Abstract
Aging is generally associated with a decline in several indices of cardiac function. The cellular mechanisms for this decline are not completely understood. The ability of the myocardium to perform external work (power output) is a critical aspect of ventricular function. The purpose of this study was to determine the effect of aging on loaded shortening and power output properties. We measured force–velocity properties in permeabilized (skinned) myocytes from the hearts of 9-, 24-, and 33-month-old male Fisher 344 × Brown Norway F1 hybrid rats (F344BN) during loaded contractions using a force-clamp technique. Power output was calculated by multiplying force and shortening velocity values. We found that peak power output normalized to maximal force was significantly decreased by 18% and 31% in myocytes from 24- and 33-month-old group, respectively, compared with 9-month group (p < .05). These results suggest that aging is associated with a significant decrease in the ability of the myocardium to do work.
Keywords: Aging, Power output, Loaded shortening velocity, Skinned cardiac myocytes, Myocardial function
IT is generally accepted that with age the normal mammalian heart undergoes numerous structural and functional changes (1–3). At the whole heart level, aging is associated with a decrease in ejection fraction, fractional shortening, and velocity of circumferential shortening (3–5). In vivo contractile measurements, such as working heart preparations (6,7), hemodynamic analyses (5,8), and pressure–volume measurements (9), have also demonstrated that aging decreases peak left ventricular pressure and the rate of pressure generation (+dP/dt).
Age-related decreases in cardiac function may have two separate but related mechanisms: (a) changes in myocardial structure related to the loss of functional myocytes or (b) a change in functional properties of the remaining myocytes (7,10). Numerous studies have demonstrated age-associated decreases in myocyte number as a result of apoptosis, necrosis, or both (11). Because a heart is composed of nondividing cardiac myocytes with very little potential to regenerate, even a very low level of cell death could alter the contractile properties of the myocardium. In response to this loss of functional myocytes, areas of interstitial fibrosis in the myocardium have been shown to increase with age, and this change in the composition of the ventricular wall potentially increases ventricular stiffness and increases myocardial dysfunction (2,5,7,8,10).
In addition to this age-related loss of myocytes described earlier, the surviving myocytes undergo a number of alterations in their contractile properties, and these changes in contractile properties of remaining myocytes also contribute to ventricular dysfunction associated with aging. Age-associated changes in contractile properties of cardiac muscle preparations include increases in the time to peak tension development, the half-relaxation time, and decreases in the rate of tension generation (+dP/dt) and tension decay (−dP/dt) in papillary muscle preparations (12–14). In addition, a number of studies have demonstrated that aging decreases maximal unloaded shortening velocity in papillary muscles (12), intact myocytes (15), and skinned cardiac myocytes (6).
Nearly all studies that have addressed changes in myocardial contractile properties with aging have focused on the properties of isometric tension or unloaded shortening. Under these conditions, however, the work capacity of heart, expressed as power output (work/time), is zero. The functional work capacity of the myocardium is dictated by both the pressure generating capacity in the ventricle (which is related to maximal isometric force) and the properties of myocardial shortening against a load (loaded shortening velocity). Thus, investigation of the properties of loaded shortening and power output is fundamental to our understanding of the ability of heart to move the blood throughout the circulation. However, age-related changes in these contractile properties have not been previously studied. Our purpose in this study was to determine the effect of aging on loaded shortening velocity and power output properties in permeabilized cardiac myocytes. We hypothesized that aging would decrease loaded shortening velocity and thus decrease the power output in skinned cardiac myocytes.
METHODS
Animals
Male Fisher 344 × Brown Norway F1 hybrid rats (F344BN) of 9 (n = 6), 24 (n = 6), and 33 months (n = 6) were obtained from the National Institute on Aging colony maintained by Harlan Sprague–Dawley (Indianapolis, IN). Animals were selected to represent young adults (9 months), middle age (24 months), and senescent (33 months ≈50% mortality) rats. F344BN was chosen because they have been shown to have a low incidence of age-related common pathologies and a significantly longer life span than other rat strains, such as Wistar and Fisher 344 rats (16–19). Rats were individually housed in clear plastic cages on a 12-h/12-h light/dark cycle with access to food and water ad libitum. To reduce the stress associated with shipping and acclimation to a new place, experiments were begun 1 week after rats’ arrival. Handling and euthanasia were carried out under the guidelines of University of Wisconsin—Madison Animal Use and Care Committee. Rats were anesthetized by inhalation of Halothane, and the hearts were quickly excised, weighed, and the left ventricle was cut into several pieces. The pieces were quick frozen and stored at −80°C for subsequent analysis.
