Abstract
Baboons are an ideal model for studies of human inflammatory response due to their physiological and immunological similarities to people; however; little is known about how age affects immune function in the baboon. We sought to determine if baboons show age-related innate immune changes similar to that described in people. Age was correlated with increased serum C-reactive protein and interleukin-6 or, however, no change in interleukin-10 concentration was observed (n = 120 baboons). Cytokine release from unstimulated peripheral blood mononuclear cells as well as following immune (lipopolysaccharide) stimulation increased with age. When whole blood was assayed, both lipopolysaccharide stimulated and unstimulated samples showed an age-related increase in interleukin-6 response, although the unstimulated cytokine response was reduced compared with that observed in peripheral blood mononuclear cells. Tumor necrosis factor-α response was not related to age. Cytokine response in lipopolysaccharide-stimulated whole blood was negatively correlated with serum DHEA-S concentration and positively correlated with TGF-β concentration.
Keywords: Nonhuman primate, Immunosenescence, IL-6, C-reactive protein, TGF-β
AGE-RELATED immune dysfunction leads to increased disease morbidity and mortality in the elderly individual, with both an inadequate immune response (immunosenescence) and an excessive inflammatory response (inflammaging) occurring with age (1–4). Therefore, enhancement of immune function is an attractive potential target for improving health and quality of life in the elderly individual. If rational therapeutic approaches are to be developed, a better understanding of the underlying defects that impair the immune system of the aged is needed. For this, appropriate animal models are required. Most of aging research has been performed in relatively simple organisms, such as yeast, worms, flies, and rodents. However, the genetic and physiological differences between humans and these evolutionarily divergent animals limit their usefulness. Due to their similarities to people, baboons are an attractive animal model for assessing complex processes such as aging.
The baboon is considered an excellent model for human disease and its associated immune response (5–9). Baboons are unique among non-human primates in that similar to people they have four classes of IgG and have comparable susceptibility to pathogens (10,11). However, to the authors’ knowledge, only three studies have reported the effect of age on the immune function in the baboon, all of which focused on the effect of age on the adaptive immune response. Stacy and colleagues (12) reported that aged baboons (19–24 years) retain an intact ability to generate a protective immunoglobulin response to a primary and secondary antigenic challenge compared with young (2.5 years old) animals. This is in contrast to what is observed in people and rodent. Attanasio and colleagues (13) reported an age-associated increase in autoantibody concentration over life span, although it was not clear how many of animals in the study were old (≥19 years). Lymphocyte subtype populations were also found to change over life span, with a decrease in total lymphocyte count, a decrease in the percentage of B cells, and an increase in the percentage of both CD4 and CD8 T cells (14). Serum IL2r-alpha concentration was negatively correlated with age. However, lymphocyte function, defined as IgG concentration for B cells or lymphocyte proliferative activity for T cells, did not change with age. A limitation of this study was there were an insufficient number of aged animals (≥19 years) to specifically assess the role of old age. Therefore, due to the lack of available data assessing immune function in aged baboons, the objective of the current study was to determine if captive baboons show characteristic aging changes to innate immunity similar to that described in humans, including increased serum proinflammatory cytokine (interleukin [IL]-6) and acute phase protein (C-reactive protein [CRP]) concentrations, decreased anti-inflammatory cytokine (IL-10) concentrations, and decreased cytokine (IL-6 and tumor necrosis factor [TNF-α]) response following lipopolysaccharide (LPS) stimulation.
MATERIALS AND METHODS
Population Study
Serum was collected during routine health monitoring from 120 captive baboons (Papio hamadryas anubis) from the Baboon Research Resources, Department of Comparative Medicine, University of Oklahoma Health Science Center (BBR-OUHSC). Animals ranged from 2 to 26 years of age with a mean of 12.03 ± 6.8 years and included 96 females and 23 males.
Longitudinal Study
To determine age-related changes within individual animals, paired serum was collected from 30 baboons (26 females and 4 males) at two time points, with an average of 10.6 years (range 6–15 years) between sample collection dates and a mean age at initial sampling of 8.9 ± 3.3 years compared with 19.6 ± 3.7 years at second sampling. Archived samples were stored at −80°C until assayed. To ensure that degradation of cytokines had not occurred during long-term storage, the mean concentration of IL-6 in the archived samples (IL-6: 16.2 ± 7.1 pg/mL, n = 29; mean age = 8 years) was compared with the mean IL-6 concentration of age-matched samples from the population study (IL-6: 15.6 ± 2.0 pg/mL, n = 83; mean age = 9 years) and found to be similar (p = .9).
LPS Stimulation
Whole blood was collected from baboons for peripheral blood mononuclear cell (PBMC; n = 62) and whole-blood stimulation assays (n = 59). PBMC were isolated by Ficoll density gradient centrifugation (Histopaque-1077; Sigma, St. Louis, MO) as recommended by the supplier. Cells were washed twice in phosphate-buffered saline (PBS) and plated at a density of 5 × 106 cells/mL in RPMI complete media. Cells were incubated at 37°C, 5% CO2 in the presence of either 1 μg/mL of LPS (O111:B4, Sigma,, St. Louis MO) or an equal volume of PBS for 4 hours at which time media was collected for cytokine analysis and cells collected for RNA extraction.
