Abstract
Chronic pain is a common neurological disease involving lasting, multifaceted maladaptations from gene modulations to synaptic malfunctions and to emotional disorders. Sustained pathological stimuli in many diseases alter output activities of certain genes through epigenetic modifications, but it is unclear how epigenetic mechanisms operate in the development of chronic pain. We demonstrate here that, in the rat brainstem nucleus raphe magnus, which is important for central mechanisms of chronic pain, persistent inflammatory and neuropathic pain epigenetically suppresses gad65 activities through histone deacetylase (HDAC)-mediated histone hypoacetylation, resulting in impaired GABA synaptic inhibition. gad65 knockout mice display similar sensitized pain behavior and impaired GABA synaptic function in the brainstem neurons. HDAC inhibitors overwhelmingly increase gad65 activities, restore GABA synaptic function and relieve the sensitized pain behavior, but not in gad65 knockout mice. These findings suggest GAD65 and HDACs as potential therapeutic targets in an epigenetic approach to the treatment of chronic pain.
INTRODUCTION
Environmental factors such as pathological conditions can alter activities of many genes through modifications of chromatin structure, including DNA methylation and histone acetylation, resulting in stable phenotypes1,2. Chromatin remodeling dynamically modulates, either positively or negatively, the transcriptional activity of target genes3. Histone acetylation increases gene activity by de-condensing chromatin structure, allowing increased accessibility of transcriptional machinery to DNA for transcriptional activation4. Epigenetic mechanisms are implicated in adaptive responses to many neurological disorders where persistent neurochemical stimuli are present5,6. For example, histone acetylation critically regulates synaptic plasticity and memory formation7, and drugs of abuse alter chromatin structure through histone acetylation and phosphorylation, leading to maladaptive changes in behaviors of drug addiction8-10.
Chronic pain is a neurological disease caused by nerve injury and persistent tissue inflammation under various pathological conditions such as cancer and neurodegenerative diseases11. Distinct from acute pain, chronic pain could induce long-term synaptic and cellular maladaptive changes, involve dynamic memory processes and cause characteristic emotional disorders including depression, stress and anxiety11-14. The molecular mechanisms underlying chronic pain development remain poorly understood. The characteristics of chronic pain are strongly suggestive of epigenetic modulations. Evidence is emerging in animal pain models, showing antinociceptive effects of histone deacetylase (HDAC) inhibitors15,16 and epigenetic regulation of C-fiber dysfunction in hypoesthesia17. However, how epigenetic mechanisms operate and what are the target genes in chronic pain development are largely unknown. In this study, we explored persistent pain-induced histone modifications in animal models of inflammatory and neuropathic pain. Whereas spinal adaptive mechanisms are important in chronic pain, our study focused on the brainstem nucleus raphe magnus (NRM), a critical supraspinal site for maintenance of pain hypersensitivity in behavioral states of chronic pain18,19.
RESULTS
Inflammatory pain increases global histone acetylation
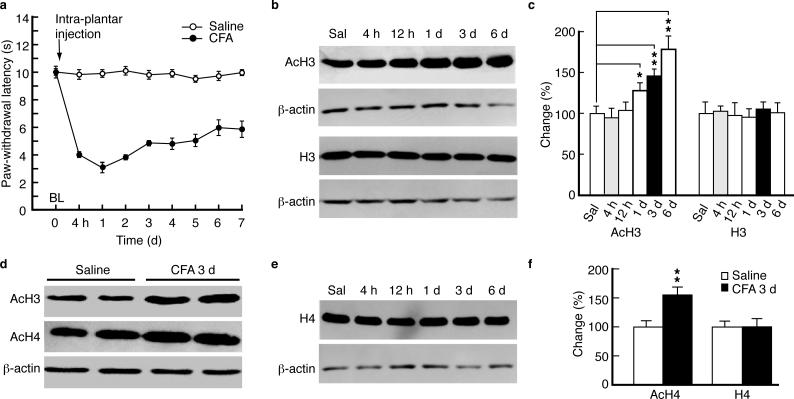
We first examined global histone acetylation levels in rats with persistent inflammatory pain induced by complete Freund's adjuvant (CFA)20. CFA induced persistent pain sensitization (hyperalgesia) (Fig. 1a). Sampling NRM tissues at different time points (4 h, 12 h, 1 d, 3 d and 6 d post-CFA injection), we found that global histone H3 acetylation was unchanged until 1 d when it displayed a continued increase for 6 d (Fig. 1b,c). Total H3 protein levels were unchanged during this period. In tissues taken at 3 d (representing persistent pain), both histone H3 and H4 acetylation levels were increased (Fig. 1d,f), but not the total H4 protein (Fig. 1e,f). Similar results were obtained by ELISA for H3 acetylation at 3 d post-injection (171.4 ± 34.1% increase, n = 7, p < 0.05).
Figure 1.
Persistent inflammatory pain induces time-dependent hyperacetylation of histones H3 and H4. (a) Time course for the development of persistent pain sensitization induced by complete Freund's adjuvant (CFA) and for saline controls, measured by the paw-withdrawal test (n = 6 rats in each group). (b,c) Western blot lanes (b) and summarized data (c, n = 5–9 rats for each group) of global acetylated histone H3 (AcH3) and total H3 proteins, normalized to β-actin, in tissues of rat nucleus raphe magnus (NRM) taken at various time points after CFA injection. (d) Western lanes of AcH3 and AcH4 3 d after CFA injection. (e,f) Western lanes (e) and summarized results (f, n = 7 rats for each group) of AcH4 and total H4 after CFA injection. Data are expressed as mean ± SEM. * p < 0.05, ** p < 0.01. BL, baseline. Sal, saline.
These results suggest that persistent pain (>1 d), but not acute pain (hours), involves global histone hyperacetylation in NRM.
