Abstract
One new dimeric diterpenoid, 8(14)-enyl-pimar-2′(3′)-en-4′(18′)-en-15′(16′)-endolabr- 16,15,2′,3′-oxoan-16-one (1) and five known terpenoids: Tagalsin C (2), Tagalsin I (3), lup-20(29)-ene-3β,28-diol (4), 3-oxolup-20(29)-en-28-oic acid (5) and 28-hydroxylup- 20(29)-en-3-one (6) were isolated from the roots of the mangrove plant Ceriops tagal. Their structures and relative stereochemistry were elucidated by means of extensive NMR, IR and MS analysis. The antifouling activity against larval settlement of the barnacle Balanus albicostatus were evaluated using capsaicin as a positive control. All these terpenoids exhibited antifouling activity against cyprid larvae of the barnacle without significant toxicity. The structure-activity relationship results demonstrated that the order of antifouling activity was diterpenoid (Compound 2) > triterpenoid (Compounds 4, 5 and 6) > dimeric diterpenoid (Compounds 1 and 3). The functional groups on the C-28 position of lupane triterpenoid significantly affect the antifouling activity. The diterpenoid dimmer with two identical diterpenoid subunits might display more potent antifouling activity than one with two different diterpenoid subunits. The stability test showed that Compounds 2, 4, 5 and 6 remained stable over 2-month exposure under filtered seawater.
Keywords: terpenoids, antifouling activity, barnacle, Ceriops tagal, structure-activity relationship
1. Introduction
It is well known that marine fouling organisms, settling on ship hulls and other artificial surfaces submerged in seawaters, cause technical and economic problems [1,2]. Antifouling paints, containing organotin compounds such as tributyltin (TBT) and tributyltin oxide (TBTO), are very effective in controlling these fouling organisms. However, these substances have been found to be toxic to many non-target marine organisms and to pollute the marine ecosystem [2,3]. These have resulted in the total ban of the application of organotin-based antifouling paints in January 2008 in many countries [2]. Currently, some booster biocides are used for antifouling paints, but they may also pollute the aquatic environments [4]. Thus, there is an urgent demand for finding new antifouling agents which are effective and environmentally friendly. In many studies, it is suggested that marine organisms have both physical and chemical methods to protect themselves from the harmful process of biofouling [5–7]. The key chemical antifouling mechanism of marine organisms occurs via the production of secondary metabolites (also known as natural products) which deter foulers [2,8–14]. Based on recent research, terpenoids, which are an important natural product, may act as defensive compounds for a variety of organisms in the marine world [15,16]. Ceriop tagal, a mangrove plant species in the Family of Rhizophoraceae, has been proved to possess a series of antifouling terpenoids with unique structural diversity [9,14]. Moreover, our previous work has demonstrated that pimarane diterpenoids isolated from the C. tagal can significantly inhibit larval settlement of the barnacle Balanus albicostatus, which is one important fouling organism in East Asian coastal waters [9]. The structure–activity relationship (SAR) analysis of these pimarane diterpenoids suggested that the functional groups on the C-15 and C-16 position of pimarane diterpenoid were responsible for the antifouling activity [9]. This was further confirmed by the result obtained from our SAR studies on synthetic pimarane diterpenoids [17].
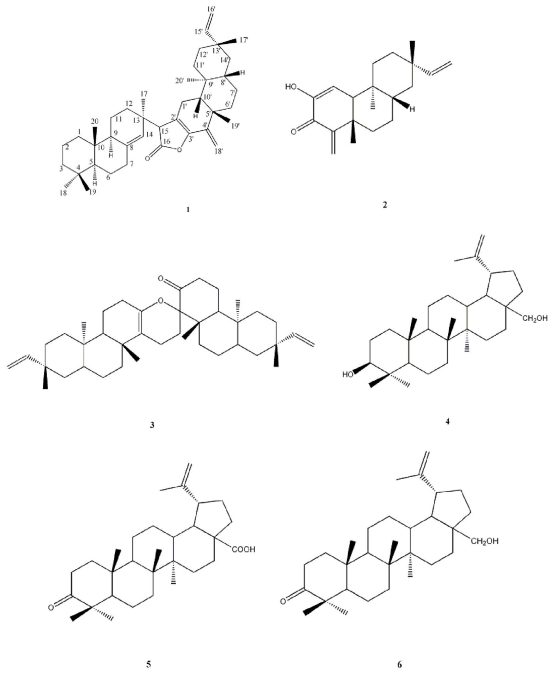
Due to the highly structural diversity of terpenoids, the structure–activity of mangrove terpenoids merits further investigation. We obtained six terpenoids from C. tagal, and then evaluated their antifouling activity against larval settlement of the barnacle B. albicostatus using capsaicin as a positive control. These antifouling terpenoids included one new dimeric diterpenoid, 8(14)-enyl-pimar- 2′(3′)-en-4′(18′)-en-15′(16′)-en-dolabr-16,15,2′,3′-oxoan-16-one (1) and five known terpenoids, designated as Tagalsin C (2), Tagalsin I (3), lup-20(29)-ene-3β,28-diol (4), 3-oxolup-20(29)-en-28-oic acid (5) and 28-hydroxylup-20(29)-en-3-one (6) (Figure 1). The isolation, structural elucidation and antifouling activity of these compounds are described in the present study.