Cardiac Myocyte Preparation, Force–Velocity, and Force–Power Measurement
The experimental apparatus used for contractile measurements on single myocyte-sized preparations have been described previously (20). Briefly, single myocyte-sized preparations were obtained by mechanical disruption of frozen ventricular tissue, and the myocytes were permeabilized with Triton X-100. Permeabilized (skinned) cardiac myocyte preparations were attached between a capacitance-gauge transducer (model 403; Aurora Scientific) and a DC torque motor (model 308; Aurora Scientific) by placing the ends of the preparation into stainless steel troughs. The ends were then secured to the troughs by overlaying a ∼0.5 mm length of 4–0 monofilament suture over each end and then tying the suture to the trough using a loop of 10–0 monofilament suture. The temperature of the experimental chamber was maintained to 15 ± 1°C using a Peltier device (Cambion, Cambridge, MA) connected to a water bath. The entire mechanical apparatus was mounted on a pneumatic vibration isolation table with a cut-off frequency of 1 Hz. The length of the preparation was adjusted so that sarcomere length was set to 2.3 μm in relaxing solution, and sarcomere length was monitored using video microscopy throughout the experiment to determine that it did not change significantly during activation.
The solutions and protocol for force–velocity and power–load measurements have been previously described (21). Briefly, the shortening velocity of skinned myocytes was determined at varied loads. The myocytes were transferred into activating solution (pCa 4.5), and steady tension was allowed to develop. The computer then switched the motor from length control mode to force control mode by applying a 5 V logic pulse. The myocytes were rapidly stepped to a predetermined force (expressed as a fraction of that cell’s maximal isometric force), which was maintained for 250 ms while changes in myocyte length were monitored. Following this force clamp, the myocytes were slackened to reduce the force to zero to allow measurement of the relative force during the isotonic shortening period. Measurements at several different force levels were carried out on each myocyte (about 8–10 force clamps). Force was expressed normalized to the peak force generated by that cell during a given activation (P/Po). If the maximal force declined by below 80% during the experimental protocol, that cell was discarded and the data were not used.
Analysis of Myosin Heavy Chain Isoform Contents
The relative amount of myosin heavy chain (MHC) isoform of ventricular homogenates was determined with sodium dodecyl sulfate–polyacrylamide gel electrophoresis technique using N-N′-Diallyltartardiamide (DATD) as described previously (22).
Data Analysis and Statistical Analysis
Length traces, force–velocity curves, and force–power curves were analyzed as previously described (21). Briefly, force and velocity data were fitted to the Hill equation (23):
where P is force at velocity V, Po is the peak isometric force, and a and b are constants with dimensions of force and velocity, respectively. The maximum shortening velocity was determined from the y-intercept of the best-fit line, extrapolating a force–velocity curve based on raw data. The Force–power curve was then constructed as the product of force value and velocity value at each load on the force–velocity curve. The force at which power output is optimal (Fopt) was obtained by using the equation (24)
Once Fopt was determined for each cell, we calculated the velocity at that force and used that value as Vopt (the velocity at which power is optimal).
Data were fit to equations using commercial software (SigmaPlot; Jandel Scientific). Peak power output in each myocyte was taken from the highest power value on the fitted line. Power output in single myocyte was normalized to cell mass using the following formula based on an elongated elliptical shape of the myocytes: muscle mass = volume (V) × density (D); V = L × W2/2667 pl/μm3, where V is in picoliter; L (length) and W (width) are in μm; D = 1.065 (25). Power output was also normalized for maximal force, and this value (P/Po × ML/sec) is given in Table 2.
Table 1.
Effect of Aging on Physical Characteristics F344BN Rats
| 9 Months (n = 6) | 24 Months (n = 6) | 33 Months (n = 6) | |
| Body weight (g) | 409.3 ± 41.7 | 575.2 ± 33.1* | 581.9 ± 50.6* |
| Ventricular weight (g) | 0.95 ± 0.11 | 1.32 ± 0.12* | 1.42 ± 0.17* |
| Tibia length (mm) | 41.3 ± 0.76 | 43.4 ± 0.38* | 46.2 ± 0.43* |
| Heart weight/body weight × 1,000 | 2.32 ± 0.03 | 2.34 ± 0.23 | 2.44 ± 0.33 |
| Heart weight/tibia length × 1,000 | 23.00 ± 2.66 | 30.41 ± 2.08* | 30.74 ± 3.68* |
Notes: Values are means ± SD. n = number of animals.
*Significantly different from 9 months at p < .05.