Persistent pain decreases GABAergic synaptic function
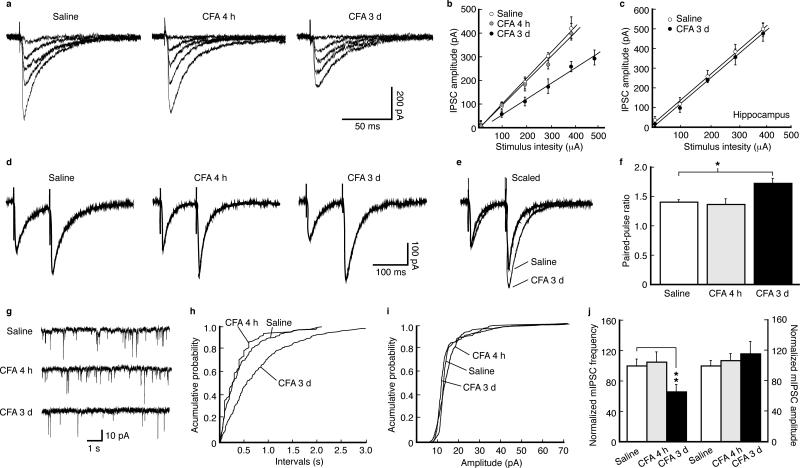
Chronic pain is presumably caused partly by sustained activation of descending pain-facilitatory pathways from NRM18. This neuronal hyper-activation could result from loss of inhibitory GABA functions in NRM. In NRM neurons from CFA-injected rats, we found that the slope of input-output curve for GABAergic inhibitory post-synaptic currents (IPSCs) was similar to controls at 4 h post-injection (for acute pain), but decreased at 3 d (for persistent pain) (Fig. 2a,b). No difference was observed in IPSC slopes of hippocampal neurons (Fig. 2c).
Figure 2.
Persistent pain decreases GABAergic synaptic function by inhibiting presynaptic GABA release. (a) Representative traces of GABA inhibitory post-synaptic currents (IPSCs) evoked by various stimulation intensities in NRM neurons from a saline-injected rat and a CFA-injected rat at 4 h and 3 d post-injection. (b) A plot of input-output curves for IPSC amplitudes in neurons of the three treatment groups as in (a). Slopes: saline, 90.4 ± 11.5 pA 0.1 mA–1, n = 13; CFA 4 h, 88.7 ± 12.9 pA 0.1 mA–1, n = 26, p > 0.05; CFA 3 d, 53.6 ± 10.5 pA 0.1 mA–1, n = 35, p < 0.01. (c) A similar input-output plot of IPSCs from hippocampal CA1 neurons. Slopes: saline, 98.1 ± 14.8 pA 0.1mA–1, n = 15; CFA 3 d, 102.7 ± 16.2 pA 0.1mA–1, n = 16, p > 0.05. (d) Representative IPSC pairs evoked by two stimuli (100 ms apart) in NRM neurons from the three groups of rats. (e) Two representative IPSC pairs, from the two indicated groups, superimposed and scaled to the amplitude of the first IPSC. (f) Group data of changes in the paired-pulse ratios in the three groups (n = 15–35 cells for each group). (g) Representative traces of spontaneous miniature IPSCs (mIPSCs) in neurons from the three groups. (h–j) Distribution plots of mIPSC frequency (h) and amplitude (i) from neurons of each group and their group data (j, n = 16–20 cells).
For the synaptic site of this decrease, we found that the paired-pulse ratio, which is inversely related to presynaptic neurotransmitter release21,22, was unchanged at 4 h, but increased at 3 d (Fig. 2d–f), and miniature IPSC (mIPSC) frequency, but not amplitude, was reduced at 3 d, but not at 4 h (Fig. 2g–j), indicating decreased presynaptic GABA release. Thus, persistent pain decreases presynaptic function of GABA synapses in NRM neurons.
Persistent pain epigenetically reduces GAD65 expression
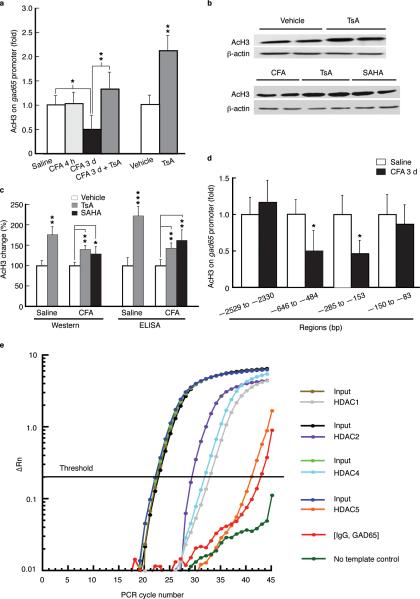
Glutamic acid decarboxylase 65 (GAD65) is a GABA synthetic enzyme that preferentially synthesizes GABA in synaptic terminal for vesicle release whereas GAD67 preferentially synthesizes cytoplasmic GABA23,24. We used Chromatin immunoprecipitation (ChIP) assays to determine H3 acetylation levels in gad65 promoter regions under the pain conditions. We found that H3 acetylation in the region of –646 to –484 bp upstream of the transcription start site (TSS) was reduced in rats at 3 d post-CFA, but not at 4 h (Fig. 3a). Systemic treatment with histone deacetylase (HDAC) inhibitors trichostatin A (TsA) or suberoylanilide hydroxamic acid (SAHA), which increases histone acetylation non-selectively25, increased the pain-reduced H3 acetylation level in the gad65 promoter region and global H3 acetylation in NRM from saline- and CFA-injected rats (Fig. 3a–c). Acetylated H3 was also reduced in gad65 promoter region of –285 to –153 bp, , but not in regions of <150 bp and >2 kb upstream (Fig. 3d).
Figure 3.