Figure 1.
Structures of the isolated terpenoids 1–6.
2. Results and Discussion
2.1. Structural Elucidation of Terpenoids
Compound 1 was obtained as yellow oil, and its molecular composition of C40H58O2 was established by HR-ESI-MS (m/z 571.4751 [M + H+]+, C40H58O2+). The ESI-MS of 1 showed quasimolecular ion peaks at m/z 571.3 [M + 1]+ and 592.7 [M + Na]+, respectively. The IR absorptions at 1724 1650, 1237, 1001 and 798 cm–1 showed the presence of carbalkoxy and double groups. Analyses of the 1H-NMR, 13C-NMR, DEPT and HMQC spectra revealed not only the presence of 7 methyls, 15 methylenes, 7 methines and 11 quaternary carbon (including one carbalkoxy group at δC 177.0, d), but also the presence of one tetrasubstituted double bond, one tetrasubstituted double bond and two terminal double bonds (Tables 1). Four methyl singlets (δC 24.7, q; 33.7, q; 22.1, q; 15.4, q) and one trisubstituted double bond (δC 137.7, s; 128.5, d) suggesting that a pimarane-type diterpenoid structure might be presented [9,18–21]. Meanwhile, three methyl singlets (δC 23.0, q; 33.1, q; 11.8, q) and two terminal double bonds (δC 139.5, s; 105.9, t; 151.0, d; 109.0, t) might indicate the structure of a dolabradiene-type diterpenoid [22–25]. Therefore, Compound 1 seemed to be a dimeric diterpenoid composed of two moieties, one being a pimarane-type diterpenoid (part A) and the other a dolabradiene-type diterpenoid (part B).
Table 1.
1H-NMR Data (500 MHz) and 13C-NMR Data (125 MHz) for Compound 1 (CDCl3).
| No. | δC | δH | No. | δC | δH |
|---|---|---|---|---|---|
| 1 | 39.2 t | 0.95 (m) | 1′ | 22.2 t | 2.28 (m) |
| 1.71 (m) | 2.56 (dd, J = 7.8, 14.4 Hz) | ||||
| 2 | 19.0 t | 1.53 (m) | 2′ | 113.6 s | |
| 3 | 42.1 t | 1.40 (m) | 3′ | 148.6 s | |
| 1.19 (m) | 4′ | 139.5 s | |||
| 4 | 33.3 s | 5′ | 39.1 s | ||
| 5 | 55.0 d | 1.06 (m) | 6′ | 36.8 t | 2.17 (m) |
| 6 | 22.7 t | 1.58 (m) | 1.54 (m) | ||
| 1.34 (m) | 7′ | 25.6 t | 1.19 (m) | ||
| 7 | 36.23 t | 2.05 (m) | 8′ | 43.0 d | 1.47 (m) |
| 2.36 (d, J = 15 Hz) | 9′ | 38.1 s | |||
| 8 | 137.7 s | 10′ | 53.3 d | 1.38 (m) | |
| 9 | 49.8 d | 1.74 (m) | 11′ | 35.2 t | 1.60 (m) |
| 10 | 38.87 s | 1.34 (m) | |||
| 11 | 18.6 t | 1.60 (m) | 12′ | 32.0 t | 1.54 (m) |
| 1.10 (m) | 1.31 (m) | ||||
| 12 | 32.4 t | 1.68 (m) | 13′ | 36.18 s | |
| 1.22 (m) | 14′ | 38.93 t | 1.36 (m) | ||
| 13 | 38.0 s | 1.04 (m) | |||
| 14 | 128.5 d | 5.55 (s) | 15′ | 151.0 d | 5.81 (dd, J = 12.6, 21 Hz) |
| 15 | 56.2 d | 3.20 (s) | 16′ | 109.0 t | 4.82 (d, J = 13.2 Hz) |
| 4.92 (d, J = 21 Hz) | |||||
| 16 | 177.0 s | 17′ | 23.0 q | 1.01 (s) | |
| 17 | 24.7 q | 0.99 (s) | 18′ | 105.9 t | 5.26 (s) |
| 18 | 33.7 q | 0.89 (s) | 5.08 (s) | ||
| 19 | 22.1 q | 0.85 (s) | 19′ | 33.1 q | 1.14 (s) |
