Abstract
Crystal structures of the lactose permease of Escherichia coli (LacY) reveal twelve, mostly irregular transmembrane α-helices surrounding a large cavity open to the cytoplasm and a tightly sealed periplasmic side (inward-facing conformation). However, LacY is highly dynamic, and binding of a galactopyranoside causes closing of the inward-facing cavity with opening of a complementary outward-facing cavity. Therefore, the coupled, electrogenic translocation of a sugar and an H+ across the cytoplasmic membrane via LacY very likely involves a global conformational change that allows alternating access of sugar- and H+-binding sites to either side of the membrane. Here the various biochemical/biophysical approaches that provide strong support for the alternating access mechanism are reviewed. Evidence is also presented indicating that opening of the periplasmic cavity is probably the limiting step for binding as well as transport.
Keywords: LacY, membrane proteins, symport, site-directed alkylation, fluorescence, double electron-electron resonance, thiol cross-linking
Typical of many transport proteins from organisms as widely separated evolutionarily as Archaea and Homo sapiens, the lactose permease of Escherichia coli (LacY), a member of the Major Facilitator Superfamily (MFS) (1), catalyzes the coupled, stoichiometric translocation of a galactopyranoside and an H+ (sugar/H+ symport) across the cytoplasmic membrane (2, 3). Since transport is obligatorily coupled, uphill transport of sugar against a concentration gradient is achieved by transduction of free energy released from the downhill movement of H+ with the electrochemical H+ gradient (; interior negative and/or alkaline). Conversely, downhill sugar translocation by LacY drives uphill H+ translocation with the generation of , the polarity of which depends on the direction of the sugar concentration gradient (reviewed in 4, 5, 6). Thus, LacY and other ion-coupled transporters are energy transducers and not pumps (7), which generally refer to proteins that utilize the energy released by ATP hydrolysis to generate a solute concentration gradient.
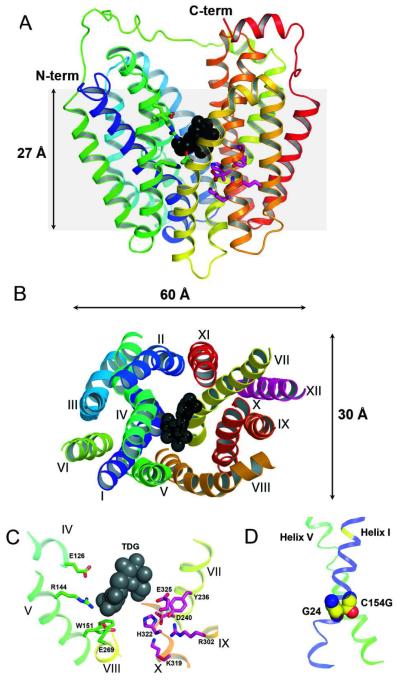
LacY functions as a monomer (reviewed in 5), and a single purified polypeptide is fully functional after reconstitution into proteoliposomes (reviewed in 8). Furthermore, x-ray crystal structures of the conformationally restricted mutant C154G (helix V) exhibit an inward-facing structure (9, 10), and a subsequent crystal structure of WT LacY (11), as well as a single-Cys mutant (12), have the same conformation. All structures to date have twelve, mostly irregular transmembrane α-helices organized into two pseudo-symmetrical six-helix bundles surrounding a large interior hydrophilic cavity open to the cytoplasm (Fig. 1A, B). The sugar-binding site and the residues involved in H+ binding are distributed at the approximate middle of the molecule at the apex of the hydrophilic cavity (Fig. 1A, C). The side chains important for sugar recognition are predominantly in the N-terminal six-helix bundle, and the side chains that form an H+-binding site(s) are in the C-terminal six-helix bundle. The amino acids involved in H+ translocation do not form a pathway through the membrane and are organized in a tightly interconnected H-bond/salt bridge cluster, which is responsible for a remarkably high pKa of approximately 10.5 that has been determined for sugar binding affinity to LacY (see 5, 13, 14). The periplasmic side of LacY is tightly sealed, and the sugar-binding site is inaccessible from the external (periplasmic) side of the molecule (Fig. 1A). Clearly, an alternative outward-facing conformation open to the periplasmic side is required for substrate transport across the membrane. An outward-facing model for LacY was proposed on the basis of thiol cross-linking studies as well as accessibility of Cys replacements for site-directed alkylation, and an alternating-access mechanism of transport was proposed to describe lactose/H+ symport catalyzed by LacY (9, 15). This mechanism, which is probably typical for other MFS transporters, has found a strong support from the crystal structures of other related transporters. A similar inward-facing structure has been observed for GlpT (16), which has little or no sequence homology with LacY and catalyzes exchange of inorganic phosphate for glycerol-3-P across the membrane. Later, occluded intermediates (17, 18) and an outward-facing (19) structure have also been observed for other MFS family members. Molecular modeling based on existing LacY structures, as well as a wealth of biochemical data to be described below, has led to a reasonable model for the outward-facing conformation of LacY (20). First molecular dynamics (MD) simulations of LacY embedded in phospholipid bilayer reported partial closing on cytoplasmic side and minor periplasmic conformational changes (21, 22) that were significantly smaller than those estimated from experimental studies. Recently, two-step hybrid simulation approach led to a model that has opening on periplasmic side with pore diameter of 7 Å and closure on cytoplasmic side triggered by sugar binding (23) that agrees with alternating access mechanism based on the experimental data. A novel approach based on structural repeats in the transmembrane domains of LacY suggests a model highly comparable with an outward-facing crystal structure solved for FucP (24).
Figure 1.
X-ray structure of LacY with transmembrane helices rainbow colored from blue (helix I) to red (helix XII) and bound TDG presented as back spheres. Residues in sugar- and proton-binding sites are shown as green and pink sticks, respectively. (A) View parallel to the membrane (PDB ID 1PV7). Hydrophilic cavity is open to cytoplasm. Grey area represents the approximate thickness of the membrane phospholipid bilayer. (B) Cytoplasmic view showing dimensions of the LacY molecule and spatial packing of transmembrane helices. The loop regions are omitted for clarity. (C) Detailed view from cytoplasm showing residues in the sugar- and H+-binding sites. (D) Transmembrane helices I and V are tightly packed in the C154G mutant viewed parallel to the membrane (from LacY structure PDB ID 2CFQ). Gly residues 24 and 154 are shown as spheres.
WT LacY molecule is highly dynamic. Proton/deuterium exchange of virtually all of the backbone amide protons in WT LacY occurs at a very rapid rate with this hydrophobic protein (~75% hydrophobic side chains) embedded in either a detergent micelle (25, 26) or a phospholipid membrane (27). Moreover, sugar binding by WT LacY is mostly entropic in nature (28), inducing widespread conformational changes, (reviewed in 4, 5, 15). Although an outward-open conformation of LacY has not yet been obtained crystallographically, various biochemical/biophysical approaches reviewed here provide converging evidence that binding of galactosidic sugars increases the open probability of a wide hydrophilic cleft on the periplasmic side of the molecule with closure of the cytoplasmic cavity. By this means, sugar- and H+-binding sites in the middle of LacY become alternatively accessible to either side of the membrane as a result of reciprocal opening and closing of hydrophilic pathways on either side of the membrane. Importantly, the periplasmic cleft must open, as well as close, for translocation of sugar across the membrane to occur (29-32).