Persistent pain induces histone hypoacetylation at the gad65 promoter. (a) Summarized data (n = 5–6 rats each group) of acetylated H3 levels in the gad65 promoter region [(–646 to –484 bp upstream of the transcription start site (TSS)] in NRM tissues from the indicated groups of CFA-injected rats and controls. (b,c) Immunoblotting of acetylated H3 (b) and summarized results of Western analyses and ELISA (c) in saline- and CFA-injected rats (n = 5–7 for each group) treated with TsA or SAHA. (d) Normalized levels of acetylated H3 in the indicated sequence regions upstream of the TSS in the gad65 gene in NRM tissues from saline- and CFA-injected rats at 3 d post-injection (n = 5–6 rats each group). (e) Representative real-time PCR data from ChIP with HDAC-specific antibodies at the gad65 promoter region (–646 to –484 bp) in NRM tissues of rats (n = 5) at 3 d after CFA injection, showing amplification curves of input, immunoprecipitates with antibodies to HDAC1, HDAC2, HDAC4 and HDAC5, and non-immune IgG as negative control.
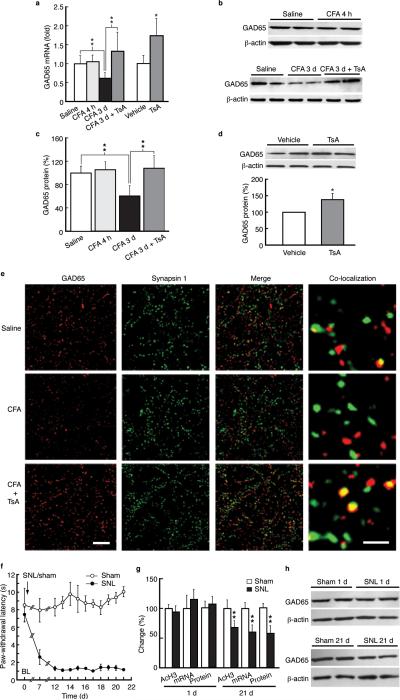
We determined relative levels of HDAC1 and HDAC2 of class I and HDAC4 and HDAC5 of activity-dependent class II26,27 present on gad65 promoter in NRM chromatin preparations from rats at 3 d post-CFA, and found that chromatin-associated HDAC1, HDAC2 and HDAC4 marks, but not HDAC5, showed significant levels in gad65 promoter (Fig. 3e). Additionally, GAD65 mRNA level was decreased and so was GAD65 protein (Fig. 4a–c). No change in GAD65 expression was observed 4 h post-CFA. These reductions in GAD65 transcriptional and translational activities were completely overcome by TsA (Fig. 4a–c). TsA also increased GAD65 protein in control rats to a less extent (Fig. 4d), suggesting a global TsA effect. Anatomically, persistent pain decreased co-localization of GAD65 and terminal protein synapsin I by 46 ± 10%, and the co-localization displayed a ~2-fold increase by TsA in NRM neurons (Fig. 4e).
Figure 4.
Persistent inflammatory and neuropathic pain reduces GAD65 expression. (a) Effects of CFA and TsA on GAD65 mRNA levels in NRM tissues from control and CFA-injected rats (n = 5–7 each group). (b,c) Western lanes (b) and group data (c, n = 5– 7 each group) of GAD65 proteins in saline- and CFA-injected rats 4 h and 3 d post-injection without or with the TsA treatment in vivo. (d) Western lanes of GAD65 proteins in NRM tissues from normal rats. (e) Micrographs of immunohistochemical staining for GAD65 (red), the synaptic terminal protein synapsin I (green), their merged images and co-localization of GAD65 and synapsin I (yellow) in saline- (top row) and CFA- (bottom two rows) injected rats without or with the TsA treatment (n = 5–6 rats each group and 4–6 randomly selected sections were analyzed and compared). Scale bars = 50 μm (left three columns) and 2 μm (right column). (f) Time course of changes in pain threshold in rats after spinal nerve ligation (SNL, n = 6) or sham surgery (n = 5) performed on day 0 (arrow). (g) Normalized changes in levels of acetylated H3 at gad65 promoter (–646 to – 484 bp), GAD65 mRNA and protein in NRM tissues from sham and SNL rats at 1 d and 21 d after surgery (n = 5–6 each group). (h) Representative Western lanes of GAD65 and β-actin proteins from a sham rat and an SNL rat at 1 d and 21 d.
These results indicate that CFA-induced persistent hyperalgesia epigenetically suppresses gad65 output activities in NRM and HDAC inhibitor-induced global histone hyperacetylation can overwhelm this pain effect, increasing acetylation at gad65 promoter and its output activities.
Next, we examined gad65 activities in rats with spinal nerve ligation (SNL), another rodent model of chronic neuropathic pain that lasts for months28. NRM tissues were harvested from SNL rats and sham-operated control rats at 1 d (acute pain) and 21 d (prolonged pain) after surgery (Figure 4f). We found that acetylated H3 level in gad65 promoter displayed no change at 1 d, but was reduced at 21 d (Fig. 4g). Moreover, both GAD65 mRNA and protein levels were decreased at 21 d, but not at 1 d (Fig. 4g,h). Thus, both prolonged sensitization of neuropathic pain and inflammatory hyperalgesia epigenetically reduce gad65 activities.