| 20 | 15.4 q | 0.82 (s) | 20′ | 11.8 q | 0.57 (s) |
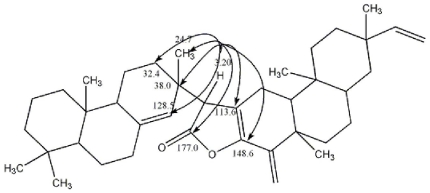
The HMBC correlations of Me18 (δH 0.89, s) with C-5 (δC 55.0, d), C-3 (δC 42.1, t), C-4 (δC 33.3, s) and C-19 (δC 22.1, q); Me19 (δH 0.85, s) with C-5 (δC 55.0, d), C-3 (δC 42.1, t), C-4 (δC 33.3, s) and C-18 (δC 33.7, q); Me20 (δH 0.82, s) with C-9 (δC 49.8, d), C-1 (δC 39.2, t); Me17 (δH 0.99, s) with C-14 (δC 128.5, d), C-15 (δC 56.2, d), C-13 (δC 38.0, s) and C-12 (δC 32.4, t); H-5 (δH 1.06, m) with C-20 (δC 15.4, q), C-6 (δC 22.7, t); H-9 (δH 1.74, m) with C-8 (δC 137.7, s), C-14 (δC 128.5, d), C-20 (δC 15.4, q), C-11 (δC 18.6, t)and C-10 (δC 38.87, s); H-14 (δH 5.55, s) with C-9 (δC 49.8, d), C-13 (δC 38.0, s), C-7 (δC 36.23, t), C-12 (δC 32.4, t) and C-17 (δC 24.7, q) confirmed that part A belonged to a pimarane-type diterpenoid. The HMBC correlations of Me-20′ (δH 0.57, s) with C-10′ (δC 53.3, d), C-8′ (δC 43.0, d), C-9′ (δC 38.1, s ) and C-11′ (δC 35.2, t); Me-19′ (δH 1.14, s) with C-4′ (δC 139.5, s), C-10′ (δC 53.3, d), C-5′ (δC 39.1, s ) and C-6′ (δC 36.8, t); Me-17′ (δH 1.01, s) with C-15′ (δC 151.0, d), C-13′ (δC 36.18, s) and C-12′ (δC 32.0, t); H-15′ (δH 5.81, dd, J = 12.6, 21 Hz) with C-14′ (δC 38.93, t), C-13′ (δC 36.18, s), C-12′ (δC 32.0, t) and C-17′ (δC 23.0, q); H-16′ (δH 4.82, d, J = 13.2 Hz) with C-15′ (δC 151.0, d) and C-13′ (δC 36.18, s); H-1′ (δH 2.28, m) with C-3′ (δC 148.6, s), C-2′ (δC 113.6, s), C-10′ (δC 53.3, d), C-9′ (δC 38.1, s) and C-5′ (δC 39.1, s); H-1′ (δH 2.56, dd, J = 7.8, 14.4 Hz) with C-3′ (δC 148.6, s), C-2′ (δC 113.6, s), C-10′ (δC 53.3, d) and C-9′ (δC 38.1, s); H-10′ (δH 1.38, m) with C-5′ (δC 39.1, s), C-9′ (δC 38.1, s), C-11′ (δC 35.2, t), C-19′ (δC 33.1, q) and C-1′ (δC 22.2, t); H-18′ (δH 5.26, s) with C-5′ (δC 39.1, s), C-3′ (δC 148.6, s) and C-4′ (δC 139.5, s); H-18′ (δH 5.08, s) with C-5′ (δC 39.1, s), C-3′ (δC 148.6, s) and C-4′ (δC 139.5, s) confirmed that part B belonged to a dolabradiene-type diterpenoid. Then the chemical shift of δC 56.2 (d, C-15), δC 177.0 (s, C-16), δC 148.6 (s, C-3′) were compatible with a bond between C-3′ and the lactone O-atom, which was further confirmed by the HR-ESI-MS spectrum. The HMBC correlations from H-15 (δH 3.20, s) to C-3′ (δC 148.6, s), C-14 (δC 128.5, d), C-2′ (δC 113.6, s), C-13 (δC 38.0, s), C-12 (δC 32.4, t), C-17 (δC 24.7, q) and C-16 (δC 177.0, s) established the connection between part A and part B at the site of C-15, C-2′ and C-3′ (Figure 2). Furthermore, other HMBC correlations along with the comparison of its NMR data and the known pimarane-type diterpenoid and dolabradiene-type diterpenoid comprehensively confirmed that the planar structure of 1 was assigned as a novel dimeric diterpenoid [18–26].
