Abstract
Background
For patients who present with synchronous colorectal carcinoma and colorectal liver metastasis (CRLM), a reversed treatment sequence in which the CRLM are resected before the primary carcinoma has been proposed (liver-first approach). The aim of the present study was to assess the feasibility and outcome of this approach for synchronous CRLM.
Methods
Between 2005 and 2010, 22 patients were planned to undergo the liver-first approach. Feasibility and outcomes were prospectively evaluated.
Results
Of the 22 patients planned to undergo the liver-first strategy, the approach was completed in 18 patients (81.8%). The main reason for treatment failure was disease progression. Patients who completed treatment and patients who deviated from the protocol had a similar location of the primary tumour, as well as comparable size, number and distribution of CRLM (all P > 0.05). Post-operative morbidity and mortality were 27.3% and 0% following liver resection and 44.4% and 5.6% after colorectal surgery, respectively. On an intention-to-treat-basis, overall 3-year survival was 41.1%. However, 37.5% of patients who completed the treatment had developed recurrent disease at the time of the last follow-up.
Conclusions
The liver-first approach is feasible in approximately four-fifths of patients and can be performed with peri-operative mortality and morbidity similar to the traditional treatment paradigm. Patients treated with this novel strategy derive a considerable overall-survival-benefit, although disease-recurrence-rates remain relatively high, necessitating a multidisciplinary approach.
Keywords: synchronous colorectal liver metastasis
Introduction
Up to one-fourth of patients who present with colorectal cancer have concomitant colorectal liver metastasis (CRLM).1,2 Patients with these synchronous CRLM are thought to have a worse prognosis compared with patients with metachronous CRLM.3 While surgical resection of CRLM is regarded the only potential for a cure,4,5 the simultaneous presentation of primary and secondary disease provides a unique chance in deciding the optimal therapy sequence in these patients.
The classic approach for patients with synchronous CRLM encompasses resection of the primary tumour, followed by optional adjuvant chemotherapy and eventually succeeded by liver surgery. Potential advantages of this approach are primary tumour oriented and include prevention of local ingrowth, bowel obstruction or bleeding from the colorectal carcinoma. However, major disadvantages include progression of the CRLM beyond resectability, especially after a delay in the treatment paradigm owing to morbidity associated with the colorectal surgery. Moreover, in case of chemotherapy, serious hepatoxicity can occur6,7 or the hepatic lesions can disappear.8 While some authors have therefore supported a simultaneous resection of the primary tumour and concurrent liver disease,9–11 others advocated a staged approach in which the liver is operated on before the primary tumour and increasingly frequently preceded by induction chemotherapy.12–14 Particularly for rectal primaries, the latter approach is regarded as more natural as the post-radiation required waiting period offers ample time for performance of a liver resection. Additional rationale for this reversed sequence of therapy is that CRLM are the main cause of death and that it is therefore important to eliminate these lesions first. With a liver-first approach, the treatment of the CRLM is by no means interrupted by possible complications after resection of the primary tumour. A concurrent advantage could be that administration of pre-operative chemotherapy provides a chance to evaluate a response and thereby define the tumour biology of the CRLM. Moreover this strategy provides a certain window during which possibly latently present extrahepatic metastases have a chance to declare themselves.15,16
Currently, there are very little data available regarding the liver-first approach.12,14,17,18 Therefore, the aim of the present study was to describe the experience with the liver-first approach in a tertiary referral centre. Moreover, we sought to not only examine the feasibility and short-term outcomes after the liver-first approach, but also to assess the influence of this sequence in therapy on the oncological outcome.
Methods
Prospectively collected data on patients who underwent liver surgery from 1st January 2005 to 31st December 2010 were queried from the hepatectomy databases at a large hepatobiliary centre (Maastricht University Medical Centre). All patients with synchronous CRLM were identified. In 22 of these patients, a liver-first approach was planned and these patients are the scope of the present study.
