Abstract
Background
Fetal Alcohol Syndrome is a leading cause of neurodevelopmental impairments (NDI) in developed countries. Sensory deficits can play a major role in NDI, yet few studies have investigated the effects of prenatal alcohol exposure on sensory function. In addition, there is a paucity of information on the life-long effects of prenatal alcohol exposure. Thus, we sought to investigate the effects of prenatal alcohol exposure on auditory function across the life span in an animal model. Based on prior findings with prenatal alcohol exposure and other forms of adverse prenatal environments, we hypothesized that animals prenatally exposed to alcohol would show an age-dependent pattern of (A) hearing and neurological abnormalities as post-weanling pups, (B) a substantial dissipation of such abnormalities in young adulthood, and (C) a resurgence of such abnormalities in middle-aged adulthood.
Method
Pregnant rats were randomly assigned to an untreated control (CON), a pair-fed control (PFC) or an alcohol treated group (ALC). The ALC dams were gavaged with 6 mg/kg alcohol daily from gestation day (GD) 6 to 21. The PFC dams were gavaged daily from GD6-21 with an isocaloric and isovolumetric water-based solution of Maltose-Dextrins and pair-fed to the ALC dams. The CON dams were the untreated group to which the ALC and CON groups were compared. Hearing and neurological functions in the offspring were assessed with the Auditory Brainstem Response (ABR) at the postnatal ages of 22, 220 and 520 days of age.
Results & Conclusions
In accord with our hypothesis, ABR abnormalities were first observed in the post-weanling pups, largely dissipated in young adulthood, and then resurged in middle-aged adulthood. This age-related pattern suggests that the ALC pups had a developmental delay that dissipated in young adulthood and an enhanced age-related deterioration that occurred in middle-aged adulthood. Such a pattern is consistent with the fetal programming hypothesis of adult-onset diseases (the Barker Hypothesis). Our findings have important clinical implications for the assessment and management of (A) childhood hearing disorders and their co-morbidities (i.e., speech-and-language, learning, and attention deficit disorders) and (B) enhanced age-related hearing and neurological degeneration in middle-aged adulthood that can result from prenatal alcohol exposure. We recommend hearing evaluation be a part of any long-term follow-up for FAS patients and patients exposed to any adverse prenatal environment.
Keywords: Auditory Brainstem Response (ABR), Barker Hypothesis, Fetal Alcohol Syndrome (FAS), Hearing Disorders, Prenatal Undernutrition, Presbycusis
INTRODUCTION
Fetal Alcohol Syndrome (FAS), a leading cause of mental retardation in developed countries, is characterized by craniofacial anomalies and neurodevelopmental impairments (NDIs) such as cognitive and learning disabilities, language deficits and behavioral disorders (e.g., attention deficits, hyperactivity and social misconduct) (Astley et al., 2009, Carr et al., 2010). Sensory deficits can play a major role in these NDIs, yet few studies have investigated the effects of prenatal alcohol exposure on sensory deficits. Those few studies found deficits in the visual (Stromland, 1987, Stromland and Pinazo-Duran, 1994, Medina et al., 2005, Burden et al., 2009, Zajac et al., 1988), balance (Roebuck et al., 1998, Connor et al., 2006), olfactory (Bonthius and West, 1991, Chen et al., 1999, Maier and West, 2001), somatosensory (Miller, 2006, Roebuck et al., 1998, Chappell et al., 2007) and hearing systems (Church et al., 1997, Church and Gerkin, 1988, Church et al., 1987, Church et al., 1996, Kaneko et al., 1996, Rossig et al., 1994). Our particular interest concerns hearing disorders. Human studies on hearing function found that FAS children have high rates of mild to moderate sensorineural hearing loss, recurrent otitis media (Church et al., 1997, Church and Gerkin, 1988, Rossig et al., 1994) and central auditory processing disorders (Church et al., 1997). Animal studies on hearing function found that prenatal alcohol exposure can cause sensorineural hearing loss and abnormal cortical processing of auditory information (Church et al., 1987, Church et al., 1996). Although informative, such findings provide just a preliminary view of the possible gamut of hearing disorders that can be caused by prenatal alcohol toxicity. Further investigation of hearing deficits will provide a better understanding of the learning, language and behavioral disorders of FAS children, thereby improving the clinical management and outcome of such children (Cone-Wesson, 2005, Church et al., 1997).
In addition to the paucity of information on hearing deficits, there is also a dearth of information on the life-long effects of prenatal alcohol exposure. A few human FAS studies have found a persistence of intellectual impairments and behavioral disorders from early childhood into adolescence and young adulthood (Astley et al., 2009, Carr et al., 2010). There are no published data on FAS beyond young adulthood. A few animal studies on prenatal alcohol exposure have explored long-term effects. These studies found shortened life span in adult rats (Abel et al., 1987), reduced somatosensory cortex whisker vibrissae barrel field size in 7 month-old adult rats (Chappell et al., 2007), altered behavior in aged rats (Abel and Dintcheff, 1986, Abel and Berman, 1994, Janicke and Coper, 1993, Markel et al., 1995), enhanced memory loss in aged mice (Dumas and Rabe, 1994), altered hypothalamic-pituitary-adrenal (HPA) axis function and depression/anxiety disorders in adult rodents (Weinberg et al., 2008), diabetes in adult rats (Pennington et al., 2002, Yao et al., 2006), and preliminary evidence of abnormal auditory function in rats aged 6 to 18 months (Church et al., 1996). Life-long effects are of interest because the Barker Hypothesis states that an adverse prenatal environment can program the embryo or fetus for adult-onset diseases such as coronary heart disease, hypertension, type II diabetes, enhanced age-related neural degeneration, depression/anxiety and other psychiatric disorders, and a shortened life span (Barker, 2004). Thus, fetal alcohol exposure seems to be an important factor in the fetal programming of adult onset diseases and knowing the life-long effects of prenatal alcohol toxicity is important for the long-term clinical management of this patient group (Weinberg et al., 2008).
With these issues in mind, our aims were to further investigate hearing and lifelong disorders in an animal model of FAS. Specifically, we sought to investigate prenatal alcohol's life-long effects as evidenced by the auditory brainstem response (ABR). The ABR is a sensitive measure of brain development and sensory function that is used to assess various aspects of both hearing and neurological function. For the present study, we used the ABR to assess hearing loss, neural transmission times, and responsivity to a stimulus stressor throughout the animal's life span. We also assessed the animals’ longevity and growth. We hypothesized that animals prenatally exposed to alcohol would exhibit ABR evidence of hearing loss, impaired neural responsivity to a stimulus stressor, prolonged neural transmission times, and enhanced age-related neural degeneration. Based on prior longevity studies of prenatal toxicity, we predicted that ABR abnormalities would show an age-related pattern characterized by strong effects in pre-adolescence which would largely dissipate in young adulthood and then recur in middle-aged adulthood (Church et al., 2010, Janicke and Coper, 1993, Barone et al., 1995, Dumas and Rabe, 1994, Janicke and Coper, 1994, Markel et al., 1995, Martin, 1986, Schallert, 1983, Wallace et al., 1972). We also predicted reduced body weights and a shortened life span as consequences of prenatal alcohol exposure (Church et al., 2010, Abel et al., 1987, Church et al., 2004b).
METHODS
Animal Husbandry
Wayne State University's animal investigation committee approved the procedures for this study. Institutional and NIH guidelines were followed.
The CD strain of Sprague Dawley rats, ~10 weeks of age, were purchased from Charles River Laboratories (Portage, Michigan 49024, USA) and mated individually in breeding cages. The presence of a sperm plug was designated as gestational day one (GD1). On GD1, females were placed individually in polycarbonate cages (25 × 45 × 20 cm) and randomly assigned to one of the three treatment groups described below. Food consumption and maternal weights were assessed daily. Animals were housed at ~53% relative humidity and at ~22°C. Day of birth was designated postnatal day one (PND1). Within 24 hours after delivery, pups were counted and weighed. On PND2, each litter was culled to 10 pups consisting of 5 male and 5 female offspring when possible. Rather than placed with untreated surrogate dams, pups were kept with their natural mothers to conserve animal and financial resources and because this mimics the usual human situation. Pups were weaned on PND21.
Treatment Conditions and Group Sizes
The three treatment groups consisted of an alcohol treated group (ALC), a pair-fed control group (PFC) and an untreated control group (CON). ALC dams were orally gavaged daily from GD6 to GD21 with 6 g/kg of alcohol, given as a 15% w/v solution of alcohol/water. Half this dose (3 g/kg) was given in the morning (~0900 hr) and the other half (3 g/kg) was given in the afternoon (~1500 hr). We did not measure blood alcohol concentration (BAC) in the current study; but in a previous study, we found that a slightly higher dose of 3.5 g/kg twice daily gave maternal peak BACs of 210 and 310 mg/100 ml after the first and second injections (Church et al., 1990). Others reported maternal peak BACs of 300 to 355 mg/100ml from a single daily oral dose of 6 g/kg alcohol (Janicke and Coper, 1993). ALC dams were weighed each morning before calculating the amount of solution to be given that day. GD6 was selected as the first treatment day because treating the dams earlier might disrupt embryo implantation and because the embryonic inner ear starts to develop at this stage (Kotch and Sulik, 1992). Treatment was stopped after GD21 because treating dams later might disrupt the birthing process. The daily alcohol dose of 6 g/kg was chosen because it has been used successfully by others studying long-term effects on rat offspring (Chappell et al., 2007, Janicke and Coper, 1993) and our previous studies suggested too much alcohol toxicity from a higher dose (Church et al., 1990, Abel et al., 1987). The PFC dams were orally gavaged daily from GD6 to GD21 with a water-based solution containing Maltose-Dextrins. This solution was isocaloric and isovolumetric to the alcohol-containing solution given to the ALC dams. The PFC dams were also pair-fed to the ALC dams from GD6 to GD21 following standard procedure (Church et al., 2004b, Church et al., 1987, Chappell et al., 2007). The PFC group served as a control for maternal under-nutrition and handling stress. The CON dams received no treatment and were unhandled except for cage changing and weighing. The CON group served as the normal controls to which the other groups were compared. All animals had ad lib access to water. Four pregnant ALC dams died prior to delivery due to either a bad gavage injection or alcohol toxicity. All other pregnant dams produced viable litters. The study produced group sizes of N = 28, 24 and 21 litters for the CON, PFC and ALC groups.
