Abstract
ATP-dependent chromatin remodeling factors of the SNF2 family are key components of the cellular machineries that shape and regulate chromatin structure and function. Members of this group of proteins have broad and heterogeneous functions ranging from controlling gene activity, facilitating DNA damage repair, promoting homologous recombination to maintaining genomic stability. Several chromatin remodeling factors are critical components of nucleosome assembly processes, and recent reports have identified specific functions of distinct chromatin remodeling factors in the assembly of variant histones into chromatin. In this review we will discuss the specific roles of ATP-dependent chromatin remodeling factors in determining nucleosome composition and, thus, chromatin fiber properties.
Keywords: chromatin, histone variant, chromatin remodeling factor, centromere, linker histone, chromatin assembly
1. Introduction
Chromatin is an extremely complex structure that serves to compact eukaryotic DNA in order to comply with the size restrictions of the nucleus. In addition, the way in which chromatin is organized and in which its arrangement is modulated endows it with an extraordinary regulatory potential. At its most basic level of organization, chromatin consists of repeating spherical particles termed nucleosomes. Nucleosomes are formed by the wrapping of 147 bp of DNA in 1.7 left-handed superhelical turns around a core of small, evolutionary conserved, highly basic histone proteins [1]. Two molecules each of the histones H3 and H4 interact via the so-called “histone-fold” domains to generate a protein tetramer, which associates with two heterodimers of the histones H2A and H2B to form the nucleosome core [1]. Nucleosomes are connected by short stretches of linker DNA resulting in a fiber with a diameter of ~10 nm that has a beads-on-a-string-like appearance [2,3]. Although this structure may seem uniform from a superficial perspective, a tremendous amount of research during the past decades has provided ample evidence that nucleosomes can differ from each other with respect to their structure, the type of histones that they contain as well as the nature and extent of chemical modifications on both the DNA and histones. In addition, the positioning of the nucleosomes along the DNA can show striking variation, including regular arrangements with constant spacing (e.g., in constitutive heterochromatin), irregular arrays of nucleosomes (typically in active genes) or regions that are devoid or depleted of nucleosomes (e.g., at enhancers and promoters) [4,5]. Importantly, chromatin structure is not static. On the contrary, the organization and composition of chromatin is constantly changing thereby facilitating or preventing access for DNA-utilizing proteins to their substrate. In this review we will discuss some of the mechanisms that contribute to the shaping of chromatin structure not only at the level of the 10 nm fiber but also in higher-order levels of chromatin organization. We will give special attention to the ATP-dependent chromatin remodeling machines and their diverse roles in modulating the composition of nucleosomes and chromatin fibers.
2. Chromatin Remodeling Machines and Their Impact on Nucleosome Structure
Chromatin organization is regulated on various levels and by a multitude of diverse proteins and non-coding RNAs. On one hand, enzyme complexes that use DNA for transcription, replication, recombination or repair actively contribute to changing chromatin structure. For instance, RNA and DNA polymerases travel along the DNA double helix and by doing so introduce torsional stress that can promote the loss of histones ahead of them and facilitate the reassembly of nucleosomes in their wake [6]. Although most of this stress is constantly released by the action of topoisomerases, it is likely that DNA-utilizing processes exert distinct effects on local as well as regional chromatin structure. Other mechanisms that profoundly affect chromatin structure are posttranslational modifications of nucleosomal histones, the incorporation of so-called variant histone proteins and of other non-histone architectural proteins, such as high mobility group (HMG) proteins, as well as the energy-consuming remodeling of nucleosomes by ATP-dependent remodeling machines [7–10]. ATP-dependent chromatin remodeling factors typically are large protein complexes that contain an ATPase subunit, which belongs to the sucrose non-fermenting 2 (SNF2) family of ATPases/helicases [11,12]. SNF2-like ATPases can be grouped into 23 subclasses according to sequence differences in their ATPase domains and the presence of additional protein motifs [11]. The best-studied chromatin remodeling factors belong to the SWI/SNF (switch/sucrose non-fermenting), the ISWI (imitation switch), the CHD (chromo helicase DNA binding) and the INO80 (inositol auxotroph 80) subfamilies [10,13–17].
2.1. The Role of ATP-Dependent Chromatin Remodeling Factors in Nucleosome Positioning
Several recent studies that mapped the positions of nucleosomes at a genome-wide level in different organisms and cell types have reported the existence of rather well conserved patterns of nucleosome occupancy in particular at the 5′ and 3′ ends of genes (e.g., [18–22]). Using micrococcal digestion combined with deep-sequencing technology, it was shown for yeast, Drosophila and humans that promoters are commonly marked by a nucleosome-free or depleted region (NDR) upstream of the transcriptional start site (TSS). Furthermore, the first nucleosome downstream of the TSS (+1 nucleosome) usually occupies a distinct position, which is ~50 bp downstream of the TSS in yeast and at ~ +135 bp in Drosophila and humans [4,5]. Another NDR appears to be distinctive of 3′-ends of genes. Upstream of this NDR a positioned nucleosome is usually detected although the latter appears not to be universally conserved [4,5,23]. Although DNA sequence is likely to influence some of the nucleosome positions, in particular the NDRs, it was postulated that ATP-dependent chromatin remodeling machines play an important role in determining nucleosome positions in vivo [4,5,24]. This is especially likely for nucleosomes that occupy energetically unfavorable positions.
Chromatin remodeling enzymes are well equipped to carry out this task. In many elegant in vitro studies, it has been demonstrated that by using the energy derived from hydrolyzing ATP, these enzymes can break and/or establish histone-DNA contacts. The results of these actions are manifold and dependent on the type of remodeler as well as on the functional context [10,25–27]. Numerous studies exploring the effects of deletion or knock-down of chromatin remodelers have found wide-spread gene regulation defects [28]. These effects can at least in part be attributed to a role of these factors in positioning and remodeling of nucleosomes. Two SNF2 subfamilies in particular, the ISWI and the CHD families, have been shown to be able to move nucleosomes to different translational positions along the DNA (“sliding”) [29–34]. Consistent with this function, ISWI and CHD type enzymes have been shown to be associated with active genes [35–38]. They have roles in remodeling nucleosomes in the vicinity of the TSS [37–40], but they seem also involved in regulating nucleosome positioning at the 3′-end of genes. In yeast it was observed that loss of Isw2 resulted in increased production of non-coding transcripts. These transcripts originated from mis-oriented transcription as a result of aberrant nucleosome positioning at the 3′-end of Isw2 target genes [37]. Likewise, yeast Chd1 was shown to be involved in organizing the nucleosomal fiber at the 3′-end of genes, since deletion of CHD1 resulted in transcription termination defects and aberrant nucleosomal arrangements at the 3′-ends of the CYC1 and ASC1 genes [41]. Very recently, the Mi-2/CHD3-related ATPase Mit1 (Mi2-like protein interacting with Clr three 1), which is part of the SHREC (Snf2/Hdac-containing Repressor Complex) complex in Schizosaccharomyces pombewas shown to profoundly affect nucleosome positioning globally and at specific heterochromatic sites [23,42].