Early site-directed mutagenesis of the native Cys residues in LacY led to the isolation and characterization of a mutant with Gly in place of native Cys154 (helix V) (33, 34). Remarkably, this single mutation completely changes the functional properties and physical characteristics of LacY (35). C154G LacY binds ligand as well or better than wild-type LacY, but catalyzes very little translocation across the membrane. Moreover, while sugar binding is mostly entropic with wild-type LacY, it is enthalpic with the C154G mutant (28). Indeed, the mutant does not exhibit the long-range conformational changes observed on the periplasmic side upon ligand binding; it is also thermostable with respect to ligand binding and aggregation (35) and arrested in a partially open outward conformation(s) in the membrane (20, 36, 37). However, the x-ray structure of the mutant exhibits an open-inward conformation with a tightly sealed periplasmic side (9, 10), indicating that crystallization selects a single conformer of LacY that is not representative of the structure of the mutant in the membrane.
The x-ray crystal structures yield a clue as to why the C154G mutant is conformationally restricted (Fig. 1D). Helices V and I cross in the approximate middle of the membrane where Cys154→Gly (helix V) and Gly24 (helix I) are in close proximity (9, 10, 38), which can lead to significantly tighter helix packing (39-42), and may partly explain the lack of conformational flexibility of the C154G mutant. Indeed, when Gly24 is replaced with Cys in the C154G mutant, the G24C/C154G double mutant exhibits a marked increase in transport activity with sugar binding and low thermostability similar to WT LacY (38). Recent site-directed cross-linking studies (43) are consistent with previous observations (38) suggesting that sugar binding to LacY causes a localized scissors-like movement between helices I and V near the point where the two helices cross in the middle of the protein. The C154G mutation may interfere with movement of the helices I and V required for proper structural rearrangements during turnover.
A functional LacY molecule devoid of the eight native Cys residues (Cys-less LacY) was engineered by constructing a cassette lacY gene with unique restriction sites about every 100 bp (44). Utilizing this cassette lacY gene for Cys-scanning mutagenesis, a highly useful library of mutants with a single-Cys residue at virtually every position of LacY has been created (45). Cys is average in bulk, relatively hydrophobic and amenable to highly specific modification. Therefore, Cys-scanning mutagenesis, a technique pioneered with LacY (reviewed in 46), was and is currently being used in conjunction with biochemical and spectroscopic techniques to reveal protein topology in the membrane, accessibility of amino acid residues to the aqueous or lipid phase [some use the acronym SCAM for substituted-cysteine accessibility method (see 47)] and spatial proximity between transmembrane domains (see 48) to study LacY, as well as a wide range of other membrane proteins (see 49).
In this presentation, the experimental approaches that provide a strong case for the alternating access mechanism in LacY are reviewed.
Site-directed alkylation (SDA)
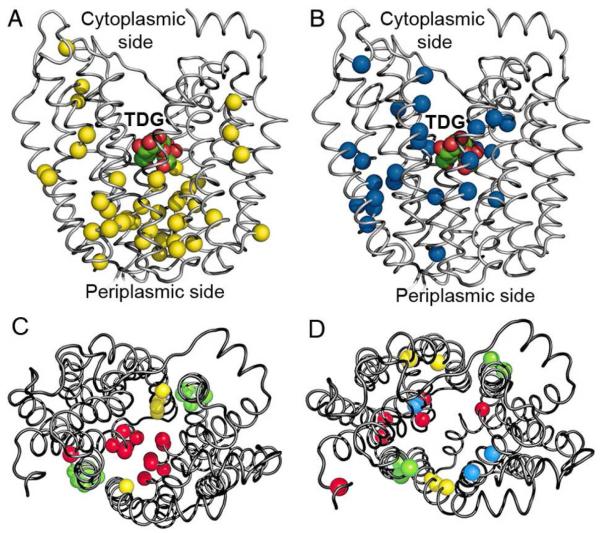
Alkylation of Cys side chains by radiolabeled N-ethylmaleimide (NEM) or fluorescent tetramethylrhodamine-5-maleimide (TMRM), which are membrane-permeant reagents, has been used extensively to study reactivity/accessibility of single-Cys residues introduced into Cys-less LacY. This approach, which is based on alkylation of single-Cys LacY in right-side-out (RSO) membrane vesicles containing single-Cys mutants, provides important information about the structure, function and dynamics of LacY (reviewed in 50, 51). The reactivity/accessibility of Cys residues depends on the surrounding environment and is limited by close contacts between transmembrane helices and/or the low dielectric of the environment. Almost every amino acid in LacY was replaced individually with Cys and tested by SDA method for reactivity with NEM, which provided initial support for an alternating access mechanism. Engineered Cys replacements located near or within the inward-facing hydrophilic cavity react well with this alkylating agent. Binding of thiodigalactoside (TDG) increases NEM reactivity of single-Cys replacements located predominantly on the periplasmic side of LacY (Fig. 2A) and decreases reactivity of replacements located predominantly on the cytoplasmic side (Fig. 2B). Furthermore, both sets of Cys replacements in the putative cavities are located at the periplasmic (increased reactivity) and cytoplasmic (decreased reactivity) ends of the same helices and distributed in a pseudo-symmetrical manner (Fig. 2C, D). The pattern is consistent with a model in which the single sugar-binding site in the approximate middle of LacY is alternatively exposed to either side of the membrane due to opening and closing of cytoplasmic and periplasmic hydrophilic pathways.
Figure 2.
Distribution of Cys replacements that exhibit changes in reactivity with NEM in the presence of TDG. Cα atoms of single Cys replacements are shown on backbone of the LacY structure in an inward-facing conformation (PDB ID 1PV7). (A and C) Positions of Cys residues that exhibit a significant increase in reactivity. (B and D) Positions of Cys residues that exhibit a significant decrease in reactivity. (A and B) Side view with bound TDG. (C and D) Cytoplasmic view demonstrating pseudo-symmetrical distribution of Cys replacements in putative translocation pathway. Residues located in symmetrically positioned helices are colored identically: I – VII (red); II – VIII (yellow); IV – X (blue); V – XI (green).
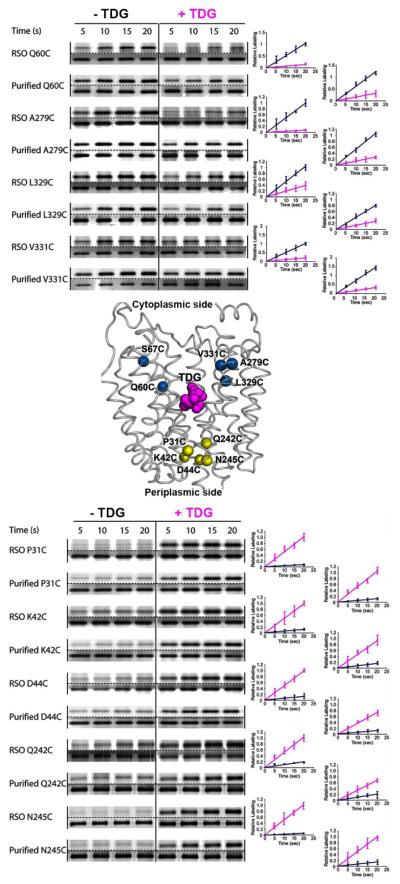
More recently, a simple, more facile SDA method with TMRM (31, 37, 51, 52) was developed to examine the effect of sugar binding on alkylation of single-Cys LacY mutants either in RSO membrane vesicles or in dodecyl-β,d-maltopyranoside (DDM) micelles (53). The experiments were carried out at 0°C, where thermal motion is restricted (54-56), and linear rates of labeling are readily obtained (Fig. 3). TMRM labeling is almost negligible with LacY containing each of five single-Cys residues at positions on the periplasmic side in RSO membrane vesicles or with purified protein solubilized in DDM. Binding of TDG markedly increases the rate of labeling (Fig. 3, lower panel). Consistently, on the cytoplasmic side, each of four single-Cys replacement mutants labels at a rapid rate in the absence of sugar both in RSO membrane vesicles and with purified protein in DDM. Binding of TDG markedly decreases the rate of TMRM labeling either in the membrane or with purified protein in DDM (Fig. 3, upper panel).