During pre-operative assessment, patients were deemed resectable only if a resection with negative margins was expected for all known disease, both intra- and extrahepatic. Moreover, only patients in whom an adequate future liver remnant was anticipated on computed tomography (CT) volumetric analysis were considered candidates for hepatic resection.19,20 Furthermore, the future liver remnant had to incorporate a minimum of two adjacent segments with sufficient vascular in- and outflow and adequate biliary drainage to be regarded suitable for resection.5,21
In short, after patients were diagnosed with synchronous CRLM, they were referred to our tertiary referral centre (coordinated by the Surgical Oncologic Network of Limburg) and were subsequently presented and discussed in a multi-disciplinary oncology meeting, during which their treatment strategy was established. Specifically, all patients with rectal cancer underwent standard locoregional staging with standard T2-weighted magnetic resonance imaging (MRI) which was in some cases extended with an additional gadofosveset-enhanced imaging sequence for lymph node staging, in accordance with our institutional protocol described previously.22 For patients with a primary tumour located in the colon, pre-operative staging was generally ascertained using a computed tomography (CT) scan. In general, all patients who were diagnosed with a rectal tumour, based on these imaging modalities, received pre-operative radiation therapy consisting of either a short course (5 × 5 Gy) of radiation or a long course of 28 fractions of 1.8 Gy radiation with capecitabine (chemoradiation, i.e. chemotherapy as a radiosensitizer, capecitabine 2× 1000 mg/m2), followed by pre-operative chemotherapy aimed at minimizing the likelihood of a (microscopically) positive resection margin after resection of the primary tumour, according to the national guidelines.23 In line with our institutional chemotherapy protocol, patients receive a total of six cycles. Moreover, in our institution, patients with a locally advanced rectal carcinoma, which was defined as a T4 tumour or a T3 tumour with involved or threatened mesorectal fascia or an N2-status or a distally located T3N1 tumour, receive chemoradiation followed by two cycles of pre-operative full-strength chemotherapy (i.e. oxaliplatin, 130 mg/m2 with or without bevacizumab 7.5 mg/kg). Patients with a non-locally advanced rectum carcinoma are generally treated with a short course of radiation therapy followed by three cycles of pre-operative chemotherapy (i.e. oxaliplatin, 130 mg/m2 with or without bevacizumab 7.5 mg/kg). This also allowed evaluation of the tumour response to chemotherapy. The remaining cycles are generally administered either after liver resection or after completion of the resection of the primary tumour. Each deviation from this protocol only takes place after careful and thorough evaluation at our weekly, institutional multi-disciplinary meeting. Reasons for not completing all cycles of chemotherapy are mainly patient based and include too severe side-effects of pre-operative chemotherapy. However, rather than treating all patients according to a strict and rigid protocol, we intend to provide each patient with a tailor-made treatment regimen.
After initial resectability of the CRLM was assessed based on the criteria described above, all patients with a primary tumour located in the rectum were considered for the liver-first approach, whereas patients with a colorectal tumour located in the colon were selected on a case-by-case basis in order to provide a tailor-made management plan.
Data collection
Apart from standard demographic data (i.e. age and gender), the following data were also collected for each patient: characteristics of the primary tumour (i.e. location and size of the primary lesions, TNM stage) as well as of the CRLM (i.e. distribution, number and size of the lesions). Moreover, data concerning treatment-related variables were collected (i.e. peri-operative receipt of radiation and/or chemotherapy, details of the hepatic surgery as well as data regarding the operation on the primary tumour). Furthermore, data regarding post-operative outcome (i.e. presence, type and severity of in-hospital or 90-day morbidity and post-operative mortality within 90 days of treatment) and data regarding disease recurrence and vital status were noted. Disease recurrence was defined as a lesion that was a biopsy proven recurrent adenocarcinoma or a lesion that was deemed suspicious on cross-sectional imaging in the setting of an elevated carcinoembryonic antigen (CEA) level.
Statistical analyses
All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Summary statistics were obtained and presented as percentages or median values. Upon comparing categorical data, the χ2-test, or if deemed appropriate Fisher's exact test, was used, while the Mann–Whitney U-test was used to compare continuous data. Recurrence-free and overall survival analyses were performed using the non-parametric product limit method.24 Overall, a P-value less than 0.05 was considered significant.
Results
Patient and tumour characteristics
Between 1st January 2005 and 31st December 2010, 186 patients underwent liver directed surgery for CRLM at our institution and were queried from our hepatobiliary database. Specifically, 90 patients presented with synchronous CRLM and in 22 of these patients (24.4%), a liver-first approach was planned during this 5-year period. These latter patients are the scope of the present study. The characteristics of these patients are detailed in Table 1. The site of the primary tumour was the rectum in most patients (n = 19; 86.4%), whereas three patients (13.6%) had a carcinoma located in the colon. The median pre-operative CEA level (range) was 15.8 µg/L (1.90–321.0).
Table 1.