ABR Procedure
Offspring Test Ages and Numbers
Offspring were tested on exactly PND22 (post-weanling pups), approximately PND220 (young adults) and approximately PND520 (middle-aged adults). By PND22, the rat ABR is well developed and the pups can tolerate the anesthesia used in the ABR testing procedure (Church et al., 1984a, Church et al., 1984b). This age is also sensitive to the detection of developmental delays in the ABR (Church et al., 2010). Because the ABR can show notable maturational changes in young pups (Church et al., 1984b), it was important to test each pup on exactly PND22 . By PND100, the rat's ABR is mature and stable (Church et al., 1984b, Crowley et al., 1973); thus testing age can be broader in adult animals. The PND220 age for young adults is similar to the age used by others (Chappell et al., 2007, Church et al., 2010, Church et al., 1987). The PND520 age for middle-aged adults was selected because health disorders typically associated with middle-aged humans emerge by this age in rats (e.g., presbycusis) and because testing at a later age would be compromised by the age-related deaths of too many animals (Church et al., 2010, Crowley et al., 1973). On PND22, one male and one female offspring from a particular litter were randomly selected for ABR testing. After this test session, the tested offspring were retained for the duration of the study. Because of work overload, it was not possible to test animals from every litter on PND22. Because of death-related attrition, it was not possible to test every animal on PND520. Consequently, animal numbers varied slightly from session to session. At the PND22 test session, the number of offspring tested were CON=38, PFC=33 and ALC=24. At the PND220 session, the numbers were CON=34, PFC=40 and ALC=39. At the PND520 session, the numbers were CON=30, PFC=32 and ALC=31. At each test session, the animal populations were essentially half male and half female (49-51%).
ABR General Procedures
Prior to ABR recording, each animal was given 100 mg/kg of the anesthetic ketamine (i.p.). Ketamine influences ABR latencies and amplitudes, but the effects are minor and the ABR quality is excellent (Church and Gritzke, 1987). Rectal temperature was monitored and maintained at ~37° C because temperature can influence the ABR (Rossi and Britt, 1984) (Model 43TD, Yellow Springs Instruments Co., Yellow Springs, Ohio 45387, USA). A water-circulating heating pad was used to regulate and maintain normothermia by raising or lowering the temperature of the circulating water (Model TP500, Gaymar Industries, Orchard Park, New York 14127, USA).
The ABR was differentially recorded between two subcutaneous platinum E-2 needle electrodes. The active electrode was inserted at the vertex, the reference electrode below the left ear, and the ground electrode below the right ear. Evoked potentials were collected by a Bio-logic Navigator (Bio-logic Corp., Mundelein, Illinois 60060, USA) and amplified 300,000 times with a digital bandpass of 300-3000 Hz. Electrode impedances ranged from 0-9 kΩ. At least 256 responses per ABR were averaged and stored on the computer's hard drive for later analysis. Recordings were made in an electrically shielded, double-walled sound attenuation chamber (Allotech, Inc., Raleigh, North Carolina 27603, USA). Binaural, ‘open field’ tone pips in the ascending order of 2000 Hz, 8000 Hz, 16000 Hz, 22000, 32000 Hz and 45000 Hz were delivered through a Telephonics Corporation (Farmingdale, NY 11735, USA) TDH-39P headphone (2000-8000 Hz) or a Tandy Corporation (Fort Worth, TX 76102, USA) Super Tweeter (16000-45000 Hz) placed in front of the animal (rise/fall time = 0.5 ms, plateau = 10.0 ms, polarity = alternating, repetition rate = 19.0/sec, stimulus intensity = 15 to 100 dB peSPL). For the rat these tone pip frequencies covered the range of greatest hearing sensitivity and beyond, permitting an assessment of frequency-dependent effects. We also used 0.1 ms click stimuli for our stress test and to determine neurotransmission times as described below.
ABR Latencies (Neural Transmission Times)
The ABR represents the summed responses of the synchronous firing of auditory nerve fibers and auditory brainstem neurons (Jewett and Williston, 1971). The rat ABR is composed of four components (labeled P1 to P4) occurring within 6 ms of stimulus onset (Church et al., 1984a, Church et al., 1984b). Although the neurogenerators of the rat's ABRs have not been determined, in the mouse they reflect neural activity chiefly from the auditory nerve (P1), the cochlear nucleus (P2), the superior olivary complex (P3), and the lateral lemniscus and/or inferior colliculus (P4) (Henry, 1979). The latency of each ABR component was measured as the time from the computer's triggering of the earphone to a wave's positive peak, including a 0.3 ms acoustic transit time between the earphone and the animal's pinnae. Two experimenters, who were ‘blind’ as to each animal's treatment condition age, gender and physical appearance, retrieved the stored ABRs and scored them for the latencies of waves P1, P2, P3 and P4. When scorers disagreed (rarely), the scores are averaged. The primary outcome variable was the P4 latency, a measure of neural transmission time along the auditory nerve and brainstem auditory pathway inclusively, in response to the 100 dB stimuli during the 16 kHz tone pip and 0.1 ms click conditions. The secondary outcomes were the P1 latency and the P1 to P4 interpeak latency (P1-P4 IPL). The P1 latency measures the auditory (peripheral) nerve's transmission time and the P1-P4 IPL measures the brainstem (central) transmission time. These secondary outcome variables allowed us to determine if treatment effects on the P4 latency had peripheral and/or central origins (Church et al., 2010, Church et al., 2004a). We used the click stimulus condition (click's peak spectral energy = 4-5 kHz) because it produces excellent ABR morphology with sharp wave peaks for highly reliable scoring.
ABR Thresholds (Hearing Acuity)
ABR thresholds were determined by the method of limits (Church et al., 2004a, Church et al., 2010). Serial ABRs were gathered over a range of stimulus intensities starting at 100dB and then descending to 80, 60, 50, 40, 35, 30, 25, 20 and 15 dB as the ABR threshold was reached and passed. To establish ABR threshold more precisely, 2 and 3 dB changes in stimulus intensity levels were tested around the ABR's threshold (as determined by visual detection) and multiple ABR traces (2 to 5) were collected at each near-threshold intensity level to enhance scoring reliability. Threshold was defined as the lowest intensity to elicit a reliably scored ABR component (usually wave P2 or P4). An experimenter, who was ‘blind’ as to each animal's treatment condition, scored the ABR thresholds. A second experimenter then checked the scoring for reliability purposes.
ABR Latency-Intensity Curves
An ABR latency-intensity (L-I) curve can help determine if a subject's hearing loss is a conductive hearing loss (CHL) or a sensorineural hearing loss (SNHL). A subject with a CHL or non-recruiting SNHL has an elevated ABR threshold and an L-I curve that is displaced upward and parallel to the normal curve. A subject with a recruiting SNHL will also have an elevated ABR threshold, however the wave latencies will typically be normal or near normal in response to loud stimulus intensities but progressively curve upward from the normal range as the stimulus intensity decreases (Hood, 1998). In rodents, one typically uses the ABR's P2 wave to derive an animal's latency-intensity (L-I) curve because the rodent's P2 wave is the largest wave and usually the last wave to disappear as the sound stimulus decreases (Church et al., 2004a, Church et al., 2010). P2 latency was measured at this waveform's positive peak. For the purpose of data reduction, we used only one tone pip condition to develop the LI curves. We selected the 16 kHz tone pip condition because it has the lowest (best) ABR threshold and thereby provided the longest and best L-I curves.
ABR Amplitude-Intensity Curves
The amplitude of the P2 waveform was used as an index of the ABR's maximum amplitude because the rodent's P2 wave is the largest wave and the last wave to disappear as the sound intensity is decreased. P2 amplitude was measured from the positive peak of wave P2 to the subsequent negative trough (labeled N2). This was done at each stimulus intensity level in order to derive amplitude-intensity (A-I) curves. Whereas the ABR amplitude is a function of neural synchrony and the number of neural units firing, the ABR amplitude can provide diagnostic information on these neural functions (Davis, 1976, Phillips and Carr, 1998, Starr et al., 2001) and the presence of hyperacusis (Church et al., 2010). We used only the 16 kHz tone pip condition to develop the A-I curves for reasons described above.
Stimulus Stress Test
Increasing the ABR's stimulus repetition rate constitutes a ‘stress test’ that is useful in detecting abnormalities in patients with brainstem abnormalities (Pratt et al., 1981), auditory neuropathy (Starr et al., 2000), multiple sclerosis (Santos et al., 2004), and perinatal asphyxia (Jiang et al., 2004). Such patients have an impaired neural responsivity that results in a diminished ability to follow fast stimulus rates (i.e., a deficit in temporal processing) (Starr et al., 2000). Accordingly, we presented our animals with 0.1 ms click stimuli at rates of 12.5, 50 and 100/s with an intensity of 80 dB peak equivalent Sound Pressure Level. We predicted that the ALC offspring would exhibit a diminished ability to follow fast stimulus rates as evidenced by progressively abnormal prolongations in ABR wave latencies and/or progressively smaller ABR wave amplitudes as the stimulus repetition rates got progressively faster. Our chief outcome variables for the stress test were P4 latency and P2 amplitude. P4 latency measures the cumulative effects on neurotransmission time along the entire peripheral and brainstem auditory pathway and is therefore the most sensitive latency measure. P2 amplitude is the largest and most reliably measured ABR amplitude and therefore the most sensitive measure of neural synchrony.
Life Span, Adult Body Weights and Necropsies
For the life span portion of our study, animals were allowed to die naturally. The exception was when an animal appeared to be suffering to an unacceptable degree as determined by the veterinarian. This usually meant that the animal was too weak to eat or drink and was losing weight rapidly. At the first sign of significant weight loss (~15% decrease), an animal would be placed on soft food prepared by moistening the food pellets in water. Frequently, an animal would respond with weight stabilization and be maintained for weeks with this procedure. When an animal's condition deteriorated to the point that it was clear that the animal would not survive more than a few days, it was humanely sacrificed. Life span was measured as the animal's age at the date of its sacrifice or the date of its natural demise. The life span study was terminated for any animal that reached the age of ~24 months (720 days). The study was terminated at this age to conserve financial resources and because group survival trends were clear by this age. Animals still alive at the end of the study were assigned an age of 720 days to enable data analyses by the Kaplan-Meier survival curves and Log Rank Test.