Whole-blood stimulation assays were performed with 100 μL of heparinized blood in a final volume of 1 mL of RPMI media supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin (RPMI complete media) with 1 μg/mL of LPS or an equal volume of PBS. Samples were incubated at 37°C, 5% CO2 for 4 hours at which time media was collected for cytokine analysis.
LPS Dose Determination
PBMC isolated from four baboons were stimulated with LPS at 10 ng/mL, 100 ng/mL, or 1 μg/mL or an equal volume of PBS for 4 hours at 37°C, 5% CO2. The concentrations of TNF-α and IL-6 in the supernatant were measured by enzyme-linked immunosorbent assay (see below). In all four baboons, the cytokine response increased in a dose-dependent manner (mean % increase cytokine concentration ± SEM: TNF-α: 100 ng/mL of LPS = 27.43 ± 13.03, 1 μg/mL of LPS = 38.35 ± 20.13; IL-6: 100 ng/mL of LPS = 59.13 ± 24.57, 1 μg/mL of LPS = 79.12 ± 33.06). Therefore, to ensure a robust response of all animals in both the PBMC and whole-blood assay, an LPS dose of 1 μg/mL was selected. This dose was consistent with reported dose ranges used in stimulation of human PBMC (15–18).
Assays
TNF-α, IL-6, IL-10, and IL-4 were measured using a non-human primate–specific enzyme-linked immunosorbent assay (U-Cytech, Utrecht) each with a lower limit of detection of 1 pg/mL. Sample with a concentration below detection were assigned a value of 0.5 pg/mL for statistical analysis. CRP was determined using a human-specific enzyme-linked immunosorbent assay (MP Biomedicals, Orangeburg, NY), with a lower limit of detection of 0.1 mg/L. Serum dehydroepiandrosterone sulfate (DHEA-S) and cortisol were measured in serum from the whole blood by radioimmunoassay (MP Biomedicals, Irvine, CA; Coat-a-Count; Siemens Diagnostics, Deerfield, IL). Serum transforming growth factor (TGF)-beta 1 was measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Human-specific assays were validated for use in baboons by demonstrating linearity of diluted baboon serum samples and linearity of diluted pooled serum samples spiked with known concentrations of substrate.
Quantitative Polymerase Chain Reaction
Quantitative polymerase chain reaction was performed on stimulated PBMC from 24 adult female animals, 7–25 years of age. RNA was extracted using Trizol (Invitrogen, Carlsbad, CA), treated with DNase (Ambion, Foster City, CA), and then reverse transcribed into complementary DNA using poly-A primers (Thermoscript RT-PCR Kit; Invitrogen). Real-time polymerase chain reaction was performed in AB7500 thermocycler (Applied Biosystems, Foster City, CA) using SYBR green methodology (Power SYBR Green Master Mix; Applied Biosystems) with primers designed for TNF-α, IL-6, and β-actin using a primer sequence design program (19). Dissociation curves were performed with each assay to confirm a single amplification product. Cytokine expression relative to β-actin expression was reported.
Statistics
Data found to be non-normally distributed using the Kolmogorov–Smirnov test were log10 (inflammatory markers) or rank (age) transformed for analysis. Backward stepwise regression was performed to assess the effect of age and gender on serum CRP, IL-6, and IL-10 concentrations. Simple linear (CRP) or multiple linear regression (IL-6 and IL-10) with the significant factors was used to describe the effect of age and gender on serum CRP, IL-6, and IL-10 concentrations. Longitudinal data were compared by paired t test. A Pearson coefficient was used to compare rank age with log cytokine response in PBMC and whole blood stimulation assays. In addition, mean cytokine concentrations following stimulation of young (<5 years), adult (5–19 years), and aged baboons (≤20 years) were compared by one-way analysis of variance with Bonferroni’s post hoc correction. Univariate analysis (Pearson coefficient) was used to determine the serum factors significantly associated with cytokine response to LPS stimulation for inclusion in the final multiple linear regression model. Data were considered significant at p < .05.
RESULTS
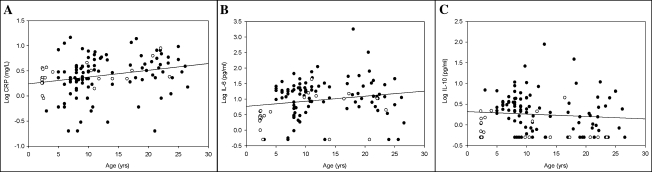
Serum CRP concentration increased with age, independent of gender (r = .3, p < .01; Figure 1A). Serum IL-6 also increased with age (r = .3, p < .05), with a greater concentration observed in female baboons according to the equation log IL6 = 1.3 + (0.003 × rank age) − (0.448 × gender), with female = 0 and male = 1 (Figure 1B). Serum IL-10, although unaffected by age, was also greater in female baboons (p < .001; Figure 1C). There was an increase of IL-6/IL-10 ratio with age (r = .3, p < .05). CRP increased in proportion to IL-6 (r = .4, p < .0001); however, there was no correlation between the anti-inflammatory cytokine, IL-10, and either proinflammatory marker, IL-6 or CRP. In the longitudinal study, serum CRP (p < .01; Figure 2A) and IL-6 (p < .01; Figure 2C) concentrations were greater in samples collected from individual animals when aged compared with samples collected from the same animal when young. There was no correlation between serum CRP or IL-6 concentration in samples collected when animals were young and samples from the same animal when aged (Figure 2B and D).
