Abstract
Cadmium is a well known nephrotoxicant; chronic exposure increases risk for chronic kidney disease. Recently, however, associations between urine cadmium and higher creatinine-based estimated glomerular filtration rate (eGFR) have been reported. Analyses utilizing alternate biomarkers of kidney function allow evaluation of potential mechanisms for these observations. We compared associations of urine cadmium with kidney function measures based on serum cystatin C to those with serum creatinine in 712 lead workers. Mean (standard deviation) molybdenum-corrected urine cadmium, Modification of Diet in Renal Disease (MDRD) eGFR and multi-variable cystatin C eGFR were 1.02 (0.65) μg/g creatinine, and 97.4 (19.2) and 112.0 (17.7) mL/min/1.73 m2, respectively. The eGFR measures were moderately correlated (rs = 0.5; p less than 0.001). After adjustment, ln(urine cadmium) was not associated with serum cystatin-C-based measures. However, higher ln(urine cadmium) was associated with higher creatinine-based eGFRs including the MDRD and an equation incorporating serum cystatin C and creatinine (beta-coefficient = 4.1 ml/min/1.73 m2; 95% confidence interval =1.6, 6.6). Urine creatinine was associated with serum creatinine-based but not cystatin-C-based eGFRs. These results support a biomarker-specific, rather than a kidney function, effect underlying the associations observed between higher urine cadmium and creatinine-based kidney function measures. Given the routine use of serum and urine creatinine in kidney and biomarker research, additional research to elucidate the mechanism(s) for these associations is essential.
Keywords: cadmium, cystatin C, kidney function, serum creatinine, urine creatinine
1. INTRODUCTION
Environmental exposure to cadmium is widespread globally. Recent publications have reported that higher cadmium dose is associated with worse kidney function (lower glomerular filtration rate measures or need for dialysis) even at lower exposure levels e.g., urine cadmium levels less than 2 μg/g creatinine (Akesson et al., 2005; Hellstrom et al., 2001; Navas-Acien et al., 2009). Therefore, in order to assess whether low-level cadmium co-exposure contributes to nephrotoxicity in lead workers, we examined associations between urine cadmium and kidney outcomes in our study of lead workers in the Republic of Korea. Unexpectedly, higher urine cadmium levels were associated with higher calculated creatinine clearance and MDRD eGFR (also based on creatinine) and lower serum creatinine (Weaver et al., 2011). The direction of these associations is opposite of those traditionally observed with cadmium nephrotoxicity but was also recently reported in children (de Burbure et al., 2006). Potential mechanisms for these findings include cadmium-related hyperfiltration; an effect of renal filtration on urine cadmium levels; a statistical effect related to the impact of adjustment for urine dilution with creatinine in models of creatinine-based kidney function measures; and an impact of cadmium on proximal tubule creatinine secretion.
In order to further evaluate these hypotheses, we examined associations of urine cadmium with glomerular filtration rate (GFR) measures based on an alternate marker of kidney function, serum cystatin C, in the same population in whom the previously reported associations were observed (Weaver et al., 2011). Serum creatinine and cystatin C have different sources (muscle and all nucleated cells, respectively) and are handled differently by the kidney. Both are filtered at the glomeruli but creatinine is secreted in the proximal tubules and excreted in the urine whereas cystatin C is reabsorbed and catabolized in the proximal tubules. Despite these differences, both biomarkers provide correlated measures of kidney function. Therefore if opposite direction associations are also observed with cystatin C outcomes, an effect involving kidney function, such as hyperfiltration or reverse causation, is suggested. In contrast, if associations are not observed with cystatin C, a biomarker specific mechanism is more likely. Examples include a cadmium effect on proximal tubule creatinine secretion or a statistical effect related to the impact of adjustment for urine dilution with urine creatinine in models of serum creatinine-based kidney function measures in populations in whom urine and serum creatinine are associated..
2. MATERIALS AND METHODS
2.1 Study overview and design
We performed a cross-sectional analysis of data from the fourth evaluation in a longitudinal study of current and former lead-exposed workers. Evaluations were performed between April 8, 2004 and September 24, 2005. Participation in the study was voluntary and all participants provided written, informed consent. The study protocol was approved by Institutional Review Boards at the SoonChunHyang University School of Medicine and the Johns Hopkins University Bloomberg School of Public Health.
2.2 Study population
As previously described (Schwartz et al., 2001; Weaver et al., 2011; Weaver et al., 2003), participants in the initial cohort of this study were recruited between 1997 and 1999 (phase I study participants) and followed longitudinally for three annual evaluations. In 2004, recruitment for three additional annual evaluations was begun; 498 of the 803 (62%) lead workers in the original cohort were re-enrolled (due to the economic conditions in Asia during the late 1990s, many workers in the initial study cohort were laid off and lost to follow-up). In addition, 279 new participants were recruited (phase II study participants). Inclusion criteria included occupational lead exposure and, for new participants, age 40 years or older in order to enrich the study with participants at greater risk for adverse kidney outcomes. No medical exclusionary criteria were used. At the end of this second enrollment phase (September 24, 2005), 778 current and former lead workers had completed the fourth of six evaluations in the overall longitudinal study. In order to optimize study data for both cross-sectional and longitudinal cadmium analyses while addressing funding constraints, urine cadmium was measured in fourth evaluation samples in the subset of 712 workers who came to both the fourth and fifth evaluations.