All results were expressed as means ± SD. Statistical significance was tested with analysis of variance followed by Fisher’s least significant difference for multiple group comparisons. The p < .05 was regarded as significant among groups.
RESULTS
Significantly increases in body weight (BW), heart weight (HW), tibial length (TL), and HW normalized to TL (HW/TL × 1,000) were found in both 24- and 33-month-old rats compared with 9-month-old rats. However, no effect of aging was observed in HW normalized to BW (HW/BW × 1,000) in any group (see Table 1). These results support the idea that body weight measures, as a method of assessing myocardial hypertrophy, may be unreliable because body weight appears to be quite variable in response to perturbations such as exercise, hormonal treatment, or aging (26).
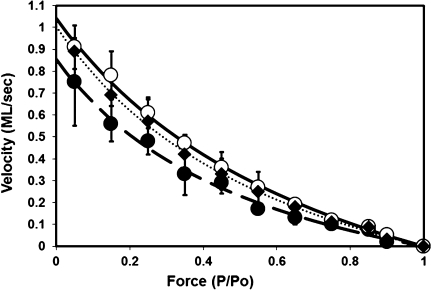
We characterized force–velocity and force–power relationship in skinned cardiac myocytes from the hearts of 9-, 24-, and 33-month-old rats in two ways as shown in Table 2 and Figures 1 and 2. First, the data for each cell were fitted to the Hill equation as described in Methods. This analysis resulted in a value for Vmax (maximal unloaded shortening velocity) and a value for a/Po (a measure of the curvature of the F–V relationship). The force–velocity values (both measured values and the fitted line to the Hill equation) were converted to power output values by multiplying force and velocity. The maximum power value from the fitted line was taken as peak power output for each cell. We then calculated values for peak power output (normalized for cell mass), peak power output (normalized for maximal force), Fopt (the relative force at which peak power was reached), and Vopt (the relative velocity at which peak power was reached). All of these values were then summed for each cell from the three age groups. These data are presented in Table 2 along with maximal isometric tension data. Compared with that seen at 9 months, peak power output (normalized to maximal force) was decreased by 18% and 31% in myocytes from the 24- and 33-month-old rats, respectively (p < .05; Table 2). However, differences in maximal force (kN/m2) and Vmax (ML/s, where ML is muscle length at a sarcomere length [SL] of 2.3 μm) were not statistically significant among any group (Table 2).
Table 2.
Mechanical Properties of Myocytes Isolated From 9-, 24-, and 33-Months-Old Rats
| Maximal Force (kN/m2) | Vmax (ML/s) | a/Po | Peak Power Output (μW/mg) | Peak Power Output (normalized to max force; P/Po × ML/s) | Fopt(P/Po) | Vopt (V/Vmax) | |
| 9 months (n = 60) | 13.87 ± 9.14 | 0.87 ± 0.21 | 0.36 ± 0.10 | 2.69 ± 1.42 | 0.085 ± 0.025 | 0.31 ± 0.08 | 0.22 ± 0.05 |
| 24 months (n = 49) | 14.53 ± 10.26 | 0.78 ± 0.30 | 0.24 ± 0.08* | 2.49 ± 2.16 | 0.070 ± 0.022* | 0.29 ± 0.06 | 0.14 ± 0.04* |
| 33 months (n = 55) | 14.84 ± 11.27 | 0.73 ± 0.39 | 0.19 ± 0.15* | 2.71 ± 1.83 | 0.059 ± 0.012*,† | 0.29 ± 0.07 | 0.13 ± 0.04* |
Notes: Values are means ± SD. n = number of myocytes; ML= muscle length (measured after attachment); a/Po = measure of the curvature of force–velocity relationship with lower values indicating a greater curve; Peak power output was the highest power determined from best-fit line; P/Po = force relative to maximum isometric force; Fopt = relative force at which power output was optimal, Vopt = relative velocity at which power output was optimal.
*Significantly different from 9 months at p < .05.
†Significantly different from 24 months.
Figure 1.
Composite force–velocity curves for myocytes from different aged rats. Data were compiled from 60, 49, and 55 myocytes for 9-, 24-, and 33-month-old rats, respectively. Isotonic shortening velocity values at each load were averaged from all myocytes in each group. Data points are presented as mean ± SD. • and dashed line = 33 months old; ♦ and dotted line = 24 months old; ○ and solid line = 9 months old. Lines are the best-fit regression line using the Hill equation as described in Methods.
Figure 2.