Figure 1.
Effect of age and gender on serum C-reactive protein (CRP), interleukin (IL)-6, and IL-10 concentrations in baboons. Serum CRP concentration (A) increased with age (p < .01) independent of gender. Serum IL-6 concentration (B) increased with age (p < .05) with a greater concentration in female baboons (closed circles). Serum IL-10 concentration (C), although not correlated with age, was higher in female baboons (p < .001).
Figure 2.
Longitudinal analysis of serum C-reactive protein (CRP) and interleukin (IL)-6 concentrations. In a longitudinal study of 30 baboons, serum CRP concentration (A) and IL-6 concentration (C) were increased in samples collected from animals when aged (mean age: 19.6 ± 3.7 years) compared with the concentration in samples collected from the same animal when young (mean age: 8.9 ± 3.3 years). CRP and IL-6 concentrations when young were not correlated with the concentration from that animal when aged (B and D), indicating that early-life cytokine concentration did not predict late life concentration. **p < .01.
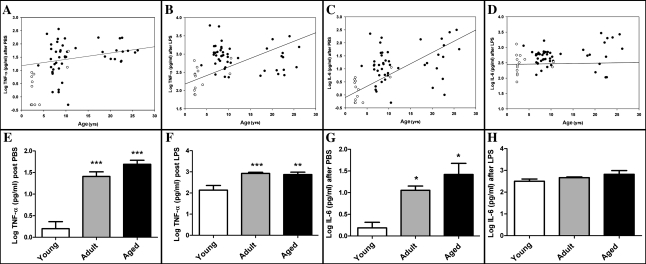
In vitro cytokine mRNA expression increased with age in baboons in unstimulated (TNF-α: n = 24, r = .45, p < .05; IL-6: n = 24, r = .58, p < .01; Figure 3) but not in LPS-stimulated PBMC (TNF-α: n = 24, r = .15, p = .5; IL-6: n = 24, r = .4, p = .06). Cytokine release from PBMC also increased with age in baboons when treated with PBS (TNF-α: n = 61, r = .6, p < .001 and IL-6: n = 61, r = .5, p < .0001; Figure 4A and C) and more weakly with LPS (TNF-α: n = 61, r = .4, p < .01 and IL-6: n = 61, r = .3, p < .05; Figure 4). However, gender distribution was age biased in this population, with the majority of the young baboons (<5 years) being males. To further evaluate the effect of age on cytokine stimulation, baboons were grouped by age (young < 5 years, n = 13; adult 5–19 years, n = 37; aged ≥ 20 years, n = 11) and compared using one-way analysis of variance (Figure 4E–H). Aged animals did not differ from adult animals for either cytokine.
Figure 3.
Tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 mRNA expression following lipopolysaccharide (LPS) stimulation of peripheral blood mononuclear cells (PBMC) from adult baboons. TNF-α and IL-6 mRNA relative expression of phosphate-buffered saline (PBS; A and B) but not LPS-treated (C and D) PBMC increases with age. (A) TNF-α after PBS: r = .45, p < .05; (B) IL-6 after PBS: r = .6, p < .01; (C) TNF-α after LPS: r = .15, p = .5; (D) IL-6 after LPS: r = .4, p = .06.
Figure 4.
Cytokine release (tumor necrosis factor-α [TNF-α] and interleukin [IL-6]) following lipopolysaccharide (LPS) stimulation of peripheral blood mononuclear cells (PBMC) from adult baboons. PBMC from baboons had an age-related increase in TNF-α and IL-6 release after phosphate-buffered saline (PBS; A and C) or LPS treatment (B and D). There was a greater mean cytokine release following PBS treatment (E and G) in adult (5–19 years of age) and aged baboons (≥20 years of age) compared with young baboons (<5 years of age). After LPS treatment, TNF-α release (F) was greater in adult and aged baboons but IL-6 release did not differ from that observed in young baboons (H). There was no difference between adult and aged baboons. (A) TNF-α after PBS: r = .6, p < .0001; (B) TNF-α after LPS: r = .4, p < .01; (C) IL-6 after PBS: r = .6, p < .0001; (D) IL-6 after LPS: r = .3, p < .05; (E–G) *denotes difference from young group; *p < .05, **p < .01, ***p < .001.