In addition to birth weights, offspring were weighed at the age of 28 days, 84 days, and then at three-month intervals. If an animal died before 720 days of age, its weight on the day of death was recorded. Its death weight was not entered at each subsequent weighing session. It was considered as missing datum instead, resulting in a reduced population size for that particular weighing session and all subsequent sessions. Animals that died or were terminated in old age were given necropsies to help ascertain the cause of death. The animals’ kidneys, livers, lungs, spleen, heart and mammary tumors were grossly inspected for pathology. The choice of the surveyed organs was influenced by prior rat life span studies (Church et al., 2004b, Church et al., 2010).
Data Analyses
For the maternal variables, the individual dam was the unit of measure. For offspring birth weights and postnatal mortality, the litter was the unit of measure. Treatment effects on these variables were analyzed by one-way analyses of variances (ANOVA), except that postnatal mortality was analyzed by the Kruskal-Wallis Test and life span was analyzed by Kaplan-Meier survival curves and the Log Rank Test (Church et al., 2004b). For the ABR variables, the individual pup (one male and one female per litter) was the unit of measure. Most, but not all, offspring were ABR-tested at each of the three age categories. Consequently, we used a linear mixed model (LMM) design to test the main effects of Age (a within-subjects measure) and its interactions with the other independent variables. Simple effect tests for each of the three Age categories were subsequently performed if Age or Group had significant main or interactive effects for a particular ABR outcome variable. For simple effect tests, analyses of variances (ANOVA) were used to assess the Treatment, Sex and Tone Pip Frequency (or Stimulus Intensity or Stimulus Repetition Rate) factors. Unless otherwise stated in the Results section, Sex had no significant main effect or interaction. Tone Pip Frequency (or Intensity or Rate) was a within-subject measure. The Greenhouse-Geisser corrections for the degrees of freedom and probability statistics were used for main effects and interactions involving within-subject factors. Frequency (or Intensity or Rate) had no significant interactions unless stated. If there was a significant Treatment-by-Frequency (or Intensity or Rate) interaction, univariate ANOVAs were done for each level of the Frequency (or Intensity or Rate) factor. If an ANOVA indicated a significant Treatment main effect or interaction, Bonferroni tests were used to make pairwise comparisons between treatment groups. For post-weaning body weights and necropsy results, the individual offspring (one male and one female per litter) was the unit of measure. The criterion for statistical significance was p < 0.05. We predicted that (A) the ALC group would differ significantly from the CON group and (B) the PFC group, because of adverse effects from maternal handling stress and under-nutrition (Church et al., 2010), would fall between the ALC and CON groups. We also predicted a specific age-dependent pattern described earlier (see Introduction).
RESULTS
Maternal and Offspring Outcomes
The maternal and offspring variables are summarized in Table 1. There were no significant group differences in gestation length, the numbers of uterine implantation sites, embryo resorptions, litter sizes (live + still births), and live births. The ALC and PFC groups had lower maternal weight gains, maternal food consumption and pup birth weights than the CON group. Although postnatal mortality (PND2 to PND21) was low for all groups, the ALC group nonetheless had significantly higher postnatal mortality than the CON and PFC groups. There was no group difference in the maturational milestone of eye opening age, but the ALC male and female offspring did weigh less than their CON cohorts as post-weanling pups on PND28.
Table 1.
Maternal and offspring outcomes as a function of Treatment Group (mean±SE)
| Treatment group |
||||
|---|---|---|---|---|
| CON | PFC | ALC | P value | |
| Litters (#) | 28 | 24 | 21 | |
| Maternal weight gains GD6 to GD21 (g) | 120±5 | 70±4a | 73±4a | 0.000 |
| Maternal food consumptions GD6 to GD21 (g) | 377±5 | 229±1a | 226±8a | 0.000 |
| Gestation length (day) | 21.9±0.1 | 22.0±0.0 | 22.0±0.0 | NS |
| Uterine implantation sites (#/dam) | 16.3±0.5 | 16.5±0.5 | 15.1±0.6 | NS |
| Embryo resorptions (#/dam) | 1.9±0.5 | 1.3±0.3 | 1.5±0.4 | NS |
| Litter sizes (# pups/litter) | 14.4±0.6 | 15.2±0.6 | 13.6±0.7 | NS |
| Live births (# pups/litter) | 14.4±0.7 | 15.1±0.6 | 13.5±0.7 | NS |
| Pup birth weights (g) | 6.3±0.1 | 5.9±0.1a | 6.0±0.1a | 0.002 |
| Eye opening (day) | 14.1±0.1 | 14.1±0.1 | 14.4±0.1 | NS |
| Postnatal deaths (%) | 0.04 | 1.25 | 5.42b | 0.000 |
| Male PND28 weight (g) | 94.8±1.4 | 93.2±1.8 | 87.3±3.1c | 0.032 |
| Female PND28 weight (g) | 87.7±1.3 | 86.0±1.5 | 79.8±2.0c | 0.002 |
| Life span (day)d | 640±15 | 606±24 | 647±17 | NS |
NS=not significant, GD=gestation day, PND=postnatal day
ALC and PFC < CON
ALC > CON and PFC
ALC < CON
Animal numbers for Life span determination were CON=30, PFC=32 and ALC=31
Neural Transmission Times
The LMM analysis for ABR neurotransmission times (P4 latencies) indicated significant effects for Age: F(2, 236)=227.74, p<0.001, Group: F(2, 216)=5.36, p=0.005) but not their interaction: F(4, 239)=0.787, (p=0.54). There was a significant effect for Sex (p=0.016). The interpretations of these effects are described below by age category.
In response to the click stimuli for the PND22 pups (rate=12.5 clicks/s), the ALC group had longer P4 latencies than the PFC and CON groups (respective means±SE = 4.23±0.04, 4.19±0.04 and 4.14±0.04 ms). These differences were not statistically significant however (p=0.274). Of the 0.09 ms difference in P4 latencies between the ALC and CON groups, half of it was due to a prolongation in P1 latency (peripheral effect) and half was due to a prolongation in the P1-P4 IPL (central effect). For the PND220 young adults, the P4 latencies were essentially identical for the three treatment groups (respective means±SE = 3.67±0.02, 3.68±0.02 and 3.64±0.02 ms; p=0.240). For the PND520 middle-aged adults, the ALC group had longer P4 latencies than the PFC and CON groups (respective means±SE = 3.83±0.03, 3.79±0.02 and 3.73±0.03 ms). These differences were significant: F(2, 93)=4.32, p=0.016. Post hoc analyses indicated that the ALC group differed significantly from the CON group (p<0.05) and that the PFC did not differed from their ALC and CON cohorts. Of the 0.10 ms difference in P4 latencies between the ALC and CON groups, essentially all of it was due to a prolongation in P1 latency (peripheral effect) and none was due to a prolongation in the P1-P4 IPL (central effect). Females had shorter P4 latencies than males when data from all age categories were combined (means=3.98±0.01 and 4.02±0.01 ms, respectively).
To assess the validity of these findings, we also evaluated the P4 latencies in response to the 16 kHz tone pip condition (100 dB). The results were essentially the same as those reported above for the click stimulus condition.
ABR Thresholds
The LMM analysis for ABR thresholds indicated significant effects for Age: F(2, 804)=143.52, p<0.001, Group: F(2, 67)=67.35, p<0.001) and their interaction: F(4, 765)=13.32, (p<0.001). There were also significant effects for Frequency, Sex and all other interactions (p=0.021 to <0.001). The interpretations of these effects are described below by age category.
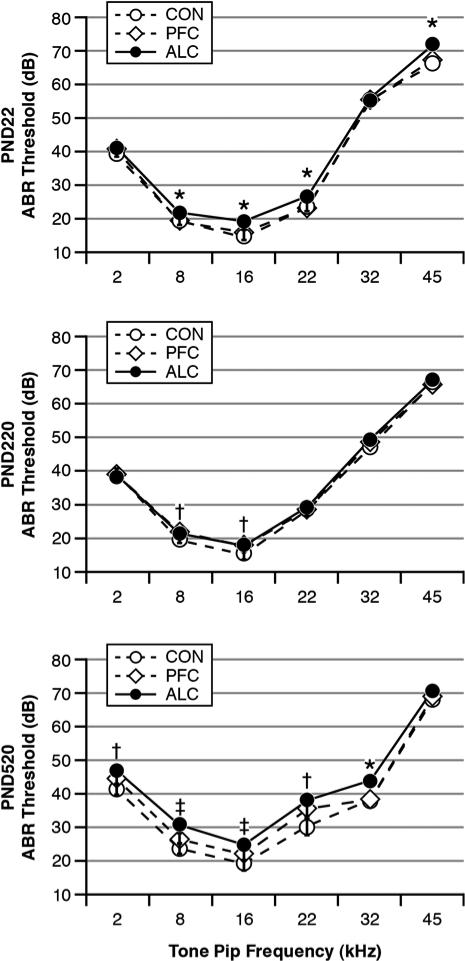
Fig. 1 shows ABR thresholds as functions of treatment Group and tone pip Frequency at each testing age. For the PND22 pups, there was a significant Group effect: F(2, 89)=7.16, p=0.001. There was also a significant Group-by-Frequency interaction (p=0.002). Post hoc analyses showed that the ALC pups had significantly higher (worse) ABR thresholds than the CON and PFC pups at the tone pip frequencies of 8, 16, 22 and 45 kHz (p<0.05), but not at 2 and 32 kHz. The CON and PFC pups did not differ from each other. There was a significant effect for Frequency (p=0.000), reflecting that rats have their lowest ABR thresholds (best hearing acuity) in the vicinity of 16 kHz (Church et al., 2004a, Church et al., 2007). There were no male-female differences in ABR thresholds for the PND22 pups.
Fig. 1.
ABR thresholds as functions of tone pip frequencies, treatment group and test age. There was an age-related pattern whereby ALC offspring typically had higher (worse) ABR thresholds than their CON and PFC cohorts as PND22 pups, which largely dissipated by PND220 young adulthood, and then reappeared by PND520 middle-aged adulthood. Group differences: *ALC > CON = PFC, †ALC = PFC > CON, ‡ALC > PFC > CON.
Fig. 1 shows that the PND22 treatment Group differences in ABR thresholds mostly disappeared in the PND220 young adults. For the PND220 young adults, there was a significant Group effect: F(2, 107)=4.022, p=0.021. There were also significant effects for Sex (p=0.009), Frequency (p=0.000), the Frequency-by-Sex interaction (p=0.001) and a nearly significant effect for the Frequency-by-Group interaction (p=0.054). Post hoc analyses indicated that the ALC and PFC groups differed from the CON group at the 8 and 16 kHz Frequencies only (p=0.025 and 0.001). The ALC and PFC groups did not differ from each other at any tone pip frequency. Females had lower ABR thresholds than males.