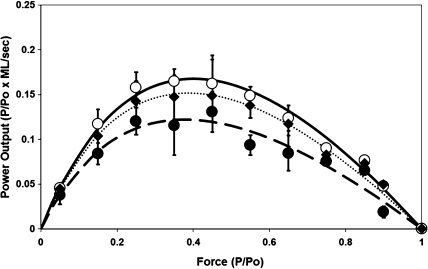
Force–power curve constructed from force–velocity data. In each myocyte at each load, force values (expressed as P/Po) were multiplied times mean velocity values (expressed ML/s) to result in a value of power output for that load. Data points are means ± SD for all cells at that age. • and dashed line = 33 months old; ♦ and dotted line = 24 months old; ○ and solid line = 9 months old. Lines are the best-fit regression line using the Hill equation as in Methods. Peak power output was taken from the highest point in the best-fit line.
To give a measure of the cell-to-cell variability for force–velocity and force–power relationship, the data was analyzed as shown in Figures 1 and 2. Figure 1 presents composite force–velocity curves for myocytes from the three age groups with the mean ± SD velocity values at each relative force value. This mean force and velocity data were then fitted using the Hill equation (shown by solid, dashed, and dotted lines) resulting in cumulative Vmax and a/Po. This analysis yielded estimated Vmax values of 1.03, 0.99, and 0.85 ML/s for 9-, 24-, and 33-month-old myocytes, respectively. A force–power curve (see Figure 2) was then constructed by multiplying for each cell the velocity by the force values for each force clamp. The resulting power output values for a predetermined range of forces were then summed for all myocytes in a given age group, and Figure 2 shows the resulting mean ± SD values for normalized power output plotted against the relative force values for these.
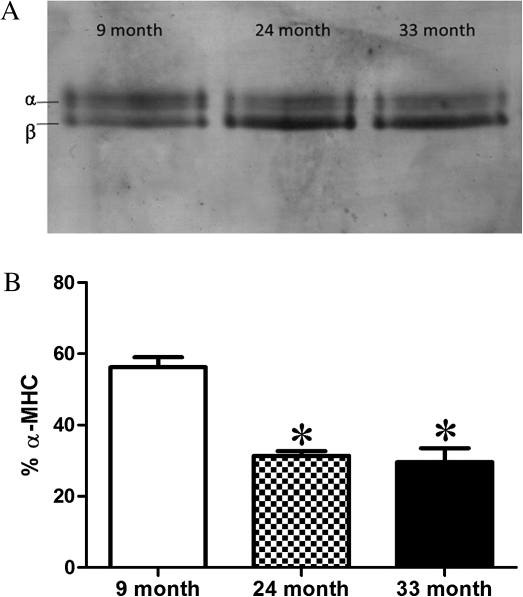
Figure 3a shows a representative gel from the sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis of MHC isoforms in ventricular homogenates from 9-, 24-, and 33-month-old F344BN rats. Figure 3b shows the summary of the MHC results, in bar graph form, from n = 6 animals per age group. We found a significant shift in MHC isoform from α-MHC to β-MHC with aging. The percent α-MHC content was significantly decreased in both 24- and 33-month-old groups compared with 9-month-old group (p < .05).
Figure 3.
(a) Representative 6% sodium dodecyl sulfate–polyacrylamide gel electrophoresis showing the distribution of myosin heavy chain (MHC) isoforms in ventricular homogenates from 9-, 24-, and 33-month-old rats. We found significant differences in MHC isoform content in the 24- and 33-month-old rats compared with the 9-month-old rats. (b) Bar graph representation of the MHC isoform distribution in heart homogenates from 9-, 24-, and 33-month-old rats. Values are expressed as the % of total MHC that is αMHC. *Significantly different (p < .05) from 9 month.
DISCUSSION
The primary conclusion from this study was that peak power output normalized to maximal force (P/Po × ML/s) was decreased significantly (18% and 31%, respectively) in 24- and 33-month-old myocytes compared with 9-month-old myocytes (Table 2). This is the first direct study of the effect of aging on properties of force–velocity and power output in skinned myocytes, and these results provide evidence that aging results in a decrease in the capacity of the myocardium to perform external work that is related to alterations in the functional properties of remaining myocytes.
Aging has been associated with decreased ventricular function during multiple phases of the cardiac cycle (9). The loss of functional myocytes and the subsequent increase in fibrosis in the myocardium (2,5,7,8,10) contributes to increased ventricular stiffness with age. This decrease in compliance (7) affects left ventricular end-diastolic pressure (7), isovolumic relaxation time (5), and filling in early diastole (1). Although the diastolic properties of aging heart have been extensively studied, the effects of aging on the ejection phase of the cardiac cycle, in which the myocytes shorten against a load in order to eject blood from the ventricle, have not been adequately explored at the cellular level. Age-associated decreases in ejection fraction or fractional shortening (3–5) have been demonstrated in whole heart preparations, but the cellular mechanism(s) for this effect have not been determined. Unloaded shortening velocity has been shown to be decreased with aging in papillary muscles (12), intact myocytes (15), and skinned cardiac myocytes (6), but the properties that regulate unloaded shortening and loaded shortening may be different and thus respond differently to aging.