In whole blood, both LPS-stimulated and unstimulated samples showed an age-related increase in IL-6 response, although the correlation was weaker compared with that observed in PBMC (LPS treated: n = 59, r = .33, p = .01; PBS treated: n = 59, r = .24, p = .07; Figure 5). TNF-α response in whole blood was not related to age (LPS treated: n = 59, r = .16, p = .2; PBS treated: n = 59, r = .07, p = .6; Figure 5). When baboons were grouped by age (young < 5 years, n = 13; adult 5–19 years, n = 37; aged ≥ 20 years, n = 11) and compared using one-way analysis of variance (Figure 5E–H), aged animals did not differ from adult animals for either cytokine. The percent increase in cytokine concentration in PBMC following LPS stimulation was not correlated with the increase in whole blood for either cytokine (TNF-α: r = .23, p = .1; IL-6: r = −.1, p = .5).
Figure 5.
Cytokine release (tumor necrosis factor-α [TNF-α] and interleukin [IL-6]) following lipopolysaccharide (LPS) stimulation of whole blood from adult baboons. Whole blood IL-6 response to LPS (D) and phosphate-buffered saline (PBS) treatment (C) increased modestly with age. Whole blood TNF-α response following PBS or LPS treatment was not correlated with age (A and B). Compared with cytokine concentrations in young baboons (<5 years of age), there was a greater mean concentration of IL-6 following PBS treatment (G) or TNF-α following LPS treatment (F) in adult (5–19 years of age) but not in aged baboons (≥20 years of age). Both adult and aged baboons had a greater concentration of IL-6 compared with young baboons after LPS treatment (H). No affect of age was observed in TNF-α concentration following LPS treatment. (A) TNF-α after PBS: r = .07, p = .6; (B) TNF-α after LPS: r = .16, p = .2; (C) IL-6 after PBS: r = .24, p = .07; (D) IL-6 after LPS: r = .33, p = .01; (E–G) *denotes difference from young group; *p < .05, **p < .01, ***p < .001.
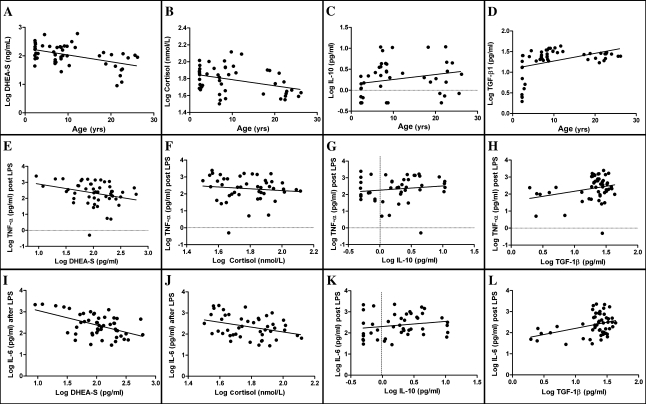
To assess whether anti-inflammatory cytokines or immunoregulatory hormone concentrations may have influenced proinflammatory cytokine response to LPS in whole blood, serum DHEA-S, cortisol, IL-10, TGF-β, and IL-4 concentrations were measured in the samples used for the stimulation assays. Serum IL-4 concentration was only detectable in six samples, so no further analysis was performed. Serum DHEA-S (r = −.4, p < .01), TGF-β (r = .6, p < .0001), and IL-10 (r = .3, Pp< .05) varied with age, whereas cortisol (r = −.27, p = .07) failed to reach significance (Figure 6). Serum DHEA-S and cortisol concentrations were negatively correlated with the concentration of IL-6 following LPS stimulation (DHEA-S: r = −.5, p < .001; cortisol: r = −.35, p < .05; Figure 6), whereas TGF-β was positively correlated (r = .4, p < .01). DHEA-S concentration was also negatively correlated with TNF-α concentration following LPS stimulation (r = −.3, p < .05; Figure 6). IL-10 concentration was not associated with cytokine response in the whole-blood assay. IL-6 concentration following LPS stimulation was predicted by the equation: log IL-6 = 2.998 − (0.642 × log DHEA-S) + (0.521 × TGF-β). TNF-α concentration was predicted by simple regression equation: log TNF-α = 3.448 − (0.556 × DHEA-S). Age was not retained in either equation.
Figure 6.
Effect of serum anti-inflammatory hormone and cytokine concentration on cytokine response to lipopolysaccharide (LPS) stimulation of whole blood. (A–D) Serum DHEA-S (A: r = −.4, p < .01) and cortisol (B: r = −.27, p = .07) decreased with age; transforming growth factor (TGF)-β; D: r = .6, p < .0001) increased with age; interleukin (IL)-10 was not correlated with age (C: r = .26, p = .08). (E–L) IL-6 release following LPS stimulation was negatively correlated with DHEA-S concentration (I: r = −.5, p < .01) and cortisol (J: r = −.35, p < .05) and positively correlated with TGF-β (H: r = .4, p < .01). TNF-α release following LPS stimulation was negatively correlated only with DHEA-S concentration (E: r = −.3, p < .05).