Fig.1 shows that the PND520 middle-aged adults had a resurgence of treatment group differences. There was a significant Group effect: F(2, 87)=10.37, p=0.000. There were also significant effects for Sex (p=0.002), Frequency (p=0.000) and Group-by-Frequency (p=0.035). Post hoc tests indicated that the ALC group had higher ABR thresholds than the CON group at all tone pip frequencies except 45 kHz. The ALC group had higher ABR threshold than the PFC group at 8, 16 and 32 kHz. The PFC group had higher thresholds than the CON group at 2, 8, 16 and 22 kHz. To determine the proportion of animals with usual amounts of hearing loss, we used a cut point criterion of 2 standard deviations above the CON group's mean thresholds for at least two tone pip conditions. This criterion indicated that 1 of 30 CON (3.3%), 7 of 32 PFC (21.9%) and 12 of 31 ALC (38.7%) animals in the PND520 age group had elevated hearing thresholds relative to their CON peers: Fisher Exact Test Chi Square (2)=11.31, p=0.004. Females had lower ABR thresholds than males.
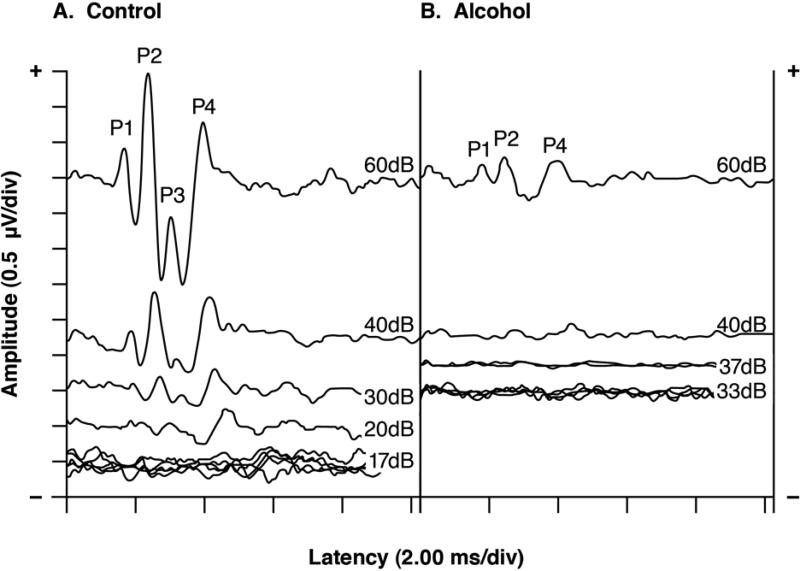
Fig. 2 shows serial ABRs elicited by 16 kHz tone pips from representative PND520 middle-aged adults in the CON and ALC groups. The CON and ALC animals had their ABRs disappear below 20 and 40 dB respectively. The ALC animal therefore had an elevated ABR threshold (defined as ≥2 standard deviations above the CON group's mean), indicating hearing loss of ~20 dB.
Fig. 2.
Serial ABRs from typical middle-aged (PND520) rats in the CON and ALC groups. For the CON rat, the ABR was still present at a stimulus intensity of 20 dB but not at 17 dB, giving a threshold score of 20 dB. In contrast, the ALC rat had a threshold score of 40 dB, indicating a threshold elevation (hearing loss) of 20 dB. Note the marked ABR amplitude differences between the CON and ALC animals at 60 dB. Multiple traces were taken at the lower stimulus intensities to confirm reproducibility. Latencies include a 0.3 ms stimulus transit time. Stimuli=16 kHz tone pips.
ABR L-I Curves
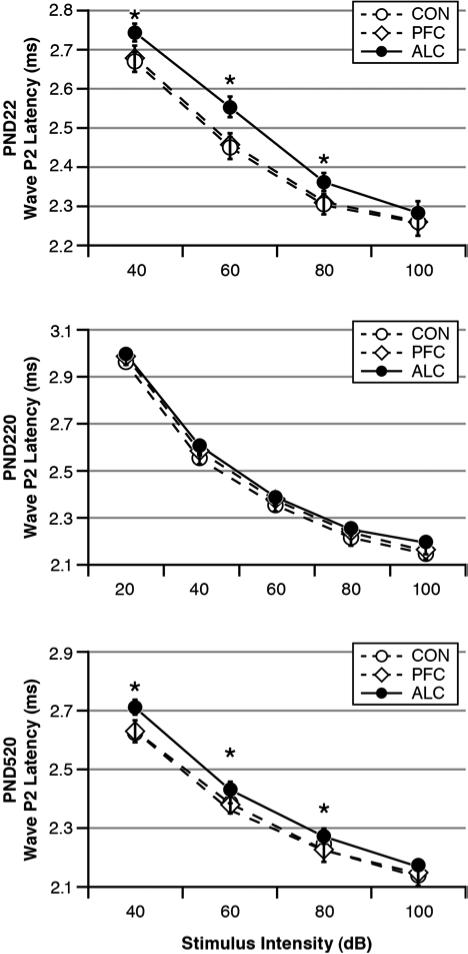
The LMM analysis for the P2 L-I curves indicated significant effects for Age: F(2, 791)=165.96, p<0.001, Group: F(2, 904)=42.30, p<0.001) and their interaction: F(4, 786)=3.23, (p=0.012). There were also significant effects for Intensity, Sex and Age-by-Sex (p<0.001). The interpretations of these effects are described below by age category.
Fig. 3 shows the ABR's wave P2 L-I curves as functions of treatment Group and stimulus Intensity at each testing age in response to the 16 kHz tone pips. For the PND22 pups, there was a significant Group effect: F(2, 92)=4.52, p=0.013. There were also significant effects for Intensity (p=0.000) and the Group-by-Intensity interaction (p=0.015). Post hoc analyses indicated that the ALC pups had P2 latencies that were similar to the CON and PFC pups in response to the highest stimulus intensity of 100 dB. At lower stimulus intensities, however, the ALC pups had P2 latencies that were significantly prolonged in comparison to the CON and PFC pups. The L-I pattern shown by the ALC pups is the same pattern seen with recruiting SNHL (i.e., prolonged latencies in response to lower intensities but normal ABR latencies in response to loud stimulus intensities). The CON and PFC pups did not differ from each other.
Fig. 3.
Latency-Intensity (L-I) curves for ABR wave P2 as functions of treatment group and test age. There was an age-related pattern whereby the ALC offspring had prolonged L-I curves as PND22 pups, which mostly dissipated by PND220 young adulthood, and then reappeared by PND520 middle-aged adulthood. Latencies include 0.3 ms acoustic transit time. Stimuli = 16 kHz tone pips. Group differences: *ALC > CON = PFC.
Fig. 3 shows that the PND22 treatment Group differences in the wave P2 L-I curves largely dissipated in the PND220 young adults. Nonetheless, there was a significant Group effect for the PND220 animals: F(2, 106)=4.23, p=0.017. There were also significant effects for Intensity (p=0.000) and Sex (p=0.000). Even though there was no significant Group-by-Sex interaction (p=0.220), post hoc analyses found significant Group differences in the females: F(2, 53)=6.16, p=0.004 but not the males: F(2, 53)=0.35, p=0.705. Post hoc tests indicated that the ALC and PFC females had slightly but significantly prolonged P2 latencies in comparison to their CON cohorts at all stimulus intensities except 20 dB. The PND220 females had shorter P2 latencies than the males.
Fig. 3 shows that the PND520 middle-aged adults had a resurgence of treatment group differences. There was a significant Group effect: F(2, 87)=6.52, p=0.002. There was also a significant Intensity effect (p=0.000). The ALC group's L-I curve, like the curve seen when they were PND22 pups, showed a pattern suggestive of recruiting hearing loss. There were also significant effects for Sex (p=0.044), Intensity-by-Group (p=0.029), and Intensity-by-Sex (p=0.003) where males had longer P2 latencies than females especially at the highest stimulus intensity and the ALC group had longer P2 latencies than the CON and PFC groups except at the highest stimulus intensity.
ABR A-I Curves
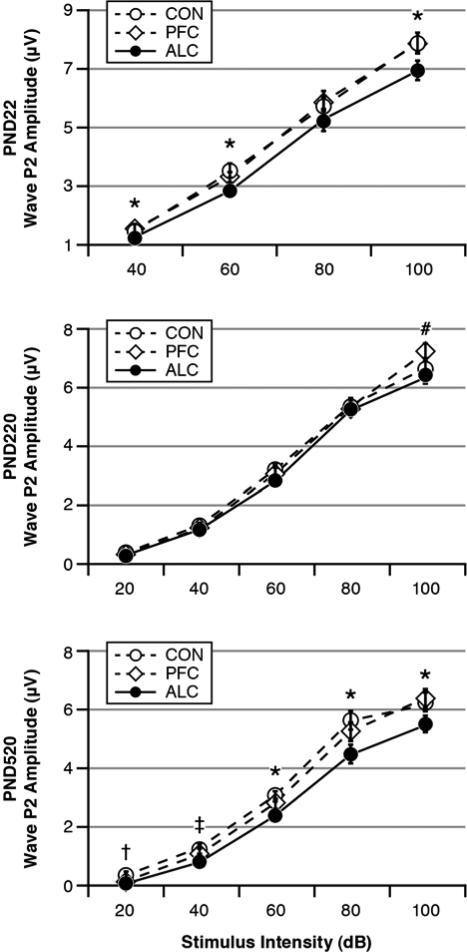
The LMM analysis for the P2 A-I curves indicated significant effects for Age: F(2, 309)=61.61, p<0.001, Group: F(2, 428)=38.30, p<0.001) and their interaction: F(4, 308)=3.40, (p=0.010). There were also significant effects for Intensity, Sex and Age-by-Sex (p<0.001). The interpretations of these effects are described below by age category.
Fig. 4 shows the ABR's wave P2 amplitudes as functions of treatment Group and stimulus Intensity at each testing age in response to the 16 kHz tone pips. For the PND22 pups, there was a significant Group effect: F(2, 92)=4.02, p=0.021. Post hoc tests indicated that the ALC pups had lower P2 amplitudes than their CON and PFC cohorts. There was also a significant Intensity effect, indicating that P2 amplitudes were larger for higher stimulus intensities (p=0.000).
Fig. 4.