2.3 Data collection
As previously described (Weaver et al., 2011), a standardized, interviewer-administered questionnaire was used to elicit information on demographics; medical history; medications; current and past smoking and alcohol use; education; income; and occupational history. Blood pressure was measured with the IntelliSense™ blood pressure monitor (Model HEM-907; Omron; Vernon Hills, IL) using a standardized protocol. Data and biologic specimens also included: height and weight measurements; a blood specimen (for serum creatinine, cystatin C, and blood lead); four-hour urine collection (for cadmium and creatinine); and tibia lead.
2.4 Laboratory methods
As previously described (Weaver et al., 2011), urine cadmium was measured in the Trace Elements section of the Laboratory of Inorganic and Nuclear Chemistry at the New York State Department of Health’s Wadsworth Center (Albany, NY, USA). The analysis was carried out using an inductively coupled plasma-mass spectrometer (ICP-MS; Sciex ELAN DRC II, PerkinElmer Life and Analytical Sciences, Shelton, CT) equipped with dynamic reaction cell technology. The ICP-MS was operated according to a standard operating procedure optimized for multiple elements in urine (Minnich et al., 2008). Multi-element calibration standards were prepared from National Institute of Science and Technology traceable stock solution (High Purity Standards, Charleston, SC). Pooled human urine was used to matrix-match the calibration standards. Calibration solutions, reagents and urine samples were prepared under conditions (Clean Room and Class IIB Biosafety Cabinet) certified as Class 100 or better. Urine 114cadmium was measured in standard mode along with molybdenum to correct for a potential polyatomic interference from 98Mo16O+ at m/z 114 (Jarrett et al., 2008). The laboratory participates successfully in four External Quality Assessment Schemes specifically for trace elements in urine (Weaver et al., 2011). Urine-based internal quality control materials were included in each analytical run along with the study samples. The method detection limit for cadmium in urine, calculated as three times the standard deviation (SD) from a base level internal quality control sample over 20 runs, was 0.02 μg/L, while the limit of quantitation, calculated as 10 times the SD, was 0.07 μg/L.
Blood lead was measured (Fernandez, 1975) with an Hitachi 8100 Zeeman background-corrected atomic absorption spectrophotometer (Hitachi Ltd. Instruments, Tokyo, Japan) at the Institute of Industrial Medicine, a certified reference laboratory for lead in South Korea. Tibia lead levels were assessed via a 30-minute measurement of the left mid-tibia diaphysis using 109cadmium in a back-scatter geometry to fluoresce the K-shell X-rays of lead. The lead X-rays were recorded with a radiation detector and then quantified and compared to calibration data to estimate the concentration of lead in bone (Todd, 2000; Todd and Chettle, 2003; Todd et al., 2002).
Serum cystatin C was measured from samples stored at −80°C using an automated Dade Behring nephelometry assay on a Dimension Vista Lab System (Siemens Healthcare Diagnostics, Deerfield, IL, USA). For quality control purposes, the original cystatin C results were ordered by concentration and five percent were selected sequentially for duplication. The median coefficient of variation for these 41 samples run in duplicate was 6.1%; duplicate concentrations were approximately 90% of the original values consistent with variability in calibration standards available.
Kidney outcome measures included cystatin C and three estimates of GFR (kidney filtering ability) based on it using the equations shown below (Stevens et al., 2008):
single variable cystatin C eGFR = 76.7 × serum cystatin C−1.19
multi-variable cystatin C eGFR = 127.7 × serum cystatin C−1.17 × age−0.13 × 0.91 if female
Dual biomarker (cystatin C/creatinine) eGFR = 177.6 × serum creatinine−0.65 × serum cystatin C−0.57 × age−0.20 × 0.82 if female
Serum and urine creatinine were measured via a Dimension® clinical chemistry system using a Flex reagent cartridge in a modified kinetic Jaffe assay (model RxL; Dade Behring, Glasgow, DE, USA). The fifth kidney outcome measure, creatinine-based eGFR, in mL/min/1.73 m2, was estimated using the abbreviated Modification of Diet in Renal Disease (MDRD) formula: 186.3 × (serum creatinine)−1.154 × (age)−0.203 × 0.742 (if the participant was female) (Levey et al., 1999; Levey et al., 2000).
2.5 Statistical analysis
The goals of the analysis were to: 1) compare and contrast associations of urine cadmium levels with serum cystatin C, and eGFRs based on it, to those based on serum creatinine, while controlling for covariates; and 2) to evaluate whether these associations differed across eGFR tertiles, while also controlling for covariates. Statistical analysis was completed using SAS/STAT and SAS/GRAPH software, Version 9.2 of the SAS System for Windows (SAS Institute Inc, Cary, NC, USA).
Initially, variable distributions were examined. Cadmium was right skewed and thus was ln-transformed to minimize the influence of outliers. In linear regression models, associations of urine cadmium with outcomes were evaluated in two ways: the traditional approach, in which cadmium concentration is adjusted for urine dilution by dividing by urine creatinine; and a more recent approach, in which urine cadmium and creatinine are both included as separate covariates in the model (Barr et al., 2005). The latter approach (Barr et al., 2005) has been recommended for study populations that include groups likely to differ by muscle mass such as men and women across a range of ages.