DISCUSSION
The objective of the current study was to determine whether similar inflammatory changes occur in baboons with age as have been reported to occur in humans. Serum biomarkers of inflammation (CRP, IL-6, IL-6/IL-10) increased with age in a population of adult baboons, with CRP showing the greatest change irrespective of gender. These data suggest that the acute phase protein CRP may be a more robust early biomarker of aging compared with inflammatory cytokines, which is consistent with previous reports by others (20–22). Evaluation of the balance between proinflammatory and anti-inflammatory cytokine concentrations has been suggested to provide a better assessment of the systemic inflammatory state than measurement of a single proinflammatory cytokine (23–26), and characterization of proinflammatory and anti-inflammatory responses together predicted survival in an aged population better than either response alone (26). In the present study, although IL-6/IL-10 ratio increased with age, the strength of the correlation was not greater than that of IL-6 concentration alone, and both measures were only weakly (r ≤ .3) correlated with age. The weak relationship of IL-6 and IL-6/IL-10 with age may reflect the relatively small sample size (n = 120) compared with the more robust sample sizes used in human aging population studies. IL-6 was reported to increase with age and predict mortality in the Iowa Rural Health Study with 1,293 individuals (27), the MacArthur Research Network on Successful Aging Community Study with 870 individuals (28), and Framingham Heart Study that evaluated inflammatory markers in 525 subjects (29).
Although many previous reports cite an increase in CRP and inflammatory cytokine concentration with age, others have suggested that acute phase protein and cytokine expression increases not as a function of aging but in response to age-associated disease or frailty (20,30–34). In a study of octogenarians by Brinkley and colleagues (20), there was no difference in serum CRP or IL-6 between 80- and 20-year-old individuals, provided the individuals were living independently . However, both markers were increased in 80-year-old individuals that required continuous care. Hand strength, a proxy for overall muscle strength and physical functionality, has been shown to be negatively associated with IL-6 and CRP concentration, independent of disease (32). IL-6 concentration has recently been shown to predict gait speed and gait speed decline in an aged population (33). Gait speed is an indicator of frailty and a known predictor of falls, dementia, and mortality (34). To differentiate age-related immune changes from immune alterations due to age-related disease, strict inclusion criteria, known as the SENIEUR protocol, have been developed for use in human studies (35). This protocol eliminates participants with any clinical or biochemical evidence of disease, unhealthy lifestyle, or use of medication. When the protocol is applied, only ∼10% of participants ≥ 65 years qualify for the study. In the current study, baboons were not evaluated for concurrent disease, so it is possible that age-associated diseases may have contributed to the increase in inflammation observed.
Many additional factors beyond disease and age can affect plasma cytokine concentration, including gender, body mass index, and lifestyle. The specific influence of gender on plasma cytokine concentration is unclear due to a lack of consistent findings among studies (27–29,32,36–38). This in part may reflect a difficulty isolating gender effects from other physiological effects and sociological influences. A limitation of the current study is the significant gender bias in the baboon population, with a greater proportion being female and the majority of males being young. In addition, data on body mass index, rank in social hierarchy, and phase in estrus cycle were not collected but may have contributed to inflammatory status.
Inflammatory markers have also been associated with a diet deficient in antioxidants. Inflammatory cytokine expression was reduced in rats fed a high antioxidant supplement (39,40), and serum CRP was reduced by 70% in baboons supplemented with vitamin E and coenzyme Q10 (41). The captive baboons of this study all received a commercial monkey diet (High Protein Monkey Diet 5045; Purina LabDiet, St Louis, MO) supplemented daily with fresh fruits and vegetables. It is possible that the baboons in this study showed relatively mild inflammatory changes compared with what has been reported in humans because their diet was healthier than the typical Western diet of humans.
To increase statistical strength, a population of aged baboons was identified for which archived serum samples collected 6–15 years earlier were available. Using paired samples from individual baboons, a marked increase in both serum IL-6 and CRP concentrations was observed when the animals were aged. The significant inter-animal variation in cytokine concentrations observed indicates that, whenever feasible, longitudinal rather than cross-sectional studies should be used to study inflammaging.
To assess the ability of aged baboons to respond to an inflammatory stimulus, cytokine response from mononuclear cells and whole blood were measured following LPS stimulation. In PBMC, cytokine response increased with age both at the RNA and protein level with the exception of TNF-α expression following LPS stimulation for which an age-related increase was only observed at the protein level. This may be a function of sample timing, as TNF-α expression is an earlier response to LPS stimulation than IL-6, and in vitro mRNA stability is likely transient, whereas proteins secreted into the media will persist.
Whole-blood cytokine response to LPS stimulation is considered to better represent an in vivo response than stimulation of isolated cells (42,43). In contrast, in vitro assays better detect changes in intracellular signaling pathways because cells are removed from the influence of the whole blood environment (43). Therefore, we elected to perform both in this study. The correlation between age and cytokine release in whole blood was significantly reduced (IL-6) or absent (TNF-α) compared with that observed in PBMC. These data suggest that in the aged baboon, mononuclear cells are proinflammatory and that factors present in the whole blood modify this exaggerated inflammatory response.