Chromatin remodeling complexes of the SWI/SNF family have also been extensively characterized in vitro and in vivo. One salient feature of this type of remodeler is its ability to disrupt nucleosome structure more profoundly than ISWI and CHD enzymes (e.g., [43–46]). SWI/SNF enzymes can eject histones from nucleosomes, they can transfer dimers and tetramers to other DNA molecules (e.g., [43,47–49]) and they can catalyze nucleosome sliding reactions [10,50]. Thus, in vivo SWI/SNF ATPases have been identified as crucial regulators of gene activation, and they have been shown to be able to generate NDRs [51].
Almost all SNF2-type motors are part of (large) protein complexes. The accessory subunits can gravely impact on the biochemical properties of a remodeler complex. For instance, association of the ISWI motor protein with the ATP-dependent chromatin assembly factor 1 (Acf1) subunit, strongly stimulates the efficiency by which it can assemble and remodel nucleosomes [52]. In a similar manner the chromatin remodeling activity of the SWI/SNF ATPases BRG1 (brahma related gene 1) and hBRM (human brahma) are significantly enhanced by the INI1 (integrase interactor 1) and the brahma-associated factors BAF155 and BAF170 complex subunits [53]. Nevertheless, a recent study demonstrated that the ATPases themselves exhibit strikingly different characteristics with respect to their nucleosome sliding properties. When Drosophila ISWI and CHD1 as well as human Snf2H, Brg1 and Mi-2 (dermatomyositis specific autoantigen Mi-2) were tested side by side in an in vitro sliding assay, each remodeler moved the nucleosome to different positions although the underlying DNA sequence was the same in all cases [34]. Hence, it is conceivable that in vivo different chromatin remodeling factors may establish specific local nucleosome positions in addition to histone displacement. The action of these enzymes, therefore, will not only facilitate but also impede the access of factors to their binding sites on the DNA.
2.2. Chromatin Remodeling Factorsin Replication-Coupled Nucleosome Assembly
During S-phase, when the DNA is replicated, chromatin is completely disassembled and nucleosomes are reformed at the nascent daughter strands. Thereby, newly synthesized histones must be incorporated to complement the “old” histones that are reused in the newly established nucleosomes [54,55]. ATP-dependent factors are likely to adopt a critical position within the DNA replication process. They are known to not only slide and restructure existing nucleosomes but also to mediate the formation of new nucleosomes or change the histone composition of nucleosomes [8,56]. ISWI-containing remodeling complexes, such as ACF (ATP-dependent chromatin assembly and remodeling factor)and RSF (remodeling and spacing factor), and CHD1 have been demonstrated to be able to generate nucleosome arrays in vitro from purified histones and DNA. ACF and CHD1 perform this reaction in conjunction with the histone chaperone NAP-1 (nucleosome assembly protein 1), while RSF does not require a chaperone [52,57–60].
Despite the well-characterized biochemical activities of chromatin remodeling factors it is rather surprising that information about their involvement in replication-coupled chromatin assembly in vivo is still limited. To date, only ISWI-type enzymes have been linked to nucleosome formation during S-phase. In Drosophila the inactivation of the ACF complex by deletion of its Acf1 subunit resulted in an acceleration of S-phase caused by a shortening of heterochromatin replication timing [61]. Similarly, the human ISWI homolog SNF2h was proposed to play a role in replication-coupled heterochromatin assembly [62–64]. In this case, two different SNF2h-containing complexes appear to be important, since knock-down of the ACF1 subunit of the human ACF complex inhibited progression through S-phase [63], while a complex containing Snf2h and the Williams syndrome transcription factor (WSTF) targeted SNF2h to heterochromatin by interaction with proliferating cell nuclear antigen (PCNA), which is a processivity factor of DNA polymerase [62,64]. Thus, ISWI enzymes appear to be involved in replication-coupled heterochromatin assembly. However, in light of more recent studies implicating ISWI in the incorporation of the linker histone H1 (see below), the above-mentioned observations might not fully support this conclusion. A recent report identified the mammalian SNF2-type ATPase SMARCAD1 as an important regulator of global DNA replication-associated histone deacetylation. As a consequence of SMARCAD1 knock-down, heterochromatin establishment, in particular histone H3 lysine 9 trimethylation and HP1 binding was perturbed [65]. Thus, while SMARCAD1 appears to play a crucial role in thedeacetylation of newly incorporated histones, which are acetylated, it seems not to be directly involved in histone deposition. Therefore, to date no chromatin remodeler has been unequivocally demonstrated to mediate the reassembly of either heterochromatin or euchromatin in the course of DNA replication in vivo.
2.3. Incorporation of Linker Histone H1
The linker histone H1 associates with DNA at nucleosome entry/exit sites and thereby affects the folding of the 10 nm nucleosomal fiber into higher-order structures with a diameter of about 30 nm [66,67]. It is assumed that the 30 nm fiber makes chromatin less accessible to DNA binding factors and is thus largely refractory for processes such as transcription. Although several recent studies have made considerable progress in elucidating the structure of in vitro reconstituted 30 nm fibers [68–71], their in vivo organization appears to be heterogeneous and is still poorly understood [72,73]. This may be due in part to the highly dynamic behavior of H1 in vivo. While the core histones H3 and H4 typically remain bound to the chromatin over several cell generations, H1 turn-over occurs within seconds [74–77].
Several lines of evidence point to a critical role for ATP-dependent chromatin remodelers in H1 assembly. First, it was shown that in vitro ACF and ISWI but not the CHD-type factor CHD1 can generate periodic H1-containing nucleosome arrays [58,78,79]. Second, in Drosophila, deletion of ISWI resulted in global decondensation of the transcriptionally hyperactive single X chromosome in salivary glands of male larvae, and overexpression of a dominant negative allele of ISWI led to striking alterations in the appearance of autosomes as well as sex chromosomes. These changes were accompanied by a decrease in chromosomal H1 levels [80,81]. These findings suggest that ISWI is required for the incorporation of H1 into chromatin in vivo. ISWI is part of multiple chromatin remodeling complexes, and in a study of the largest subunit of the ISWI-containing NURF (nucleosome remodeling factor) complex, Nurf301, it was shown that Nurf301 mutant alleles resulted in a decondensation phenotype of the male X chromosome similar to that of an Iswi mutation [82,83] suggesting that NURF might be involved in H1 incorporation. However, Nurf301 mutation also causes the upregulation of roX RNA, which is a central component of the male specific lethal (MSL) complex, which is required for dosis compensation in Drosophila males. Mutations of roX suppressed the puffing phenotype of the Nurf301 mutants [83], and it is not clear at this point whether the derepression of roX in Nurf301 mutants and/or H1 incorporation defects are responsible for the distortions in chromatin structure observed in the absence of functional Nurf301. There is also evidence that another ISWI-containing complex, ACF, might contribute to H1 incorporation. In mutants for the signature subunit of ACF, Acf1, a global shortening of nucleosomal repeat length was observed [61]. Such changes also occur when H1 levels are strongly reduced [77,84,85] and therefore might argue for an involvement of ACF in H1 assembly. Given that Acf1 mutants do not exhibit structural defects on the male X chromosome, it is possible that H1 loading is achieved by the combined actions of different ISWI complexes. Regardless of the type of complex, these studies provide an example that ATP-dependent chromatin remodeling not only affects the structure of the basic nucleosome fiber but also has important functions in modulating higher-order chromatin folding.