Patients and tumour characteristics
| Variable | No. of patients (%), n = 22 |
|---|---|
| Patient characteristics | |
| Median age [range], year | 65 [41–86] |
| Sex (male) | 16 (72.7) |
| Median pre-operative CEA level [range], µg/L | 15.8 [1.90–321.0] |
| Primary tumour site | |
| Location of the primary tumour | |
| Colon | 3 (13.6) |
| Rectum | 19 (86.4) |
| Symptoms caused by the primary tumour | |
| Symptoms at the time of presentation | |
| None | 7 (31.8) |
| Rectal blood loss | 9 (40.9) |
| Changes in bowel habits | 4 (18.2) |
| Bowel obstruction | 3 (13.6) |
| Onset of symptoms during the course of the illness | |
| None | 5 (22.7) |
| Bowel obstruction | 2 (9.1) |
| AJCC T-stage on pathologya | |
| ypT1/ypT2 | 2 (12.5) |
| ypT3/ypT4 | 14 (87.5) |
| Lymph node status on pathologya | |
| ypN1/ypN2 | 6 (37.5) |
| ypN0 | 10 (62.5) |
| Hepatic metastasis | |
| Size of largest metastasis (median [range]), cm | 1.7 [0.4–6.6] |
| No. of metastasis (median [range]) | 2 [1–7] |
| Location (unilobular) | 10 (45.5) |
Excluding patients who did not undergo resection of their primary colorectal tumor (n = 6).
At the time of presentation, 15 patients (68.2%) complained of primary tumour-associated symptoms. Furthermore, another two patients (12.5%) developed symptoms caused by the primary tumour during the course of their illness, but before their liver-directed surgery. Both of these patients developed an obstruction of the bowel as a result of their colorectal carcinoma and subsequently underwent a laparotomy during which a diverting ostomy was constructed, before their liver-directed surgery.
Details of radiation and chemotherapeutic therapy
In total, 21 patients (95.5%) received radiation therapy of their primary tumour. Eight patients (36.4%) received a short course of radiation therapy, of whom seven patients (31.8%) also received multiple cycles of pre-operative chemotherapy before surgery. Moreover, 13 patients (59.1%) underwent a long course of radiotherapy with a chemo-sensitizer (chemo-radiation therapy). Nine of these patients (40.9%) received additional cycles of pre-operative systemic chemotherapy (i.e. not as chemo-sensitizer). Furthermore, one patient (4.5%) did not receive radiation therapy of the primary tumour, but this patient did receive pre-operative chemotherapy. All of the patients who received pre-operative chemotherapy received an oxaliplatin-based chemotherapy regimen. Moreover, the majority of patients (n = 10) received one or more cycles including biologicals (i.e. bevacizumab).
Four patients (18.2%) received interval chemotherapy in the surgical window between hepatic and colorectal surgery, excluding those patients who did not undergo resection of their primary. Nine patients (40.9%) who completed the curative intent treatment plan received adjuvant chemotherapy after completion of both operations. For those patients who received chemotherapy after their liver resection (either interval of adjuvant), this generally also consisted of an oxaliplatin-based regimen.
Details of surgery
Of the 22 patients in whom a liver-first approach was planned, in one patient (4.5%) no liver resection was performed owing to a per-operative diagnosis of locoregional perihilar lymph nodal disease. These lymph nodes were located adjacent to the vena cava, making performance of a lymphadenectomy impossible. The operative characteristics of the 21 patients who did undergo a liver resection are detailed in Table 2. Importantly, one patient (4.5%) underwent a planned two-stage approach for his bilateral CRLM. This patient also underwent portal vein ligation (PVL) of the right portal vein during the first stage of his treatment.
Table 2.
Details of surgical procedures
| Variable | No. of patients (%), n = 22 |
|---|---|
| Type of liver resection | |
| None | 1 (4.5) |
| Single stage resection | |
| <Hemihepatectomy | 11 (50.0) |
| Hemihepatectomy | 8 (36.4) |
| Plus additional minor resection | 4 (18.2) |
| Central hepatectomy | 1 (4.5) |
| Two-stage resection | |
| Triple metastasectomy and subsequent hemihepatectomy | 1 (4.5) |
| Type of colorectal resection | |
| None | 6 (27.3) |
| Watchful wait | 2 (9.1) |
| Extensive metastatic disease | 4 (18.2) |
| Transanal endoscopic microsurgery | 1 (4.5) |
| Low anterior resection | 9 (40.9) |
| With diverting ostomy | 6 (27.3) |
| Hartmann's resection | 2 (9.1) |
| With revision ostomy | 1 (4.5) |
| Right hemicolectomy | 1 (4.5) |
| Rectum extirpation with end ostomy | 2 (9.1) |
| Rectosigmoid resection with end ostomy | 1 (4.5) |
| Median of lymph nodes harvested [range] | 12 [4–14] |
After a median interval of 3 months (range: 1–11), 16 patients (72.7%) underwent surgery for their primary tumour. The operative characteristics of these 16 patients are detailed in Table 2. Of the five patients who did not undergo surgery of their primary tumour, three patients (13.6%) developed extensive metastatic disease and were therefore not thought to be candidates for resection. Specifically, in two of these latter patients, the extrahepatic metastases were diagnosed during attempted colorectal resection. Furthermore, in two patients (9.1%) the primary tumour had disappeared as a result of the chemo-radiation therapy and therefore in these patients a wait-and-see approach was conducted, in accordance with a watchful wait protocol currently executed at our institution. A complete response of the primary tumour was diagnosed by endoscopic examination and was pathologically confirmed by a biopsy of the region of the lesion. Altogether, the total number of patients not treated according to the protocol was four (18.2%), whereby excluding the two patients who are presently included in the follow-up protocol for complete responders to chemo-radiation. (Fig. 1)
Figure 1.