Covariate selection utilized a priori variables (age, sex, and body mass index [BMI; weight in kilograms divided by the square of height in meters]) in modeling that included urine cadmium and creatinine with other covariates added in separate models. Additional covariates assessed included diabetes and hypertension (both based on participant report of physician diagnosis or medication use); regular analgesic use (based on questionnaire data on medication usage); self-reported work status (current vs. former lead worker); study status (phase I vs. II study participant), systolic and diastolic blood pressure (average of three measurements); tobacco use (smoking status: never, former, current; smoking dose [cigarettes per day × years of smoking] in quartiles for current smokers and dichotomized for former smokers); alcohol consumption (never, former, current); education (less than middle school graduate, less than high school graduate, high school graduate, greater than high school) and annual income (less than or equal to 10, 10–20, 20–30, 30–40, and greater than 40 million won). Variables were retained in the final model if, for any of the kidney outcomes, they substantially affected either the urine cadmium regression coefficient or the explanatory value (r2) of the model; or were relevant based on a priori knowledge or hypotheses inherent to this study (e.g., blood and tibia lead). Blood and tibia lead were added to final models after all other covariates were selected.
In the recently reported cadmium analysis (Weaver et al., 2011), associations between urine cadmium and kidney outcomes were examined in three groups stratified by tertile of eGFR in order to determine whether associations were potentially consistent with reverse causality (this process implies that urinary cadmium excretion is decreased as a result of decreased kidney function so associations would be observed only in the group with the worst kidney function). Following that approach in these analyses, tertile cutpoints by kidney function measure were 0.69 and 0.76 mg/L for serum cystatin C and, in ml/min/1.73 m2, 107.2 and 120.1 for single variable cystatin C eGFR; 105.4 and 119.2 for multi-variable cystatin C eGFR; 99.4 and 112.5 for dual biomarker eGFR; and 88.9 and 103.0 for MDRD eGFR.
As in previous analyses (Weaver et al., 2003), models were evaluated for linear regression assumptions and the presence of outlying data points using added variable plots (Weisberg, 1985), which are graphical summaries of the relation between Y and a particular X, adjusted for all of the other covariates. Each plot displays residuals and two lines: the regression line, and a line determined by a cubic spline scatterplot smoothing method (Reinsch, 1967). When applicable, models were repeated without outliers. Models were also assessed for collinearity through examination of variance inflation factors and condition indices.
3. RESULTS
3.1 Selected Demographics, Exposure, and Health Outcome Measures
Information on demographics, cadmium and lead biomarkers, kidney function measures, and selected covariates from the fourth evaluation in 712 lead workers is presented in Table 1. Women comprised 149 (20.9%) of the population. Mean (SD) molybdenum-corrected urine cadmium and blood and tibia lead levels were 1.02 (0.65) μg/g creatinine, 23.1 (14.1) μg/dL, and 26.6 (28.9) μg Pb/g bone mineral, respectively. Mean values for serum cystatin-C-based eGFRs were higher than those based on serum creatinine. Although mean values were normal, the range for each kidney measure included abnormally high and low values.
Table 1.
Selected demographic, exposure, and health outcome measures from the fourth evaluation in 712 current and former lead workersa
| Characteristic | All Participants |
|---|---|
| N (%) | |
| Female | 149 (20.9) |
| Diabetes | 27 (3.8) |
| Hypertension | 86 (12.1) |
| Former lead workers | 234 (32.9) |
| Smoking | |
| Never | 242 (34.0) |
| Current | 310 (43.5) |
| Former | 160 (22.5) |
| Alcohol use | |
| Never | 109 (15.3) |
| Current | 571 (80.2) |
| Former | 32 (4.5) |
| Education | |
| Less than middle school graduate | 180 (25.3) |
| Less than high school graduate | 182 (25.6) |
| High school graduate | 294 (41.3) |
| Median | Mean (SD) | Range | |
|---|---|---|---|
| Age, years | 46.7 | 47.6 (7.9) | 24.1–71.3 |
| BMI, kg/m2 | 24.2 | 24.2 (2.8) | 15.6–33.3 |
| Systolic blood pressure, mm Hg | 121.5 | 123.7 (15.5) | 90.5–213.3 |
| Diastolic blood pressure, mm Hg | 74.3 | 75.2 (12.0) | 46.0–147.0 |
| Urine cadmium, μg/g creatininea | 0.84 | 1.02 (0.65) | 0.17–4.63 |
| Urine cadmium, μg/Lb | 0.65 | 0.89 (0.73) | 0.06–5.4 |
| Urine creatinine, mg/dL | 82.2 | 94.4 (58.1) | 11.8–342.7 |
| Blood lead, μg/dL | 21.4 | 23.1 (14.1) | 1.9–74.4 |
| Tibia lead, μg Pb/g bone mineral | 19 | 26.6 (28.9) | −12–231 |
| Lead job duration, years | 13.2 | 13.1 (7.3) | 0.23–37.4 |
| Serum creatinine, mg/dl | 0.87 | 0.87 (0.15) | 0.42–1.53 |
| Serum cystatin C, mg/L | 0.72 | 0.73 (0.12) | 0.50–2.35 |
| Single var. cystatin C eGFR, mL/min/1.73 m2 | 113.9 | 113.7 (16.7) | 27.8–176.3 |
| Multi-var. cystatin C eGFR, mL/min/1.73 m2 | 112.8 | 112.0 (17.7) | 28.1–186.3 |
| Dual biomarker eGFR, mL/min/1.73 m2 | 105.8 | 106.1 (17.5) | 24.3–174.5 |
| MDRD eGFR, mL/min/1.73 m2 | 95.5 | 97.4 (19.2) | 23.6–189.7 |
modified from Weaver et al., 2011
molybdenum-corrected
All five outcome measures were significantly inter-correlated (Table 2). The Spearman correlation coefficient for serum creatinine and cystatin C was 0.37. eGFR correlations ranged from 0.47 for single variable cystatin C eGFR and MDRD eGFR to 0.93 for the two cystatin-C-based eGFR measures. A scatterplot of the relation between the multi-variable cystatin C eGFR and the MDRD eGFR is shown in Figure 1.