2.4. Incorporation of Variant Histones
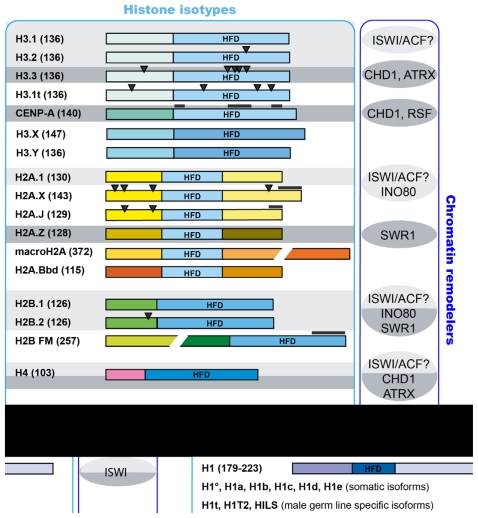
A major manifestation of chromatin dynamics is the constant turn-over of chromosomal histones. Even in post-mitotic cells histones are continually exchanged. During replication-coupled assembly the so-called “canonical” histones are incorporated. These histones are encoded by multiple gene copies in higher eukaryotes, and their expression is tightly controlled to reach its maximum in S-phase [86]. Canonical histones are not incorporated by replication-independent mechanisms. To this end, variant histones are used [87]. Consequently, in post-mitotic cells canonical histones are gradually replaced with histone variants. For instance, measurements in long-lived neurons have shown that ~80% of all H3 histones are of the H3.3 variant type [88]. An important process that requires the replication-independent assembly of histones is transcription. It has been shown that fast and profound histone loss occurs at highly transcribed genes, such as the heat shock protein 70 (Hsp70) genes in Drosophila [39]. Moreover, measurements of the incorporation of GFP-tagged histone H3.3 have revealed that H3.3 accumulates at transcriptionally active sites [89,90]. Some histone variants are highly similar in sequence to their replication-coupled counterparts. For instance, H3.3 differs from H3.2 with only four amino acids. On the other hand, there are histone isotypes, such as macroH2A or H2A.Bbd (H2A Barr body deficient), whose sequence deviates considerably from the canonical histone type (Figure 1) [91–93].
Figure 1.
Schematic representation of mammalian histone isotypes (left panel) and of the respective remodeling enzymes that have been linked to their incorporation (right panel). Numbers in parenthesis represent the amino acid sequence lengths of the histone proteins. Identical colors indicate identical amino acid sequences. Replication-coupled histone incorporation is denoted by light grey shading, replication-independent assembly is indicated by dark grey shading [91–93].
Multiple studies have shown that assembly and exchange of histones require the concerted action of histone chaperones and ATP-dependent chromatin remodeling factors in the context of both replication-coupled as well as replication-independent processes [56,94]. It has also become apparent in recent years that in vivo the incorporation of individual histone variants requires distinct types of ATP-dependent factors together with specialized histone chaperones. Although the mechanisms of incorporation of a number of variants still await discovery, considerable progress has been made in elucidating the critical factors involved in the incorporation of variant histones, such as H3.3, the centromere-specific H3 variant CenH3 and the H2A variant H2A.Z [8,92,95,96].
Regardless of the mechanisms of incorporation, histone variant composition of chromatin correlates with its functional properties and affects chromatin dynamics locally or in a global manner [92]. For instance, H3.3 and the H2A variant H2A.Bbd colocalize predominantly with transcriptionally active chromatin, CenH3 is only found at centromeres, where it generates a chromatin structure suitable to the formation of the kinetochore, and the variant histone macroH2A is distinctive of the transcriptionally silent X-chromosome of female mammals [92]. Different histone isotypes may affect nucleosome and chromatin structure and dynamics in various ways. They may be subject to distinct posttranslational modifications [97], alter interactions with components of the DNA-utilizing machineries, or they may affect the structural properties of variant-containing nucleosomes in a way that makes the underlying DNA sequence more or less permissible for a certain functional state.
2.5. Chromatin Remodelers and H3.3
A recent crystal structure analysis revealed that incorporation of H3.3 into nucleosomes instead of the replication-coupled H3.1 and H3.2 forms does not lead to obvious structural effects on the nucleosome [98,99]. Nevertheless, nucleosomes purified from chicken erythroid cells were found to be less stable when containing H3.3 [99]. Therefore, it was proposed that a destabilization of nucleosomes by H3.3 may promote the accessibility of active genes and regulatory regions [99]. Consistent with this idea is the observation that increased levels of H3.3 are detected over active genes and at transcription factor binding sites [100–102]. Despite its broad distribution in nuclear chromatin, H3.3 is not essential for viability of Drosophila [103,104]. Yet it is required for germ cell development, and male and female flies with mutated H3.3 are sterile [103,104].
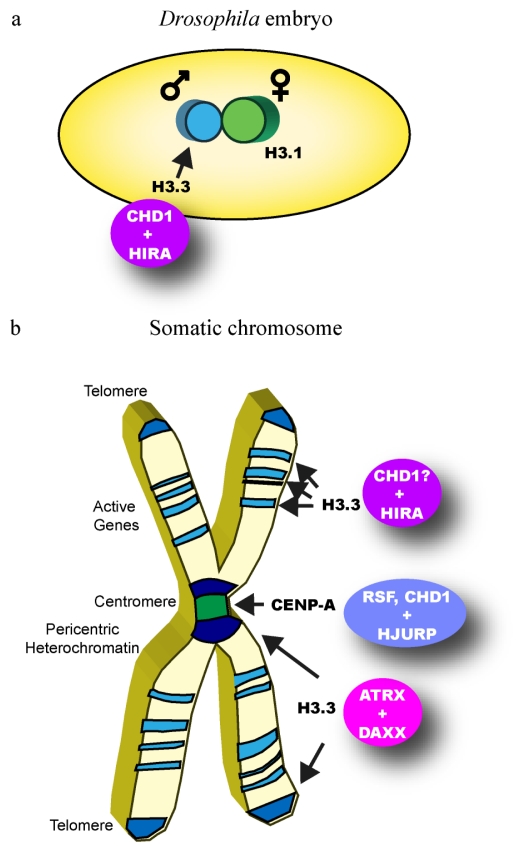
H3.3 is predominantly incorporated co-transcriptionally with the exception of an early instance in development. At fertilization the chromatin of the paternal pronucleus requires drastic reorganization. In this situation, global reassembly of nucleosomes must occur in order to replace sperm-specific DNA packaging proteins, the protamines, that are responsible for organizing sperm chromatin. It has been shown in Drosophila and mouse embryos that during paternal pronuclear rearrangement the histone variant H3.3 but not H3.1 is loaded onto the DNA [105,106]. In Drosophila, the protamine/histone exchange takes place prior to the onset of DNA replication and transcription and thus, H3.3 deposition must be independent of a transcription-linked process. The histone chaperone HIRA (histone cell cycle regulation defective homolog A) was identified as a crucial factor for the loading of H3.3 in this process, since mutation of HIRA abrogated the incorporation of H3.3 into the paternal chromatin [105,107]. Similar but not identical defects were observed when the ATP-dependent chromatin remodeler CHD1 was deleted in the fly. The absence of CHD1 resulted in the accumulation of H3.3 at the nuclear periphery of paternal pronuclei indicating that CHD1 is required for correct deposition of H3.3 [108]. Thus, CHD1 and HIRA appear to work together in the transcription-independent incorporation of H3.3 at this specific developmental instance, a notion that is corroborated by the observation that both factors physically interact in early Drosophila embryos [108] (Figure 2a).