Figure 3.
TMRM labeling of cytoplasmic (top panel) or periplasmic (bottom panel) single-Cys mutants in RSO membrane vesicles or as the purified proteins in DDM. Labeling of cytoplasmic single-Cys LacY mutants, Q60C, A279C, L329C, and V331C or periplasmic single-Cys LacY mutants Q31C, K42C, D44C, Q242C and N245C was performed with 40 μM TMRM (RSO membrane vesicles) or 4 μM TMRM (with purified proteins in DDM) for given time at 0 °C in the absence of TDG (-TDG; blue plots) or pre-incubated for 10 min with TDG prior to addition of TMRM (+TDG; pink plots). Relative TMRM labeling rates were calculated as described in (53); the data are plotted relative to the 20 s points. For SDS/PAGE gels shown for each mutant, the upper gel displays the results of TMRM labeling; the lower gel is the silver stained protein.
The observations are certainly consistent with the interpretation that WT LacY in the native bacterial membrane is in a conformation similar to that of the x-ray crystal structures in the absence of ligand (9-11). The periplasmic side is tightly closed, and an open cavity is present facing the cytoplasm (the inward-facing conformation). Sugar binding leads to closure of the cytoplasmic-facing cavity with opening of a cavity on the periplasmic side. The average increase in periplasmic TMRM labeling observed in the presence of TDG in RSO vesicles is ~10-fold, and the average cytoplasmic decrease in the presence of TDG is very similar (~9-fold) (Table 1). With purified single-Cys proteins in DDM, comparable averages are ~6-fold and ~5-fold. Therefore, the change in TMRM labeling induced by sugar on opposite faces of LacY is about the same in RSO vesicles or with the purified mutants in DDM.
Table 1.
TDG effect on the rate of TMRM labeling of single-Cys mutants.
| LacY mutant | Helix | Fold change in TMRM labeling in RSO vesicles |
Fold change in TMRM labeling in DDM |
|
|---|---|---|---|---|
| periplasmic | P31C | I | 13.6 | 8.9 |
| K42C | II | 8.5 | 5.9 | |
| D44C | II | 8.2 | 6.2 | |
| Q242C | II | 5.2 | 3.2 | |
| N245C | VII | 14.9 | 6.9 | |
|
| ||||
| cytoplasmic | Q60C | II | −7.2 | −3.8 |
| S67C | II | −14.7 | −8.3 | |
| A279C | IX | −13.1 | −3.9 | |
| L329C | X | −2.6 | −3.0 | |
| V331C | X | −5.1 | −4.3 | |
Rates of TMRM labeling were obtained from the time courses shown in Figure 3 as described (53). For each mutant, the ratio of the estimated initial rate of TMRM labeling in the presence of TDG relative to that observed in the absence of TDG was calculated. Positive numbers indicate an increase in the relative labeling rate due to addition of TDG (periplasmic cysteines) and negative numbers indicate a decrease in the relative labeling rate due to addition of TDG (cytoplasmic cysteines).
The data obtained by SDA with TMRM not only provide further evidence that sugar binding markedly increases the open probability on the periplasmic side, but that sugar binding also increases the probability of closing on the inside, the implication being that opening and closing may be reciprocal. However, reciprocity may not be obligatory. The reactivity/accessibility of periplasmic Cys replacements in C154G LacY is very high in the absence or presence of sugar confirming that the periplasmic pathway is arrested in an open conformation in the C154G mutant, but the cytoplasmic cavity is still able to close and open (37). It has also been demonstrated (32) that replacement of Asp68 with Glu at the cytoplasmic end of helix II blocks sugar-induced opening of the periplasmic cavity, but has little or no effect on closing of the cytoplasmic cavity.
Single molecule fluorescence resonance energy transfer (sm-FRET)
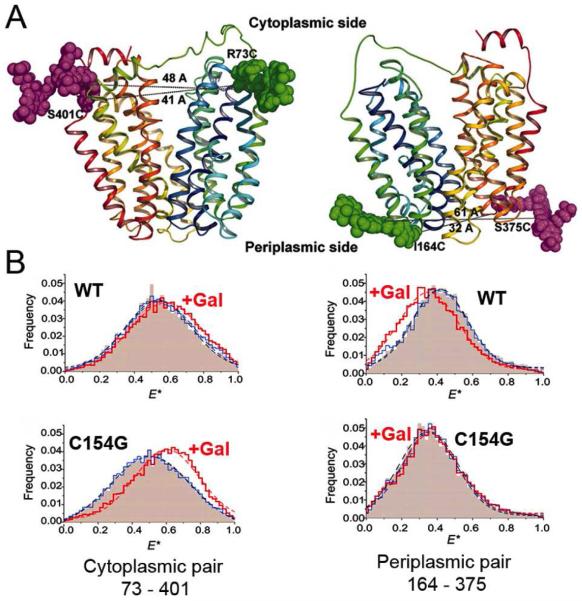
sm-FRET determined by alternating laser excitation spectroscopy was used in collaboration with Devdoot Majumdar and Shimon Weiss to study ligand-induced distance changes on the cytoplasmic and periplasmic sides of LacY diffusing freely in detergent micelles (36). The alternating access model was tested with WT LacY and the conformationally restricted mutant C154G. Pairs of Cys residues at the ends of two helices on the cytoplasmic or periplasmic sides were labeled with appropriate donor and acceptor fluorophores (Fig. 4A), smFRET was determined in the absence and presence of sugar, and distance changes were estimated from apparent energy transfer efficiency E* (Fig. 4B). With WT LacY, addition of a galactopyranoside, but not a glucopyranoside, results in a decrease in distance on the cytoplasmic side and an increase in distance and in distance distribution on the periplasmic side. In contrast, with the C154G mutant, a more pronounced decrease in distance and in distance distribution is observed on the cytoplasmic side, but there is no change on the periplasmic side. The results are consistent with the alternating access model and indicate that the functional defect in the mutant is due to impaired ligand-induced flexibility on the periplasmic side.
Figure 4.
Inter-helical distance changes on the cytoplasmic and periplasmic sides of LacY probed by smFRET. (A) LacY backbone with Alexa fluorophores attached at the ends of transmembrane helices on the cytoplasmic (left) or periplasmic (right) sides. Donor (Alexa 488) and acceptor (Alexa 647) are shown as magenta, or green space-filled models, respectively. (B) Ligand-induced effects on the smFRET efficiency distribution (E*) measured with WT LacY (upper panels) or C154G mutant (lower panels). Higher E* corresponds to shorter distance. Pink area – no sugar; blue line, addition of glucopyranoside; red line, addition of galactopyranoside.