Histone hyperacetylation increases GABA synaptic function
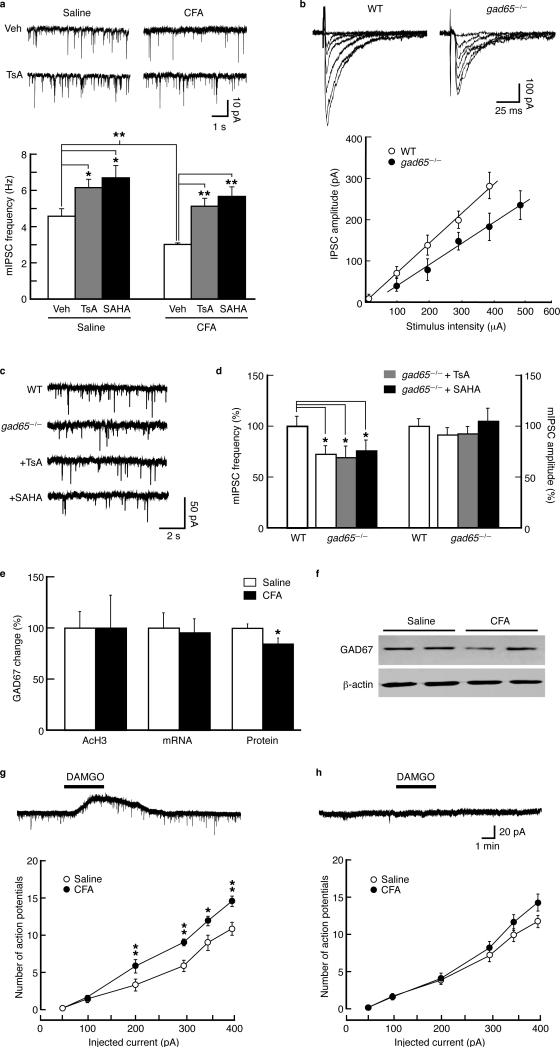
As expected from above results of pain-reduced GABA synaptic function by epigenetic hypoacetylation at gad65, TsA or SAHA augmented GABA neurotransmission, increasing mIPSC frequency in neurons from CFA- and saline-injected rats (Fig. 5a). This suggests that GAD65 expression is important for GABA neurotransmission and its epigenetic repression by persistent pain decreases, and its pharmacological augmentation by HDAC inhibitors enhances, GABA synaptic transmission.
Figure 5.
Histone hyperacetylation overcomes pain-induced reduction in GABA synaptic function through GAD65. (a) Spontaneous mIPSC traces (top) and summarized data (bottom) of effects of TsA and SAHA on mIPSC frequency in NRM neurons from saline- and CFA-injected rats (n = 12–17 cells each group). (b) Representative traces of GABA IPSCs evoked by stimuli of increasing intensities in an NRM neuron from a wild type (WT) and from a gad65–/– mouse (top), and an input-output plot of GABA IPSCs in neurons from WT (n = 23 cells) and gad65–/– mice (n = 15 cells) (bottom). (c,d) Representative mIPSC traces (c) and group data (normalized to WT) of mIPSC frequency and amplitude (d) in NRM neurons from WT and gad65–/– mice without or with the TsA or SAHA treatment (n = 15–26 cells for each group). (e) Summarized data of the normalized levels of acetylated H3 in the gad67 promoter region (–374 to –273 bp), GAD67 mRNA and GAD67 protein in NRM tissues from saline- and CFA-injected rats at 3 d post-injection (n = 4–6 for each group). (f) Representative Western blots of GAD67 protein from a saline- or CFA-injected rat. (g, h) Representative membrane current traces with response to the mu-opioid receptor (MOR) agonist DAMGO (1 μM) (top) and graphs of evoked action potential firing (bottom) in MOR-expressing NRM neurons (g) and in MOR-lacking NRM neurons (h) from saline- and CFA-injected rats. * p < 0.05, ** p < 0.01. Veh, vehicle.
Considering non-selective, acetylation-promoting effects of HDAC inhibitors, we analyzed GABA IPSCs in NRM of gad65 knockout mice. The slope of IPSC input-output curve was found reduced in neurons from gad65–/– mice when compared with wild-type mice (Fig. 5b). This indicates gad65 deletion-induced reduction in GABA synaptic function, consistent with the pain effects (Fig. 2b). Supporting presynaptic function of GAD65, mIPSC frequency, but not amplitude, was lower in neurons from gad65–/– mice than those from WT mice (Fig. 5c,d), indicating impaired presynaptic GABA release and also consistent with the pain effects (Fig. 2g–j). Furthermore, in gad65–/– mice, TsA failed to increase mIPSC frequency, neither did SAHA (Fig. 5c,d). The mIPSC amplitude was unaffected. Thus, it appears that HDAC inhibitors enhance GABA neurotransmission (Fig. 5a) by promoting histone acetylation at gad65 gene, as they lost this effect in gad65–/– mice.
Examining potential GAD67 roles in pain-reduced presynaptic GABA function despite its predominant cytoplasmic localization, we found that acetylated H3 in the three gad67 sequence regions examined was unchanged at 3 d post-CFA (Fig. 5e and Supplementary Fig. 1). Neither changed was GAD67 mRNA level. However, CFA produced a small, but significant decrease in GAD67 protein expression (Fig. 5e,f). TsA increased GAD67 proteins in CFA- and saline-injected rats at 3 d (Supplementary Fig. 2). Thus, it seems unlikely that CFA has significant effects on gad67 activity through histone acetylation, but cytosolic GAD67 may play some role in CFA-induced pain sensitization.
Histone hyperacetylation relieves pain
We examined neuronal excitability of previously characterized two classes of NRM neurons: mu-opioid receptor (MOR)-lacking and MOR-expressing cells29. The latter presumably comprises the descending pain-facilitatory system19,30. We found that MOR-expressing cells, identified by hyperpolarization with MOR agonist DAMGO (1 μM, Fig. 5g, top), displayed a larger number of depolarization-evoked action potentials in CFA-injected rats 3 d post-injection than those in control rats (Fig. 5g, bottom). This difference was not observed in MOR-lacking cells (Fig. 5h and Supplementary Figure 3). Thus, the increased excitability of MOR-expressing NRM cells may underlie the cellular mechanism for CFA-induced activation of descending pain facilitation.
To determine whether GAD65-promoting HDAC inhibitors could alleviate pain, we conducted behavioral experiments in vivo. TsA infused repeatedly into NRM dose-dependently attenuated CFA-induced hyperalgesia, so did SAHA (Fig. 6a–c). Single TsA infusion was ineffective (data not shown). Repeated TsA pretreatment before CFA injection failed to alter CFA effect at 4 h (Fig. 6d), excluding a TsA effect on the acute effect of CFA.
Figure 6.