Figure 2.
Replication-independent assembly of histone H3 variants. (a) The chromatin remodeler CHD1 cooperates with the H3.3-specific histone chaperone HIRA to incorporate H3.3 into the paternal pronucleus at fertilization in Drosophila embryos. The maternal pronucleus does not require chromatin reorganization and contains predominantly H3.1. (b) Different chromatin remodeling complexes in conjunction with specific histone chaperones incorporate H3.3 and CENP-A at distinct chromosomal sites. Dark blue shading indicates H3.3 incorporation into telomeric and pericentric heterochromatin, respectively. Lighter blue shading indicates H3.3 assembly at genic locations. Green shading denotes CENP-A incorporation into chromatin at the centromere.
CHD1 may also have a role in the transcription-dependent incorporation of H3.3. This idea is supported by the finding that in Chd1-defective Drosophila embryos, aberrant H3.3 localization is detected in transcriptionally active syncytial nuclei [108]. In addition, knock-down of CHD1 in mouse embryonic stem (ES) cells resulted in compromised pluripotency probably due to an observed decrease of euchromatin and a concomitant spreading of heterochromatin [109]. Although H3.3 incorporation was not tested in this study, the fact that H3.3 normally is enriched in euchromatin may point to a defect in generating proper H3.3-containing nucleosomes. Interestingly, whole-genome H3.3 mapping experiments have revealed that mammalian HIRA is necessary for H3.3 enrichment at active and repressed genes [100,110,111] (Figure 2b).
A number of recent reports have implicated another SNF2-type chromatin remodeling factor, the α-thalassemia/mental retardation syndrome X-linked (ATRX) protein, in H3.3 incorporation into chromatin. ATRX was shown to be required for loading of H3.3 into chromatin at telomeres in mouse ES cells [100,110,111] and at pericentric heterochromatin in mouse embryonic fibroblasts [112] (Figure 2b). Thorough biochemical analyses revealed that ATRX cooperates with a novel H3.3-specific histone chaperone termed DAXX (death domain associated protein) [100,111,112]. Thus, to date two different ATP-dependent chromatin remodeling factors have been implicated in H3.3 incorporation. Both factors function together with distinct chaperones (CHD1 with HIRA, ATRX with DAXX) to generate H3.3-containing nucleosomes in specific nuclear neighborhoods or developmental occasions reflecting a highly complex assembly machinery that enables the formation of functionally distinct chromatin areas (Figure 2b).
2.6. Chromatin Remodelers and the Assembly of Centromeric Chromatin
The histone H3 variant CenH3 (also known as CENP-A, CID, Cnp1, Cse4) is incorporated into chromatin at the centromeres in a transcription-independent fashion [113,114]. Its presence at the centromere is thought to identify the region for kinetochore assembly, since centromeric DNA sequences are not conserved between organisms and therefore not likely to contribute to this task [113,115]. The assembly of centromeric chromatin appears to involve a great number of proteins. Despite considerable research efforts over the past years it is still not entirely clear as to which factors are directly involved in CenH3 assembly and which ones act in an indirect manner [113–116]. For example, a recent study showed that the yeast SWI/SNF complex acts to remove the yeast centromeric histone Cse4 from nucleosomes outside of the centromere. Thus, it acts to confine Cse4 to the single centromeric nucleosome that defines centromeres in Saccharomyces but is not involved in the loading of Cse4 [117]. A large step towards elucidating centromeric chromatin assembly has been made with the discovery of a CenH3-specific histone chaperone, termed HJURP (Holliday junction recognition protein) [118,119]. HJURP has been demonstrated to directly interact with soluble CenH3. Moreover, knock-down of HJURP led to the loss of CenH3 signals at the centromere [118,119]. Interestingly, HJURP is distantly related to the Saccharomyces cerevisiae Scm3 protein, which also has been shown to act as a CenH3 chaperone [120,121], but does not have any apparent homologs in Drosophila [122].
Among the ATP-dependent factors the ISWI-containing complex RSF was reported to interact with CenH3-containing mononucleosomes in human cells and to play a role in the incorporation of human CenH3 [123]. However, as the effects of RSF knock-down are relatively mild, it is likely that there are additional proteins involved [123]. Indeed, in chicken DT40 cells as well as in fission yeast, CHD1 and its homolog Hrp1, respectively, have been linked to a role in the assembly of CenH3-containing nucleosomes [124,125]. In contrast, no such role could be demonstrated for CHD1 in Drosophila [126]. Similarly, no centromere defects have been reported for Drosophila Rsf1 mutants [127]). Thus, flies appear to neither possess a bona fide HJURP homolog, nor are the roles of CHD1 and RSF in CenH3 incorporation conserved. Instead, a Drosophila-specific factor, termed CAL1 (chromosome alignment defect 1), was demonstrated to interact with CenH3 and to be required for its loading to chromatin [128]. To date, no ATP-dependent factor was found to participate in this process. These results indicate that different organisms might use different mechanisms and factors to ensure CenH3 assembly at centromeres.
2.7. Chromatin Remodelers and H2A.Z Exchange
The replacement of H2A/H2B dimers in nucleosomes with dimers containing the variant histone H2A.Z/H2B is a common event in all eukaryotes. Its importance is emphasized by the fact that H2A.Z is essential for viability in Drosophila, Tetrahymena and mouse [129–131]. Incorporation of H2A.Z into nucleosomes does not result in large structural alterations, but nevertheless causes some intriguing changes. On one hand, H2A.Z-containing nucleosomes possess a larger acidic patch at the surface of the octamer that was proposed to serve in the interaction with the H4 N-terminal tail of a neighboring nucleosome [132]. Indeed, in vitro H2A.Z-containing nucleosome arrays were shown to be more tightly compacted than H2A-containing nucleosomes [133]. These observations are consistent with the results from genome-wide analyses of H2A.Z distribution that found that H2A.Z is present in heterochromatic areas of the genome [134,135].