Unloaded shortening velocity is thought to be limited primarily by the rate of ATP hydrolysis by myosin which then determines the rate that cross bridges can cycle. Isometric tension development in the myocardium is determined by the number of active cross bridges and so is affected by the absolute number of cross bridges as well as by the extent of activation of the myocytes. Loaded shortening and hence power and work output are governed by some combination of these two parameters (ie, number of active cross bridges and rate of cross-bridge cycling).
In the current study, the decrease in peak power output with age was not accompanied by a change in maximal force in skinned cardiac myocytes. This result is consistent with data reported from measurements in skinned left ventricular trabeculae (27,28), intact papillary muscles (12,29), and skinned myocytes (6). Maximal unloaded shortening velocity (Vmax) has been previously shown to be decreased significantly with aging (6,12), but we did not observe a significant change in Vmax in the present study. The age-related shift in MHC isoform content from α-MHC to β-MHC that we observed would certainly imply that we should have seen a decrease in Vmax in the older myocytes, so we suggest that our lack of significant effect is likely due to variability in our measurements. Our Vmax values are obtained by extrapolation from loaded shortening values and thus are greatly affected by small internal load and measurement errors at low forces (30). Previous reports indicating an age-related decrease in unloaded shortening velocity measured Vmax more directly using the slack test (6,12).
In our present study, we described age-related effects on properties of loaded shortening, irrespective of effects on unloaded shortening velocity. We saw a significant age-related decreases in both a/Po (a measure of the curvature of the force–velocity relationship) as well as Vopt (the shortening velocity at which peak power output is reached). Both a/Po and Vopt decreased in an age-related way. The decrease in a/Po suggested an age-associated increase in the curvature of the force–velocity relationship, whereas the decrease in Vopt indicated that, with age, the shortening velocity at which power is maximal decreases. Striated muscle is hypothesized to operate at shortening velocities near Vopt in vivo, and thus, this decrease in Vopt may have implications for ventricular function in the intact heart. Both a/Po and Vopt have previously been shown to be highly influenced by the MHC isoform expression in skeletal muscle (24) and, in particular, by the level of β-MHC isoform in cardiac myocytes (31), which is consistent with our result regarding MHC shifts (discussed below).
As a result of these decreases in loaded shortening velocity at intermediate forces, aging significantly decreased peak power output in single myocytes (Figure 2), and this decline in power output has significant implications for the work output of the myocardium. Although peak power output normalized to maximal force (P/Po × ML/s) decreased in the myocytes from both 24-month-old and 33-month-old animals, we did not observe a difference in peak power output when normalized to mass (Table 2). This is likely due to variability in our estimates of muscle mass in our samples. Estimation of single myocyte size and/or mass is difficult due to the irregular shape of the myocyte preparations. We approximated myocyte mass values using a formula described in Methods, which is based on the elongated elliptical shape of rat myocytes, but this method is subject to error in the estimation of myocyte mass, and this error leads to significant variability in these values. The peak power output normalized to maximal force (P/Po × ML/s), in which variations in force were accounted for by normalizing to maximal force (Po) in that cell and variations in velocity were accounted for by normalizing to muscle length (ML) in that cell, gives a more sensitive indication of age-related changes in peak power output.