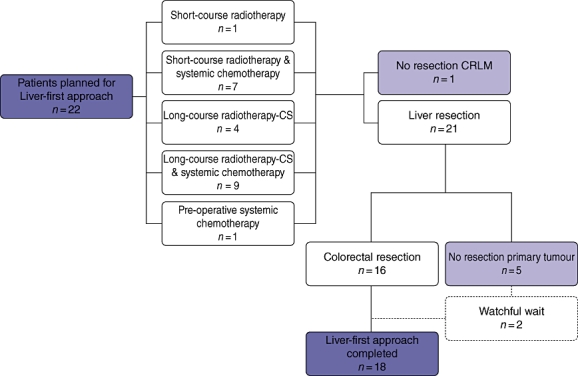
Flow chart of the 22 patients included in the present study. CRLM, colorectal liver metastasis; CS, chemo-sensitizer
Upon comparing patients who completed their per-protocol treatment and patients who deviated from the protocol, no differences were observed with regard to the location of the primary tumour, size and the number of CRLM or the distribution of the lesions (all P > 0.05). Moreover, there was no statistically significant difference in the likelihood of succeeding between patients who had a symptomatic primary lesion and patients who did not (P = 0.63).
Pathological characteristics
The median number of CRLM resected was 2 (range: 1–7), with a median size of the largest lesion of 1.7 cm (range: 0.4–6.6). On final pathological analysis, 20 patients (95.2%) had negative hepatic margins (R0).25 Moreover, in one patient (4.8%), no viable tumour was found.
Excluding patients who did not undergo resection of their primary colorectal tumour and therefore did not have pathological staging of their primary tumour, most patients (n = 14; 87.5%) had a ypT3 (i.e. invasion through the muscularis propria into pericolorectal tissues) or a ypT4 (i.e. direct invasion in adjacent organs or structures) primary tumour.26 Six patients (37.5%) were found to have positive lymph nodes, therefore 10 patients (62.5%) had node negative disease however all in the setting of pre-operative chemotherapy (ypN0).
Post-operative and oncological outcome
The complication rate during hospital admittance after liver-directed surgery, or the 90-day post-operative period, was 27.3% (n = 6), all of which were major complications (Clavien grade ≥3).27 The specifics of post-operative morbidity are detailed in Table 3. Importantly, there was no significant difference in the interval between hepatic surgery and the resection of the primary tumour when patients who did develop complications [median: 3.4 months (range: 3.3–9.8)] were compared with patients who did not [median: 2.3 months (range: 1.0–10.9)] (P = 0.095). Moreover, eight patients (44.4%) developed post-operative complications after (attempted) resection of their primary tumour (n = 18), of whom half (n = 4; 22.2%) had also previously developed post-operative morbidity after liver-directed surgery. While two patients (11.1%) developed minor complications (Clavien grade <3), six patients (33.3%) developed major complications (Clavien grade ≥3).27 Importantly, one patient (5.4%) died within 90 days of operation for the primary tumour, on post-operative day 74.
Table 3.