Figure 2.
Key HMBC of Compound 1.
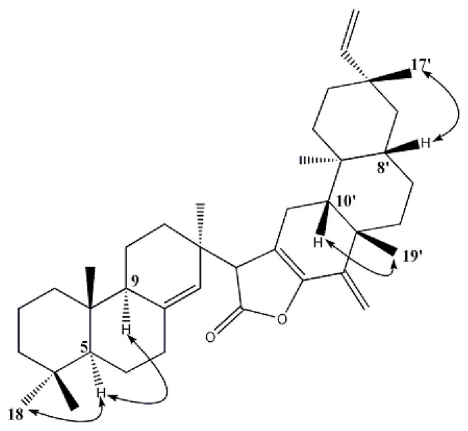
The relative stereochemistry of 1 was established by NOE spectrum. Comparison of the NMR data of 1 with the pimarane-type diterpenoid and dolabradiene-type diterpenoid showed that the Me-17 (δC 24.7, q), Me-18 (δC 33.7, q) and Me-20′ (δC 11.8, q) were in a relative α-orientation, while Me-19 (δC 22.1, q), Me-20 (δC 15.4, q), Me-19′ (δC 33.1, q) and Me-17′ (δC 23.0, q) were in a relative β-orientation [18–26]. NOE correlations between Me-18/H-5 and H-5/H-9 indicated that H-5 and H-9 were on the β-side of 1. NOE correlations between H-10′/Me-19′ and Me-17′/H-8′ indicated that they were oriented on another side of the nucleus (Figure 3).
Figure 3.
Key NOE correlations of Compound 1.
Five known terpenoids, Tagalsin C (2), Tagalsin I (3), lup-20(29)-ene-3β,28-diol (4), 3-oxolup- 20(29)-en-28-oic acid (5) and 28-hydroxylup-20(29)-en-3-one (6), were identified by comparison of their physical and spectral data with those in the literature [23,27–31].
2.2. Antifouling Activity of Terpenoids
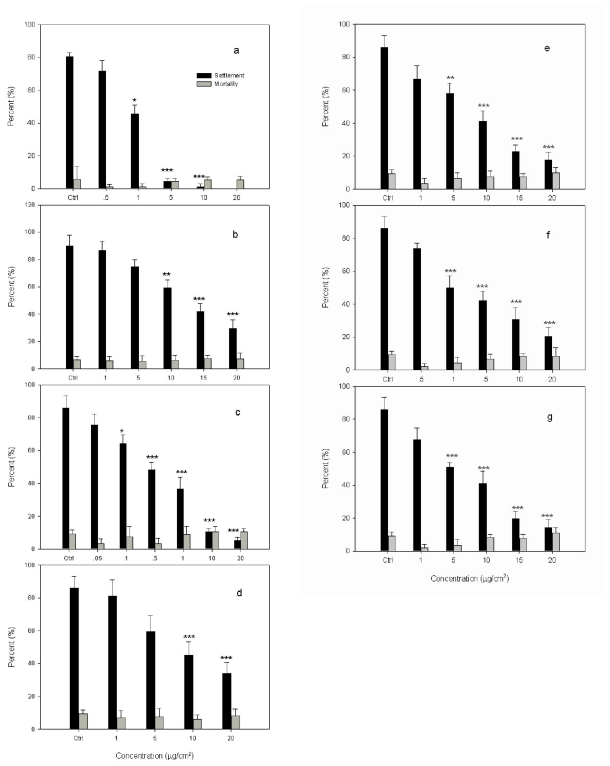
The effects of terpenoids on settlement and mortality of B. albicostatus cyprids are shown in Figure 4 and their EC50 and LC50 values are summarized in Table 2. Capsaicin was used as a positive standard with EC50 value of 1.32 μg/cm2 (Figure 4 and Table 2). Filtered seawater (FSW) was used as a negative control. Comparison of means using ANOVA and Dunnet’s test showed that all of the terpenoids significantly inhibited settlement compared with the negative control (P < 0.001) without significant toxicity (P > 0.05). From Dunnet’s test, capsaicin significantly reduced larval settlement at 5 μg/cm2 (P < 0.001), and Compounds 1–6 significantly reduced larval settlement at 0.5–15 μg/cm2 (P < 0.001). Moreover, the non-toxicity compounds were defined as those that do not directly kill fouling organisms at or near the levels at which they deter fouling [32]. In the present study, the LC50 values of these terpenoids were all above 250 μg/cm2. The lower EC50/LC50 ratio indicated that all these terpenoids had the ability to inhibit larval settlement in a non-toxic way. In addition, the stability test showed that Compounds 1 and 3 were degraded while Compounds 2, 4, 5 and 6 remained stable over 2-month exposure under FSW.