Table 2.
Spearman correlation coefficients for urine cadmium, urine creatinine and kidney function measures in 712 lead workers
| Urine creatinine | Urine cadmium μg/g | Serum cystatin C | Serum creatinine | Single variable cystatin C eGFR | Multi-variable cystatin C eGFR | Dual eGFR | MDRD eGFR | |
|---|---|---|---|---|---|---|---|---|
| Unadjusted urine cadmium μg/L | 0.69c | 0.51c | 0.13c | −0.12c | −0.13c | −0.18c | −0.06 | 0.03 |
| Urine creatinine, mg/dL | −0.22c | 0.08a | 0.13c | −0.08a | 0.004 | 0.02 | 0.03 | |
| Urine cadmium μg/g | 0.07 | −0.34c | −0.07 | −0.24c | −0.10b | 0.01 | ||
| Serum cystatin C, mg/L | 0.37c | −1.0c | −0.93c | −0.76c | −0.47c | |||
| Serum creatinine, mg/dL | −0.37c | −0.19c | −0.59c | −0.73c | ||||
| Single variable cystatin C eGFR, mL/min/1.73 m2 | 0.93c | 0.76c | 0.47c | |||||
| Multi-variable cystatin C eGFR, mL/min/1.73 m2 | 0.80c | 0.50c | ||||||
| Dual biomarker eGFR, mL/min/1.73 m2 | 0.90c |
p-value less than 0.05;
p-value less than 0.01;
p-value less than 0.001
Figure 1.
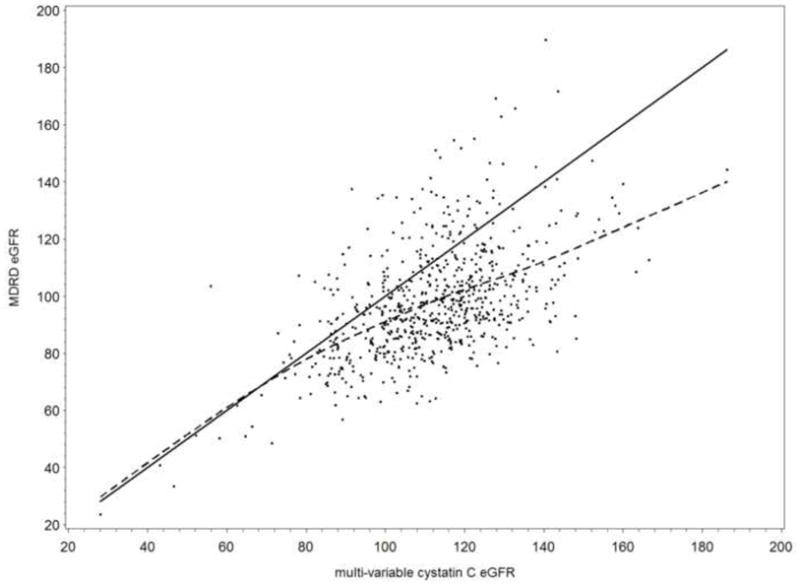
Scatterplot between MDRD eGFR and multi-variable cystatin C eGFR in 712 Korean lead workers. The line of equivalency (solid) and a smoothed line (dotted), which was estimated using the scatterplot smoothing method (SAS/GRAPH software), are shown.
3.2 Associations of Urine Cadmium with Kidney Function Measures
In adjusted analyses, ln(urine cadmium) was not associated with cystatin C or the two eGFR measures based solely on it (single variable cystatin C eGFR and multi-variable cystatin C eGFR; Table 3; Fully-adjusted model). In contrast, higher ln(urine cadmium) was associated with higher eGFR using the two equations that included serum creatinine (MDRD eGFR and the dual biomarker eGFR; Table 3; Fully-adjusted model). Consistent association patterns were observed in simpler a priori models that adjusted for ln(urine creatinine), age, sex, and BMI (data not shown). However, in models that included ln(urine cadmium) and ln(urine creatinine) without other covariates, ln(urine cadmium) was negatively associated (p less than 0.05) with all eGFR measures except MDRD eGFR, and borderline positively associated (p equal to 0.06) with serum cystatin C. Results were consistent with these findings when ln(urine cadmium) was entered as a covariate in μg/g creatinine in the three increasingly adjusted sets of models (data not shown). Higher ln(urine creatinine) was associated with lower eGFR only in measures that included serum creatinine (Table 3). In models without adjustment for urine dilution, ln(urine cadmium) was associated only with MDRD eGFR (Table 3; Model Without Ln(urine) Creatinine). Associations between urine cadmium and kidney measures were not substantially altered by adjustment for lead dose (data not shown).
Table 3.