The findings of the present study indicated that, while the age-related loss of myocytes may have significant consequences to ventricular performance, age-related changes in the contractile properties of the surviving myocytes are also important in determining myocardial function. Surviving myocytes in aging myocardium have been shown to have altered Ca2+ handling properties (32–34) as well as altered mitochondrial function (35), and these cellular changes likely affect the tension-generating capabilities of the myocardium. Related to our findings of decreased power output in single myocytes, there is also an age-related change in contractile protein isoform expression in the myocardium. It is generally recognized that, in rodents, aging shifts MHC isoform from α-MHC to β-MHC (4,6,15,27). β-MHC has a lower myosin ATPase activity than the α-MHC form and thus changes in MHC isoform content are likely to lead to alterations in cross-bridge cycling rate and subsequent altered contractile properties. Previous studies have demonstrated that changes in MHC isoform expression in the myocardium are closely related to changes in contractile properties (29,36,37) including maximal shortening velocity, loaded shortening velocity, and peak power output (38,39). In agreement with previous findings, our result indicated that β-MHC isoform content relative to the total MHC increased in 24- and 33-month-old groups compared with 9-month-old group. However, the transition from α- to β-MHC begins to occur at relatively young age and increases continually through a senescence (6,27). Our results are consistent with these earlier findings, as, in our study, β-MHC isoform content increased significantly between 9 and 24 months but did not increase significantly thereafter (ie, 33 months). Thus, our study suggests that decreased power output in myocytes from the old age groups (24 and 33 months) may be partially due to the age-related increase in β-MHC isoform content, but this increase in β-MHC isoform content cannot solely be the mechanism responsible for the decrease in power output with aging because we did not find any significant differences in MHC composition between 24- and 33-month-old groups, but power output was further decreased in 33-month-old group compared with 24-month-old group (Figure 2). More work remains to determine other possible age-related changes in contractile proteins that may explain some of the decrease in myocytes power output.
It has been suggested that the accumulation of advanced glycation end products may account for some of the age-associated cardiac contractile dysfunction (40,41). Previous studies have demonstrated that the levels of myosin glycation in cardiac muscle are increased with aging (41) and may account for the decreased myofibrillar ATPase activity (42), although no data regarding the effects of increased glycation of myosin on force–velocity or power output properties have been obtained. When myosin from skeletal muscle was incubated with glucose, the speed of actin filament sliding was significantly decreased over time (43), indicating a potential effect of glycation on shortening velocity. Thus, combination of age-associated increase in β-MHC expression and glycation of myosin may contribute to the decreased contractile properties with age in cardiac myocytes. Further investigation is needed to fully understand the mechanisms underlying the decrease in power output in 33-month-old group compared with 24-month-old group.
In summary, we have demonstrated for the first time that power output properties of rat cardiac myocytes are decreased by aging. These alterations are characterized by a decrease in normalized peak power output and indicate that altered contractile properties of remaining myocytes can contribute to age-associated decreases in cardiac function. This decline in work capacity of myocytes in 33 months is likely, in part, due to a shift in the MHC isoform content, from α-MHC to β-MHC.
FUNDING
This work was supported by the NIH (AG030423).
References
- 1.Brenner DA, Apstein CS, Saupe KW. Exercise training attenuates age-associated diastolic dysfunction in rats. Circulation. 2001;104(2):221–226. doi: 10.1161/01.cir.104.2.221. [DOI] [PubMed] [Google Scholar]
- 2.Wanagat J, Wolff MR, Aiken JM. Age-associated changes in function, structure and mitochondrial genetic and enzymatic abnormalities in the fisher 344 x brown Norway F1 hybrid rat heart. J Mol Cell Cardiol. 2002;34:17–28. doi: 10.1006/jmcc.2001.1483. [DOI] [PubMed] [Google Scholar]