Double electron-electron resonance (DEER)
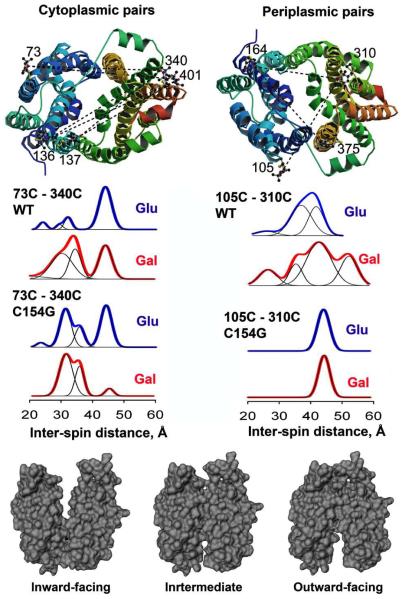
Four-pulse DEER (57, 58), combined with site-directed spin labeling is highly sensitive to distance change within 20-60 Å range in proteins, which is compatible with the size of LacY. The nitroxide probes are much smaller than the Alexa dyes used in smFRET and quantify distance changes more accurately. Nitroxide-labeled paired-Cys replacements at the ends of transmembrane helices on the cytoplasmic or periplasmic sides of WT LacY and C154G mutant were used for distance measurements in collaboration with Christian Altenbach and Wayne Hubbell (20). Six cytoplasmic and three periplasmic pairs were individually tested for distance changes in the presence of galactosidic or non-galactosidic sugars (Fig. 5, upper panel). Remarkably, binding of galactosidic sugars causes conformational rearrangements on both sides of wild type LacY. On the cytoplasmic side, each nitroxide-labeled pair exhibits decreased inter-spin distances. Distance distributions shifted toward shorter distances (from 4 to 21 Å for different pairs) in the presence of galactopyranoside (compare blue and red plots for the 73-340 pair in Fig. 5, middle panel). Conversely, on the periplasmic side, each of three spin-labeled pairs shows increased distances ranging from 4 to 14 Å for WT-based protein (Fig. 5; blue and red plots for the 105-310 pair). Thus, in the presence of the galactopyranoside specifically, the inward-facing cytoplasmic cavity closes and a cavity opens on the tightly sealed periplasmic side. In the C154G mutant, sugar-induced closure is observed on the cytoplasmic side (Fig. 5; blue and red plots for the 73-340 pair in the C154G mutant), but little or no change occurs on periplasmic side, which is partially open in the absence of sugar (Fig. 5; blue and red plots for the 105-310 pair in the C154G mutant). The DEER measurements in conjunction with molecular modeling based on the x-ray structures provide strong support for the alternating access model and suggest a structure for the outward-facing conformer of LacY, which agrees remarkably well with the model proposed by Radestock & Forrest (24). In addition, the measurements are consistent with the presence of intermediate conformation(s) in LacY (see Fig. 5, lower panel), since multiple distance distributions have been typically observed for the WT symporter with or without bound sugar.
Figure 5.
Sugar binding effect on inter-helical distances of LacY in DEER experiments. (Top) Disulfide-linked nitroxide chains are modeled on the LacY X-ray structure (PDB ID 1PV7) viewed from cytoplasmic (left) and periplasmic (right) sides. Nitroxides attached to LacY are shown as balls and sticks. Individual pairs used in DEER experiments are connected with dotted lines. (Middle) DEER characterization of sugar-binding effects on interspin distances of nitroxide-labeled double Cys mutants located on the cytoplasmic side (73-340 pair on WT or C154G background) or the periplasmic side (105-310 pair on WT or C154G background). Protein samples mixed with given sugars were frozen in liquid N2 and measurements were carried out at 50 K. Distance distributions obtained by Tikhonov regularization are shown for LacY with no sugar bound (glucosidic sugar, blue) and with bound sugar (galactosidic, sugar, red). Multi-Gaussian fits (black lines) demonstrate relative distributions of conformational populations. (Bottom) Molecular modeling of major conformations of LacY based on DEER distance measurements. Space filling representations of conformers are shown with helices II and VIII removed to illustrate openings on the cytoplasmic or periplasmic sides.
Site-directed cross-linking
As discussed above, the side chains essential for sugar recognition and H+ binding are located at the apex of the cytoplasmic cavity and inaccessible from the outside (Fig. 1A). On the periplasmic side, helices I/II and VII from the N- and C-terminal six-helix bundles, respectively, participate in sealing the cavity from the outside. Three double-Cys mutants (I40C/N245C, T45C/N245C, and I32C/N245C) located in the interface between helices I/II and VII on the periplasmic side of LacY were constructed with a factor Xa protease cleavage site in the loop between helices IV and V (Fig. 6A) for detection of cross-linking (29). All three pairs cross-link quantitatively and reversibly with flexible homo-bifunctional thiol reagents (Fig. 6B, C). Strikingly, relatively short reagents (less than 10 Å) block lactose transport in all three mutants, whereas full or partial activity is observed when cross-linking is mediated by flexible reagents greater than about 10 Å in length (Fig. 6D). Rigid cross-linking reagent naphthyl dimaleimide (NDM) practically eliminates transport, although it is sufficiently long for the partial activity to occur (Fig. 6). Therefore, the transport mechanism of LacY must involve closing, as well as opening, of a large periplasmic cavity. Satisfyingly, 17 Å is the minimum length of cross-linker required for maximum transport activity, a distance similar to that obtained from DEER measurements for opening of periplasmic side in the presence of sugar.
Figure 6.
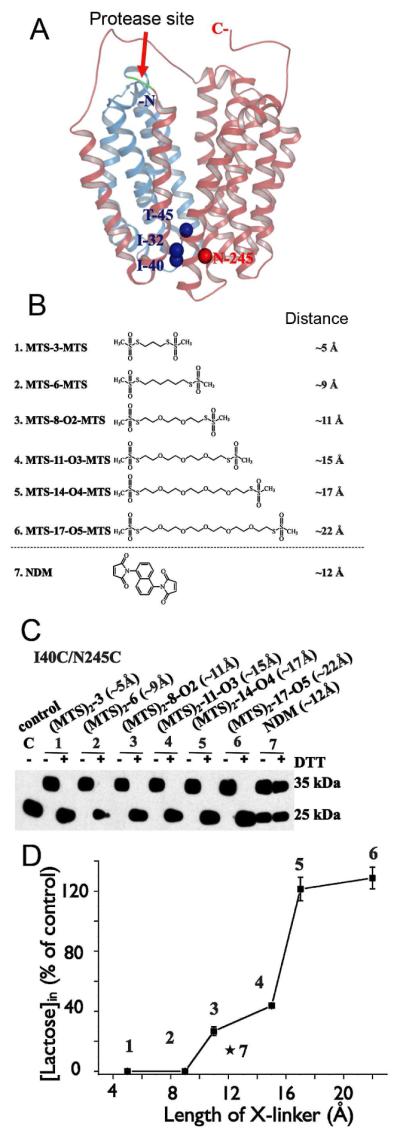
Effect of cross-linking at the periplasmic side of LacY on lactose transport. (A) Structural model of LacY with close periplasmic pathway. Cα atoms of the residues on periplasmic side used for Cys replacements are shown as blue (helices I and II) or red (helix VII) spheres. Red arrow indicates cleavage site for factor Xa protease located between helices IV and V. Helices I-IV and V-XII are colored in blue and pink, respectively. (B) Homo-bifunctional cross-linking reagents with approximate S-S distances between bridging sulfur atoms in the chains are shown. (C) Western blot analysis with anti-C-terminal antibody in cross-linking experiments after factor Xa protease digestion. Control – I40C/N245C mutant without addition of cross-linkers; 1 – 7, results of cross-linking with indicated reagents and effect of reducing agent (DTT). (D) Effect of cross-linking by different length reagents on lactose transport with mutant I40C/N245C. All experiments were performed with RSO vesicles.