Amplitude-Intensity (A-I) curves for ABR wave P2 as functions of treatment group and test age. There was an age-related pattern whereby the ALC offspring had decreased amplitudes as PND22 pups, which largely dissipated by PND220 young adulthood, and then reappeared by PND520 middle-aged adulthood. Stimuli = 16 kHz tone pips. Group differences: *ALC < CON = PFC, #ALC < PFC, †ALC = PFC < CON, ‡ALC < PFC < CON.
Fig. 4 shows that the PND22 treatment Group differences in the wave P2 A-I curves mostly disappeared in the PND220 young adults. For the PND220 young adults, there was a nearly significant Group effect: F(2, 106)=3.00, p=0.054 (2-tailed test). There were significant effects for Intensity (p=0.000), the Group-by-Intensity interaction (p=0.000), Sex (p=0.000) and the Intensity-by-Sex interaction (p=0.000). Post hoc tests indicated that the ALC young adults had smaller P2 amplitudes than their PFC cohorts primarily at the stimulus intensity of 100 dB and only in the male offspring. The ALC and CON groups did not differ from each other, and the CON and PFC groups did not differ from each other. Females had larger P2 amplitudes than males in response to the higher stimulus intensities.
Fig. 4 shows that the PND520 middle-aged adults had a resurgence of treatment group differences. There was a significant Group effect with the ALC group having the smallest P2 amplitudes: F(2, 88)=9.37, p=0.000. There were significant effects for Intensity (p=0.000), Sex (p=0.000), Intensity-by-Group (p=0.000) and Intensity-by-Sex (p=0.000), indicating that ABR amplitudes were larger for higher stimulus intensities and for females and that group differences varied as a function of stimulus intensity.
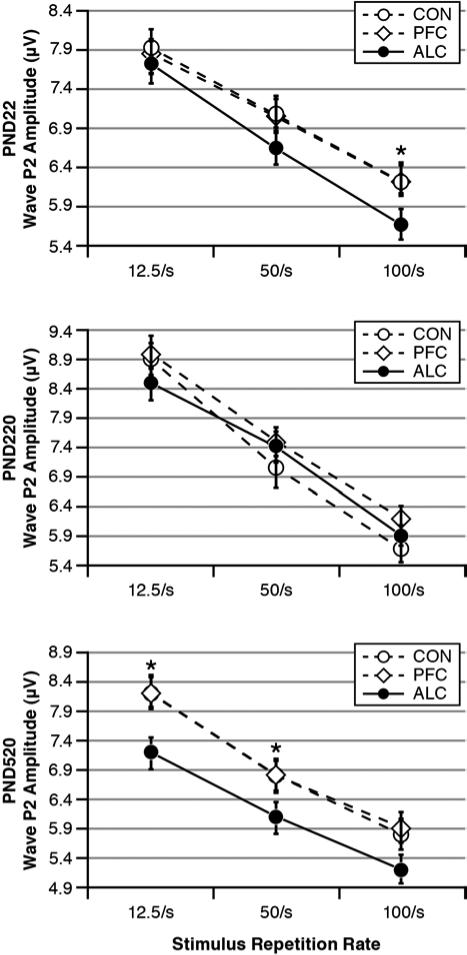
Stimulus Stress Test
Wave P2 Amplitudes
The LMM analysis for P2 amplitudes indicated significant effects for Age: F(2, 519)=13.69, p<0.001, Group: F(2, 811)=9.75, p<0.001) and marginally their interaction: F(4, 522)=2.44, (p=0.046). There were also significant effects for Rate, Sex and Age-by-Sex (p<0.001). The interpretations of these effects are described below by age category.
Fig. 5 shows the ABR's wave P2 amplitudes as functions of treatment Group and stimulus repetition Rate at each testing age. During the stress test, the PND22 pups showed no significant Group main effect; but more importantly for our hypothesis, there was a significant Group-by-Rate interaction: F(2, 200)=3.23, p=0.030. Post hoc tests indicated that there were no significant Group differences in ABR amplitudes until the stimulus repetition rate reached the fastest rate of 100/s. Here the ALC pups had smaller P2 amplitudes than their CON and PFC cohorts. For the PND220 young adults, this Group difference completely disappeared. For the PND520 middle-aged adults, there was a re-emergence of Group differences, but with a different pattern from that seen in the PND22 pups. There was a significant Group effect and Group-by-Rate interaction: F(2, 88)= 2.80, p=0.033 and F(2, 176)=2.61, p=0.034 respectively. As seen in Fig. 5, the ALC group had smaller ABR amplitudes than the CON and PFC groups and the magnitudes of these differences were similar at all three stimulus repetition rates. Overall, females had larger P2 amplitudes than males.
Fig. 5.
Wave P2 amplitudes as functions of stimulus repetition rate, treatment group and test age. There was an age-related pattern whereby the ALC offspring had decreased P2 amplitudes in response to the fastest stimulus repetition rate (100/s), which disappeared by PND220 young adulthood, and then reappeared by PND520 middle-aged adulthood but now with reduced amplitudes largely independent of stimulus repetition rate. Stimulus = 0.1 ms clicks. Group differences: *ALC < CON = PFC.
Wave P4 Latencies
The LMM analysis for P4 latencies indicated significant effects for Age: F(2, 725)=630.10, p<0.001, Group: F(2, 685)=8.48, p<0.001) but not their interaction: F(4, 760)=0.16, (p<0.164). There were also significant effects for Rate, Sex and Age-by-Group-by-Sex (p<0.001). The interpretations of these effects are described below by age category.
During the stress test, the wave P4 latencies of the PND22 pups and the PND220 young adults showed no significant effects for Group or Sex or any of their interactions. For the PND520 middle-aged adults, there was a trend for a Group effect (p=0.074) and there was a significant Group-by-Rate interaction: F(4, 176)=3.23, p=0.030. Post hoc tests indicated that the ALC group had longer P4 latencies than the CON group, but this difference reached statistical significance (p=0.016) only at the slowest stimulus repetition rate of 12.5/s. As described earlier (see section on Neural transmission times), the ALC middle-aged adults had longer P4 latencies in response to the 12.5/s clicks and this effect was secondary to a prolongation of P1 latency. All three age groups had significant effects for Rate (p=0.000), indicating that P4 latencies prolonged with increasingly faster stimulus repetition rates. Females had shorter P4 latencies than males at PND220 and PND520, but not PND22.
Life Span, Adult Body Weights and Necropsies
There was no Group difference in estimated life span when the study was terminated on PND720, at which time 39.1% of the offspring were still alive: Log Rank Chi Square (2)=2.23, p=0.327. Each group's average life span is presented in Table 1.
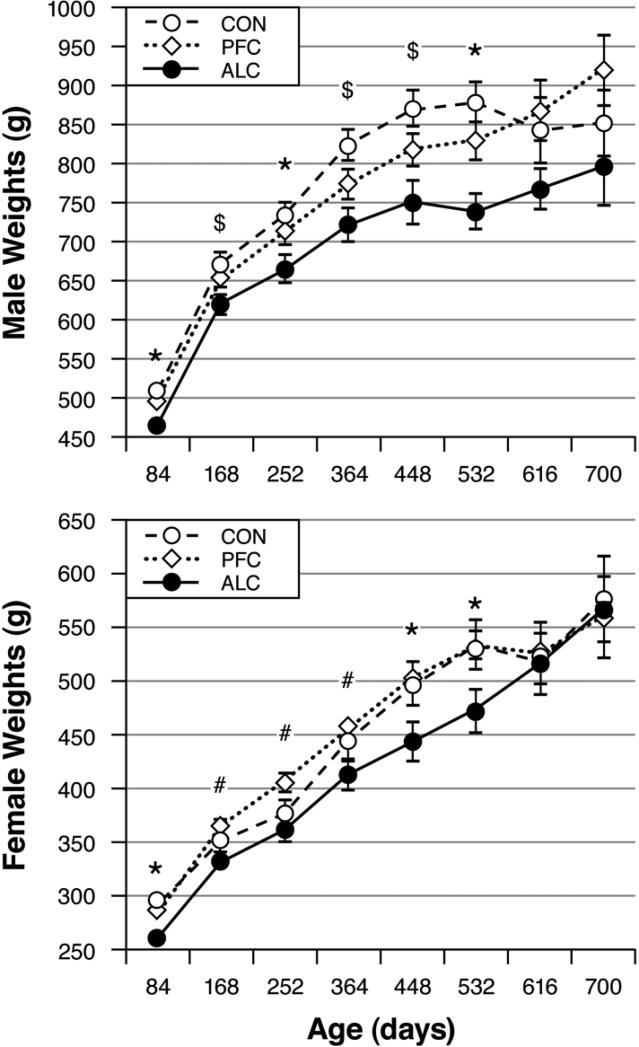
Fig. 6 shows that the ALC male and female offspring weighed significantly less than their CON cohorts throughout most of adulthood. The PFC male and female adults had weights that were intermediate to their ALC and CON cohorts. There were no significant group differences in death weights (means±SE) where males in the CON, PFC and ALC groups weighed 696±45, 700±40 and 664±40g and females weighed 437±52, 446±49 and 394±64g, respectively.
Fig. 6.
Offspring body weights as functions of treatment group, sex and adult age. The ALC male and female offspring typically weighed less than their CON and/or PFC cohorts. Group differences: *ALC < CON = PFC, #ALC < PFC, $ALC < CON.
There were no treatment group differences in our necropsy findings. For the CON, PFC and ALC groups respectively, kidney pathology (55%, 54% and 35%), liver pathology (73%, 91% and 85%), spleen pathology (55%, 47% and 38%), lung pathology (53%, 56% and 59%) and mammary tumors (41%, 40% and 35%) were the most common findings. Diseased kidneys typically had chronic interstitial glomerulonephropathy (enlarged glomeruli, thickened glomerular capsules and basement membranes). Diseased livers usually had lipidosis. Spleen disorders were characterized by enlargement (splenomegaly), probably secondary to infection, a metabolic disorder or anemia. Diseased lungs typically had chronic histiocytic pneumonia (alveolar macrophages, lymphocytic infiltrates). There were also no significant group differences in heart weights (means±SE) where male CON, PFC and ALC hearts weighed 3.1±0.2, 3.0±0.2 and 2.5±0.2g and female hearts weighed 1.7±0.3, 1.7±0.2 and 1.8±0.2g, respectively.