On the other hand, however, the interface between H2A.Z/H2B and H3/H4 dimers in the crystal structure was found to be slightly less stable than in H2A-nucleosomes and thus may render these nucleosomes more prone to disruption [132]. Aside from heterochromatic sites, H2A.Z is particularly enriched in nucleosomes at the transcription start sites of genes [99,136]. In line with the predictions of the crystal structure analysis, H2A.Z-containing nucleosomes isolated from chicken erythroid cells displayed reduced stability [99,136]. However, H2A.Z-containing nucleosomes were only less stable when they simultaneously contained H3.3 but not when they contained H3 [99,136]. Hence, it appears that the combination of H2A.Z with either H3.3 or H3.1 confers quite distinct properties to these nucleosomes. This may in part explain the seemingly contradictory presence of H2A.Z in heterochromatin and euchromatin.
H2A.Z replacement is carried out by a dedicated ATP-dependent remodeler, termed SWR1 [137–141] (Figure 1). SWR1 belongs to the INO80 subclass of chromatin remodelers and is characterized by a split ATPase domain [15]. It is part of a multiprotein complex, which also contains subunits necessary for H2A.Z recognition and for binding to acetylated H3/H4 [142]. A recently published study demonstrated that the second member of the INO80 subfamily, INO80, also affects H2A.Z-containing nucleosomes [143] (Figure 1). Interestingly, INO80 performs the opposite reaction to SWR1 by catalyzing the exchange of H2A.Z/H2B dimers for H2A/H2B. Deletion of INO80 in yeast resulted in aberrant localization of H2A.Z in promoter and coding regions. Moreover, replication fork progression defects of δino80 mutants were alleviated by reduced expression of H2A.Z, suggesting that the misincorporation of H2A.Z in the absence of INO80 causes the observed defect [143].
A number of histone chaperones have been implicated in H2A.Z dynamics. Nap1 was shown to enable H2A.Z/H2B dimer exchange in an in vitro reaction [144] and was also detected in purified SWR1 complex fractions [137]. In S. cerevisiae, another H2A.Z-specific chaperone, termed Chz1, was identified, which together with Nap1 represents the two major H2A.Z/H2B chaperones in this organism [145]. Interestingly in the absence of both Chz1 and Nap1 additional proteins, such as the FACT complex, the karyopherin Kap114 and two peptidylprolyl cis-trans isomerases termed Fpr3 and Fpr4, were shown to interact with H2A.Z/H2B dimers [145]. Further studies in yeast indicated that NAP-1 is important for chaperoning the soluble pool of H2A.Z, whereas Chz1 does not interact with H2A.Z in the cytoplasm [146].
3. Do Chromatin Remodeling Factors Incorporate Non-Histone Chromosomal Proteins?
As discussed above, ATP-dependent remodeling factors are crucial components of the machineries that deposit histones and generate patterns of nucleosomes with diverse composition (Figure 1). Apart from histones and their variants, however, there are other abundant non-histone architectural proteins, such as HMG proteins or heterochromatin protein 1 (HP1), that associate with the chromatin and shape its structure and dynamics. Do these factors also require motor proteins to bind correctly to the nucleosome fiber? Although this intriguing question has not been investigated in great detail so far, some recent studies provide evidence that suggests this may indeed be the case.
The HMG proteins are among those non-histone architectural proteins that have been studied most extensively [9,147,148]. HMG proteins generally act to decrease the compactness of the chromatin fiber and therefore render chromatin more accessible to regulatory factors [147,149]. They bind to chromatin in a highly dynamic and reversible way either by directly contacting the nucleosome and/or via co-factors. There are three subfamilies of HMG proteins, termed HMGA, HMGB and HMGN [9,148]. Members of the HMGN group, in particular, have been shown to bind to nucleosomes at the entry/exit sites of the DNA and therefore compete with the linker histone H1 for nucleosome binding sites [150,151]. They also exhibit exchange dynamics that are similar to those of H1 [152]. As detailed above, H1 incorporation into chromatin is strongly dependent on the ISWI chromatin assembly factor [61,81]. By analogy, HMGN proteins might also require an ATP-dependent factor for efficient chromatin association. In a recent study addressing the effects of HMGN1 and HMGN2 on chromatin remodeling by the ATP-dependent factors ACF and the SWI/SNF-family protein BRG1, it was shown that ACF can assemble extended periodic nucleosome arrays containing HMGN proteins in vitro [153]. Although in vivo studies have not yet been carried out, these experiments provide an intriguing hint for a possible function of ACF and potentially other chromatin remodeling factors in the assembly of not only histones but also of non-histone architectural proteins into chromatin.
Another candidate remodeling factor for the incorporation of non-histone chromosomal proteins may be ATRX. As discussed above, ATRX has recently been characterized to be required for the incorporation of histone H3.3 into pericentric and telomeric chromatin [100,110–112]. Yet, ATRX has also been shown to physically interact with HP1, which is an abundant protein localized in heterochromatin [154–157]. Two recent studies provided evidence for a function of ATRX in the loading of HP1 to chromatin. In Drosophila, deletion of ATRX resulted in the loss of HP1α from pericentricβ-heterochromatin [157]. Along the same lines, depletion of ATRX in mouse ES cells led to a strong decrease of HP1α localization at telomeric chromatin [110]. Although these findings point to a role of ATRX in the association of HP1α with heterochromatin, biochemical studies will be necessary to determine, if indeed ATRX uses its catalytic activity to incorporate HP1 or if the observed phenotypes are the result of recruitment defects. Previous in vitro experiments have demonstrated that the ACF remodeling factor greatly stimulates the association of HP1 with reconstituted nucleosome arrays. This stimulation, however, was found to be dependent on the Acf1 subunit of the complex and did not involve ATP-hydrolysis [158]. Nevertheless, although the evidence is somewhat circumstantial at the moment, it will be interesting to elucidate whether non-histone architectural proteins are actual targets of ATP-dependent chromatin remodeling machines.
4. Conclusion
Nucleosome assembly is not only necessary for preserving chromatin structure and, thus, genome integrity, it is also a process that strongly impacts on the functional properties of the nucleosomal fiber. In particular, the incorporation of specific histone variants into nucleosomes on one hand serves to modulate the biophysical properties of nucleosomes but can also endow nucleosomes with distinct abilities to interact with regulatory factors or to receive specific posttranslational modification marks. Moreover, the presence of histone variants at functionally distinct regions in the genome has been postulated to serve as a means to transmit epigenetic information across cell generations [159,160]. With the discovery of several novel histone incorporation pathways over the past years, it has become clear that ATP-dependent chromatin remodeling factors in conjunction with specific histone chaperones act at the center of these processes. It will be interesting to see in the future, whether dedicated partnerships of ATP-dependent motor proteins with histone chaperones exist for the assembly of all histone variants and even non-histone architectural proteins and what the biological consequences of their actions are.
Acknowledgments
We apologize to all colleagues whose work we could not cite due to space limitations. We are grateful to Peter Loidl for critical reading of the manuscript. Research in the authors’ lab is supported by the Austrian Science Fund (FWF):START Y275-B12; F4408-B19.