Factors in whole blood that might differentially influence the immune response in aged animals would include cellular or serum factors such as immunomodulatory hormones (eg, cortisol, DHEA-S) or cytokines (IL-10, IL-4 sIL-6R, sTNF-R, TGF-β). In rodents, basal concentrations of glucocorticoids increase with age (44–46). The effect of age on cortisol concentration in human and non-human primates is less well understood and has been reported to increase as well as decrease (47–49). Due to the episodic, pulsatile, and seasonal nature of cortisol secretion, interpretation of single point cortisol samples is difficult. Dehydroepiandrosterone and its precursor, dehydroepiandrosterone sulfate (DHEA-S), have clearly been shown to decrease with age in people, although data in baboons are limited (50). DHEA modulates immune function by enhancing IL-2 release and decreasing TNF-α and IL-6 release following immune stimulation (51–53). In the present study, cortisol and DHEA-S concentration was measured in the sera of animals used for whole-blood assays to determine if these might have influenced the response to LPS stimulation. Both DHEA-S and cortisol decreased with age, although cortisol failed to reach significance. Similar to previous studies in people, in baboons, DHEA-S was negatively correlated with TNF-α and IL-6 release following LPS stimulation (Figure 6), whereas cortisol was negatively correlated with IL-6 release only. TGF-β is another immune modulator that acts in a dose-, time-, and cell type–dependent manner to inhibit TNF-α and IL-1 β release (54). Alterations in TGF-β signaling have been identified in multiple age-related disease models (55–59). Although TNF-α release from PBMC is inhibited by treatment with TGF-β, TGF-β induces IL-6 release in a TNF-α–independent manner (55). We observed a similar response in the baboon whole-blood assay; TNF-α did not increase relative to TGF-β, whereas IL-6 did. Because both TNF-α and IL-6 increased in response to LPS treatment in the PBMC assay, this differential response is unlikely to be an effect of time or dose. Although the role of IL-10 in aging has not been extensively studied, several studies have suggested that aging is associated with an increase in IL-10 production capacity following LPS stimulation in humans (60), monkeys (61), and mice (62–64). In the current study, serum IL-10 concentration was not correlated with either age or cytokine release from whole blood.
In addition to the presence of serum factors, the cell populations differ in whole-blood and PBMC assays and may have contributed to the disparity in cytokine responses observed in the two assays. Similar to what occurs in people, baboons have been shown to have an age-related modulation in lymphocyte populations (14). Further studies are needed to determine the role of age-associated alterations of cell populations in inflammaging in baboons.
In summary, the baboon may provide an appropriate animal model to study inflammaging as similar, albeit milder inflammatory changes were observed as have been described in people. Whole blood and PBMC cytokine response to LPS stimulation in aged baboons is similar to that observed in adult baboons. Changes in serum hormones and cytokines, including an age-related decrease in DHEA-S and increase in TGF-β, may modulate innate response in aged baboons. Further studies in baboons may help characterize the pathological events that lead to inflammaging and identify potential targets for interventions.
FUNDING
This work was supported by grants from the National Institutes of Health/National Center for Research Resources: K01 RR023946 (D.M), R24 RR16556, and P40 RR12317 (G.L.W.).
References
- 1.Sansoni P, Vescovini R, Fagnoni F, et al. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Grubeck-Loebenstein B, Della BS, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009;21:201–209. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- 3.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy RC, Shearer MH, Hildebrand W. Nonhuman primate models to evaluate vaccine safety and immunogenicity. Vaccine. 1997;15:903–908. doi: 10.1016/s0264-410x(96)00277-0. [DOI] [PubMed] [Google Scholar]
- 6.Locher CP, Witt SA, Herndier BG, Tenner-Racz K, Racz P, Levy JA. Baboons as an animal model for human immunodeficiency virus pathogenesis and vaccine development. Immunol Rev. 2001;183:127–140. doi: 10.1034/j.1600-065x.2001.1830111.x. [DOI] [PubMed] [Google Scholar]
- 7.Murthy KK, Salas MT, Carey KD, Patterson JL. Baboon as a nonhuman primate model for vaccine studies. Vaccine. 2006;24:4622–4624. doi: 10.1016/j.vaccine.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui AA, Ahmad G, Damian RT, Kennedy RC. Experimental vaccines in animal models for schistosomiasis. Parasitol Res. 2008;102:825–833. doi: 10.1007/s00436-008-0887-6. [DOI] [PubMed] [Google Scholar]
- 9.Wolf RF, Papin JF, Hines-Boykin R, et al. Baboon model for West Nile virus infection and vaccine evaluation. Virology. 2006;355:44–51. doi: 10.1016/j.virol.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Shearer MH, Dark RD, Chodosh J, Kennedy RC. Comparison and characterization of immunoglobulin G subclasses among primate species. Clin Diagn Lab Immunol. 1999;6:953–958. doi: 10.1128/cdli.6.6.953-958.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attanasio R, Jayashankar L, Engleman CN, Scinicariello F. Baboon immunoglobulin constant region heavy chains: identification of four IGHG genes. Immunogenetics. 2002;54:556–561. doi: 10.1007/s00251-002-0505-1. [DOI] [PubMed] [Google Scholar]