DISCUSSION
Our study's results on the life-long effects of prenatal alcohol exposure were mostly supportive of our hypotheses. In general, the ALC offspring exhibited ABR evidence of hearing loss, impaired neural responsivity to a stimulus stressor, enhanced age-related neural degeneration, reduced body weights, and an age-related pattern of treatment group differences in post-weanling pups that largely dissipated in young adulthood and recurred in middle-aged adulthood. Contrary to one hypothesis, our ALC group did not have a shortened life span.
Maternal and Offspring Outcomes
Our prenatal alcohol treatment resulted in reduced maternal food consumption, reduced maternal weight gain, reduced birth weights, and increased postnatal mortality. These results are similar to those reported previously (Church et al., 1991, Abel, 1980, Pennington et al., 2002). Unlike some other studies (Church et al., 1991, Abel, 1980), we did not observe an increase in embryonic resorptions or a developmental delay in the maturational milestone of eye opening.
ABR Findings
The ALC offspring showed a variety of ABR abnormalities that varied as a function of postnatal age. As PND22 pups, the ALC offspring showed signs suggestive of sensorineural hearing loss as evidenced by (A) elevated ABR thresholds, (B) an L-I curve characterized by normal ABR latencies in response to our loudest stimulus intensity but prolonged latencies in response to softer stimulus intensities (i.e., recruiting hearing loss), and (C) reduced ABR amplitudes. As PND220 young adults, these ABR abnormalities largely disappeared. As PND520 middle-aged adults, ABR abnormalities reappeared and were stronger than when the offspring were PND22 pups. We believe that the ABR effects in the PND22 pups were chiefly developmental delays secondary to pre- and postnatal growth retardation. The nearly normal ABR findings in the PND220 young adults indicated a catch-up in maturation. In contrast, the effects seen in the PND520 middle-aged adults reflected an early or more severe onset of age-related sensory degeneration. This interpretation is consistent with another study which reported premature aging in rats prenatally exposed to alcohol as evidenced by a variety of behavioral tests (Janicke and Coper, 1993) and a preliminary ABR study by our group (Church et al., 1996).
When hearing loss occurs in old age as a degenerative process, it is referred to as presbycusis (Nelson and Hinojosa, 2006, Gates and Mills, 2005). Presbycusis is a multifactorial disorder that can involve sensory receptor, stria vascularis, neural and/or middle ear mechanisms. An analysis of individual ALC animals in the PND520 age category indicated that 38.7 % had significant hearing loss in comparison to their CON cohorts and that most of these hearing loss animals (~73%) had L-I patterns suggestive of recruiting hearing loss. Recruiting sensorineural hearing loss is most typically due to loss of the outer hair cells (sensory receptor cells) in the cochlea (inner ear) but can also include pathology of other cochlear tissues such as the stria vascularis, spiral ganglion, and basilar membrane (Nelson and Hinojosa, 2006, Gates and Mills, 2005). A non-recruiting hearing loss can be either a conductive (middle ear) hearing loss or an non-recruiting sensorineural hearing (Hood, 1998). Conductive hearing loss can result from the aging process wherein the tympanic membrane stiffens and/or the middle ear ossicles become arthritic (Gratton et al., 2008, Ozcan et al., 2002). A non-recruiting sensorineural hearing can be due to either cochlear or auditory nerve pathology (Hood, 1998, Nelson and Hinojosa, 2006, Gratton et al., 2008). Future studies using histology and oto-acoustic emission procedures can better distinguish between these possibilities. Regardless of the pathogenic basis, the resurgence of ABR abnormalities in the ALC middle-aged adults suggests an early or more severe onset of age-related hearing and neural degeneration.
As PND22 pups, the ALC group had smaller ABR amplitudes than the CON and PFC pups across a broad range of stimulus intensities. As PND220 young adults, this group difference dissipated and subsequently reappeared in the PND520 middle-aged adults. The A-I curves are useful in detecting hyperacusis which is characterized by under-responding to soft auditory stimuli and over-responding to loud auditory stimuli (i.e., an abnormal growth in loudness perception). While we reported hyperacusis in rats perinatally exposed to a form of nutritional toxicity (Church et al., 2010), we did not see this phenomenon in relation to prenatal alcohol toxicity. We conclude that the reduced ABR amplitudes in the ALC offspring were secondary to their elevated ABR thresholds (i.e., hearing loss) and that these animals were not afflicted with the neuropathology of hyperacusis.
F o r our stimulus stress test, we specifically predicted a Group-by-Rate interaction. As predicted, the PND22 pups from the ALC group showed an abnormal decrease in ABR amplitudes in concert with increasingly faster stimulus repetition rates. This result indicates impaired processing of temporal information (i.e., impaired temporal acuity) caused by poor neural synchrony (Starr et al., 2000, Schneider et al., 1994) that is possibly central in origin (Phillips et al., 2010). This effect disappeared in the PND220 young adulthood, suggesting that the ALC pups were experiencing a developmental delay. Decreased ABR amplitudes re-emerged in the PND520 middle-aged adults from the ALC group, but the ABR amplitude decreases were now independent of the stimulus repetition rates. Thus, the reduced ABR amplitudes in these lattermost animals were likely due to age-related hearing loss rather than a temporal processing disorder. Our stress test did not show a Group-by-Rate interaction for the ABR latencies at any test age, indicating the neural transmission time was not affected by the stressor. Thus, we conclude that (A) the PND22 pups in the ALC group experienced poor neural synchrony in our stress test but with no influence on neural transmission time and (B) this effect was a developmental delay.
We had mixed results with neurotransmission time assessments. The ALC group as PND22 pups had wave P4 latencies that were 0.10 ms longer than their CON cohorts. However, this difference was not statistically significant and half of this effect was due to a prolongation of wave P1 (peripheral effect) and half was due to a prolongation of the P1-P4 IPL (central effect). This group difference disappeared in the PND220 young adults. The PND520 middle-aged adult ALC offspring had prolonged P4 latencies, but this was secondary to cochlear hearing loss. We conclude that our alcohol treatment had no noteworthy effects on neural transmission times along the peripheral and brainstem auditory pathways.
Life Span, Adult Body Weights and Necropsies
Unlike our previous study (Abel et al., 1987), we did not see a shortened life span. Our previous study used a higher alcohol dose of 7 g/kg/day (3.5 g/kg twice daily). A study by another research group using the same alcohol dose used by our current study (i.e., 6 g/kg/day), likewise did not find a reduction in life span (Janicke and Coper, 1993). Thus, these differing life span results are likely due to differences in the alcohol dose. The absence of increased embryonic resorptions, no delay in eye opening and the rather modest 5% reduction in birth weight in our ALC group, suggests that our current study achieved only a modest level of prenatal alcohol toxicity. Yet, our ALC offspring were still vulnerable to an adult onset disorder such as presbycusis.
ALC offspring weighed less than their CON and PFC cohorts throughout most of adulthood. These differences dissipated in very old adulthood (i.e., PND616 and older) mostly because the underweight ALC animals tended to die early. There were no significant group differences in death weights. There were no treatment group differences in our gross necropsy findings.
Adverse Prenatal Environments and Adult-Onset Disorders
We found that prenatal alcohol (ALC group), and to some extent prenatal stress and under-nutrition (PFC group), caused a distinctive aged-dependent pattern of abnormalities that has been reported previously. Although there are only a handful of animal studies that examined the life-long effects of an adverse prenatal/neonatal environment, they frequently report a pattern of offspring abnormalities in very young animals, a dissipation of abnormalities in young adulthood and a resurgence or enhancement of abnormalities in old adulthood. Such a pattern has been reported with prenatal and/or neonatal exposure to triethyltin (Barone et al., 1995), brain lesions (Schallert, 1983), irradiation (Wallace et al., 1972), hypoxia (Janicke and Coper, 1994), alcohol (Abel et al., 1987, Janicke and Coper, 1993, Markel et al., 1995, Church et al., 1996, Dumas and Rabe, 1994), cocaine (Church et al., 2004b), nutritional toxicity (Church et al., 2010), under-nutrition and low birth weight (Barker, 2004), tobacco, amphetamine and barbiturate (Martin, 1986). Our specific finding of enhanced age-related hearing loss (presbycusis) as a consequence of an adverse prenatal/neonatal environmental also has some precedence. Increased risk for presbycusis was found in men who were born small for gestational age (Barrenas et al., 2005) and in rats perinatally exposed to a form of nutritional toxicity (Church et al., 2010). Both studies suggested that mechanisms linked to the Barker's fetal programming hypothesis played a significant role in causing presbycusis. Our current study's findings of enhanced age-related hearing loss in the ALC and PFC groups are consistent with this hypothesis. Combined, these studies suggest that adverse prenatal environments are heretofore unrecognized risk factors for presbycusis.
Mechanisms
There are several mechanisms that may be responsible for the observed ABR effects. Prenatal alcohol impairs neural development by interfering with myelination, synaptogenesis and cell migration and by enhancing cell death (Bonthius and West, 1991, Chen et al., 1999, Miller, 2006, Maier and West, 2001). Regarding the auditory system specifically, prenatal alcohol exposure induces cell damage in the embryonic inner ear (otic placode) (Kotch and Sulik, 1992), reduces the size of auditory brainstem structures, and causes auditory sensory receptor cell damage (Church and Kaltenbach, 1997). In addition, adverse prenatal environments, including prenatal alcohol exposure (Weinberg et al., 2008, Zhang et al., 2005), result in epigenetic modifications, altered hypothalamic-pituitary-adrenal (HPA) axis activity, and elevated corticosteroid levels in the mother and fetus. These events then program the fetus for adult-onset diseases in accord with the Barker Hypothesis or the fetal origin of adult diseases (FOAD) (Barker, 2004). These adult-onset diseases include type 2 diabetes, hypertension and enhanced neural degeneration. Diabetes (Mitchell et al., 2009), hypertension and neural degeneration are risk factors for presbycusis (Gates and Mills, 2005, Nelson and Hinojosa, 2006). Thus, elevated maternal and fetal corticosteroid levels likely played a role in our results.
Maternal under-nutrition and handling stress are known to elevate maternal and fetal corticosteroid levels and thereby cause fetal programming effects (Barker, 2004). Maternal under-nutrition and handling stress played a partial role in two outcome variables. First, the PFC offspring as PND520 middle-aged adults showed an increased incidence of presbycusis. Second, the PFC offspring showed some reduction in birth weights and adult male body weights. These effects were not as strong as those seen in the ALC offspring, however. These differences, as well as various treatment effects seen in the ALC offspring but not the PFC offspring, indicate that the ALC treatment had effects substantially above and beyond those caused by maternal under-nutrition and stress alone.