Associations between urine cadmium and kidney function measures in 712 lead workersa
| Fully-adjusted Model | Model Without Ln(urine) Creatinine | |||
|---|---|---|---|---|
| β coeffe(95 % CI) | Model r2 | β coeffe(95 % CI) | Model r2 | |
| Serum Cystatin C, mg/l | ||||
| Ln(urine cadmium) μg/L | 0.005 (−0.008, 0.018) | 0.16 | 0.006 (−0.002, 0.015) | 0.16 |
| Ln(urine creatinine), mg/dL | 0.002 (−0.013, 0.017) | NA | ||
| Single variable cystatin C eGFR, ml/min/1.73 m2 | ||||
| Ln(urine cadmium) μg/L | −0.26 (−2.8, 2.3) | 0.16 | −0.85 (−2.4, 0.7) | 0.16 |
| Ln(urine creatinine), mg/dL | −0.9 (−3.8, 2.0) | NA | ||
| Multi-variable cystatin C eGFR, ml/min/1.73 m2 | ||||
| Ln(urine cadmium) μg/L | −0.33 (−2.8, 2.1) | 0.31 | −0.91 (−2.4, 0.6) | 0.31 |
| Ln(urine creatinine), mg/dL | −0.8 (−3.6, 1.9) | NA | ||
| Dual biomarker eGFR, ml/min/1.73 m2 | ||||
| Ln(urine cadmium) μg/L | 4.1 (1.6, 6.6) c | 0.24 | 1.0 (−0.6, 2.6) | 0.23 |
| Ln(urine creatinine), mg/dL | −4.5 (−7.4, −1.6) c | NA | ||
| MDRD eGFR, ml/min/1.73 m2 | ||||
| Ln(urine cadmium) μg/L | 7.2 (4.2, 10.2) d | 0.17 | 2.4 (0.53, 4.3) b | 0.15 |
| Ln(urine creatinine), mg/dL | −7.0 (−10.3, −3.6) d | NA | ||
Models also adjusted for age, sex, BMI, work status (current vs. former lead worker), smoking dose (cigarettes per day × years of smoking) in quartiles for current smokers and ex-smoker status, alcohol consumption (never, former, current); education (less than middle school graduate, less than high school graduate, high school graduate, greater than high school), annual income (less than or equal to 10, 10–20, 20–30, 30–40, and greater than 40 million won), study phase (phase I vs. II study participant), and blood and tibia lead.
p-value less than 0.05;
p-value less than 0.01;
p-value less than 0.001
beta (regression) coefficient indicates change in outcome for a one unit increase in ln(urine cadmium)
3.3 Urine Cadmium Associations in eGFR Subgroups
In models stratified by outcome tertile (Table 4), higher ln(urine cadmium) was associated with higher eGFR in participants in the lowest and highest dual biomarker eGFR tertiles, in a pattern similar to results with MDRD eGFR, although of only borderline significance (p less than 0.1). Ln(urine cadmium) was positively associated with multi-variable cystatin C eGFR in the highest tertile (p less than 0.1). In contrast, ln(urine cadmium) was positively associated with serum cystatin C in the highest tertile and negatively associated with multi-variable cystatin C eGFR in the lowest tertile (p equal to 0.05).
Table 4.
Associations between urine cadmium and kidney function measures in models stratified by outcome tertile (n = 712)a
| Kidney Function Measure | Lowest Tertileb | Middle Tertileb | Highest Tertileb | |||
|---|---|---|---|---|---|---|
| β coeffc(95 % CI) | p-value | β coeffc(95 % CI) | p-value | β coeffc(95 % CI) | p-value | |
| Serum Cystatin C, mg/l | ||||||
| Ln(urine cadmium) μg/L | −0.005 (−0.017, 0.006) | 0.36 | 0.003 (−0.003, 0.009) | 0.36 | 0.019 (−0.000, 0.038) | 0.05 |
| Ln(urine creatinine), mg/dL | −0.0003 (−0.013, 0.014) | 0.96 | 0.003 (−0.004, 0.009) | 0.43 | −0.015 (−0.037, 0.007) | 0.19 |
| Single variable cystatin C eGFR, ml/min/1.73 m2 | ||||||
| Ln(urine cadmium) μg/L | −2.1 (−4.9, 0.7) | 0.14 | −0.6 (−1.7, 0.6) | 0.32 | 0.8 (−2.2, 3.9) | 0.58 |
| Ln(urine creatinine), mg/dL | 1.2 (−2.1, 4.4) | 0.48 | −0.5 (−1.8, 0.7) | 0.43 | 0.6 (−2.9, 4.0) | 0.75 |
| Multi-variable cystatin C eGFR, ml/min/1.73 m2 | ||||||
| Ln(urine cadmium) μg/L | −2.7 (−5.5, 0.005) | 0.05 | 0.8 (−0.5, 2.0) | 0.23 | 3.1 (−0.1, 6.2) | 0.06 |
| Ln(urine creatinine), mg/dL | 2.4 (−0.8, 5.5) | 0.14 | −0.9 (−2.2, 0.5) | 0.21 | −2.4 (−6.0, 1.1) | 0.18 |
| Dual biomarker eGFR, ml/min/1.73 m2 | ||||||
| Ln(urine cadmium) μg/L | 2.2 (−0.4, 4.7) | 0.09 | 0.3 (−0.9, 1.4) | 0.66 | 3.2 (−0.3, 6.6) | 0.07 |
| Ln(urine creatinine), mg/dL | −2.0 (−4.8, 0.8) | 0.16 | −0.4 (−1.8, 0.9) | 0.52 | −2.3 (−6.4, 1.9) | 0.29 |
| MDRD eGFR, ml/min/1.73 m2 | ||||||
| Ln(urine cadmium) μg/L | 3.0 (0.8, 5.3) | <0.01 | 0.50 (−0.7, 1.7) | 0.42 | 6.5 (2.1, 10.9) | <0.01 |
| Ln(urine creatinine), mg/dL | −3.3 (−5.7, −0.9) | <0.01 | −0.3 (−1.6, 1.1) | 0.71 | −6.0 (−11.3, −0.7) | 0.03 |
Models also adjusted for age, sex, BMI, work status (current vs. former lead worker), smoking dose (cigarettes per day × years of smoking) in quartiles for current smokers and ex-smoker status, alcohol consumption (never, former, current); education (less than middle school graduate, less than high school graduate, high school graduate, greater than high school) and annual income (less than or equal to 10, 10–20, 20–30, 30–40, and greater than 40 million won), study phase (phase I vs. II study participant), and blood and tibia lead.