Specifics of direct post-operative outcomes
| Variable | No. of patients (%), n = 22 |
|---|---|
| Liver-directed surgery | |
| Post-operative complications (any) | 6 (27.3) |
| Minor (Clavien grade < 3) | 0 |
| Major (Clavien grade ≥ 3) | 6 (27.3) |
| Post-operative mortality (within 90 days) | 0 |
| Specific complications | |
| Biloma | 3 (13.6) |
| Intra-abdominal abscess | 1 (4.5) |
| Iatrogenic bowel perforation | 1 (4.5) |
| Stroke | 1 (4.5) |
| Surgery on primary colorectal tumoura | |
| Post-operative complications (any) | 8 (44.4) |
| Minor (Clavien grade < 3) | 2 (11.1) |
| Major (Clavien grade ≥ 3) | 6 (33.3) |
| Post-operative mortality (within 90 days) | 1 (5.6) |
| Specific complications | |
| Intra-abdominal abscess | 4 (22.2) |
| Anastomotic leakage | 1 (5.6) |
| Re-bleeding | 1 (5.6) |
| Pneumonia | 2 (11.1) |
| Fascia dehiscence | 1 (5.6) |
| Urinary tract infection | 1 (5.6) |
| Surgical site infection | 1 (5.6) |
Excluding patients in whom no resection for a primary tumour was attempted (n = 4).
At the time of last follow-up, one-third (n = 6; 33.3%) of the patients who underwent the complete curative paradigm or in whom a wait-and-see approach was conducted had developed recurrent disease. Specifically, none of the two patients in whom a wait-and-see approach was conducted experienced a local recurrence. The median recurrence-free survival after hepatic surgery was 14.5 months. The pattern of recurrence was combined intra- and extrahepatic recurrence in four patients (25.0%), whereas another two patients (12.5%) developed recurrent disease outside of the liver only. No patient developed recurrent disease solely in the liver. On an intention-to-treat basis, the estimated overall 1- and 3-year survival calculated from the time of liver surgery were 74.2% and 41.1%, respectively, with a median survival of 35.5 months. (Fig. 2)
Figure 2.
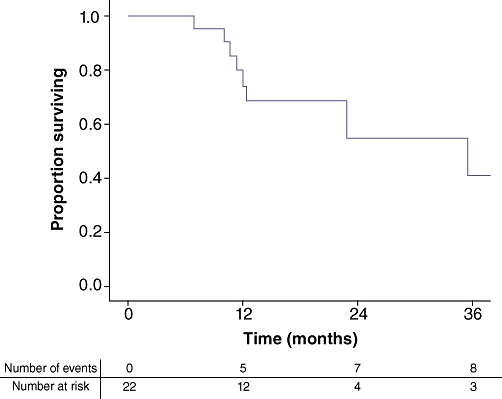
Kaplan–Meier curve showing overall survival of the 22 patients who were planned to undergo the liver-first approach (intention-to-treat analysis)
Discussion
Based on serious potential pitfalls of the traditional approach for synchronous CRLM, it has been proposed to reverse the order of the staged approach for these patients i.e. the liver-first approach. However, most data on this sequential in the treatment of patients with CRLM comes from a small series. Mortensen et al.28 described 26 patients in whom the CRLM were resected before the primary tumour. However, in only a small portion of these patients, this therapeutic sequence was a planned strategy. Mentha et al.12,17 have reported on their experience on several occasions with the largest series including 35 patients, whereas Verhoef et al.18 reported on 23 patients in whom a liver-first approach was planned. Moreover, this latter group reported on a cohort of 20 patients who all completed their liver-first strategy within a larger study on patients with synchronous CRLM.29 Nonetheless, in this previous study, the specific characteristics of these 20 patients were not described in great detail.
As previously mentioned, our institution has adopted the liver-first strategy several years ago, particularly in all patients who present with synchronous CRLM from a rectal origin but also in selected patients with synchronous CRLM from a primary tumour located in the colon, when an indication for pre-operative radiation therapy or chemotherapeutic regimens is present. In the current series, we report on 22 patients who were planned to undergo a liver-first approach during the 5 years of inclusion. Specifically, 18 patients (81.8%) were treated per protocol and completed the treatment plan for liver and primary tumours. This percentage of feasibility is higher than the rate of patients who completed the formalized treatment plan reported by Verhoef et al. (69.6%).18 Moreover, in the most recent series by Mentha et al.12 the authors report that 14% of patients did not complete the curative paradigm, while an additional 20% of patients had their primary tumour removed at the same time as the hepatic resection (i.e. a simultaneous approach). Therefore, in this previous study, merely 66% of patients were treated according to protocol. The feasibility of being able to complete the entire treatment protocol under the liver-first approach has been of some concern. As it has been stated that surgical management of CRLM is associated with an increased quality of life compared with chemotherapeutic therapy alone,30 an aggressive approach seems warranted. In our cohort about one-fifth of patients were unable to complete their treatment paradigm, as four patients (18.2%) deviated from the protocol as a result of the development of extrahepatic disease during the course of their treatment (i.e. under chemotherapy). It has been shown that tumour progression of CRLM under pre-operative chemotherapy is associated with a poor outcome, even after all (visible) metastatic disease is completely removed during curative intent surgery.15,16 This supports the potential benefit of the surgery-free interval as patients can be ‘saved’ an operation in the case of disease progression.
