- McMillan TM. Minor head injury. Current Opinion in Neurology. 1997;10(6):479–83. doi: 10.1097/00019052-199712000-00008. [DOI] [PubMed] [Google Scholar]
- Nejad AB, Ebdrup BH, Siebner HR, Rasmussen H, Aggernææs B, Glenthøøj BY, Baaréé WFC. Impaired temporoparietal deactivation with working memory load in antipsychotic-naïïve patients with first-episode schizophrenia. World Journal of Biological Psychiatry. 2011;12(4):271–281. doi: 10.3109/15622975.2010.556199. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Cole MA, Demery JA, Seignourel PJ, Dixit NK, Larson MJ, Briggs RW. Parametric manipulation of working memory load in traumatic brain injury: Behavioral and neural correlates. Journal of the International Neuropsychological Society. 2004;10:724–741. doi: 10.1017/S1355617704105110. [DOI] [PubMed] [Google Scholar]
- Rama P, Linnankoski I, Tanila H, Pertovaara A, Carlson S. Medetomidine, atipamezole and guanfacine in delayed response performance of aged monkeys. Pharmacology, Biochemistry & Behavior. 1996;55(3):415–22. doi: 10.1016/s0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Ramos B, Stark D, Verduzco L, van Dyck C, Arnsten A. Alpha-2A-Adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learning and Memory. 2006;13(6):770–6. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. 2nd ed. Neuropsychology Press; Tucson, AZ: 1993. [Google Scholar]
- Sirvio J, Riekkinen P, Jr., Vajanto I, Koivisto E, Riekkinen PJ. The effects of guanfacine, alpha-2 agonist, on the performance of young and aged rats in spatial navigation task. Behav Neural Biol. 1991;56(1):101–7. doi: 10.1016/0163-1047(91)90327-m. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests(eds) New York; Oxford UP: 1998. Controlled Oral Word Association; pp. 447–467. [Google Scholar]
- Steere JC, Arnsten AF. The alpha-2A noradrenergic receptor agonist guanfacine improves visual object discrimination reversal performance in aged rhesus monkeys. Behavioral Neuroscience. 1997;111(5):883–91. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- Tanila H, Rama P, Carlson S. The effects of prefrontal intracortical microinjections of an alpha-2 agonist, alpha-2 antagonist and lidocaine on the delayed alternation performance of aged rats. Brain Res Bull. 1996;40(2):117–9. doi: 10.1016/0361-9230(96)00026-3. [DOI] [PubMed] [Google Scholar]
- The Psychological Corporation . WAIS-III Wechsler Adult Intelligence Scale-Third Edition WMS-III Wechsler Memory Scale-Third Edition Technical Manual. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: An intra-subject fMRI study at 4 Tesla. Human Brain Mapping. 2006;27(8):694–705. doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos B, Paspalas C, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Whyte J, Schuster K, Polansky M, Adams J, Coslett HB. Frequency and duration of inattentive behavior after traumatic brain injury: effects of distraction, task and practice. Journal of the International Neuropsychological Society. 2000;6(1):1–11. doi: 10.1017/s1355617700611013. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test (WRAT3): Administration Manual. Wide Range, Inc.; Wilmington, DE: 1993. [Google Scholar]