HDAC inhibitors relieve pain through GAD65. (a, b) Changes in pain threshold in CFA-injected rats with repeated NRM infusions (arrowheads) of vehicle (n = 5) or TsA (a, n = 8), and vehicle (n = 5) or SAHA (b, n = 6 each group). (c) Effects of TsA at indicated doses (n = 5–6 for each data point). (d) Effect of TsA pretreatment by NRM infusions (arrowheads) on CFA-induced acute hyperalgesia. (e) Averaged baseline pain threshold in WT (n = 10) and gad65–/– mice (n = 7), and in gad65–/– mice treated with NRM infusions of vehicle (n = 7) or TsA (n = 7). (f) Pain threshold changes in saline- and CFA-injected WT and gad65–/– mice. (g) Effects of single NRM infusion of saline or the GABAA receptor agonist muscimol (50 ng) on CFA-induced hyperalgesia (n = 5 rats each group). (h) Pain threshold changes following repeated NRM infusions of saline (n = 5) or the proinflammatory cytokine interleukin 1 (IL-1β, 3 μg, n = 5) (arrows), and following single NRM infusion of IL-1β plus the IL-1 receptor antagonist IL-1Ra (17 μg, n = 6). (i,j) Western blots (i) and summarized data (j) of GAD65 protein normalized to β-actin in NRM tissues from normal rats after repeated NRM infusions of saline or IL-1β, and from CFA-injected rats treated with similar 4-day NRM infusions of saline or IL-1Ra (17 μg). *, compared to saline group; #, compared to IL-1β + L-1Ra group. MPE, maximum possible effect.
Histone hyperacetylation-induced pain relief requires GAD65
Additional evidence supporting the GAD65 role in the pain mechanism was obtained from behavioral experiments on gad65–/– mice. Compared to WT mice, gad65–/– mice exhibited lower baseline pain threshold, indicating a sensitized basal pain state (basal hyperalgesia) (Fig. 6e), consistent with CFA-induced hyperalgesia through epigenetic inhibition of GAD65 expression. Furthermore, in gad65–/– mice, similar NRM infusions of TsA could no longer ameliorate the sensitized pain behavior (Fig. 6e), further supporting the GAD65 role in histone hyperacetylation-induced pain relief. To determine how gad65–/– mice might respond to CFA differently, we treated those mice with CFA and found that, on top of the basal hyperalgesia in gad65–/– mice, CFA induced further pain sensitization to a level similar in amplitude to that in WT mice at 1 d (Fig. 6f). This indicates that this acute CFA effect is independent of GAD65 and may be mediated by yet unidentified mechanisms. Interestingly, at 3 d, the amplitude of hyperalgesia remained unchanged in WT mice, but was partially recovered in gad65–/– mice (Fig. 6f). The underlying mechanisms remain to be investigated.
Next, we reasoned that, if GAD65 suppression-induced loss of GABA synaptic inhibition contributed to the pain hypersensitivity, pharmacologically promoting GABA inhibition under those conditions should alleviate the hyperalgesia. As predicted, in rats 3 d post-CFA injection, acute NRM infusion of the GABAA receptor agonist muscimol induced an antinociceptive effect (Fig. 6g). Therefore, like histone hyperacetylation-mediated upregulation of GAD65 activity and GABA function, pharmacological activation of inhibitory GABA function also can relieve the pain.
Depression and proinflammatory cytokines
Chronic pain is often associated with psychophysiological disorders such as depression31. To determine potential effects of depression, we treated SNL rats or sham rats with the antidepressant drug fluoxetine for 21 d, a treatment that reverses depressive behavior in rodents32. We found that fluoxetine had no effect on SNL-reduced expression level of NRM GAD65 protein (Supplementary Fig. 4), likely excluding a general effect of depression on GAD65 expression in NRM.
CFA is known to release pain-facilitating proinflammatory cytokines, including interleukin 1 (IL-1)33. We examined IL-1β effect on NRM GAD65 expression and pain threshold. IL-1β infused into NRM decreased pain threshold in naïve rats acutely for about 4 h (Fig. 6h). Co-infusion of IL-1β and the IL-1 receptor antagonist IL-1Ra largely blocked the IL-1β effect. However, unlike CFA-induced hyperalgesia, repeated NRM infusions of IL-1β did not produce lasting hyperalgesia over 3 d although its acute effect remained (Fig. 6h). In NRM tissues from rats treated repeatedly with IL-1β, we found no change in GAD65 protein (Fig. 6i,j). Furthermore, repeated NRM infusions of IL-1Ra failed to block CFA-induced reduction in GAD65 protein (Fig. 6i,j). These results indicate that, although proinflammatory cytokines are important in chronic pain development33,34, NRM IL-1 is unlikely to play a significant role in CFA-induced modulation of GAD65 expression and related pain mechanisms.
DISCUSSION
In animal models of chronic pain, we have shown that persistent pain, but not acute pain, epigenetically suppresses the output activities of gad65 gene and consequently causes impaired inhibitory function of GABAergic synapses in central pain-modulating neurons, contributing to the development of persistent pain sensitization. These results are supported by observations in gad65–/– mice showing impaired GABA synaptic function in the same neurons and sensitized pain behavior. In addition, histone hyperacetylation overcomes these molecular and synaptic changes by promoting gad65 output activities, thereby relieving the sensitized behavior of persistent pain.
Chronic pain involves altered expression of many genes through unknown mechanisms35. In drug addiction, histone H3 and H4 acetylation modulates the expression of several genes that regulate transcriptional activities, including Cdk5, c-fos, CREB and ΔFosB8. In nerve injury-induced hypoesthesia, the C-fiber dysfunction is reportedly mediated by epigenetic upregulation of the transcriptional repressor neuron-restrictive silencer factor (NRSF), but the pain sensitization does not seem to involve NRSF upregulation17. Interestingly, HDAC inhibitors reduce inflammatory pain by upregulating spinal metabotropic glutamate 2 receptors16. The present study identifies gad65 as an important target gene of histone modifications induced by persistent pain conditions, providing a potential epigenetic mechanism for the development of chronic pain.