References
- 1.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Olins AL, Olins DE. Spheroid chromatin units (v bodies) Science. 1974;183:330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- 3.Woodcock CL, Safer JP, Stanchfield JE. Structural repeating units in chromatin. I. Evidence for their general occurrence. Exp. Cell Res. 1976;97:101–110. doi: 10.1016/0014-4827(76)90659-5. [DOI] [PubMed] [Google Scholar]
- 4.Jiang C, Pugh BF. Nucleosome positioning and gene regulation: advances through genomics. Nat. Rev. Genet. 2009;10:161–172. doi: 10.1038/nrg2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radman-Livaja M, Rando OJ. Nucleosome positioning: how is it established, and why does it matter? Dev. Biol. 2010;339:258–266. doi: 10.1016/j.ydbio.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavelle C. Transcription elongation through a chromatin template. Biochimie. 2007;89:516–527. doi: 10.1016/j.biochi.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Jin J, Cai Y, Li B, Conaway RC, Workman JL, Conaway JW, Kusch T. In and out: histone variant exchange in chromatin. Trends Biochem. Sci. 2005;30:680–687. doi: 10.1016/j.tibs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Hock R, Furusawa T, Ueda T, Bustin M. HMG chromosomal proteins in development and disease. Trends Cell Biol. 2007;17:72–79. doi: 10.1016/j.tcb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- 11.Flaus A, Martin DM, Barton GJ, Owen-Hughes T. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006;34:2887–2905. doi: 10.1093/nar/gkl295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorbalenya AE. Helicases: amino acid sequence comparisons and structure-function relationships. Curr. Opin. Struct. Biol. 1993;3:419–429. [Google Scholar]
- 13.Eberharter A, Becker PB. ATP-dependent nucleosome remodelling: factors and functions. J. Cell Sci. 2004;117:3707–3711. doi: 10.1242/jcs.01175. [DOI] [PubMed] [Google Scholar]
- 14.Lusser A, Kadonaga JT. Chromatin remodeling by ATP-dependent molecular machines. Bioessays. 2003;25:1192–1200. doi: 10.1002/bies.10359. [DOI] [PubMed] [Google Scholar]
- 15.Bao Y, Shen X. INO80 subfamily of chromatin remodeling complexes. Mutat. Res. 2007;618:18–29. doi: 10.1016/j.mrfmmm.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marfella CG, Imbalzano AN. The Chd family of chromatin remodelers. Mutat. Res. 2007;618:30–40. doi: 10.1016/j.mrfmmm.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21:396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan GC, Liu YJ, Dion MF, Slack MD, Wu LF, Altschuler SJ, Rando OJ. Genome-scale identification of nucleosome positions in S. cerevisiae. Science. 2005;309:626–630. doi: 10.1126/science.1112178. [DOI] [PubMed] [Google Scholar]
- 19.Mavrich TN, Ioshikhes IP, Venters BJ, Jiang C, Tomsho LP, Qi J, Schuster SC, Albert I, Pugh BF. A barrier nucleosome model for statistical positioning of nucleosomes throughout the yeast genome. Genome Res. 2008;18:1073–1083. doi: 10.1101/gr.078261.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mavrich TN, Jiang C, Ioshikhes IP, Li X, Venters BJ, Zanton SJ, Tomsho LP, Qi J, Glaser RL, Schuster SC, et al. Nucleosome organization in the Drosophila genome. Nature. 2008;453:358–362. doi: 10.1038/nature06929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valouev A, Ichikawa J, Tonthat T, Stuart J, Ranade S, Peckham H, Zeng K, Malek JA, Costa G, McKernan K, et al. A high-resolution, nucleosome position map of C. elegans reveals a lack of universal sequence-dictated positioning. Genome Res. 2008;18:1051–1063. doi: 10.1101/gr.076463.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schones DE, Cui K, Cuddapah S, Roh TY, Barski A, Wang Z, Wei G, Zhao K. Dynamic regulation of nucleosome positioning in the human genome. Cell. 2008;132:887–898. doi: 10.1016/j.cell.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lantermann AB, Straub T, Stralfors A, Yuan GC, Ekwall K, Korber P. Schizosaccharomyces pombe genome-wide nucleosome mapping reveals positioning mechanisms distinct from those of Saccharomyces cerevisiae. Nat. Struct. Mol. Biol. 2010;17:251–257. doi: 10.1038/nsmb.1741. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Wippo CJ, Wal M, Ward E, Korber P, Pugh BF. A packing mechanism for nucleosome organization reconstituted across a eukaryotic genome. Science. 2011;332:977–980. doi: 10.1126/science.1200508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flaus A, Owen-Hughes T. Mechanisms for ATP-dependent chromatin remodelling: the means to the end. FEBS J. 2011;278:3579–3595. doi: 10.1111/j.1742-4658.2011.08281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langst G, Becker PB. Nucleosome remodeling: one mechanism, many phenomena? Biochim. Biophys. Acta. 2004;1677:58–63. doi: 10.1016/j.bbaexp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Morettini S, Podhraski V, Lusser A. ATP-dependent chromatin remodeling enzymes and their various roles in cell cycle control. Front. Biosci. 2008;13:5522–5532. doi: 10.2741/3096. [DOI] [PubMed] [Google Scholar]
- 28.Moshkin YM, Mohrmann L, van Ijcken WF, Verrijzer CP. Functional differentiation of SWI/SNF remodelers in transcription and cell cycle control. Mol. Cell. Biol. 2007;27:651–661. doi: 10.1128/MCB.01257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guschin D, Wade PA, Kikyo N, Wolffe AP. ATP-Dependent histone octamer mobilization and histone deacetylation mediated by the Mi-2 chromatin remodeling complex. Biochemistry (Mosc. ) 2000;39:5238–5245. doi: 10.1021/bi000421t. [DOI] [PubMed] [Google Scholar]
- 30.Brehm A, Langst G, Kehle J, Clapier CR, Imhof A, Eberharter A, Muller J, Becker PB. dMi-2 and ISWI chromatin remodelling factors have distinct nucleosome binding and mobilization properties. EMBO J. 2000;19:4332–4341. doi: 10.1093/emboj/19.16.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockdale C, Flaus A, Ferreira H, Owen-Hughes T. Analysis of nucleosome repositioning by yeast ISWI and Chd1 chromatin remodeling complexes. J. Biol. Chem. 2006;281:16279–16288. doi: 10.1074/jbc.M600682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langst G, Bonte EJ, Corona DF, Becker PB. Nucleosome movement by CHRAC and ISWI without disruption or trans- displacement of the histone octamer. Cell. 1999;97:843–852. doi: 10.1016/s0092-8674(00)80797-7. [DOI] [PubMed] [Google Scholar]