Figure 4.
Effects of compounds on settlement and survival of B. albicostatus cyprids. a: Capsaicin; b: 8(14)-enyl-pimar-2′(3′)-en-4′(18′)-en-15′(16′)-en-dolabr-16,15,2′,3′-oxoan- 16-one (1); c: Tagalsin C (2); d: Tagalsin I (3); e: lup-20(29)-ene-3β,28-diol (4); f: 3-oxolup-20(29)-en-28-oic acid (5); g: 28-hydroxylup-20(29)-en-3-one (6). Data were analyzed using one-way ANOVA, where *P < 0.05; **P < 0.01 and ***P < 0.001 were significantly different from the negative control.
Table 2.
Antifouling activity of the investigated terpenoids against B. albicostatus cyprid larvae.
| Compound | Skeleton structure | EC50 (μg/cm2) | LC50 (μg/cm2) |
|---|---|---|---|
| Capsaicin (positive control) | - | 1.32 ± 0.02 b | > 250 |
| 8(14)-enyl-pimar-2′(3′)-en-4′(18′)-en-15′(16′)-en-dolabr-16,15,2′,3′-oxoan-16-one (1) | Dimeric diterpenoid | 13.94 ± 0.81 f | > 250 |
| Tagalsin C (2) | Diterpenoid | 0.65 ± 0.02 a | > 250 |
| Tagalsin I (3) | Dimeric diterpenoid | 11.67 ± 0.47 e | > 250 |
| lup-20(29)-ene-3β,28-diol (4) | Triterpenoid | 9.27 ± 0.30 d | > 250 |
| 3-oxolup-20(29)-en-28-oic acid (5) | Triterpenoid | 3.50 ± 0.24 c | > 250 |
| 28-hydroxylup-20(29)-en-3-one (6) | Triterpenoid | 8.73 ± 0.43 d | > 250 |
Values with different letters in the same column of EC50 are significantly different at p < 0.05 level.
As shown in Table 2, Compounds 1–6 exhibited antifouling activity against cyprid larvae of the barnacle B. albicostatus, with EC50 values ranging from 0.65 to 13.9 μg/cm2. Compound 2 was more active than capsaicin, while Compounds 1, 3, 4, 5 and 6 showed antifouling activity weaker than capsaicin. Among these terpenoids, diterpenoid (Compound 2) showed the best antifouling activity against cyprid larvae of the barnacle B. albicostatus, followed by triterpenoid (Compounds 4, 5 and 6), and then dimeric diterpenoid (Compounds 1 and 3). In addition, the antifouling activity of Compound 3 was stronger than Compound 1, which suggested that the diterpenoid dimmer with two identical diterpenoid subunits might display more potent antifouling activity than the one with two different diterpenoid subunits. By comparing the EC50 value of Compound 5 and Compound 6 and considering that the only difference between the structures of these two compounds is the difference group on the C-28 of lupane triterpenoid, it is evident that the carboxyl group at the C-28 of lupane triterpenoid might have a more potent antifouling activity than the oxygenated methylene group. However, when carbonyl group at the C-3 position in lupane triterpenoid (Compound 6) is substituted by hydroxyl group, the resulting compound (Compound 4) showed almost the same antifouling activity as Compound 6. This result indicated that the hydroxyl group might be another important functional group expressing potent antifouling activity in lupane triterpenoid.
3. Experimental Section
3.1. General
1H-NMR spectra were measured with Varian UNITY PLUS 500 spectrometer (USA). 1H-, 13C- and 2D-NMR spectra were measured with Varian INOVA 600 spectrometer (USA). High-resolution ESI mass spectra data were measured with a Waters Q-TOF MicroTM spectrometer (USA) and were provided by Zhengzhou University, China. Low-resolution ESI mass spectra data were recorded on an AB 3200Q TRAP spectrometer (USA). IR spectra were measured using a Nicolet 380 FT-IR spectrophotometer (USA). The optical rotation data were obtained on a Rudolph Autopol IV polarimeter (USA). 200–300 mesh Silica gel (Qingdao Marine Chemical Factory, Qingdao, China) were used for column chromatography. Compounds were monitored by TLC on GF 254 silica gel (Qingdao Marine Chemical Factory, Qingdao, China). ODS and Sephadex LH-20 were obtained from Pharmacia Co. Capsaicin was purchased from Sigma Chemical Co., USA, with purity above 95%.