- 12.Stacy S, Pasquali A, Sexton VL, Cantwell AM, Kraig E, Dube PH. An age-old paradigm challenged: old baboons generate vigorous humoral immune responses to LcrV, a plague antigen. J Immunol. 2008;181:109–115. doi: 10.4049/jimmunol.181.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attanasio R, Brasky KM, Robbins SH, Jayashankar L, Nash RJ, Butler TM. Age-related autoantibody production in a nonhuman primate model. Clin Exp Immunol. 2001;123:361–365. doi: 10.1046/j.1365-2249.2001.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayashankar L, Brasky KM, Ward JA, Attanasio R. Lymphocyte modulation in a baboon model of immunosenescence. Clin Diagn Lab Immunol. 2003;10:870–875. doi: 10.1128/CDLI.10.5.870-875.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabriel P, Cakman I, Rink L. Overproduction of monokines by leukocytes after stimulation with lipopolysaccharide in the elderly. Exp Gerontol. 2002;37:235–247. doi: 10.1016/s0531-5565(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 16.Leng SX, Yang H, Walston JD. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin Exp Res. 2004;16:249–252. doi: 10.1007/BF03327392. [DOI] [PubMed] [Google Scholar]
- 17.Pietschmann P, Gollob E, Brosch S, et al. The effect of age and gender on cytokine production by human peripheral blood mononuclear cells and markers of bone metabolism. Exp Gerontol. 2003;38:1119–1127. doi: 10.1016/s0531-5565(03)00189-x. [DOI] [PubMed] [Google Scholar]
- 18.Rees D, Miles EA, Banerjee T, et al. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr. 2006;83:331–342. doi: 10.1093/ajcn/83.2.331. [DOI] [PubMed] [Google Scholar]
- 19.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 20.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev. 2007;65:S253–S259. doi: 10.1111/j.1753-4887.2007.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 22.Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63:403–411. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 23.Elenkov IJ, Chrousos GP, Wilder RL. Neuroendocrine regulation of IL-12 and TNF-alpha/IL-10 balance. Clinical implications. Ann N Y Acad Sci. 2000;917:94–105. doi: 10.1111/j.1749-6632.2000.tb05374.x. [DOI] [PubMed] [Google Scholar]
- 24.Jerin A, Pozar-Lukanovic N, Sojar V, Stanisavljevic D, Paver-Erzen V, Osredkar J. Balance of pro- and anti-inflammatory cytokines in liver surgery. Clin Chem Lab Med. 2003;41:899–903. doi: 10.1515/CCLM.2003.136. [DOI] [PubMed] [Google Scholar]
- 25.Ng PC, Li K, Wong RP, et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Child Fetal Neonatal Ed. 2003;88:F209–F213. doi: 10.1136/fn.88.3.F209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wijsman CA, Maier AB, de Craen AJ, et al. An unopposed proinflammatory response is beneficial for survival in the oldest old. Results of the Leiden 85-plus Study. J Gerontol A Biol Sci Med Sci. 2011;66:393–399. doi: 10.1093/gerona/glq212. [DOI] [PubMed] [Google Scholar]
- 27.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 28.Reuben DB, Cheh AI, Harris TB, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50:638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- 29.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med. 2009;122:605–613. doi: 10.1016/j.amjmed.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16:153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 32.Tiainen K, Hurme M, Hervonen A, et al. Inflammatory markers and physical performance among nonagenarians. J Gerontol A Biol Sci Med Sci. 2010;65:658–663. doi: 10.1093/gerona/glq056. [DOI] [PubMed] [Google Scholar]
- 33.Verghese J, Holtzer R, Oh-Park M, et al. Inflammatory markers and gait speed decline in older adults. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr099. doi:10.1093/gerona/glr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verghese J, LeValley A, Hall CB, et al. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006;54:255–261. doi: 10.1111/j.1532-5415.2005.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ligthart GJ, Corberand JX, Fournier C, et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev. 1984;28:47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 36.Bruunsgaard H, Pedersen AN, Schroll M, et al. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clin Exp Immunol. 1999;118:235–241. doi: 10.1046/j.1365-2249.1999.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jha HC, Divya A, Prasad J, et al. Plasma circulatory markers in male and female patients with coronary artery disease. Heart Lung. 2010;39:296–303. doi: 10.1016/j.hrtlng.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Von Aulock S, Deininger S, Draing C, et al. Gender difference in cytokine secretion on immune stimulation with LPS and LTA. J Interferon Cytokine Res. 2006;26:887–892. doi: 10.1089/jir.2006.26.887. [DOI] [PubMed] [Google Scholar]