Tertile cutpoints by outcome were 0.69 and 0.76 mg/L for serum cystatin C and, in ml/min/1.73 m2, were 107.2 and 120.1 for the single variable cystatin C eGFR; 105.4 and 119.2 for the multi-variable cystatin C eGFR; 99.4 and 112.5 for the dual biomarker eGFR and 88.9 and 103.0 for the MDRD eGFR.
beta (regression) coefficient indicates change in outcome for a one unit increase in ln(urine cadmium)
4. DISCUSSION
In this cross-sectional analysis, we compared associations of urine cadmium with serum cystatin C and four eGFR measures: the widely used serum creatinine-based abbreviated MDRD eGFR; two serum cystatin-C-based estimates; and an estimate incorporating both serum creatinine and cystatin C. In 712 lead workers, all kidney function measures were correlated (rs = 0.47 or higher). However, despite correlated outcomes, ln(urine cadmium) was only associated with serum-creatinine-based eGFR measures.
Cadmium, at higher levels of exposure, is a well established nephrotoxicant associated with decreased glomerular filtration and chronic kidney disease (Kido et al., 2003). However, we recently reported associations between higher ln(urine cadmium) and higher calculated creatinine clearance and the MDRD eGFR and lower serum creatinine (Weaver et al., 2011) in lead workers with low-level cadmium exposure. Analyses of kidney outcome measures that are correlated with but not based on serum creatinine provide additional information on the potential mechanism(s) for these associations, which are in the opposite direction from those traditionally reported with cadmium nephrotoxicity. Cystatin C is the most relevant biomarker for this purpose. It is a cysteine protease inhibitor that is freely filtered at the glomerulus and reabsorbed and catabolized in the proximal tubules and it is secreted by all nucleated cells (Fried, 2009), thus avoiding the muscle mass confounding with serum creatinine. These characteristics have led to recent research to assess cystatin C and eGFR based on it as kidney outcome measures (Dharnidharka et al., 2002; Madero et al., 2006; Roos et al., 2007; Tidman et al., 2008). To date, such research has revealed associations of cystatin C with age, sex, race/ethnicity, diabetes, body size/composition, and inflammatory markers that persisted after adjustment for GFR, which indicates that, like creatinine, factors other than kidney function affect cystatin C levels (Stevens et al., 2009).
Few publications have reported associations between cadmium and cystatin C or eGFR based on it. Consistent with our work, higher urine cadmium was associated with lower serum creatinine, but was not significantly associated with serum β2 microglobulin or cystatin C, in a study in European children (de Burbure et al., 2006). In the children, blood cadmium was not associated with any of these measures but higher blood lead was associated with lower serum levels of all three, consistent with hyperfiltration. Both blood and urine cadmium were associated with lower creatinine clearance and serum cystatin-C-based eGFR in 820 Swedish women (Akesson et al., 2005) however neither blood nor urine cadmium was significantly associated with serum cystatin C in 200 adolescents (Staessen et al., 2001).
The data herein allow additional consideration of the previously published hypotheses (Weaver et al., 2011) for observed associations in the opposite direction from those traditionally reported in cadmium nephrotoxicity. Since creatinine and cystatin C are correlated measures of kidney function, associations present only with one marker suggest a biomarker-specific rather than a kidney function effect. In this population, urine creatinine, used to adjust urine cadmium levels for urine dilution, is positively associated with serum creatinine which was used to estimate GFR. This may create associations between cadmium and creatinine-based kidney function measures that are statistical rather than biological. In a priori and fully adjusted models, urine creatinine was significantly associated with serum creatinine but not cystatin-C-based eGFRs.
A cadmium effect on renal handling of creatinine must also be considered. In addition to being filtered at the glomeruli, creatinine is secreted by the proximal tubules. If cadmium increases creatinine secretion, the observed associations could result. Factors that affect creatinine secretion, such as medications, generally compete with creatinine for secretion resulting in decreased secretion (Rose and Post, 2010; Stevens and Perrone, 2010). Cation transporters have been implicated in creatinine secretion (Urakami et al., 2004). However, recent studies in mice have also implicated anion transporters (Eisner et al., 2010) and cadmium has been reported to increase transport of p-aminohippurate (a classic organic anion substrate) in low-level exposure but decrease transport at higher exposure levels (Van Kerkhove et al., 2010).