Conclusions, Clinical Implications, and Future Directions
Our finding of impaired processing of auditory information as a consequence of prenatal alcohol exposure has important clinical implications. Impaired hearing is associated with impaired speech and language acquisition during childhood and is associated with the co-morbidities of learning disabilities, attention deficit disorders, conduct disorders and poor sound discrimination (Cone-Wesson, 2005). Thus, FAS children need to be assessed and managed for auditory processing disorders and their co-morbidities so they can reach their full intellectual and behavioral potentials. It is important to take childhood developmental disorders seriously. Even though they may dissipate by young adulthood, they are harbingers of health disorders in middle-aged adulthood. Thus children with low birth weights or developmental delays, whether they are prenatal alcohol children or not, should be monitored and clinically managed for adult-onset disorders.
The age-related enhancements of presbycusis by prenatal alcohol exposure and undernutrition have particularly important implications. They indicate that prenatal alcohol exposure, undernutrition and maternal stress can program the offspring for enhanced sensory and neural degeneration in late adulthood. In another study, we found that perinatal nutritional toxicity likewise caused age-related hearing loss in middle-aged adulthood (Church et al., 2010). Combined, these findings suggest that (A) adverse prenatal environments are risk factors for enhanced or early onset of age-related sensory degeneration in accord with the Barker Hypothesis, (B) adverse prenatal environments are previously unrecognized causes of presbycusis and (C) presbycusis should be considered as one of the adult-onset disorders in the morbidity rosters of the Fetal Alcohol Syndrome, prenatal undernutrition, and the Barker Hypothesis on the fetal origins of adult diseases.
Our study used only one alcohol dosing regimen, so dose-dependency was not assessed. We did not assess critical periods of exposure, maternal and fetal glucocorticoid levels, maternal lactation and care, surrogate or cross-fostering procedure, adult offspring diabetes, hypertension, cochlear histopathology and epigenetic modifications. These would be important considerations for future studies.
ACKNOWLEGDMENTS
This project was supported by grants from the National Institutes of Health (AA09991-03 and GM58905-08).
Footnotes
There were no conflicts of interest to report.
REFERENCES
- ABEL EL. Fetal alcohol syndrome: behavioral teratology. Psychol Bull. 1980;87:29–50. [PubMed] [Google Scholar]
- ABEL EL, BERMAN RF. Long-term behavioral effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol. 1994;16:467–70. doi: 10.1016/0892-0362(94)90124-4. [DOI] [PubMed] [Google Scholar]
- ABEL EL, CHURCH MW, DINTCHEFF BA. Prenatal alcohol exposure shortens life span in rats. Teratology. 1987;36:217–20. doi: 10.1002/tera.1420360209. [DOI] [PubMed] [Google Scholar]
- ABEL EL, DINTCHEFF BA. Effects of prenatal alcohol exposure on behavior of aged rats. Drug Alcohol Depend. 1986;16:321–30. doi: 10.1016/0376-8716(86)90066-9. [DOI] [PubMed] [Google Scholar]
- ASTLEY SJ, OLSON HC, KERNS K, BROOKS A, AYLWARD EH, COGGINS TE, DAVIES J, DORN S, GENDLER B, JIRIKOWIC T, KRAEGEL P, MARAVILLA K, RICHARDS T. Neuropyschological and behavioral outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Can J Clin Pharmacol. 2009;16:178–201. [PMC free article] [PubMed] [Google Scholar]
- BARKER DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- BARONE S, JR., STANTON ME, MUNDY WR. Neurotoxic effects of neonatal triethyltin (TET) exposure are exacerbated with aging. Neurobiol Aging. 1995;16:723–35. doi: 10.1016/0197-4580(95)00089-w. [DOI] [PubMed] [Google Scholar]
- BARRENAS ML, JONSSON B, TUVEMO T, HELLSTROM PA, LUNDGREN M. High risk of sensorineural hearing loss in men born small for gestational age with and without obesity or height catch-up growth: a prospective longitudinal register study on birth size in 245,000 Swedish conscripts. J Clin Endocrinol Metab. 2005;90:4452–6. doi: 10.1210/jc.2005-0385. [DOI] [PubMed] [Google Scholar]
- BONTHIUS DJ, WEST JR. Acute and long-term neuronal deficits in the rat olfactory bulb following alcohol exposure during the brain growth spurt. Neurotoxicol Teratol. 1991;13:611–9. doi: 10.1016/0892-0362(91)90044-w. [DOI] [PubMed] [Google Scholar]
- BURDEN MJ, ANDREW C, SAINT-AMOUR D, MEINTJES EM, MOLTENO CD, HOYME HE, ROBINSON LK, KHAOLE N, NELSON CA, JACOBSON JL, JACOBSON SW. The effects of fetal alcohol syndrome on response execution and inhibition: an event-related potential study. Alcohol Clin Exp Res. 2009;33:1994–2004. doi: 10.1111/j.1530-0277.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- CARR JL, AGNIHOTRI S, KEIGHTLEY M. Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcohol Clin Exp Res. 2010;34:1022–32. doi: 10.1111/j.1530-0277.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- CHAPPELL TD, MARGRET CP, LI CX, WATERS RS. Long-term effects of prenatal alcohol exposure on the size of the whisker representation in juvenile and adult rat barrel cortex. Alcohol. 2007;41:239–51. doi: 10.1016/j.alcohol.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN WJ, PARNELL SE, WEST JR. Effects of alcohol and nicotine on developing olfactory bulb: loss of mitral cells and alterations in neurotransmitter levels. Alcohol Clin Exp Res. 1999;23:18–25. [PubMed] [Google Scholar]
- CHURCH MW, ABEL EL, DINTCHEFF BA, GERKIN KP, GRITZKE R, HOLLOWAY JA. Brain-stem and cortical auditory evoked potentials in rats chronically exposed to alcohol in utero. Electroencephalogr Clin Neurophysiol Suppl. 1987;40:452–60. [PubMed] [Google Scholar]
- CHURCH MW, ABEL EL, DINTCHEFF BA, MATYJASIK C. Maternal age and blood alcohol concentration in the pregnant Long-Evans rat. J Pharmacol Exp Ther. 1990;253:192–9. [PubMed] [Google Scholar]
- CHURCH MW, ABEL EL, KALTENBACH JA, OVERBECK GW. Effects of prenatal alcohol exposure and aging on auditory function in the rat: preliminary results. Alcohol Clin Exp Res. 1996;20:172–9. doi: 10.1111/j.1530-0277.1996.tb01061.x. [DOI] [PubMed] [Google Scholar]
- CHURCH MW, BLAKLEY BW, BURGIO DL, GUPTA AK. WR-2721 (Amifostine) ameliorates cisplatin-induced hearing loss but causes neurotoxicity in hamsters: dose-dependent effects. J Assoc Res Otolaryngol. 2004a;5:227–37. doi: 10.1007/s10162-004-4011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH MW, ELDIS F, BLAKLEY BW, BAWLE EV. Hearing, language, speech, vestibular, and dentofacial disorders in fetal alcohol syndrome. Alcohol Clin Exp Res. 1997;21:227–37. [PubMed] [Google Scholar]
- CHURCH MW, GERKIN KP. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics. 1988;82:147–54. [PubMed] [Google Scholar]
- CHURCH MW, GRITZKE R. Effects of ketamine anesthesia on the rat brain-stem auditory evoked potential as a function of dose and stimulus intensity. Electroencephalogr Clin Neurophysiol. 1987;67:570–83. doi: 10.1016/0013-4694(87)90060-5. [DOI] [PubMed] [Google Scholar]
- CHURCH MW, HOLMES PA, OVERBECK GW, TILAK JP, ZAJAC CS. Interactive effects of prenatal alcohol and cocaine exposures on postnatal mortality, development and behavior in the Long-Evans rat. Neurotoxicol Teratol. 1991;13:377–86. doi: 10.1016/0892-0362(91)90086-c. [DOI] [PubMed] [Google Scholar]
- CHURCH MW, HOLMES PA, TILAK JP, HOTRA JW. Prenatal cocaine exposure influences the growth and life span of laboratory rats. Neurotoxicol Teratol. 2004b;26:429–41. doi: 10.1016/j.ntt.2004.02.004. [DOI] [PubMed] [Google Scholar]
- CHURCH MW, JEN K-LC, STAFFERTON T, HOTRA JW, ADAMS BR. Reduced auditory acuity in rat pups from excess and deficient omega-3 fatty acid consumption by the mother. Neurotoxicol Teratol. 2007;29:203–10. doi: 10.1016/j.ntt.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH MW, JEN KL, ANUMBA JI, JACKSON DA, ADAMS BR, HOTRA JW. Excess omega-3 fatty acid consumption by mothers during pregnancy and lactation caused shorter life span and abnormal ABRs in old adult offspring. Neurotoxicol Teratol. 2010;32:171–81. doi: 10.1016/j.ntt.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH MW, KALTENBACH JA. Hearing, speech, language, and vestibular disorders in the fetal alcohol syndrome: a literature review. Alcohol Clin Exp Res. 1997;21:495–512. doi: 10.1111/j.1530-0277.1997.tb03796.x. [DOI] [PubMed] [Google Scholar]
- CHURCH MW, WILLIAMS HL, HOLLOWAY JA. Brain-stem auditory evoked potentials in the rat: effects of gender, stimulus characteristics and ethanol sedation. Electroencephalogr Clin Neurophysiol. 1984a;59:328–39. doi: 10.1016/0168-5597(84)90050-9. [DOI] [PubMed] [Google Scholar]
- CHURCH MW, WILLIAMS HL, HOLLOWAY JA. Postnatal development of the brainstem auditory evoked potential and far-field cochlear microphonic in non-sedated rat pups. Brain Res. 1984b;316:23–31. doi: 10.1016/0165-3806(84)90005-1. [DOI] [PubMed] [Google Scholar]