3.2. Extraction and Isolation
The roots of C. tagal were collected from Hainan Province, China in July 2005. The air-dried and powdered material (4.1 kg) was extracted with 95% EtOH at room temperature. The extract was concentrated and partitioned sequentially with petroleum ether, ethyl acetate and H2O. The petroleum ether extract (11.5 g) was subjected to a silica gel column, eluted with a petroleum ether–ethyl acetate gradient to obtain 13 fractions (A–M). Fraction C (0.135 g) was chromatographed over silica gel and Sephadex LH-20 repeatedly, and the major fraction obtained was then purified by reversed-phase semi-preparation HPLC (elution with 1:1 hexane-CHCl3) to yield Compound 1 (50 mg). Fraction K (1.059 g) was chromatographed over silica gel and Sephadex LH-20 repeatedly (elution with 30:1 petroleum ether–acetone) to yield Compound 4 (5 mg) and Compound 5 (10 mg). Fraction J (5.3 g) was chromatographed over silica gel repeatedly (elution with 30:1 petroleum ether–acetone) to yield Compound 6 (6 mg). Moreover, the ethyl acetate extract (73.6 g) was subjected to a silica gel column, eluted with a CHCl3–MeOH gradient to obtain 12 fractions (A2-L2). Fraction B2 (8.32 g) was chromatographed over silica gel and Sephadex LH-20 repeatedly. Compound 2 (10 mg) was further separated by silica gel (elution with 40:1 petroleum ether–ethyl acetate) and Compound 3 (53 mg) was purified by silica gel (elution with 2.5:1 hexane-CHCl3).
8(14)-enyl-pimar-2′(3′)-en-4′(18′)-en-15′(16′)-en-dolabr-16,15,2′,3′-oxoan-16-one (1): C40H58O2; yellow oil; [α]D25 = +68.25 (c = 0.50, CHCl3); UV (CHCl3): 214 (2.5905), 224 (3.0367), 234 (2.929), 244 (2.7263). IR νmax (KBr): 1724,1650, 1237, 1001, 798 cm–1; ESI-MS, m/z 571.3 ([M + 1]+), 592.7 [M + Na]+, HR-ESI-MS at m/z 571.4751 ([M + H+]+, C40H58O2+calc. 571.4741 ); 1H-NMR and 13C-NMR data for 1 are listed in Table 1.
3.3. Antifouling Assay
Antifouling efficacies of the six terpenoids isolated from C. tagal were evaluated by the settlement inhibition assay with cyprid larvae of B. albicostatus. B. albicostatus adults were collected from intertidal rocks in Xiamen, China. Adults released the nauplius I and nauplius II stages upon immersion in seawater after dried for 12 h [8]. Naupilar larvae actively swimming towards the light were collected using a pipette. They were cultured in filtered seawater (FSW, 0.22 μm, salinity 30‰, and temperature 25 °C) by feeding with the diatom Chaetoceros muelleri at a concentration of 2.0 × 105 cells·mL−1. The FSW and diet were renewed everyday. After 5~6 days, most of the nauplii had metamorphosed to cyprids, and then the cyprids were collected within 24 h. Antifouling efficacies of the six terpenoids were investigated according to the method of Hellio et al. with a minor modification [33]. The tested compounds were introduced to the glass dishes using CH2Cl2 as carrier solvent. After evaporation of the solvent at room temperature, 30 cyprids and 10 mL FSW were added to each glass dishe. There were three replicates for FSW negative control and each concentration of the tested compounds. Dishes were incubated in dark at 25 °C for 48 h. After that, numbers of larvae which settled, died or did not settle in each replicate were enumerated under a stereomicroscope. Cyprids that did not move and did not respond to a touch with a metal probe were regarded as dead [34]. Cyprids that permanently attached and metamorphosed were counted as settled [33,35]. In addition, the stability of terpenoids was tested using the method of thin layer chromatography (TLC), which were identified by comparison of the spots and Rf values of these terpenoids before and after 2-month exposure in FSW control [17]. Three replicates were set up for each of FSW control. The tested terpenoids were dissolved in CH2Cl2 and evaporated after 2-month exposure in FSW (three replicates). Compounds that showed the same spots and Rf values with before were regarded as stability. Compounds that showed the different spots and Rf values with before were regarded as degradation. Moreover, statistical calculations were performed with the SPSS software package. One-way ANOVA followed by a Dunnett's test for multiple comparisons of treatment means with a negative control was used for the settlement or mortality percentages analysis. The significance level was defined as p < 0.05. Both EC50 (the concentration that reduces the settlement rate by 50% relative to the negative control) and LC50 (the concentration that results in 50% mortality relative to the negative control) were estimated using the Spearman-Karber method with a 95% confidence interval [36,37].