In two patients (9.1%), a complete response of the primary lesion owing to radiation therapy was observed. While the disappearance of the primary lesion has been shown to be of concern, with adequate follow-up and strict surveillance including regular evaluation of the CEA-level and appropriate cross-sectional imaging, patients can still be treated under a curative paradigm.31 Therefore, these patients were not classified as treatment failures in the present study. Furthermore, although obstruction by the primary tumour during the course of treatment occurred in five patients (22.7%), in all of these a diverting ostomy was constructed and in none of these patients this was a reason to divert from the planned strategy.
On another note, the potential surgical morbidity in managing patients with synchronous CRLM has also been an area of ongoing concern. Specifically, under the liver-first protocol, a complication after hepatic resection can result in a delay with regard to the treatment of the primary tumour. In our series, six patients (27.3%) developed complications after liver-directed surgery, whereas the post-operative mortality was zero. These numbers corroborate the incidence of post-operative morbidity reported in other, larger series.32,33 Importantly, the interval between the operations for the hepatic and primary disease was similar for patients who did and patients who did not develop complications after liver surgery. Moreover, for patients in whom a too small future remnant is anticipated and who are therefore at an increased risk of developing hepatic failure, portal vein embolization (PVE) or PVL could be undertaken to enlarge the future liver remnant by means of hypertrophy,34,35 especially as patients treated with the liver-first approach are generally regarded to have advanced disease and undergo several cycles of pre-operative chemotherapy which could have subsequently caused hepatocyte damage. After surgery on the primary tumour (n = 18), post-operative morbidity occurred in eight patient (44.4%). Notably, one patient (5.6%) died during the 90-day post-operative period. These complication rates are similar to the post-operative morbidity rates reported in cohorts of patients who had not previously undergone a liver resection.36,37
Apart from the short-term results of the liver-first approach, it is important to also emphasize the influence on long-term outcomes (i.e. recurrence and survival) in these patients. In the current series, on an intention-to-treat basis, the estimated overall 1- and 3-year survival calculated from the time of liver surgery were 74.2% and 41.1%, respectively, with a median survival of 35.5 months. These statistics are corroborated by other, albeit limited, series on patients operated for synchronous CRLM, treated using the traditional approach3,38 or the liver-first strategy.12 However, owing to advances in medical oncology (e.g. modern chemotherapy regimens)39,40 as well as surgical oncology (e.g. performance of repeat resections41 and improved peri-operative outcome),42 the overall survival for all patients who present with CRLM has improved dramatically. Therefore, it has become increasingly important to assess disease recurrence in order to draw conclusions with regard to the curative potential of the proposed treatment paradigm. In the current cohort, six (33.3%) of the patients who were treated per-protocol had developed recurrent disease at the time of the last follow-up. Available data on recurrence after the liver-first approach are scarce and range from 25.0%18 to 66.7%12 at the time of the last follow-up. However, as recurrence is associated with time passed since surgery, it is preferred to investigate this variable in a time-to-event modus rather that comparing the crude probabilities. In the present cohort, we found a median recurrence-free interval of 14.5 months. While other studies on the liver-first approach did not report a median recurrence-free interval, this interval is in concordance with those reported in assorted cohorts of patients with CRLM.43,44 Moreover, although the recurrence rates reported in the series on the liver-first approach varied greatly, they do show that disease recurrence is not uncommon and therefore, especially in the proposed treatment sequence, there seems to be a role for a multidisciplinary approach.
The present study has several limitations associated with the retrospective nature of the study. This limited number of patients reflects the highly selected nature of the cohort of patients with synchronous CRLM who were considered for this comparatively novel approach. As a result of the small sample size, the study has limited statistical power and therefore the statistical analyses are also limited.
In conclusion, the present study shows that resection of hepatic metastasis before resection of the primary colorectal tumour can be performed with acceptable peri-operative mortality and morbidity rates, similar to the traditional treatment paradigm for synchronous CRLM. Especially when the primary tumour requires a neo-adjuvant treatment strategy that provides a window for the liver-first approach, this sequence of therapy should be considered. Although over one-fifth of patients were not able to complete the entire treatment plan, an important finding of this series is that the anticipated pitfalls of this approach (i.e. growth of CRLM beyond resectability and bowel obstruction by the primary tumour) were not found to alter the proposed treatment strategy. Moreover, the here presented data show that patients with synchronous CRLM treated with the liver-first strategy derive a considerable overall-survival benefit, although disease-recurrence rates remain relatively high. Especially in light hereof, the selection of patients for this novel strategy should be individualized and incorporated in a multidisciplinary approach to achieve the best outcomes.