GAD65 is preferentially targeted to presynaptic terminals of central neurons for GABA synthesis of synaptic vesicles and is required for active GABA synaptic release23,24,36. Impaired GABA release would cause loss of GABAergic inhibition, leading to neuronal hyper-activation. For instance, nerve injury induces a loss of GABA inhibition in spinal neurons and enhancing GABA synaptic inhibition is effective in relieving injury-induced pain37-39. Our results from rats and GAD65-deficient mice suggest that persistent pain of inflammation and neuropathy induces down-regulation of GAD65 activities, causing impairment of GABA synaptic inhibition in NRM and increasing the excitability of presumably pain-facilitating neurons. This is in line with recent reports that gad65–/– mice display sensitized pain behavior40 and viral delivery of gad65 gene produces orofacial analgesia41. Given the multifaceted mechanisms of chronic pain, it is likely that other genes, in addition to gad65, also are targets of chronic pain-induced chromatin remodeling. This likely accounts for our observation of increased global histone acetylation by CFA, indicating increased activities of other genes to be investigated. While the pain-induced changes in GAD65 activities and GABA synaptic function indicate a likely neuronal locus, further studies are necessary to verify the localization of neuronal nuclei for the pain-induced histone modification.
GAD67 is a GABA-synthesizing enzyme preferentially for cytoplasmic GABA and its tonic release from neurons23. Our data demonstrate a major role of presynaptic GAD65 in the pain mechanism, but that does not preclude the role of cellular GAD67, particularly in pain-induced adaptive changes in neuronal excitability for sensitized pain behaviors. While our results do not indicate pain-induced epigenetic modulation of gad67 gene through histone acetylation, GAD67 could participate in the pain mechanisms by decreasing tonic inhibition among neurons through reduced expression (Fig. 5e,f) for cellular GABA, by reducing synaptic GABA through some presynaptic functions, and by compensatory changes in response to GAD65 deficiency. Detailed mechanisms for the GAD67 roles warrant further studies.
How functionally distinct populations of NRM neurons adapt to chronic pain conditions and mediate sensitized pain behaviors in chronic pain remains unclear. Under normal conditions, opioids produce analgesia partly by reducing basal GABA transmission in NRM neurons, thereby activating the descending pain-inhibition system29,42. Consistently, NRM-applied GABAA receptor antagonists induce antinociception whereas GABAA receptor agonists produce pain sensitization43,44. However, under chronic pain conditions, considerable adaptive changes may have occurred both in GABA input activities onto different classes of NRM neurons and in GABAA receptor properties. Our results (Fig. 5g,h) indicate that the pain-induced impairment of GABA synaptic inputs may preferentially affect and consequently hyper-activate MOR-expressing neurons. Activation of this neuron class presumably facilitates spinal pain transmission29,30, contributing to the sensitized pain behaviors. Pain-induced decrease in GABA neurotransmission has also been reported recently in amygdala neurons from a rat model of arthritic pain45. This GABA impairment-induced pain sensitization is further supported by our behavioral results that enhancing GABA inhibition by activating NRM GABAA receptors produces an antinociceptive effect (Fig. 6g). Detailed molecular and cellular adaptations in GABA and glutamate synapses under chronic pain conditions are subjects of ongoing research.
Proinflammatory cytokines, released into the peripheral and central circulation by immune cells and glia in response to tissue inflammation and trauma, cause augmented pain33,34, but the underlying cellular and molecular mechanisms remain unclear, particularly under chronic pain states. Proinflammatory cytokines may contribute to the development of chronic pain by sustained release from their sources and by their sensitized signaling mechanisms in nociceptors and central neurons to augment pain responses after healing. Our observations of both the relatively acute hyperalgesic effect of IL-1β and the ineffectiveness of repeated IL-1β administration in NRM on GAD65 expression indicate that this proinflammatory cytokine at least in NRM is not significantly involved in the GAD65-mediated pain mechanisms for the prolonged pain behaviors induced by SNL and CFA.
A common clinical problem at present is the transition from analgesic-responsive acute pain to chronic pain, of which some types are poorly responsive to currently available analgesics and often lead to long-term neuropsychiatric disorders such as depression, stress and drug addiction10,12,46,47. While multiple forms of neuronal plasticity have been identified for the pathogenesis of chronic pain13, the mechanisms underlying this critical transition from acute pain to chronic pain remain poorly understood. Our findings of the chromatin modifications emerging only after pain development for days indicate that the epigenetic mechanism of gad65 modulation might underlie the persistent phase of these pain conditions and this, together with regulations of multiple sets of other gene activities, could be an important initial step in this transition that leads to the development of chronic pain and associated disorders. In this regard, drugs such as HDAC inhibitors that overcome the effects of persistent pain on the output activities of gad65 and other target genes may serve as a new promising class of analgesics48, as they could collectively block the upstream cause of pain-induced cascade alterations that lead to multiple system malfunctions and clinical symptoms in chronic pain development.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH National Institute on Drug Abuse grants DA023069 and DA027541. We thank L. Gao of Baylor College of Medicine for his generous gifts of GAD67 primers.
METHODS
Animals
Male Wistar rats, 9–14 d old or 200–300 g, were used. gad65–/– mice were obtained from the Jackson Laboratories. All procedures involving the use of animals conformed to the guidelines by MD Anderson Cancer Center Animal Care and Use Committee.
Pain models
Complete Freund's adjuvant (CFA, 40 μl) was injected into a hindpaw to induce hyperalgesia of inflammatory pain20. Mechanical allodynia of neuropathic pain was induced by ligating the left L5 and L6 spinal nerves as described by Kim & Chung28. Pain tests were conducted as described before21. Trichostatin A (TsA, 4 mg kg–1, in alcohol) or suberoylanilide hydroxamic acid (SAHA, 40 mg kg–1, in DMSO) was injected i.p. once daily for 4 d. NRM tissues were harvested 4 h after the last injection.