Given the lack of associations with cystatin-C-based kidney function measures, hyperfiltration and reverse causality/kidney filtration (hypotheses discussed in more detail in (Weaver et al., 2011) seem less likely although cannot be entirely excluded since opposing associations of serum creatinine and cystatin C have been observed with diabetes, C-reactive protein, white blood cell count, serum albumin and abnormal thyroid status (Manetti et al., 2005; Stevens et al., 2009). Cadmium has been reported to decrease albumin reabsorption in the proximal tubules via downregulation of megalin channels (Gena et al., 2010). This could also decrease cystatin C reabsorption (Kaseda et al., 2007), but, because cystatin C is metabolized in the proximal tubules, this would affect urine, rather than serum, cystatin C levels and should not explain our findings. Limited ability to accurately assess kidney function in this population might also be a factor. The MDRD estimating equation underestimates GFR in the normal range which is relevant for most occupational populations, including these lead workers, and none of the equations we used was developed for Korean populations. The opposite direction cadmium associations are not a secondary effect due to lead-related hyperfiltation since they were observed even in former lead workers in whom higher blood lead was associated with worse kidney function (Weaver et al., 2011). In addition, lead confounding was addressed by adjustment for both blood and tibia lead in the models.
5. CONCLUSIONS
In all participants, ln(urine cadmium) was not associated with serum cystatin C or the two eGFR measures based on it. However, consistent with associations observed with creatinine-based measures of glomerular filtration rate, higher ln(urine cadmium) was associated with higher levels of GFR estimated with an equation that included both serum cystatin C and creatinine. These results implicate a creatinine-specific rather than a kidney function mechanism underlying the unexpected associations observed between urine cadmium and creatinine-based kidney function measures. Adjustment of urine biomarkers with urine creatinine is a standard practice. Similarly, serum creatinine is the standard biomarker used to assess kidney function. Thus, not only are these opposite direction associations of concern for cadmium risk assessment but this work may also have broader implications for research with other nephrotoxicants. Additional research to elucidate the mechanism(s) for these associations is needed, including study in other populations and animal and in vitro models and analyses using other urine dilution adjustment methods.
Cadmium is a well known nephrotoxicant.
However, cadmium associations with higher estimated glomerular filtration rate based on serum creatinine were recently reported
We compared models of estimated glomerular filtration rate based on serum cystatin C to those with serum creatinine
Despite correlated estimated glomerular filtration rates, cadmium was not associated with cystatin-C-based estimated glomerular filtration rate
These results support a biomarker-specific, rather than a kidney function, mechanism
Acknowledgments
Funding: This research was supported by National Institute of Environmental Health Sciences grant 2 ES007198 (Dr. Weaver) and Korea Research Foundation-2000-00545 (Dr. Lee) from the Korea Research Foundation. The funding sources had no involvement in study design; data collection, analysis and interpretation; manuscript writing; or decisions to submit the work for publication.
We are grateful to Drs. Harvey Gonick, Walter Prozialeck and Alfred Bernard for thoughtful emails and discussions regarding this manuscript.
Abbreviations
- BMI
body mass index
- eGFR
estimated glomerular filtration rate
- GFR
glomerular filtration rate
- ICP-MS
inductively coupled plasma-mass spectrometer
- MDRD
Modification of Diet in Renal Disease
- SD
standard deviation
Footnotes
Conflict of Interest statement
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akesson A, Lundh T, Vahter M, Bjellerup P, Lidfeldt J, Nerbrand C, Samsioe G, Stromberg U, Skerfving S. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect. 2005;113:1627–31. doi: 10.1289/ehp.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Burbure C, Buchet JP, Leroyer A, Nisse C, Haguenoer JM, Mutti A, Smerhovsky Z, Cikrt M, Trzcinka-Ochocka M, Razniewska G, Jakubowski M, Bernard A. Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect. 2006;114:584–90. doi: 10.1289/ehp.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–6. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- Eisner C, Faulhaber-Walter R, Wang Y, Leelahavanichkul A, Yuen PS, Mizel D, Star RA, Briggs JP, Levine M, Schnermann J. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int. 2010;77:519–26. doi: 10.1038/ki.2009.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez FJ. Micromethod for lead determination in whole blood by atomic absorption, with use of the graphite furnace. Clin Chem. 1975;21:558–61. [PubMed] [Google Scholar]
- Fried LF. Creatinine and cystatin C: what are the values? Kidney Int. 2009;75:578–80. doi: 10.1038/ki.2008.688. [DOI] [PubMed] [Google Scholar]
- Gena P, Calamita G, Guggino WB. Cadmium Impairs Albumin Reabsorption by Downregulating Megalin and ClC5 Channels in Renal Proximal Tubule Cells. Environ Health Perspect. 2010 doi: 10.1289/ehp.0901874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom L, Elinder CG, Dahlberg B, Lundberg M, Jarup L, Persson B, Axelson O. Cadmium exposure and end-stage renal disease. Am J Kidney Dis. 2001;38:1001–8. doi: 10.1053/ajkd.2001.28589. [DOI] [PubMed] [Google Scholar]