- 3.Boluyt MO, Converso K, Hwang HS, Mikkor A, Russell MW. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J Appl Physiol. 2004;96(2):822–828. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- 4.Iemitsu M, Miyauchi T, Maeda S, et al. Exercise training improves cardiac function-related gene levels through thyroid hormone receptor signaling in aged rats. Am J Physiol Heart Circ Physiol. 2004;286(5):H1696–H1705. doi: 10.1152/ajpheart.00761.2003. [DOI] [PubMed] [Google Scholar]
- 5.Hacker TA, McKiernan SH, Douglas PS, Wanagat J, Aiken JM. Age-related changes in cardiac structure, function, and morphology in Fischer 344 x Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol. 2005;290(1):H304–H311. doi: 10.1152/ajpheart.00290.2005. [DOI] [PubMed] [Google Scholar]
- 6.Fitzsimons DP, Patel JR, Moss RL. Aging-dependent depression in the kinetics of force development in rat skinned myocardium. Am J Physiol Heart Circ Physiol. 1999;276(5):H1511–H1519. doi: 10.1152/ajpheart.1999.276.5.H1511. [DOI] [PubMed] [Google Scholar]
- 7.Capasso JM, Palackal T, Olivetti G, Anversa P. Severe myocardial dysfunction induced by ventricular remodeling in aging rat hearts. Am J Physiol Heart Circ Physiol. 1990;259(4):H1086–H1096. doi: 10.1152/ajpheart.1990.259.4.H1086. [DOI] [PubMed] [Google Scholar]
- 8.Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67(4):871–885. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- 9.Pacher P, Mabley JG, Liaudet L, et al. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol. 2004;287(5):H2132–H2137. doi: 10.1152/ajpheart.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anversa P, Puntillo E, Nikitin P, Olivetti G, Capasso JM, Sonnenblick EH. Effects of age on mechanical and structural properties of myocardium of Fischer 344 rats. Am J Physiol Heart Circ Physiol. 1989;256(5):H1440–H1449. doi: 10.1152/ajpheart.1989.256.5.H1440. [DOI] [PubMed] [Google Scholar]
- 11.Kwak H-B, Song W, Lawler JM. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J. 2006;20(6):791–793. doi: 10.1096/fj.05-5116fje. [DOI] [PubMed] [Google Scholar]
- 12.Capasso JM, Malhotra A, Remily RM, Scheuer J, Sonnenblick EH. Effects of age on mechanical and electrical performance of rat myocardium. Am J Physiol Heart Circ Physiol. 1983;245(1):H72–H81. doi: 10.1152/ajpheart.1983.245.1.H72. [DOI] [PubMed] [Google Scholar]
- 13.Taffet GE, Michael LA, Tate CA. Exercise training improves lusitropy by isoproterenol in papillary muscles from aged rats. J Appl Physiol. 1996;81(4):1488–1494. doi: 10.1152/jappl.1996.81.4.1488. [DOI] [PubMed] [Google Scholar]
- 14.Tate CA, Taffet GE, Hudson EK, Blaylock SL, McBride RP, Michael LH. Enhanced calcium uptake of cardiac sarcoplasmic reticulum in exercise-trained old rats. Am J Physiol Heart Circ Physiol. 1990;258(2):H431–H435. doi: 10.1152/ajpheart.1990.258.2.H431. [DOI] [PubMed] [Google Scholar]
- 15.Wahr PA, Michele DE, Metzger JM. Effects of aging on single cardiac myocyte function in Fischer 344 x Brown Norway rats. Am J Physiol Heart Circ Physiol. 2000;279(2):H559–H565. doi: 10.1152/ajpheart.2000.279.2.H559. [DOI] [PubMed] [Google Scholar]
- 16.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 17.Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of Brown Norway, Brown Norway x Fischer 344, and Fischer 344 x Brown Norway rats with relation to age. J Gerontol. 1996;51A:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprott RL. Mouse and rat genotype choices. Exp Gerontol. 1997;32(1–2):79–86. doi: 10.1016/s0531-5565(96)00035-6. [DOI] [PubMed] [Google Scholar]
- 19.Sprott RL. Diet and calorie restriction. Exp Gerontol. 1997;32(1–2):205–214. doi: 10.1016/s0531-5565(96)00065-4. [DOI] [PubMed] [Google Scholar]
- 20.Diffee GM, Seversen EA, Titus MM. Exercise training increases the Ca2+ sensitivity of tension in rat cardiac myocytes. J Appl Physiol. 2001;91:309–315. doi: 10.1152/jappl.2001.91.1.309. [DOI] [PubMed] [Google Scholar]
- 21.Diffee GM, Chung E. Altered single cell force-velocity and power properties in exercise-trained rat myocardium. J Appl Physiol. 2003;94(5):1941–1948. doi: 10.1152/japplphysiol.00889.2002. [DOI] [PubMed] [Google Scholar]
- 22.Warren CM, Greaser ML. Method for cardiac myosin heavy chain separation by sodium dodecyl sulfate gel electrophoresis. Anal Biochem. 2003;320(1):149–151. doi: 10.1016/s0003-2697(03)00350-6. [DOI] [PubMed] [Google Scholar]
- 23.Hill AV. The heat of shortening and the dynamic constants of muscle. Proc Roy Soc B. 1938;126:136–195. [Google Scholar]