Histone proteins extraction and Western blotting
The protocol for histone protein extraction was modified from a previous report49. After tissue homogenization and lysate centrifugation, acid supernatant and nuclear pellet were separated and acetone was added to the pellet. 30 μg proteins was mixed with SDS sample buffer. Samples were transferred to a nitrocellulose membrane and incubated in histone or acetylated histone antibodies (1:1000, Cell Signaling Technology) and ®-actin antibodies (1:1000, Santa Cruz Biotechnology). The global histone H3 acetylation assays were performed according to the menu of EpiQuik™ Global Histone H3 Acetylation Assay Kit (Epigentek Group Inc). Western blotting was performed as previously described21.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were modified from the protocol of EpiQuik™ Tissue Acetyl-Histone H3 ChIP Kit (Epigentek Group Inc). NRM tissues were harvested, cross-linked and frozen. After tissue homogenization and centrifugation, the extracted chromatin was sheared by sonication into 200–500 bp fragments. After dilution, chromatin samples and “input” DNA were transferred to each well for protein and DNA immunoprecipitation. DNA release buffer containing proteinase K was added, followed by reverse buffer to dissociate DNA and histones. After cross-linked DNA reversal, binding buffer was used for DNA precipitation and purification. Elution buffer was used to elute purified DNA from the columns. We used antibodies to acetyl histone H3 targeting Lys9 and Lys14, acetyl histone H4 targeting Lys12 (Santa Cruz Biotechnology), HDAC1, HDAC2, HDAC4 and HDAC5 (Cell signaling Technology).
Quantification of DNA
Quantitative real-time PCR (Applied Biosystems) was performed as previously described50. Based on the consensus sequence of cAMP-response element (CRE) for potential binding sites of the transcription factor CREB in gad65 and gad67 promoter regions, primers were designed to amplify representative promoter regions encompassing the CRE sequence from immediately upstream to >2 kb upstream of the TSS. Fold differences were calculated by ΔCt. The following primers (Invitrogen) were used: gad65, 5'-GCCCTGACTCGAACACTCAC-3' and 5'-ACACAGGGACAGGAAACGTG-3' (–150 to –83 bp); 5'-CTTCCTCCCTCTTTGGTTCCTT-3' and 5'-ACCAGGGAGACCTTGACAATCT-3' (–285 to –153 bp); 5'-ATAAGCAGCAGCCAAGGTCAC-3' and 5'-CGCTGGAGTCTATCACTGAGGA-3' (–646 to –484 bp); and 5'-TCTGCTGCCTCCTTTGTGAA-3' and 5'-CTCCCCACTTCGGATACAGG-3' (–2529 to –2330 bp). gad67, 5'-TTGCGCCTCTAGACTTGAGAGT-3' and 5'-TCTCGGAGACAGAAGGGAAAC-3' (–212 to –66 bp); 5'-TGATCTTTTCCCTGCTGTCA-3' and 5'-TCCCATGAGTAATCCAGAACG-3' (–374 to –273 bp); and 5'-AAGAGACAGGCCTGGGATAAAC-3' and 5'-GGTCTGTCTGAGTGATGGGAAG-3' (–2841 to –2704 bp). ®-tubulin, 5'-TAGAACCTTCCTGCGGTCGT-3 ' a n d 5 '-TTTTCTTCTGGGCTGGTCTC-3' as controls.
Quantitative RT-PCR
RNA was extracted with the RNAqueous-4PCR Kit (Applied Biosystems) and reverse transcription was performed with the RETROscript Kit (Applied Biosystems). cDNA was quantified by real-time PCR50. The following primers (Invitrogen) were used: gad65, 5'-GCCCAGGCTCATCGCATTCACGTC-3' and 5'-CCTCCACCCCAAGCAGCATCCACA-3'; gad67, 5'-GGTTTCTTGCAAAGGACCAA-3' and 5'-CACCAGGGTCACTGTTTTCA-3'; and gapdh, 5'-AACGACCCCTTCATTGAC-3' and 5'-TCCACGACATACTCAGCAC-3'.
Recordings
Visualized whole-cell recordings of NRM neurons in slice preparations were performed as previously described21. Neonatal rats were used in recording experiments due to limited cell visibility and quality in NRM slices from older rats. Both similarities and differences have to be recognized between neonates and adults in the inflammatory responses of NRM neurons for data interpretation20.
Immunohistochemistry
Experiments of immunohistochemistry were conducted as previously described50. We used antibodies to synapsin I (1:200, Synaptic Systems) and GAD65 (1:1000, Millipore), and Cy3-conjuaged secondary antibodies (1:1000, Jackson ImmunoResearch Laboratories). Immunohistochemical staining for GAD65 and synapsin I, and their overlap (n = 5–6 rats each experimental group) was obtained from randomly selected sections (n = 4–6 sections from each rat) and quantitatively compared manually with the experimenter blind to treatment groups.
Microinjection
Detailed methods of repeated NRM infusions and behavioral pain tests were described previously21,51. TsA (16.5 mM in 1 μl) or SAHA (100 μM in 1 μl) was infused into NRM once daily for 4 d, as in the systemic treatment. As a standard control, TsA infusions into a site 1 mm dorsal to NRM were without effect (data not shown).
Statistical analyses and materials
ANOVA (one-way and two-way) and post hoc analysis were used to analyze data groups with multiple comparisons. Simple statistical comparisons were made with the Students’ t tests. Drugs were purchased from Sigma or Tocris Cookson except SAHA (Cayman Chemical).
Footnotes
COMPETING FINALCIAL INTERESTS
The authors declare no competing financial interests.
Additional methods. See the Supplementary Methods for detailed methodology.