- Jarrett JM, Xiao G, Caldwell KL, Henahan D, Shakirova G, Jones RL. Eliminating molybdenum oxide interference in urine cadmium biomonitoring using ICP-DRC-MS. Journal of Analytical Atomic Spectrometry. 2008;23:962–967. [Google Scholar]
- Kaseda R, Iino N, Hosojima M, Takeda T, Hosaka K, Kobayashi A, Yamamoto K, Suzuki A, Kasai A, Suzuki Y, Gejyo F, Saito A. Megalin-mediated endocytosis of cystatin C in proximal tubule cells. Biochem Biophys Res Commun. 2007;357:1130–4. doi: 10.1016/j.bbrc.2007.04.072. [DOI] [PubMed] [Google Scholar]
- Kido T, Nordberg GF, Roels HA. Cadmium-induced renal effects. In: De Broe ME, et al., editors. Clinical Nephrotoxins: Renal Injury from Drugs and Chemicals. Kluwer Academic Publishers; Dordrecht: 2003. pp. 507–530. [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Levey AS, Greene T, Kusek JW, Beck GJ, Group MS. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:A0828. [Google Scholar]
- Madero M, Sarnak MJ, Stevens LA. Serum cystatin C as a marker of glomerular filtration rate. Curr Opin Nephrol Hypertens. 2006;15:610–6. doi: 10.1097/01.mnh.0000247505.71915.05. [DOI] [PubMed] [Google Scholar]
- Manetti L, Pardini E, Genovesi M, Campomori A, Grasso L, Morselli LL, Lupi I, Pellegrini G, Bartalena L, Bogazzi F, Martino E. Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest. 2005;28:346–9. doi: 10.1007/BF03347201. [DOI] [PubMed] [Google Scholar]
- Minnich MG, Miller DC, Parsons PJ. Determination of As, Cd, Pb, and Hg in urine using inductively coupled plasma mass spectrometry with the direct injection high efficiency nebulizer. Spectrochimica Acta Part B: Atomic Spectroscopy. 2008;63:389–395. [Google Scholar]
- Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V. Blood Cadmium and Lead and Chronic Kidney Disease in US Adults: A Joint Analysis. Am J Epidemiol. 2009;170:1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsch CH. Smoothing by Spline Functions. Numerische Mathematik. 1967;10:177–183. [Google Scholar]
- Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children--a meta-analysis. Clin Biochem. 2007;40:383–91. doi: 10.1016/j.clinbiochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Rose B, Post T. Secretory pathways in proximal tubule. UpToDate; Waltham, MA: 2010. [Google Scholar]
- Schwartz BS, Lee BK, Lee GS, Stewart WF, Lee SS, Hwang KY, Ahn KD, Kim YB, Bolla KI, Simon D, Parsons PJ, Todd AC. Associations of blood lead, dimercaptosuccinic acid-chelatable lead, and tibia lead with neurobehavioral test scores in South Korean lead workers. Am J Epidemiol. 2001;153:453–64. doi: 10.1093/aje/153.5.453. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Nawrot T, Hond ED, Thijs L, Fagard R, Hoppenbrouwers K, Koppen G, Nelen V, Schoeters G, Vanderschueren D, Van Hecke E, Verschaeve L, Vlietinck R, Roels HA. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: a feasibility study of biomarkers. Lancet. 2001;357:1660–9. doi: 10.1016/s0140-6736(00)04822-4. [DOI] [PubMed] [Google Scholar]
- Stevens L, Perrone RD. Drugs that elevate the serum creatinine concentration. UpToDate; Waltham, MA: 2010. [Google Scholar]
- Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–60. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidman M, Sjostrom P, Jones I. A Comparison of GFR estimating formulae based upon s-cystatin C and s-creatinine and a combination of the two. Nephrol Dial Transplant. 2008;23:154–60. doi: 10.1093/ndt/gfm661. [DOI] [PubMed] [Google Scholar]
- Todd AC. Contamination of in vivo bone-lead measurements. Phys Med Biol. 2000;45:229–40. doi: 10.1088/0031-9155/45/1/316. [DOI] [PubMed] [Google Scholar]
- Todd AC, Chettle DR. Calculating the uncertainty in lead concentration for in vivo bone lead x-ray fluorescence. Phys Med Biol. 2003;48:2033–9. doi: 10.1088/0031-9155/48/13/314. [DOI] [PubMed] [Google Scholar]
- Todd AC, Parsons PJ, Carroll S, Geraghty C, Khan FA, Tang S, Moshier EL. Measurements of lead in human tibiae. A comparison between K-shell x-ray fluorescence and electrothermal atomic absorption spectrometry. Phys Med Biol. 2002;47:673–87. doi: 10.1088/0031-9155/47/4/309. [DOI] [PubMed] [Google Scholar]
- Urakami Y, Kimura N, Okuda M, Inui K. Creatinine transport by basolateral organic cation transporter hOCT2 in the human kidney. Pharm Res. 2004;21:976–81. doi: 10.1023/b:pham.0000029286.45788.ad. [DOI] [PubMed] [Google Scholar]
- Van Kerkhove E, Pennemans V, Swennen Q. Cadmium and transport of ions and substances across cell membranes and epithelia. Biometals. 2010;23:823–55. doi: 10.1007/s10534-010-9357-6. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Kim NS, Jaar BG, Schwartz BS, Parsons PJ, Steuerwald AJ, Todd AC, Simon D, Lee BK. Associations of low-level urine cadmium with kidney function in lead workers. Occup Environ Med. 2011;68:250–256. doi: 10.1136/oem.2010.056077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Lee BK, Ahn KD, Lee GS, Todd AC, Stewart WF, Wen J, Simon DJ, Parsons PJ, Schwartz BS. Associations of lead biomarkers with renal function in Korean lead workers. Occup Environ Med. 2003;60:551–62. doi: 10.1136/oem.60.8.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S. Applied linear regression. John Wiley & Sons; New York: 1985. [Google Scholar]