Trp fluorescence
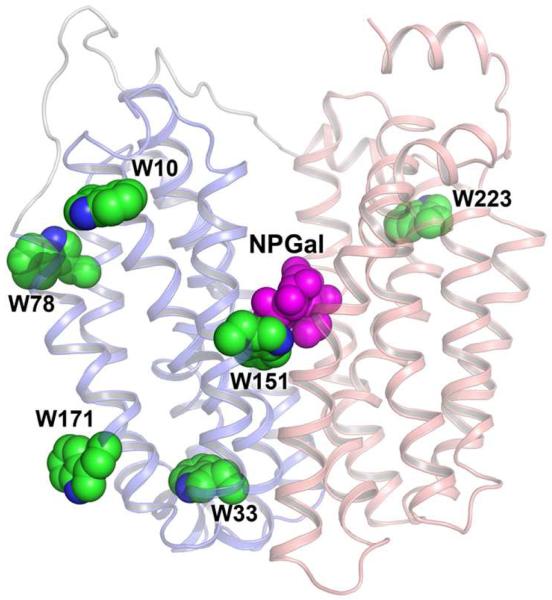
Fluorescence of intrinsic Trp residues in LacY provides a sensitive tool for functional studies using recently developed approaches that allow direct measurement of sugar binding, as well as global conformational changes in LacY (59-61). As a typical membrane transporter, LacY contains multiple Trp residues located predominantly at the interface of the phospholipid bilayer that are important for insertion and stability (62, 63). The exception is Trp151, which is a component of the sugar-binding site in LacY and the only Trp residue out of six in close proximity to bound galactopyranoside (Fig. 7). The short distance between Trp151 and the sugar is favorable for formation of a donor-acceptor pair between Trp and nitrophenyl or dansyl derivatives at the anomeric position of the galactosyl moiety resulting in fluorescence resonance energy transfer (FRET). Modeling of 4-nitrophenyl-α-d-galactopyranoside (NPG) in the binding-site of LacY places the nitrophenyl moiety ~12 Å away from Trp151, a distance commensurate with the Förster distance for a Trp-nitrophenyl pair. Indeed, NPG binding to LacY containing all six native Trp residues exhibits FRET from Trp151 (Trp151→NPG FRET) specific for the galactopyranoside, which can be measured in steady-state or stopped-flow experiments as a decrease of Trp fluorescence (59). Importantly, binding of galactopyranosides that do not absorb UV light (e.g., lactose, TDG, melibiose) has a negligible effect on the fluorescence of WT LacY, and replacement of Trp151 with Tyr completely abolishes FRET as a result of NPG binding.
Figure 7.
Location of native Trp residues in WT LacY structure. N-terminal and C-terminal 6-helix bundles are shown in blue and pink, respectively. Trp residues are presented as green spheres. NPG modeled in the sugar-binding site is shown as magenta spheres.
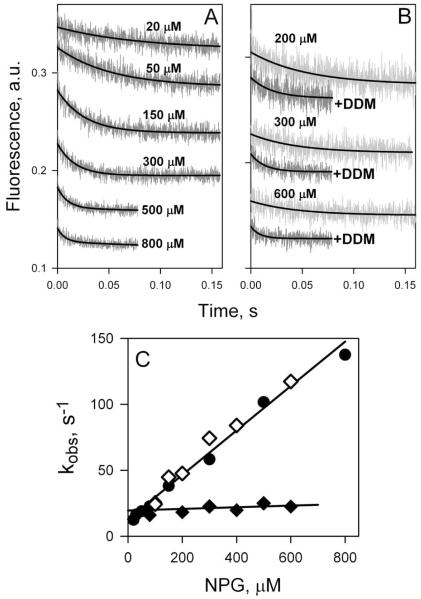
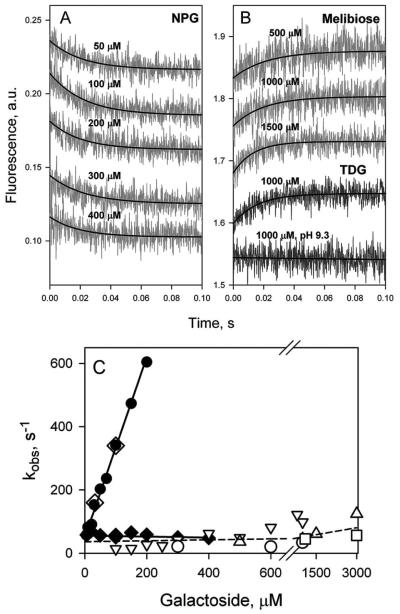
According to the alternating access mechanism, the tightly sealed periplasmic side of LacY observed in the x-ray structures, as well as in the bacterial membrane, must open in order to allow access of sugar to the binding site in the middle of the protein. Ergo the question arises as to whether or not opening of the periplasmic cavity is limiting for substrate binding and possibly transport. In an effort to address this question, rates of NPG binding were measured by Trp151→NPG FRET using stopped-flow with purified LacY in DDM, and reconstituted into proteoliposomes (61). A decrease of Trp fluorescence resulting from Trp151→NPG FRET was observed with LacY in DDM as well as in proteoliposomes (Fig 8A, B), although pre-steady state kinetics of sugar binding to solubilized LacY is completely different from that observed with the reconstituted protein. In DDM, the rate (kobs) increases linearly with increasing substrate concentration (Fig. 8C, filled circles). Thus, in an inward-facing conformation--as is the case with LacY in DDM--the sugar-binding site in LacY is readily accessible from cytoplasmic side, and binding can be described as single-step reversible process:
Figure 8.
Sugar binding rates measured as Trp151→NPG FRET with WT LacY reconstituted into proteoliposomes or solubilized in DDM. (A) Stopped-flow traces of changes in Trp fluorescence recorded after mixing of LacY in DDM with given concentrations of NPG. (B) Stopped-flow traces showing NPG binding to LacY reconstituted into proteoliposomes (light grey traces at 3 sugar concentrations), and after dissolving of proteoliposomes in 0.3% DDM (dark grey traces). (C) Concentration dependence of sugar binding rates (kobs) estimated from single-exponential fits shown in panels A and B. Data are obtained with LacY in DDM solution (●), reconstituted into proteoliposomes (◆), and after addition of DDM to proteoliposomes (◇). For protein in DDM data are fitted with linear equation (kobs□=koff + kon [NPG]) with estimated kinetic parameters koff = 13 s−1; kon = 0.2 μM−1 s−1; Kd = 65 μM. Reconstituted into proteoliposomes LacY binds NPG with kobs = 21 ± 4 s−1.
The kobs (reciprocal relaxation time 1/τ) for this reaction depends linearly on ligand concentration (kobs = koff + kon [S]).
In marked contrast, LacY reconstituted into proteoliposomes binds NPG at a slow rate that is independent of NPG concentration (Fig. 8C, filled diamonds). Addition of DDM to the same proteoliposomes dissolves the membrane and restores linear concentration dependence of NPG binding rates identical to that observed with purified protein in DDM (Fig. 8C, open diamonds). This finding suggests that in LacY reconstituted into proteoliposomes sugar-binding site is not readily accessible and that the binding reaction includes a limiting step that is likely represented by opening of the periplasmic cavity. LacY reconstituted into proteoliposomes is oriented with the periplasmic side facing the external medium (64), as in the native bacterial membrane. Therefore, as shown in the x-ray structures (9-11), as well as with RSO membrane vesicles (53), reconstituted LacY exists predominantly in an inward-facing conformation with a sealed periplasmic cavity, since sugar binding rates do not increase even at super-saturating NPG concentrations. Evidently, the periplasmic cavity leading to the sugar-binding site must open first, and this slow opening appears to be limiting for substrate binding.