- 33.Hamiche A, Sandaltzopoulos R, Gdula DA, Wu C. ATP-dependent histone octamer sliding mediated by the chromatin remodeling complex NURF. Cell. 1999;97:833–842. doi: 10.1016/s0092-8674(00)80796-5. [DOI] [PubMed] [Google Scholar]
- 34.Rippe K, Schrader A, Riede P, Strohner R, Lehmann E, Langst G. DNA sequence- and conformation-directed positioning of nucleosomes by chromatin-remodeling complexes. Proc. Natl. Acad. Sci. USA. 2007;104:15635–15640. doi: 10.1073/pnas.0702430104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stokes DG, Tartof KD, Perry RP. CHD1 is concentrated in interbands and puffed regions of Drosophila polytene chromosomes. Proc. Natl. Acad. Sci. USA. 1996;93:7137–7142. doi: 10.1073/pnas.93.14.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasan S, Armstrong JA, Deuring R, Dahlsveen IK, McNeill H, Tamkun JW. The Drosophila trithorax group protein Kismet facilitates an early step in transcriptional elongation by RNA Polymerase II. Development. 2005;132:1623–1635. doi: 10.1242/dev.01713. [DOI] [PubMed] [Google Scholar]
- 37.Whitehouse I, Tsukiyama T. Antagonistic forces that position nucleosomes in vivo. Nat. Struct. Mol. Biol. 2006;13:633–640. doi: 10.1038/nsmb1111. [DOI] [PubMed] [Google Scholar]
- 38.Sala A, Toto M, Pinello L, Gabriele A, Di Benedetto V, Ingrassia AM, Lo Bosco G, Di Gesu V, Giancarlo R, Corona DF. Genome-wide characterization of chromatin binding and nucleosome spacing activity of the nucleosome remodelling ATPase ISWI. EMBO J. 2011;30:1766–1777. doi: 10.1038/emboj.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petesch SJ, Lis JT. Rapid, transcription-independent loss of nucleosomes over a large chromatin domain at Hsp70 loci. Cell. 2008;134:74–84. doi: 10.1016/j.cell.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morettini S, Tribus M, Zeilner A, Sebald J, Campo-Fernandez B, Scheran G, Worle H, Podhraski V, Fyodorov DV, Lusser A. The chromodomains of CHD1 are critical for enzymatic activity but less important for chromatin localization. Nucleic Acids Res. 2011;39:3103–3115. doi: 10.1093/nar/gkq1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alen C, Kent NA, Jones HS, O’Sullivan J, Aranda A, Proudfoot NJ. A role for chromatin remodeling in transcriptional termination by RNA polymerase II. Mol. Cell. 2002;10:1441–1452. doi: 10.1016/s1097-2765(02)00778-5. [DOI] [PubMed] [Google Scholar]
- 42.Sugiyama T, Cam HP, Sugiyama R, Noma K, Zofall M, Kobayashi R, Grewal SI. SHREC, an effector complex for heterochromatic transcriptional silencing. Cell. 2007;128:491–504. doi: 10.1016/j.cell.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 43.Kwon H, Imbalzano AN, Khavari PA, Kingston RE, Green MR. Nucleosome disruption and enhancement of activator binding by a human SW1/SNF complex. Nature. 1994;370:477–481. doi: 10.1038/370477a0. [DOI] [PubMed] [Google Scholar]
- 44.Cairns BR, Lorch Y, Li Y, Zhang M, Lacomis L, Erdjument-Bromage H, Tempst P, Du J, Laurent B, Kornberg RD. RSC, an essential, abundant chromatin-remodeling complex. Cell. 1996;87:1249–1260. doi: 10.1016/s0092-8674(00)81820-6. [DOI] [PubMed] [Google Scholar]
- 45.Cote J, Quinn J, Workman JL, Peterson CL. Stimulation of GAL4 derivative binding to nucleosomal DNA by the yeast SWI/SNF complex. Science. 1994;265:53–60. doi: 10.1126/science.8016655. [DOI] [PubMed] [Google Scholar]
- 46.Imbalzano AN, Schnitzler GR, Kingston RE. Nucleosome disruption by human SWI/SNF is maintained in the absence of continued ATP hydrolysis. J. Biol. Chem. 1996;271:20726–20733. doi: 10.1074/jbc.271.34.20726. [DOI] [PubMed] [Google Scholar]
- 47.Lorch Y, Zhang M, Kornberg RD. RSC unravels the nucleosome. Mol. Cell. 2001;7:89–95. doi: 10.1016/s1097-2765(01)00157-5. [DOI] [PubMed] [Google Scholar]
- 48.Owen-Hughes T, Utley RT, Cote J, Peterson CL, Workman JL. Persistent site-specific remodeling of a nucleosome array by transient action of the SWI/SNF complex. Science. 1996;273:513–516. doi: 10.1126/science.273.5274.513. [DOI] [PubMed] [Google Scholar]
- 49.Dechassa ML, Sabri A, Pondugula S, Kassabov SR, Chatterjee N, Kladde MP, Bartholomew B. SWI/SNF has intrinsic nucleosome disassembly activity that is dependent on adjacent nucleosomes. Mol. Cell. 2010;38:590–602. doi: 10.1016/j.molcel.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitehouse I, Flaus A, Cairns BR, White MF, Workman JL, Owen-Hughes T. Nucleosome mobilization catalysed by the yeast SWI/SNF complex. Nature. 1999;400:784–787. doi: 10.1038/23506. [DOI] [PubMed] [Google Scholar]
- 51.Hartley PD, Madhani HD. Mechanisms that specify promoter nucleosome location and identity. Cell. 2009;137:445–458. doi: 10.1016/j.cell.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito T, Levenstein ME, Fyodorov DV, Kutach AK, Kobayashi R, Kadonaga JT. ACF consists of two subunits, Acf1 and ISWI, that function cooperatively in the ATP-dependent catalysis of chromatin assembly. Genes Dev. 1999;13:1529–1539. doi: 10.1101/gad.13.12.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phelan ML, Sif S, Narlikar GJ, Kingston RE. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol. Cell. 1999;3:247–253. doi: 10.1016/s1097-2765(00)80315-9. [DOI] [PubMed] [Google Scholar]
- 54.Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128:721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 55.Ransom M, Dennehey BK, Tyler JK. Chaperoning histones during DNA replication and repair. Cell. 2010;140:183–195. doi: 10.1016/j.cell.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lusser A, Kadonaga JT. Strategies for the reconstitution of chromatin. Nat. Methods. 2004;1:19–26. doi: 10.1038/nmeth709. [DOI] [PubMed] [Google Scholar]
- 57.LeRoy G, Loyola A, Lane WS, Reinberg D. Purification and characterization of a human factor that assembles and remodels chromatin. J. Biol. Chem. 2000;275:14787–14790. doi: 10.1074/jbc.C000093200. [DOI] [PubMed] [Google Scholar]
- 58.Lusser A, Urwin DL, Kadonaga JT. Distinct activities of CHD1 and ACF in ATP-dependent chromatin assembly. Nat. Struct. Mol. Biol. 2005;12:160–166. doi: 10.1038/nsmb884. [DOI] [PubMed] [Google Scholar]