4. Conclusions
One new dimeric diterpenoid and five known terpenoids were isolated from the roots of C. tagal. These terpenoids were able to inhibit cyprid settlement (EC50 values range of 0.65 to 13.9 μg/cm2) without significant toxicity. Moreover, the functional groups on the C-28 position of lupane triterpenoid significantly affect their antifouling activity. The diterpenoid dimmer with two identical diterpenoid subunits might display more potent antifouling activity than the one with two different diterpenoid subunits. Diterpenoid showed the strongest antifouling activity against cyprid larvae of the barnacle B. albicostatus followed by triterpenoid, and then dimeric diterpenoid. The stability test showed that Compounds 2, 4, 5 and 6 remained stable over a 2-month exposure under FSW.
Acknowledgments
This work was supported by program for New Century Excellent Talents in University (NCET-07- 0725), the National Natural Science Foundation of China (40906078).
References
- 1.Richmond MD, Seed R. A review of marine macrofouling communities with special reference to animal fouling. Biofouling. 1991;2:151–168. [Google Scholar]
- 2.Yebra DM, Kiil S, Dam-Johansen K. Antifouling technology-past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004;50:75–104. [Google Scholar]
- 3.Kwong TFN, Miao L, Li X, Qian PY. Novel antifouling and antimicrobial compound from a marine-derived fungus Ampelomyces sp. Mar. Biotechnol. 2006;8:634–640. doi: 10.1007/s10126-005-6146-2. [DOI] [PubMed] [Google Scholar]
- 4.Thomas KV. The environmental fate and behavior of antifouling paint booster biocides: A review. Biofouling. 2001;17:73–86. [Google Scholar]
- 5.Wahl M, Kröger K, Lenz M. Non-toxic protection against epibiosis. Biofouling. 1998;12:205–226. [Google Scholar]
- 6.Wahl M. Marine epibiosis. I. Fouling and antifouling: some basic aspects. Mar. Ecol. Prog. Ser. 1989;58:175–189. [Google Scholar]
- 7.Slattery M, McClintock JB, Heine JN. Chemical defenses in Antarctic soft corals: evidence for antifouling compounds. J. Exp. Mar. Biol. Ecol. 1995;190:61–77. [Google Scholar]
- 8.Maréchal JP, Hellio C. Development of new non-toxic antifouling solutions. Int. J. Mol. Sci. 2009;10:4623–4637. doi: 10.3390/ijms10114623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JD, Feng DQ, Yang ZW, Wang ZC, Qiu Y, Lin YM. Antifouling metabolites from the mangrove plant Ceriops tagal. Molecules. 2008;13:212–219. doi: 10.3390/molecules13020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian PY, Xu Y, Fusetani N. Natural products as antifouling compounds: recent progress and future perspectives. Biofouling. 2010;26:223–234. doi: 10.1080/08927010903470815. [DOI] [PubMed] [Google Scholar]
- 11.Raveendran TV, Limna Mol VP. Natural product antifoulants. Curr. Sci. India. 2009;97:508–520. [Google Scholar]
- 12.Qiu Y, Deng ZW, Xu MJ, Li QS, Lin WH. New A-nor steroids and their antifouling activity from the Chinese marine sponge Acanthella cavernosa. Steroids. 2008;73:1500–1504. doi: 10.1016/j.steroids.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZC, Lin YM, Feng DQ, Ke CH, Lin P, Yan CL, Chen JD. A new atisane-type diterpene from the bark of the mangrove. Molecules. 2009;14:414–422. doi: 10.3390/molecules14010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blunt JW, Copp BR, Hu WP, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Nat. Prod. Rep. 2009;26:170–244. doi: 10.1039/b805113p. [DOI] [PubMed] [Google Scholar]
- 15.Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007;3:408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 16.De Carvalho CCCR, Fernandes P. Production of metabolites as bacterial responses to the marine environment. Mar. Drugs. 2010;8:705–727. doi: 10.3390/md8030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JD, Yi RZ, Sun CL, Feng DQ, Lin YM. Antifouling activity of simple synthetic diterpenoids against larvae of the barnacle Balanus albicostatus Pilsbry. Molecules. 2010;15:8072–8081. doi: 10.3390/molecules15118072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lal AR, Cambie RC, Rutledge PS, Woodgate PD. Ent-Pimarane and ent-abietane diterpenes from Euphorbia fidjiana. Phytochemistry. 1990;29:2239–2246. [Google Scholar]