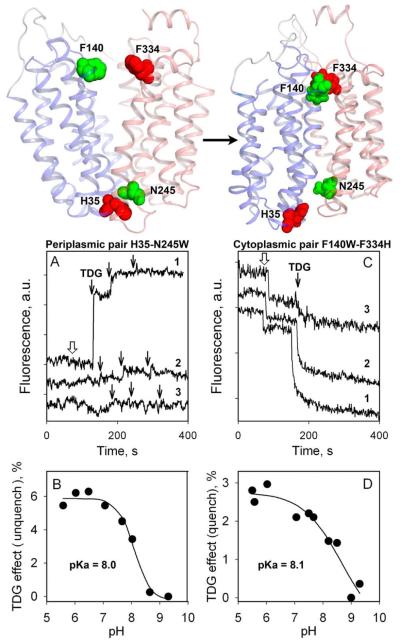
Trp fluorescence is an invaluable probe for detection of conformational changes in LacY (60, 61). Trp fluorescence in proteins is quenched by certain amino acyl side chains such as a protonated His, an amino group or a Cys residue (65). Measurements of quenching/unquenching of fluorescence of strategically introduced Trp residues allow differentiation between inward and outward-facing conformations of LacY and provide strong support for the alternating access mechanism (60). Thus, the sugar binding effect has been tested with mutants, where an additional Trp residue was introduced on either side of WT LacY far from sugar binding site, and predicted to be in close proximity to side chains of natural quenchers in either the inward- or outward-facing conformers (Fig. 9, upper panel). In mutant N245W, Trp is located on the periplasmic side of LacY (helix VII) where fluorescence is quenched by native His35 (helix I) in the inward-facing conformation. The fluorescence intensity of mutant N245W increases as a result of sugar binding (Fig. 9A, B) due to unquenching caused by an increase in distance between Trp245 and quencher His35. The opposite effect is observed in mutant F140W/F334H where Trp140 (helix V) and His334 (helix X) are located across the wide-open cytoplasmic cavity. Sugar binding leads to quenching of Trp fluorescence (Fig. 9C, D) due to direct collision between Trp140 and His334 resulting from the closing of cytoplasmic cavity. The pH dependency of Trp245 unquenching (Fig. 9B) and Trp140 quenching (Fig. 9D) exhibit a pKa of ~8, typical for a His side chain interacting with an aromatic group (66), thereby confirming His as the quencher in both mutants. The results provide yet another strong, independent line of evidence for the alternating access mechanism and demonstrate that the methodology described provides a sensitive probe to measure conformational changes.
Figure 9.
Alternating access mechanism probed by quenching/unquenching of Trp fluorescence. (Top) Backbone of the inward-facing LacY structure (left) and outward facing model (right) with N-terminal and C-terminal 6-helix bundles colored in blue and pink, respectively. Arrow indicates the conformational change resulting from sugar binding. Residues used for Trp substitutions are shown as green spheres. Residues used as the quenchers of Trp fluorescence are presented as red spheres. (Bottom) Effect of conformational change in LacY triggered by sugar binding on Trp fluorescence. Excitation and emission wavelength are 295 and 330 nm, respectively. (A and B) Unquenching of Trp fluorescence in mutant N245W after addition of TDG. (C and D) Quenching of Trp fluorescence in mutant F140W/F334H after addition of TDG. (A) Trp fluorescence change after addition of sucrose (open arrow) or TDG (black arrows) to N245W mutant at pH 6 (trace 1), and pH 9 (trace 2), or to control LacY without Trp substitution at position 245 at pH 6 (trace 3). (B) Dependence of fluorescence change on pH for the N245W mutant. (C) Trp fluorescence change after addition of sucrose (open arrow) or TDG (black arrows) to F140W/F334W mutant at pH 5.5 (trace 1), pH 8.5 (trace 2), or pH 9.0 (trace 3). (D) Dependence of fluorescence change on pH for the F140W/F334W mutant.
N245W LacY mutant with Trp introduced in periplasmic side has been used for a direct comparison between rates of opening of the periplasmic pathway and sugar-binding rates with LacY reconstituted into proteoliposomes by following either unquenching of Trp fluorescence, or Trp151→NPG FRET (61). The kinetics of sugar binding to N245W mutant (Fig. 10) is similar to that described previously for WT LacY (Fig. 8). In DDM, the rate of sugar binding exhibits a linear dependence on sugar concentration (Fig. 10C, filled circles and open diamonds). However, the mutant reconstituted into proteoliposomes binds sugar with kobs of ~60 s−1, and the rate is independent of NPG concentration (Fig. 10A, C, filled diamonds). In order to measure rates of opening of the periplasmic cavity triggered by galactoside binding, four galactosidic sugars that do not change Trp fluorescence--TDG, melibiose, octyl-α-d-galactoside, and methyl-α-d-galactoside--were used. Rates of unquenching of Trp fluorescence in mutant N245W due to binding of these galactosides to LacY in DDM (Fig. 10B) are very similar to the NPG binding rates observed in proteoliposomes (Fig. 10A). Moreover, the rates of both processes are essentially independent of sugar concentration (Fig. 10C, compare filled diamonds with open symbols fitted with dashed line). The similarity of the kinetics of two processes suggests that opening of the periplasmic cavity is likely the limiting step for sugar access to the binding site in LacY.
Figure 10.
Comparison of the rates of galactoside binding to N245W mutant reconstituted into proteoliposomes with the rates of opening of the periplasmic cavity. (A) Binding of NPG to protein reconstituted into proteoliposomes measured by Trp151→NPG FRET. Stopped-flow traces are shown for 5 sugar concentrations. Single-exponential fits are shown as black lines. B. Unquenching of Trp245 fluorescence resulting from opening of the periplasmic cavity upon sugar binding. Stopped-flow traces are recorded with the mutant in DDM after mixing with melibiose (3 upper traces) or TDG (2 lower traces) at pH 6.0, except for the trace at pH 9.3. (C) Concentration dependence of sugar binding rates and rates of periplasmic pathway opening. Binding rates (kobs) estimated for purified mutant in DDM (●); reconstituted in proteoliposomes (◆), and after dissolving proteoliposomes in DDM (◇). The reconstituted mutant binds NPG with kobs = 56 ± 7 s−1. Rates of unquenching of Trp245 fluorescence resulting from opening of the periplasmic cavity after sugar binding measured in DDM solution and presented as open symbols: TDG (▽); melibiose (△); octyl-α-d-galactoside (○); methyl-α-d-galactoside (□). The rate of opening of periplasmic cavity in DDM is 50 - 100 s−1 at the saturating concentrations of all 4 galactosides tested.
A turnover number of 16-20 s−1 was estimated for uphill lactose/H+ symport by WT LacY in RSO membrane vesicles and with the purified protein reconstituted into proteoliposomes (67). Very much the same rate (21 ± 4 s−1) is observed for sugar binding to WT LacY in proteoliposomes (Fig. 8). Therefore, opening of the periplasmic pathway may be the rate-limiting step for the overall symport mechanism catalyzed by WT LacY.