Conflicts of interest
None declared.
References
- 1.Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer. 1969;23:198–202. doi: 10.1002/1097-0142(196901)23:1<198::aid-cncr2820230126>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16:3–5. doi: 10.1016/j.suronc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Tsai MS, Su YH, Ho MC, Liang JT, Chen TP, Lai HS, et al. Clinicopathological features and prognosis in resectable synchronous and metachronous colorectal liver metastasis. Ann Surg Oncol. 2007;14:786–794. doi: 10.1245/s10434-006-9215-5. [DOI] [PubMed] [Google Scholar]
- 4.Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11:1057–1077. doi: 10.1007/s11605-006-0061-3. [DOI] [PubMed] [Google Scholar]
- 5.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 6.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 7.Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11:860–868. doi: 10.1007/s11605-007-0149-4. [DOI] [PubMed] [Google Scholar]
- 8.van Vledder MG, de Jong MC, Pawlik TM, Schulick RD, Diaz LA, Choti MA. Disappearing colorectal liver metastases after chemotherapy: should we be concerned? J Gastrointest Surg. 2010;14:1691–1700. doi: 10.1007/s11605-010-1348-y. [DOI] [PubMed] [Google Scholar]
- 9.Lyass S, Zamir G, Matot I, Goitein D, Eid A, Jurim O. Combined colon and hepatic resection for synchronous colorectal liver metastases. J Surg Oncol. 2001;78:17–21. doi: 10.1002/jso.1117. [DOI] [PubMed] [Google Scholar]
- 10.Martin R, Paty P, Fong Y, Grace A, Cohen A, DeMatteo R, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197:233–241. doi: 10.1016/S1072-7515(03)00390-9. discussion 241–2. [DOI] [PubMed] [Google Scholar]
- 11.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2009;17:171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 12.Mentha G, Roth AD, Terraz S, Giostra E, Gervaz P, Andres A, et al. ‘Liver first’ approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg. 2008;25:430–435. doi: 10.1159/000184734. [DOI] [PubMed] [Google Scholar]
- 13.Mentha G, Majno P, Terraz S, Rubbia-Brandt L, Gervaz P, Andres A, et al. Treatment strategies for the management of advanced colorectal liver metastases detected synchronously with the primary tumour. Eur J Surg Oncol. 2007;33(Suppl 2):S76–S83. doi: 10.1016/j.ejso.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Van Dessel E, Fierens K, Pattyn P, Van Nieuwenhove Y, Berrevoet F, Troisi R, et al. Defining the optimal therapy sequence in synchronous resectable liver metastases from colorectal cancer: a decision analysis approach. Acta Chir Belg. 2009;109:317–320. doi: 10.1080/00015458.2009.11680432. [DOI] [PubMed] [Google Scholar]
- 15.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. discussion 1061–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blazer DG, 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 17.Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93:872–878. doi: 10.1002/bjs.5346. [DOI] [PubMed] [Google Scholar]
- 18.Verhoef C, van der Pool AE, Nuyttens JJ, Planting AS, Eggermont AM, de Wilt JH. The ‘liver-first approach’ for patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum. 2009;52:23–30. doi: 10.1007/DCR.0b013e318197939a. [DOI] [PubMed] [Google Scholar]
- 19.Dello SA, Stoot JH, van Stiphout RS, Bloemen JG, Wigmore SJ, Dejong CH, et al. Prospective volumetric assessment of the liver on a personal computer by nonradiologists prior to partial hepatectomy. World J Surg. 2011;35:386–392. doi: 10.1007/s00268-010-0877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dello SA, van Dam RM, Slangen JJ, van de Poll MC, Bemelmans MH, Greve JW, et al. Liver volumetry plug and play: do it yourself with ImageJ. World J Surg. 2007;31:2215–2221. doi: 10.1007/s00268-007-9197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clavien PA, Emond J, Vauthey JN, Belghiti J, Chari RS, Strasberg SM. Protection of the liver during hepatic surgery. J Gastrointest Surg. 2004;8:313–327. doi: 10.1016/j.gassur.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Lambregts DM, Beets GL, Maas M, Kessels AG, Bakers FC, Cappendijk VC, et al. Accuracy of Gadofosveset-enhanced MRI for Nodal Staging and Restaging in Rectal Cancer. Ann Surg. 2011;253:539–545. doi: 10.1097/SLA.0b013e31820b01f1. [DOI] [PubMed] [Google Scholar]
- 23.Oncoline Cancer Clinical Practice Guidelines – Colon Cancer (2.0) Available at: http://oncoline.nl/index.php?pagina = /richtlijn/item/pagina.php&richtlijn_id = 598. Accessed 06-20-2011.