- 19.Ma GX, Wang TS, Yin L, Pan Y. Two pimarane diterpenoids from Ephemerantha ionchophylla and their evaluation as modulators of the multidrug resistance phenotype. J. Nat. Prod. 1998;61:112–115. doi: 10.1021/np970065o. [DOI] [PubMed] [Google Scholar]
- 20.Luo XD, Wu SH, Ma YB, Wu DG. Ent-pimarane derivatives from Dysoxylum hainanense. Phytochemistry. 2001;57:131–134. doi: 10.1016/s0031-9422(00)00482-9. [DOI] [PubMed] [Google Scholar]
- 21.Xiang Y, Zhang H, Fan CQ, Yue JM. Novel diterpenoids and diterpenoid glycosides from Siegesbeckia orientalis. J. Nat. Prod. 2004;67:1517–1521. doi: 10.1021/np0400407. [DOI] [PubMed] [Google Scholar]
- 22.Koike K, Cordell GA, Farnsworth NR. New cytotoxic diterpenes from Rondeletia panamensis (Rubiaceae) Tetrahedron. 1980;36:1167–1172. [Google Scholar]
- 23.Zhang Y, Deng Z, Gao T, Proksch P, Lin W. Tagalsins A-H, dolabranes-type diterpenes from the mangrove plant, Ceriops tagal. Phytochemistry. 2005;66:1465–1471. doi: 10.1016/j.phytochem.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Nagashima F, Tori M, Asakawa Y. Diterpenoids from the east Malaysian liverwort Schistochila aligera. Phytochemistry. 1991;30:849–851. [Google Scholar]
- 25.Ouyang XW, Wang XC, Yue QX, Hu LH. A new dolabrane-type diterpene from Ceriops tagal. Nat. Prod. Commun. 2010;5:9–12. [PubMed] [Google Scholar]
- 26.Chen JD, Qiu Y, Yang ZW, Lin P, Lin YM. Dimeric diterpenes from the roots of the mangrove plant Ceriops tagal. Helv. Chim Acta. 2008;91:2292–2298. [Google Scholar]
- 27.Zhang Y, Lu Y, Mao L, Proksch P, Lin WH. Tagalsins I and J, two novel tetraterpenoids from the mangrove plant, Ceriops tagal. Org. Lett. 2005;7:3037–3040. doi: 10.1021/ol0509843. [DOI] [PubMed] [Google Scholar]
- 28.Wang XC, Ouyang XW, Hu LH. Three new lupane-type triterpenes from Ceriops tagal. J. Asian Nat. Prod. Res. 2010;12:576–581. doi: 10.1080/10286020.2010.485566. [DOI] [PubMed] [Google Scholar]
- 29.Diqui S, Hafeez F, Begum S, Siddiqui BS. Oleanderol, a new pentacyclic triterpene from the leaves of Nerium oleander. J. Nat. Prod. 1988;51:229–233. [Google Scholar]
- 30.Kuroyanagi M, Shiotsu M, Ebihara T, Kawai H, Ueno A, Fukushima S. Chemical studies on Vibumum awabuki K. Koch. Chem. Pharm. Bull. 1986;34:4012–4017. [Google Scholar]
- 31.Tinto WF, Blair LC, Allj A. Lupane triterpenoids of Salacia cordata. J. Nat. Prod. 1992;55:395–398. [Google Scholar]
- 32.Feng DQ, Ke CH, Li SJ, Lu CY, Guo F. Pyrethroids as promising marine antifoulants: laboratory and field studies. Mar. Biotechnol. 2009;11:153–160. doi: 10.1007/s10126-008-9130-9. [DOI] [PubMed] [Google Scholar]
- 33.Hellio C, Tsoukatou M, Maréchal JP, Aldred N, Beaupoil C, Clare AS, Vagias C, Roussis V. Inhibitory effects of Mediterranean sponge extracts and metabolites on larval settlement of the barnacle Balanus amphitrite. Mar. Biotechnol. 2005;7:297–305. doi: 10.1007/s10126-004-3150-x. [DOI] [PubMed] [Google Scholar]
- 34.Rittschof D, Clare AS, Gerhart DJ, Mary SA, Bonaventura J. Barnacle in vitro assays for biologically active substance: toxicity and settlement inhibition assays using mass cultured Balanus amphitrite amphitrite Darwin. Biofouling. 1992;6:115–122. [Google Scholar]
- 35.Rittschof D, Lai CH, Kok LM, Teo SLM. Pharmaceuticals as antifoulants: concept and principles. Biofouling. 2003;19s:207–212. doi: 10.1080/0892701021000083769. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton MA, Russo RC, Thurston RV. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1977;11:714–719. [Google Scholar]
- 37.Hamilton MA, Russo RC, Thurston RV. Correction to: Hamilton, M.A., Russo, R.C., Thurston, R.V. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1978;12:417. [Google Scholar]