In view of this scenario, how does transport of sugar across the membrane occur? Based on the available biochemical and structural information, a probable description of the overall symport cycle of LacY can be formulated. In the absence of an external galactopyranoside, opening of LacY on the periplasmic side occurs spontaneously, but with low frequency. Thus, at any given time, only small population of molecules in the membrane is open on periplasmic side statistically. However, binding of a galactopyranoside from the periplasm results in formation of a high-energy intermediate in which both cavities are closed and sugar and H+ are occluded in the middle of the molecule. Depletion of the sugar-free, open-outward form of LacY shifts the equilibrium from the inward facing to the high-energy intermediate, which is in a meta-stable state and has a dramatically higher probability of opening the periplasmic cavity. To complete transport process, the intermediate with a bound sugar opens to cytoplasm, and sugar dissociates followed by dissociation of an H+. In the absence of , the limiting step of the transport is deprotonation, and in the presence of a driving force of the H+, dissociation of sugar or a conformational change becomes limiting.
References
- 1.Saier MH., Jr. Families of transmembrane sugar transport proteins. Mol Microbiol. 2000;35:699–710. doi: 10.1046/j.1365-2958.2000.01759.x. [DOI] [PubMed] [Google Scholar]
- 2.West IC. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970;41:655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]
- 3.West IC, Mitchell P. Stoichiometry of lactose-H+ symport across the plasma membrane of Escherichia coli. Biochem J. 1973;132 doi: 10.1042/bj1320587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaback HR, Sahin-Toth M, Weinglass AB. The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol. 2001;2:610–620. doi: 10.1038/35085077. [DOI] [PubMed] [Google Scholar]
- 5.Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Celma JJ, Smirnova IN, Kaback HR, Fendler K. Electrophysiological characterization of LacY. Proc Natl Acad Sci U S A. 2009;106:7373–7378. doi: 10.1073/pnas.0902471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouaux E, Mackinnon R. Principles of selective ion transport in channels and pumps. Science. 2005;310:1461–1465. doi: 10.1126/science.1113666. [DOI] [PubMed] [Google Scholar]
- 8.Viitanen P, Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- 9.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 10.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H(+) symport in LacY. Embo J. 2006;25:1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Natl Acad Sci U S A. 2007;104:15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaptal V, Kwon S, Sawaya MR, Guan L, Kaback HR, Abramson J. Crystal structure of lactose permease in complex with an affinity inactivator yields unique insight into sugar recognition. Proc Natl Acad Sci U S A. 2011;108:9361–9366. doi: 10.1073/pnas.1105687108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smirnova IN, Kasho VN, Sugihara J, Choe JY, Kaback HR. Residues in the H+ translocation site define the pKa for sugar binding to LacY. Biochemistry. 2009;48:8852–8860. doi: 10.1021/bi9011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smirnova IN, Kasho V, Kaback HR. Protonation and sugar binding to LacY. Proc Natl Acad Sci U S A. 2008;105:8896–8901. doi: 10.1073/pnas.0803577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaback HR. Structure and mechanism of the lactose permease. C R Biol. 2005;328:557–567. doi: 10.1016/j.crvi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 17.Yin Y, He X, Szewczyk P, Nguyen T, Chang G. Structure of the multidrug transporter EmrD from Escherichia coli. Science. 2006;312:741–744. doi: 10.1126/science.1125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newstead S, Drew D, Cameron AD, Postis VL, Xia X, Fowler PW, Ingram JC, Carpenter EP, Sansom MS, McPherson MJ, Baldwin SA, Iwata S. Crystal structure of a prokaryotic homologue of the mammalian oligopeptide-proton symporters, PepT1 and PepT2. EMBO J. 2011;30:417–426. doi: 10.1038/emboj.2010.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang S, Sun L, Huang Y, Lu F, Liu Y, Gong H, Wang J, Yan N. Structure of a fucose transporter in an outward-open conformation. Nature. 2010;467:734–738. doi: 10.1038/nature09406. [DOI] [PubMed] [Google Scholar]
- 20.Smirnova I, Kasho V, Choe JY, Altenbach C, Hubbell WL, Kaback HR. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci U S A. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin Y, Jensen MO, Tajkhorshid E, Schulten K. Sugar binding and protein conformational changes in lactose permease. Biophys J. 2006;91:3972–3985. doi: 10.1529/biophysj.106.085993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen MO, Yin Y, Tajkhorshid E, Schulten K. Sugar transport across lactose permease probed by steered molecular dynamics. Biophys J. 2007;93:92–102. doi: 10.1529/biophysj.107.103994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pendse PY, Brooks BR, Klauda JB. Probing the Periplasmic-Open State of Lactose Permease in Response to Sugar Binding and Proton Translocation. J Mol Biol. 2010 doi: 10.1016/j.jmb.2010.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radestock S, Forrest LR. The alternating-access mechanism of MFS transporters arises from inverted-topology repeats. J Mol Biol. 2011;407:698–715. doi: 10.1016/j.jmb.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 25.le Coutre J, Kaback HR, Patel CK, Heginbotham L, Miller C. Fourier transform infrared spectroscopy reveals a rigid alpha-helical assembly for the tetrameric Streptomyces lividans K+ channel. Proc Natl Acad Sci U S A. 1998;95:6114–6117. doi: 10.1073/pnas.95.11.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sayeed WM, Baenziger JE. Structural characterization of the osmosensor ProP. Biochim Biophys Acta. 2009;1788:1108–1115. doi: 10.1016/j.bbamem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Patzlaff JS, Moeller JA, Barry BA, Brooker RJ. Fourier transform infrared analysis of purified lactose permease: a monodisperse lactose permease preparation is stably folded, alpha-helical, and highly accessible to deuterium exchange. Biochemistry. 1998;37:15363–15375. doi: 10.1021/bi981142x. [DOI] [PubMed] [Google Scholar]
- 28.Nie Y, Smirnova I, Kasho V, Kaback HR. Energetics of Ligand-induced Conformational Flexibility in the Lactose Permease of Escherichia coli. J Biol Chem. 2006;281:35779–35784. doi: 10.1074/jbc.M607232200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Guan L, Freites JA, Kaback HR. Opening and closing of the periplasmic gate in lactose permease. Proc Natl Acad Sci U S A. 2008;105:3774–3778. doi: 10.1073/pnas.0800825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Nie Y, Kaback HR. Residues Gating the Periplasmic Pathway of LacY. J Mol Biol. 2009;394:219–225. doi: 10.1016/j.jmb.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nie Y, Zhou Y, Kaback HR. Clogging the periplasmic pathway in LacY. Biochemistry. 2009;48:738–743. doi: 10.1021/bi801976r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Madej MG, Kaback HR. Helix dynamics in LacY: helices II and IV. J Mol Biol. 2010;396:617–626. doi: 10.1016/j.jmb.2009.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Iwaarden PR, Driessen AJ, Lolkema JS, Kaback HR, Konings WN. Exchange, efflux, and substrate binding by cysteine mutants of the lactose permease of Escherichia coli. Biochemistry. 1993;32:5419–5424. doi: 10.1021/bi00071a017. [DOI] [PubMed] [Google Scholar]