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Wittekind C, Compton C, Quirke P, Nagtegaal I, Merkel S, Hermanek P, et al. A uniform residual tumor (R) classification: integration of the R classification and the circumferential margin status. Cancer. 2009;115:3483–3488. doi: 10.1002/cncr.24320. [DOI] [PubMed] [Google Scholar]
- 26.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th edn. Chicago, IL: Springer; 2010. [Google Scholar]
- 27.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortenson MM, Curley SA, Vauthey JN, Beaty K, Abdalla EK. Resection of Hepatic Metastases Before the Colorectal Primary:An Alternate Treatment Strategy for Synchronous Disease. Ann Surg Oncol. 2008;15:55–56. [Google Scholar]
- 29.van der Pool AE, de Wilt JH, Lalmahomed ZS, Eggermont AM, Ijzermans JN, Verhoef C. Optimizing the outcome of surgery in patients with rectal cancer and synchronous liver metastases. Br J Surg. 2010;97:383–390. doi: 10.1002/bjs.6947. [DOI] [PubMed] [Google Scholar]
- 30.Wiering B, Oyen WJ, Adang EM, van der Sijp JR, Roumen RM, de Jong KP, et al. Long-term global quality of life in patients treated for colorectal liver metastases. Br J Surg. 2010 doi: 10.1002/bjs.7365. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53:1692–1698. doi: 10.1007/DCR.0b013e3181f42b89. [DOI] [PubMed] [Google Scholar]
- 32.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. doi: 10.1097/01.sla.0000141195.66155.0c. discussion 708–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680. doi: 10.1001/archsurg.137.6.675. discussion 680–1. [DOI] [PubMed] [Google Scholar]
- 35.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 36.Bretagnol F, Panis Y, Rullier E, Rouanet P, Berdah S, Dousset B, et al. Rectal cancer surgery with or without bowel preparation: the French GRECCAR III multicenter single-blinded randomized trial. Ann Surg. 2010;252:863–868. doi: 10.1097/SLA.0b013e3181fd8ea9. [DOI] [PubMed] [Google Scholar]
- 37.Scabini S, Rimini E, Romairone E, Scordamaglia R, Damiani G, Pertile D, et al. Colon and rectal surgery for cancer without mechanical bowel preparation: one-center randomized prospective trial. World J Surg Oncol. 2010;8:35. doi: 10.1186/1477-7819-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Fujita S, Akasu T, Moriya Y. Resection of synchronous liver metastases from colorectal cancer. Jpn J Clin Oncol. 2000;30:7–11. doi: 10.1093/jjco/hyd002. [DOI] [PubMed] [Google Scholar]
- 39.Chibaudel B, Maindrault-Goebel F, Lledo G, Mineur L, Andre T, Bennamoun M, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol. 2009;27:5727–5733. doi: 10.1200/JCO.2009.23.4344. [DOI] [PubMed] [Google Scholar]
- 40.Dy GK, Hobday TJ, Nelson G, Windschitl HE, O'Connell MJ, Alberts SR, et al. Long-term survivors of metastatic colorectal cancer treated with systemic chemotherapy alone: a North Central Cancer Treatment Group review of 3811 patients, N0144. Clin Colorectal Cancer. 2009;8:88–93. doi: 10.3816/CCC.2009.n.014. [DOI] [PubMed] [Google Scholar]
- 41.de Jong MC, Mayo SC, Pulitano C, Lanella S, Ribero D, Strub J, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13:2141–2151. doi: 10.1007/s11605-009-1050-0. [DOI] [PubMed] [Google Scholar]
- 42.Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842–851. doi: 10.1007/s11605-008-0494-y. [DOI] [PubMed] [Google Scholar]
- 43.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 44.D'Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, et al. Effect on Outcome of Recurrence Patterns After Hepatectomy for Colorectal Metastases. Ann Surg Oncol. 2010;18:1096–1103. doi: 10.1245/s10434-010-1409-1. [DOI] [PubMed] [Google Scholar]