- 24.Woledge RC, Curtin NA, Homsher E. London: Academic Press; 1985. Energetic Aspects of Muscle Contraction; pp. 47–71. [PubMed] [Google Scholar]
- 25.Natali AJ, Wilson LA, Peckham M, Turner DL, Harrison SM, White E. Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes. J Physiol (Lond) 2002;541(3):863–875. doi: 10.1113/jphysiol.2001.013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol Heart Circ Physiol. 1982;243(6):H941–H947. doi: 10.1152/ajpheart.1982.243.6.H941. [DOI] [PubMed] [Google Scholar]
- 27.Carnes CA, Geisbuhler TP, Reiser PJ. Age-dependent changes in contraction and regional myocardial myosin heavy chain isoform expression in rats. J Appl Physiol. 2004;97(1):446–453. doi: 10.1152/japplphysiol.00439.2003. [DOI] [PubMed] [Google Scholar]
- 28.Lakatta EG, Yin FC. Myocardial aging: functional alterations and related cellular mechanisms. Am J Physiol Heart Circ Physiol. 1982;242(6):H927–H941. doi: 10.1152/ajpheart.1982.242.6.H927. [DOI] [PubMed] [Google Scholar]
- 29.Wei JY, Spurgeon HA, Lakatta EG. Excitation-contraction in rat myocardium: alterations with adult aging. Am J Physiol Heart Circ Physiol. 1984;246(6):H784–H791. doi: 10.1152/ajpheart.1984.246.6.H784. [DOI] [PubMed] [Google Scholar]
- 30.Chiu YC, Ballou EW, Ford LE. Force, velocity, and power changes during normal and potentiated contractions of cat papillary muscle. Circ Res. 1987;60(3):446–458. doi: 10.1161/01.res.60.3.446. [DOI] [PubMed] [Google Scholar]
- 31.Korte FS, Herron TJ, Rovetto MJ, McDonald KS. Power output is linearly related to MyHC content in rat skinned myocytes and isolated working hearts. Am J Physiol Heart Circ Physiol. 2005;289(2):H801–H812. doi: 10.1152/ajpheart.01227.2004. [DOI] [PubMed] [Google Scholar]
- 32.Lim CC, Apstein CS, Colucci WS, Liao R. Impaired cell shortening and relengthening with increased pacing frequency are intrinsic to the senescent mouse cardiomyocyte. J Mol Cell Cardiol. 2000;32(11):2075–2082. doi: 10.1006/jmcc.2000.1239. [DOI] [PubMed] [Google Scholar]
- 33.Howlett SE, Grandy SA, Ferrier GR. Calcium spark properties in ventricular myocytes are altered in aged mice. Am J Physiol. 2006;290(4):H1566–H1574. doi: 10.1152/ajpheart.00686.2005. [DOI] [PubMed] [Google Scholar]
- 34.Isenberg G, Borschke B, Rueckschloss U. Ca2+ transients of cardiomyocytes from senescent mice peak late and decay slowly. Cell Calcium. 2003;34(3):271–280. doi: 10.1016/s0143-4160(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 35.Ljubicic V, Menzies KJ, Hood DA. Mitochondrial dysfunction is associated with a pro-apoptotic cellular environment in senescent cardiac muscle. Mech Ageing Dev. 2010;131(2):79–88. doi: 10.1016/j.mad.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Yin FCP, Spurgeon HA, Weisfeldt ML, Lakatta EG. Mechanical properties of myocardium from hypertrophied rat hearts. A comparison between hypertrophy induced by senescence and by aortic banding. Circ Res. 1980;46(2):292–300. doi: 10.1161/01.res.46.2.292. [DOI] [PubMed] [Google Scholar]
- 37.Eisenberg BR, Edwards JA, Zak R. Transmural distribution of isomyosin in rabbit ventricle during maturation examined by immunofluorescence and staining for calcium-activated adenosine triphosphatase. Circ Res. 1985;56(4):548–555. doi: 10.1161/01.res.56.4.548. [DOI] [PubMed] [Google Scholar]
- 38.Herron TJ, Korte FS, McDonald KS. Loaded shortening and power output in cardiac myocytes are dependent on myosin heavy chain isoform expression. Am J Physiol Heart Circ Physiol. 2001;281(3):H1217–H1222. doi: 10.1152/ajpheart.2001.281.3.H1217. [DOI] [PubMed] [Google Scholar]
- 39.Herron TJ, McDonald KS. Small amounts of {alpha}-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res. 2002;90(11):1150–1152. doi: 10.1161/01.res.0000022879.57270.11. [DOI] [PubMed] [Google Scholar]
- 40.Li S-Y, Du M, Dolence EK, et al. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell. 2005;4(2):57–64. doi: 10.1111/j.1474-9728.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 41.Syrovy I, Hondy Z. Non-enzymatic glycosylation of myosin: effects of diabetes and aging. Gen Physiol Biophys. 1992;11(3):301–307. [PubMed] [Google Scholar]
- 42.Syrovy I, Hodny Z. In vitro non-enzymatic glycosylation of myofibrillar proteins. Int J Biochem. 1993;25(6):941–946. doi: 10.1016/0020-711x(93)90251-9. [DOI] [PubMed] [Google Scholar]
- 43.Ramamurthy B, Hook P, Jones AD, Larsson L. Changes in myosin structure and function in response to glycation. FASEB J. 2001;15(13):2415–2422. doi: 10.1096/fj.01-0183com. [DOI] [PubMed] [Google Scholar]