- 34.Menick DR, Sarkar HK, Poonian MS, Kaback HR. Cys154 is important for lac permease activity in Escherichia coli. Biochem Biophys Res Commun. 1985;132:162–170. doi: 10.1016/0006-291x(85)91002-2. [DOI] [PubMed] [Google Scholar]
- 35.Smirnova IN, Kaback HR. A Mutation in the Lactose Permease of Escherichia coli That Decreases Conformational Flexibility and Increases Protein Stability. Biochemistry. 2003;42:3025–3031. doi: 10.1021/bi027329c. [DOI] [PubMed] [Google Scholar]
- 36.Majumdar DS, Smirnova I, Kasho V, Nir E, Kong X, Weiss S, Kaback HR. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci U S A. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nie Y, Sabetfard FE, Kaback HR. The Cys154-->Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway. J Mol Biol. 2008;379:695–703. doi: 10.1016/j.jmb.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ermolova NV, Smirnova IN, Kasho VN, Kaback HR. Interhelical packing modulates conformational flexibility in the lactose permease of Escherichia coli. Biochemistry. 2005;44:7669–7677. doi: 10.1021/bi0502801. [DOI] [PubMed] [Google Scholar]
- 39.Cosson P, Bonifacino JS. Role of transmembrane domain interactions in the assembly of class II MHC molecules. Science. 1992;258:659–662. doi: 10.1126/science.1329208. [DOI] [PubMed] [Google Scholar]
- 40.Lemmon MA, Flanagan JM, Treutlein HR, Zhang J, Engelman DM. Sequence specificity in the dimerization of transmembrane alpha-helices. Biochemistry. 1992;31:12719–12725. doi: 10.1021/bi00166a002. [DOI] [PubMed] [Google Scholar]
- 41.Smith SO, Bormann BJ. Determination of helix-helix interactions in membranes by rotational resonance NMR. Proc Natl Acad Sci U S A. 1995;92:488–491. doi: 10.1073/pnas.92.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Javadpour MM, Eilers M, Groesbeek M, Smith SO. Helix packing in polytopic membrane proteins: role of glycine in transmembrane helix association. Biophys J. 1999;77:1609–1618. doi: 10.1016/S0006-3495(99)77009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y, Madej MG, Guan L, Nie Y, Kaback HR. An early event in the transport mechanism of LacY: Interaction between helices V and I. J Biol Chem. 2011;286:30415–30422. doi: 10.1074/jbc.M111.268433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Iwaarden PR, Driessen AJ, Menick DR, Kaback HR, Konings WN. Characterization of purified, reconstituted site-directed cysteine mutants of the lactose permease of Escherichia coli. J Biol Chem. 1991;266:15688–15692. [PubMed] [Google Scholar]
- 45.Frillingos S, Kaback HR. Cysteine-scanning mutagenesis of helix VI and the flanking hydrophilic domains on the lactose permease of Escherichia coli. Biochemistry. 1996;35:5333–5338. doi: 10.1021/bi953068d. [DOI] [PubMed] [Google Scholar]
- 46.Frillingos S, Sahin-Toth M, Wu J, Kaback HR. Cys-scanning mutagenesis: a novel approach to structure function relationships in polytopic membrane proteins. Faseb J. 1998;12:1281–1299. doi: 10.1096/fasebj.12.13.1281. [DOI] [PubMed] [Google Scholar]
- 47.Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 48.Sorgen PL, Hu Y, Guan L, Kaback HR, Girvin ME. An approach to membrane protein structure without crystals. Proc Natl Acad Sci U S A. 2002;99:14037–14040. doi: 10.1073/pnas.182552199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudnick G. Cytoplasmic Permeation Pathway of Neurotransmitter Transporters. Biochemistry. 2011;50:7462–7475. doi: 10.1021/bi200926b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaback HR, Dunten R, Frillingos S, Venkatesan P, Kwaw I, Zhang W, Ermolova N. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci U S A. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nie Y, Ermolova N, Kaback HR. Site-directed Alkylation of LacY: Effect of the Proton Electrochemical Gradient. J Mol Biol. 2007;374:356–364. doi: 10.1016/j.jmb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang X, Nie Y, Kaback HR. Site-Directed Alkylation Studies with LacY Provide Evidence for the Alternating Access Model of Transport. Biochemistry. 2011;50:1634–1640. doi: 10.1021/bi101988s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nie Y, Kaback HR. Sugar binding induces the same global conformational change in purified LacY as in the native bacterial membrane. Proc Natl Acad Sci U S A. 2010;107:9903–9908. doi: 10.1073/pnas.1004515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venkatesan P, Kaback HR. The substrate-binding site in the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1998;95:9802–9807. doi: 10.1073/pnas.95.17.9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venkatesan P, Kwaw I, Hu Y, Kaback HR. Site-directed sulfhydryl labeling of the lactose permease of Escherichia coli: helix VII. Biochemistry. 2000;39:10641–10648. doi: 10.1021/bi000438b. [DOI] [PubMed] [Google Scholar]
- 56.Venkatesan P, Liu Z, Hu Y, Kaback HR. Site-directed sulfhydryl labeling of the lactose permease of Escherichia coli: helix II. Biochemistry. 2000;39:10649–10655. doi: 10.1021/bi0004394. [DOI] [PubMed] [Google Scholar]
- 57.Pannier M, Veit S, Godt A, Jeschke G, Spiess HW. Dead-time free measurement of dipole-dipole interactions between electron spins. J Magn Reson. 2000;142:331–340. doi: 10.1006/jmre.1999.1944. [DOI] [PubMed] [Google Scholar]
- 58.Jeschke G. Distance measurements in the nanometer range by pulse EPR. Chemphyschem. 2002;3:927–932. doi: 10.1002/1439-7641(20021115)3:11<927::AID-CPHC927>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 59.Smirnova IN, Kasho VN, Kaback HR. Direct Sugar Binding to LacY Measured by Resonance Energy Transfer. Biochemistry. 2006;45:15279–15287. doi: 10.1021/bi061632m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smirnova I, Kasho V, Sugihara J, Kaback HR. Probing of the rates of alternating access in LacY with Trp fluorescence. Proc Natl Acad Sci U S A. 2009;106:21561–21566. doi: 10.1073/pnas.0911434106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smirnova I, Kasho V, Sugihara J, Kaback HR. Opening the periplsmic cavity in lactose permease is the limiting step for sugar binding. Proc Natl Acad Sci U S A. 2011;108 doi: 10.1073/pnas.1112157108. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yau WM, Wimley WC, Gawrisch K, White SH. The preference of tryptophan for membrane interfaces. Biochemistry. 1998;37:14713–14718. doi: 10.1021/bi980809c. [DOI] [PubMed] [Google Scholar]
- 63.White SH. Membrane protein insertion: the biology-physics nexus. J Gen Physiol. 2007;129:363–369. doi: 10.1085/jgp.200709741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herzlinger D, Viitanen P, Carrasco N, Kaback HR. Monoclonal antibodies against the lac carrier protein from Escherichia coli. Binding studies with membrane vesicles and proteoliposomes reconstituted with purified lac carrier protein. Biochemistry. 1984;23:3688–3693. doi: 10.1021/bi00311a018. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y, Barkley MD. Toward understanding tryptophan fluorescence in proteins. Biochemistry. 1998;37:9976–9982. doi: 10.1021/bi980274n. [DOI] [PubMed] [Google Scholar]
- 66.Loewenthal R, Sancho J, Fersht AR. Histidine-aromatic interactions in barnase. Elevation of histidine pKa and contribution to protein stability. J Mol Biol. 1992;224:759–770. doi: 10.1016/0022-2836(92)90560-7. [DOI] [PubMed] [Google Scholar]
- 67.Viitanen P, Garcia ML, Kaback HR. Purified reconstituted lac carrier protein from Escherichia coli is fully functional. Proc Natl Acad Sci USA. 1984;81:1629–1633. doi: 10.1073/pnas.81.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]