- CONE-WESSON B. Prenatal alcohol and cocaine exposure: influences on cognition, speech, language, and hearing. J Commun Disord. 2005;38:279–302. doi: 10.1016/j.jcomdis.2005.02.004. [DOI] [PubMed] [Google Scholar]
- CONNOR PD, SAMPSON PD, STREISSGUTH AP, BOOKSTEIN FL, BARR HM. Effects of prenatal alcohol exposure on fine motor coordination and balance: A study of two adult samples. Neuropsychologia. 2006;44:744–51. doi: 10.1016/j.neuropsychologia.2005.07.016. [DOI] [PubMed] [Google Scholar]
- CROWLEY DE, SCHRAMM VL, SWAIN RE, SWANSON SN. Age-related wave-form changes of 8th nerve action potentials in rats. Laryngoscope. 1973;83:264–75. doi: 10.1288/00005537-197302000-00009. [DOI] [PubMed] [Google Scholar]
- DAVIS H. Brain stem and other responses in electric response audiometry. Ann Otol Rhinol Laryngol. 1976;85:3–14. doi: 10.1177/000348947608500103. [DOI] [PubMed] [Google Scholar]
- DUMAS RM, RABE A. Augmented memory loss in aging mice after one embryonic exposure to alcohol. Neurotoxicol Teratol. 1994;16:605–12. doi: 10.1016/0892-0362(94)90038-8. [DOI] [PubMed] [Google Scholar]
- GATES GA, MILLS JH. Presbycusis. Lancet. 2005;366:1111–20. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- GRATTON MA, BATEMAN K, CANNUSCIO JF, SAUNDERS JC. Outer- and middle-ear contributions to presbycusis in the Brown Norway rat. Audiol Neurootol. 2008;13:37–52. doi: 10.1159/000107551. [DOI] [PubMed] [Google Scholar]
- HENRY KR. Auditory brainstem volume-conducted responses: origins in the laboratory mouse. J Am Aud Soc. 1979;4:173–8. [PubMed] [Google Scholar]
- HOOD LJ. Clinical Applications of the Auditory Brainstem Response. Singular Publishing Group, Inc.; San Diego: 1998. [Google Scholar]
- JANICKE B, COPER H. The effects of prenatal alcohol exposure on the behavior of rats during their life span. J Gerontol. 1993;48:B156–67. doi: 10.1093/geronj/48.4.b156. [DOI] [PubMed] [Google Scholar]
- JANICKE B, COPER H. The effects of prenatal exposure to hypoxia on the behavior of rats during their life span. Pharmacol Biochem Behav. 1994;48:863–73. doi: 10.1016/0091-3057(94)90193-7. [DOI] [PubMed] [Google Scholar]
- JEWETT DL, WILLISTON JS. Auditory-evoked far fields averaged from the scalp of humans. Brain. 1971;94:681–96. doi: 10.1093/brain/94.4.681. [DOI] [PubMed] [Google Scholar]
- JIANG ZD, YIN R, SHAO XM, WILKINSON AR. Brain-stem auditory impairment during the neonatal period in term infants after asphyxia: dynamic changes in brain-stem auditory evoked response to clicks of different rates. Clin Neurophysiol. 2004;115:1605–15. doi: 10.1016/j.clinph.2004.02.017. [DOI] [PubMed] [Google Scholar]
- KANEKO WM, EHLERS CL, PHILIPS EL, RILEY EP. Auditory event-related potentials in fetal alcohol syndrome and Down's syndrome children. Alcohol Clin Exp Res. 1996;20:35–42. doi: 10.1111/j.1530-0277.1996.tb01040.x. [DOI] [PubMed] [Google Scholar]
- KOTCH LE, SULIK KK. Patterns of ethanol-induced cell death in the developing nervous system of mice; neural fold states through the time of anterior neural tube closure. Int J Dev Neurosci. 1992;10:273–9. doi: 10.1016/0736-5748(92)90016-s. [DOI] [PubMed] [Google Scholar]
- MAIER SE, WEST JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23:49–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- MARKEL E, FELSZEGHY K, LUITEN PG, NYAKAS C. Beneficial effect of chronic nimodipine treatment on behavioral dysfunctions of aged rats exposed to perinatal ethanol treatment. Arch Gerontol Geriatr. 1995;21:75–88. doi: 10.1016/0167-4943(95)00653-3. [DOI] [PubMed] [Google Scholar]
- MARTIN JC. Irreversible changes in mature and aging animals following intrauterine drug exposure. Neurobehav Toxicol Teratol. 1986;8:335–43. [PubMed] [Google Scholar]
- MEDINA AE, KRAHE TE, RAMOA AS. Early alcohol exposure induces persistent alteration of cortical columnar organization and reduced orientation selectivity in the visual cortex. J Neurophysiol. 2005;93:1317–25. doi: 10.1152/jn.00714.2004. [DOI] [PubMed] [Google Scholar]
- MILLER MW. Effect of prenatal exposure to ethanol on glutamate and GABA immunoreactivity in macaque somatosensory and motor cortices: critical timing of exposure. Neuroscience. 2006;138:97–107. doi: 10.1016/j.neuroscience.2005.10.060. [DOI] [PubMed] [Google Scholar]
- MITCHELL P, GOPINATH B, MCMAHON CM, ROCHTCHINA E, WANG JJ, BOYAGES SC, LEEDER SR. Relationship of Type 2 diabetes to the prevalence, incidence and progression of age-related hearing loss. Diabet Med. 2009;26:483–8. doi: 10.1111/j.1464-5491.2009.02710.x. [DOI] [PubMed] [Google Scholar]
- NELSON EG, HINOJOSA R. Presbycusis: a human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope. 2006;116:1–12. doi: 10.1097/01.mlg.0000236089.44566.62. [DOI] [PubMed] [Google Scholar]
- OZCAN M, KARAKUS MF, GUNDUZ OH, TUNCEL U, SAHIN H. Hearing loss and middle ear involvement in rheumatoid arthritis. Rheumatol Int. 2002;22:16–9. doi: 10.1007/s00296-002-0185-z. [DOI] [PubMed] [Google Scholar]
- PENNINGTON JS, SHUVAEVA TI, PENNINGTON SN. Maternal dietary ethanol consumption is associated with hypertriglyceridemia in adult rat offspring. Alcohol Clin Exp Res. 2002;26:848–55. [PubMed] [Google Scholar]
- PHILLIPS DP, CARR MM. Disturbances of loudness perception. J Am Acad Audiol. 1998;9:371–9. quiz 399. [PubMed] [Google Scholar]
- PHILLIPS DP, COMEAU M, ANDRUS JN. Auditory temporal gap detection in children with and without auditory processing disorder. J Am Acad Audiol. 2010;21:404–8. doi: 10.3766/jaaa.21.6.5. [DOI] [PubMed] [Google Scholar]
- PRATT H, BEN-DAVID Y, PELED R, PODOSHIN L, SCHARF B. Auditory brain stem evoked potentials: clinical promise of increasing stimulus rate. Electroencephalogr Clin Neurophysiol. 1981;51:80–90. doi: 10.1016/0013-4694(81)91511-x. [DOI] [PubMed] [Google Scholar]
- ROEBUCK TM, SIMMONS RW, RICHARDSON C, MATTSON SN, RILEY EP. Neuromuscular responses to disturbance of balance in children with prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:1992–7. [PubMed] [Google Scholar]
- ROSSI GT, BRITT RH. Effects of hypothermia on the cat brain-stem auditory evoked response. Electroencephalogr Clin Neurophysiol. 1984;57:143–55. doi: 10.1016/0013-4694(84)90173-1. [DOI] [PubMed] [Google Scholar]
- ROSSIG C, WASSER S, OPPERMANN P. Audiologic manifestations in fetal alcohol syndrome assessed by brainstem auditory-evoked potentials. Neuropediatrics. 1994;25:245–9. doi: 10.1055/s-2008-1073029. [DOI] [PubMed] [Google Scholar]
- SANTOS MA, MUNHOZ MS, PEIXOTO MA, SILVA CS. High click stimulus repetition rate in the auditory evoked potentials in multiple sclerosis patients with normal MRI. Does it improve diagnosis? Rev Laryngol Otol Rhinol (Bord) 2004;125:151–5. [PubMed] [Google Scholar]
- SCHALLERT T. Sensorimotor impairment and recovery of function in brain-damaged rats: reappearance of symptoms during old age. Behav Neurosci. 1983;97:159–64. doi: 10.1037//0735-7044.97.1.159. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER BA, PICHORA-FULLER MK, KOWALCHUK D, LAMB M. Gap detection and the precedence effect in young and old adults. J Acoust Soc Am. 1994;95:980–91. doi: 10.1121/1.408403. [DOI] [PubMed] [Google Scholar]
- STARR A, SININGER Y, NGUYEN T, MICHALEWSKI HJ, OBA S, ABDALA C. Cochlear receptor (microphonic and summating potentials, otoacoustic emissions) and auditory pathway (auditory brain stem potentials) activity in auditory neuropathy. Ear Hear. 2001;22:91–9. doi: 10.1097/00003446-200104000-00002. [DOI] [PubMed] [Google Scholar]
- STARR A, SININGER YS, PRATT H. The varieties of auditory neuropathy. J Basic Clin Physiol Pharmacol. 2000;11:215–30. doi: 10.1515/jbcpp.2000.11.3.215. [DOI] [PubMed] [Google Scholar]
- STROMLAND K. Ocular involvement in the fetal alcohol syndrome. Surv Ophthalmol. 1987;31:277–84. doi: 10.1016/0039-6257(87)90028-2. [DOI] [PubMed] [Google Scholar]
- STROMLAND K, PINAZO-DURAN MD. Optic nerve hypoplasia: comparative effects in children and rats exposed to alcohol during pregnancy. Teratology. 1994;50:100–11. doi: 10.1002/tera.1420500204. [DOI] [PubMed] [Google Scholar]
- WALLACE RB, DANIELS CE, ALTMAN J. Behavioral effects of neonatal irradiation of the cerebellum. 3. Qualitative observations in aged rats. Dev Psychobiol. 1972;5:35–41. doi: 10.1002/dev.420050105. [DOI] [PubMed] [Google Scholar]
- WEINBERG J, SLIWOWSKA JH, LAN N, HELLEMANS KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–88. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAO XH, CHEN L, NYOMBA BL. Adult rats prenatally exposed to ethanol have increased gluconeogenesis and impaired insulin response of hepatic gluconeogenic genes. J Appl Physiol. 2006;100:642–8. doi: 10.1152/japplphysiol.01115.2005. [DOI] [PubMed] [Google Scholar]
- ZAJAC CS, BUNGER PC, MOORE JC. Neuron development in the superior colliculus of the fetal mouse following maternal alcohol exposure. Teratology. 1988;38:37–43. doi: 10.1002/tera.1420380106. [DOI] [PubMed] [Google Scholar]
- ZHANG X, SLIWOWSKA JH, WEINBERG J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp Biol Med (Maywood) 2005;230:376–88. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]