- 39.Gemma C, Mesches MH, Sepesi B, Choo K, Holmes DB, Bickford PC. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar beta-adrenergic function and increases in proinflammatory cytokines. J Neurosci. 2002;22:6114–6120. doi: 10.1523/JNEUROSCI.22-14-06114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novoselova EG, Lunin SM, Novoselova TV, et al. Naturally occurring antioxidant nutrients reduce inflammatory response in mice. Eur J Pharmacol. 2009;615:234–240. doi: 10.1016/j.ejphar.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Wang XL, Rainwater DL, Mahaney MC, Stocker R. Cosupplementation with vitamin E and coenzyme Q10 reduces circulating markers of inflammation in baboons. Am J Clin Nutr. 2004;80:649–655. doi: 10.1093/ajcn/80.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molina MAF, Gamboa EM, Tello PC, et al. Spontaneous inflammatory cytokine gene expression in normal human peripheral blood mononuclear cells. Lymphat Res Biol. 2006;4:34–40. doi: 10.1089/lrb.2006.4.34. [DOI] [PubMed] [Google Scholar]
- 43.Yaqoob P, Newsholme EA, Calder PC. Comparison of cytokine production in cultures of whole human blood and purified mononuclear cells. Cytokine. 1998;11(8):600e5. doi: 10.1006/cyto.1998.0471. [DOI] [PubMed] [Google Scholar]
- 44.DeKosky ST, Scheff SW, Cotman CW. Elevated corticosterone levels. A possible cause of reduced axon sprouting in aged animals. Neuroendocrinol. 1984;38:33–38. doi: 10.1159/000123862. [DOI] [PubMed] [Google Scholar]
- 45.Weinstein RS, Wan C, Liu Q, et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010;9:147–161. doi: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sapolsky RM, Krey LC, McEwen BS. The adrenocortical axis in the aged rat: impaired sensitivity to both fast and delayed feedback inhibition. Neurobiol Aging. 1986;7:331–335. doi: 10.1016/0197-4580(86)90159-4. [DOI] [PubMed] [Google Scholar]
- 47.Luz C, Dornelles F, Preissler T, Collaziol D, da Cruz IM, Bauer ME. Impact of psychological and endocrine factors on cytokine production of healthy elderly people. Mech Ageing Dev. 2003;124:887–895. doi: 10.1016/s0047-6374(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 48.Gust DA, Wilson ME, Stocker T, Conrad S, Plotsky PM, Gordon TP. Activity of the hypothalamic-pituitary-adrenal axis is altered by aging and exposure to social stress in female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:2556–2563. doi: 10.1210/jcem.85.7.6696. [DOI] [PubMed] [Google Scholar]
- 49.Moore RN, Schiller H, Bowden DM. Adrenocortical structure and function. In: Bowden DM, editor. Aging in Nonhuman Primates. New York, NY: Van Nostrand Reinhold Company; 1979. pp. 229–247. [Google Scholar]
- 50.Ferrari E, Cravello L, Falvo F, et al. Neuroendocrine features in extreme longevity. Exp Gerontol. 2008;43:88–94. doi: 10.1016/j.exger.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Goncharova ND, Lapin BA. Changes of hormonal function of the adrenal and gonadal glands in baboons of different age groups. J Med Primatol. 2000;29:26–35. doi: 10.1034/j.1600-0684.2000.290104.x. [DOI] [PubMed] [Google Scholar]
- 52.Hazeldine J, Arlt W, Lord JM. Dehydroepiandrosterone as a regulator of immune cell function. J Steroid Biochem Mol Biol. 2010;120:127–136. doi: 10.1016/j.jsbmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Schmitz D, Kobbe P, Wegner A, Hammes F, Oberbeck R. Dehydroepiandrosterone during sepsis: does the timing of administration influence the effectiveness. J Surg Res. 2010;163:e73–e77. doi: 10.1016/j.jss.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 54.Turner M, Chantry D, Feldmann M. Transforming growth factor beta induces the production of interleukin 6 by human peripheral blood mononuclear cells. Cytokine. 1990;2:211–216. doi: 10.1016/1043-4666(90)90018-o. [DOI] [PubMed] [Google Scholar]
- 55.van der Kraan PM, Goumans MJ, Blaney DE, et al. Age-dependent alteration of TGF-beta signalling in osteoarthritis. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1194-6. doi:10.1007/s00441-011-1194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang M, Spinetti G, Monticone RE, et al. A local proinflammatory signalling loop facilitates adverse age-associated arterial remodeling. PLoS One. 2011;6:e16653. doi: 10.1371/journal.pone.0016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doyle KP, Cekanaviciute E, Mamer LE, et al. TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation. 2010;7:62. doi: 10.1186/1742-2094-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barron DA, Strand DW, Ressler SJ, et al. TGF-beta1 induces an age-dependent inflammation of nerve ganglia and fibroplasia in the prostate gland stroma of a novel transgenic mouse. PLoS One. 2010;5:e13751. doi: 10.1371/journal.pone.0013751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pachowka M, Makula J, Korczak-Kowalska G. Diminished cytokines gene expression in lymphoid organs of healthy aged rats. Cytokine. 2011;54:24–28. doi: 10.1016/j.cyto.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 61.Mascarucci P, Taub D, Saccani S, et al. Cytokine responses in young and old rhesus monkeys: effect of caloric restriction. J Interferon Cytokine Res. 2002;22:565–571. doi: 10.1089/10799900252982043. [DOI] [PubMed] [Google Scholar]
- 62.Tateda K, Matsumoto T, Miyazaki S, et al. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64:769–774. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sierra A, Gottfried-Blackmore AC, McEwen BS, et al. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 64.Ebert EC. Endogenous inhibitory cytokines repress TNF alpha secretion. Cell Immunol. 2005;237:106–114. doi: 10.1016/j.cellimm.2005.10.003. [DOI] [PubMed] [Google Scholar]