REFERENCES
- 1.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald JL, Roskams AJ. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog. Neurobiol. 2009;88:170–183. doi: 10.1016/j.pneurobio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 4.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 5.Urdinguio RG, Sanchez-Mut JV, Esteller M. Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet Neurol. 2009;8:1056–1072. doi: 10.1016/S1474-4422(09)70262-5. [DOI] [PubMed] [Google Scholar]
- 6.Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan JS, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat. Rev. Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 9.Grayson DR, Kundakovic M, Sharma RP. Is there a future for histone deacetylase inhibitors in the pharmacotherapy of psychiatric disorders? Mol. Pharmacol. 2010;77:126–135. doi: 10.1124/mol.109.061333. [DOI] [PubMed] [Google Scholar]
- 10.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scascighini L, Sprott H. Chronic nonmalignant pain: a challenge for patients and clinicians. Nat. Clin. Pract. Rheumatol. 2008;4:74–81. doi: 10.1038/ncprheum0680. [DOI] [PubMed] [Google Scholar]
- 13.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai G, Wei D, Zou S, Ren K, Dubner R. Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol. Pain. 2010;6:51. doi: 10.1186/1744-8069-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiechio S, et al. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol. Pharmacol. 2009;75:1014–1020. doi: 10.1124/mol.108.054346. [DOI] [PubMed] [Google Scholar]
- 17.Uchida H, Ma L, Ueda H. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J. Neurosci. 2010;30:4806–4814. doi: 10.1523/JNEUROSCI.5541-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 19.Fields H. State-dependent opioid control of pain. Nat. Rev. Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Hammond DL. Cellular basis for opioid potentiation in the rostral ventromedial medulla of rats with persistent inflammatory nociception. Pain. 2010;149:107–116. doi: 10.1016/j.pain.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Pan ZZ. Synaptic mechanism for functional synergism between delta- and mu-opioid receptors. J. Neurosci. 2010;30:4735–4745. doi: 10.1523/JNEUROSCI.5968-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 23.Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol. Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- 24.Tian N, et al. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc. Natl. Acad. Sci. U S A. 1999;96:12911–12916. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finnin MS, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 26.Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J. Neurochem. 2003;85:151–159. doi: 10.1046/j.1471-4159.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- 27.Kurdistani SK, Grunstein M. Histone acetylation and deacetylation in yeast. Nat. Rev. Mol. Cell Biol. 2003;4:276–284. doi: 10.1038/nrm1075. [DOI] [PubMed] [Google Scholar]
- 28.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 29.Pan ZZ, Tershner SA, Fields HL. Cellular mechanism for anti-analgesic action of agonists of the kappa-opioid receptor. Nature. 1997;389:382–385. doi: 10.1038/38730. [DOI] [PubMed] [Google Scholar]
- 30.Pan ZZ. mu-Opposing actions of the kappa-opioid receptor. Trends Pharmacol. Sci. 1998;19:94–98. doi: 10.1016/s0165-6147(98)01169-9. [DOI] [PubMed] [Google Scholar]
- 31.Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: coincidence or consequence? J. Neuroendocrinol. 2001;13:1009–1023. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 32.Duric V, et al. A negative regulator of MAP kinase causes depressive behavior. Nat. Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watkins LR, Maier SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu. Rev. Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 34.Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 35.Lacroix-Fralish ML, Ledoux JB, Mogil JS. The Pain Genes Database: An interactive web browser of pain-related transgenic knockout studies. Pain. 2007;131:3, e1–4. doi: 10.1016/j.pain.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 36.Patel AB, de Graaf RA, Martin DL, Battaglioli G, Behar KL. Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J. Neurochem. 2006;97:385–396. doi: 10.1111/j.1471-4159.2006.03741.x. [DOI] [PubMed] [Google Scholar]
- 37.Moore KA, et al. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munro G, Ahring PK, Mirza NR. Developing analgesics by enhancing spinal inhibition after injury: GABAA receptor subtypes as novel targets. Trends Pharmacol. Sci. 2009;30:453–459. doi: 10.1016/j.tips.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Knabl J, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 40.Kubo K, et al. Thermal hyperalgesia via supraspinal mechanisms in mice lacking glutamate decarboxylase 65. J. Pharmacol. Exp. Ther. 2009;331:162–169. doi: 10.1124/jpet.109.156034. [DOI] [PubMed] [Google Scholar]
- 41.Vit JP, et al. Adenovector GAD65 gene delivery into the rat trigeminal ganglion produces orofacial analgesia. Mol. Pain. 2009;5:42. doi: 10.1186/1744-8069-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan ZZ, Williams JT, Osborne PB. Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J. Physiol. 1990;427:519–532. doi: 10.1113/jphysiol.1990.sp018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilbert AK, Franklin KB. GABAergic modulation of descending inhibitory systems from the rostral ventromedial medulla (RVM). Dose-response analysis of nociception and neurological deficits. Pain. 2001;90:25–36. doi: 10.1016/s0304-3959(00)00383-3. [DOI] [PubMed] [Google Scholar]
- 44.Heinricher MM, Kaplan HJ. GABA-mediated inhibition in rostral ventromedial medulla: role in nociceptive modulation in the lightly anesthetized rat. Pain. 1991;47:105–113. doi: 10.1016/0304-3959(91)90017-R. [DOI] [PubMed] [Google Scholar]
- 45.Ren W, Neugebauer V. Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1. Mol. Pain. 6:93. doi: 10.1186/1744-8069-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog. Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woolf CJ, Hashmi M. Use and abuse of opioid analgesics: potential methods to prevent and deter non-medical consumption of prescription opioids. Curr. Opin. Investig. Drugs. 2004;5:61–66. [PubMed] [Google Scholar]
- 48.Doehring A, Geisslinger G, Lotsch J. Epigenetics in pain and analgesia: An imminent research field. Eur. J. Pain. 2010 doi: 10.1016/j.ejpain.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Roozendaal B, et al. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J. Neurosci. 2010;30:5037–5046. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol. Pharmacol. 2006;69:1137–1145. doi: 10.1124/mol.105.019109. [DOI] [PubMed] [Google Scholar]
- 51.Ma J, Pan ZZ. Contribution of brainstem GABA(A) synaptic transmission to morphine analgesic tolerance. Pain. 2006;122:163–173. doi: 10.1016/j.pain.2006.01.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.