- 59.Torigoe SE, Urwin DL, Ishii H, Smith DE, Kadonaga JT. Identification of a Rapidly Formed Nonnucleosomal Histone-DNA Intermediate that Is Converted into Chromatin by ACF. Mol. Cell. 2011;43:638–648. doi: 10.1016/j.molcel.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson KM, Schultz MC. Replication-independent assembly of nucleosome arrays in a novel yeast chromatin reconstitution system involves antisilencing factor Asf1p and chromodomain protein Chd1p. Mol. Cell. Biol. 2003;23:7937–7946. doi: 10.1128/MCB.23.22.7937-7946.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fyodorov DV, Blower MD, Karpen GH, Kadonaga JT. Acf1 confers unique activities to ACF/CHRAC and promotes the formation rather than disruption of chromatin in vivo. Genes Dev. 2004;18:170–183. doi: 10.1101/gad.1139604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poot RA, Bozhenok L, van den Berg DL, Steffensen S, Ferreira F, Grimaldi M, Gilbert N, Ferreira J, Varga-Weisz PD. The Williams syndrome transcription factor interacts with PCNA to target chromatin remodelling by ISWI to replication foci. Nat. Cell Biol. 2004;6:1236–1244. doi: 10.1038/ncb1196. [DOI] [PubMed] [Google Scholar]
- 63.Collins N, Poot RA, Kukimoto I, Garcia-Jimenez C, Dellaire G, Varga-Weisz PD. An ACF1-ISWI chromatin-remodeling complex is required for DNA replication through heterochromatin. Nat. Genet. 2002;32:627–632. doi: 10.1038/ng1046. [DOI] [PubMed] [Google Scholar]
- 64.Bozhenok L, Wade PA, Varga-Weisz P. WSTF-ISWI chromatin remodeling complex targets heterochromatic replication foci. EMBO J. 2002;21:2231–2241. doi: 10.1093/emboj/21.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rowbotham SP, Barki L, Neves-Costa A, Santos F, Dean W, Hawkes N, Choudhary P, Will WR, Webster J, Oxley D, et al. Maintenance of silent chromatin through replication requires SWI/SNF-like chromatin remodeler SMARCAD1. Mol. Cell. 2011;42:285–296. doi: 10.1016/j.molcel.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 66.Thoma F, Koller T, Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J. Cell Biol. 1979;83:403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marsden MP, Laemmli UK. Metaphase chromosome structure: evidence for a radial loop model. Cell. 1979;17:849–858. doi: 10.1016/0092-8674(79)90325-8. [DOI] [PubMed] [Google Scholar]
- 68.Routh A, Sandin S, Rhodes D. Nucleosome repeat length and linker histone stoichiometry determine chromatin fiber structure. Proc. Natl. Acad. Sci. USA. 2008;105:8872–8877. doi: 10.1073/pnas.0802336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson PJ, Rhodes D. Structure of the “30 nm” chromatin fibre: a key role for the linker histone. Curr. Opin. Struct. Biol. 2006;16:336–343. doi: 10.1016/j.sbi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Robinson PJ, Fairall L, Huynh VA, Rhodes D. EM measurements define the dimensions of the “30-nm” chromatin fiber: evidence for a compact, interdigitated structure. Proc. Natl. Acad. Sci. USA. 2006;103:6506–6511. doi: 10.1073/pnas.0601212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schalch T, Duda S, Sargent DF, Richmond TJ. X-ray structure of a tetranucleosome and its implications for the chromatin fibre. Nature. 2005;436:138–141. doi: 10.1038/nature03686. [DOI] [PubMed] [Google Scholar]
- 72.Woodcock CL, Horowitz RA. Chromatin organization re-viewed. Trends Cell Biol. 1995;5:272–277. doi: 10.1016/s0962-8924(00)89038-8. [DOI] [PubMed] [Google Scholar]
- 73.Horowitz RA, Agard DA, Sedat JW, Woodcock CL. The three-dimensional architecture of chromatin in situ: electron tomography reveals fibers composed of a continuously variable zig-zag nucleosomal ribbon. J. Cell Biol. 1994;125:1–10. doi: 10.1083/jcb.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kimura H, Cook PR. Kinetics of core histones in living human cells: little exchange of H3 and H4 and some rapid exchange of H2B. J. Cell Biol. 2001;153:1341–1353. doi: 10.1083/jcb.153.7.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Misteli T, Gunjan A, Hock R, Bustin M, Brown DT. Dynamic binding of histone H1 to chromatin in living cells. Nature. 2000;408:877–881. doi: 10.1038/35048610. [DOI] [PubMed] [Google Scholar]
- 76.Lever MA, Th’ng JP, Sun X, Hendzel MJ. Rapid exchange of histone H1.1 on chromatin in living human cells. Nature. 2000;408:873–876. doi: 10.1038/35048603. [DOI] [PubMed] [Google Scholar]
- 77.Siriaco G, Deuring R, Chioda M, Becker PB, Tamkun JW. Drosophila ISWI regulates the association of histone H1 with interphase chromosomes in vivo. Genetics. 2009;182:661–669. doi: 10.1534/genetics.109.102053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fyodorov DV, Kadonaga JT. Chromatin assembly in vitro with purified recombinant ACF and NAP-1. Methods Enzymol. 2003;371:499–515. doi: 10.1016/S0076-6879(03)71037-4. [DOI] [PubMed] [Google Scholar]
- 79.Maier VK, Chioda M, Rhodes D, Becker PB. ACF catalyses chromatosome movements in chromatin fibres. EMBO J. 2008;27:817–826. doi: 10.1038/sj.emboj.7601902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deuring R, Fanti L, Armstrong JA, Sarte M, Papoulas O, Prestel M, Daubresse G, Verardo M, Moseley SL, Berloco M, et al. The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo. Mol. Cell. 2000;5:355–365. doi: 10.1016/s1097-2765(00)80430-x. [DOI] [PubMed] [Google Scholar]
- 81.Corona DF, Siriaco G, Armstrong JA, Snarskaya N, McClymont SA, Scott MP, Tamkun JW. ISWI regulates higher-order chromatin structure and histone H1 assembly in vivo. PLoS Biol. 2007;5:e232. doi: 10.1371/journal.pbio.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Badenhorst P, Voas M, Rebay I, Wu C. Biological functions of the ISWI chromatin remodeling complex NURF. Genes Dev. 2002;16:3186–3198. doi: 10.1101/gad.1032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bai X, Larschan E, Kwon SY, Badenhorst P, Kuroda MI. Regional control of chromatin organization by noncoding roX RNAs and the NURF remodeling complex in Drosophila melanogaster. Genetics. 2007;176:1491–1499. doi: 10.1534/genetics.107.071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fan Y, Nikitina T, Morin-Kensicki EM, Zhao J, Magnuson TR, Woodcock CL, Skoultchi AI. H1 linker histones are essential for mouse development and affect nucleosome spacing in vivo. Mol. Cell. Biol. 2003;23:4559–4572. doi: 10.1128/MCB.23.13.4559-4572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu X, Wontakal SN, Emelyanov AV, Morcillo P, Konev AY, Fyodorov DV, Skoultchi AI. Linker histone H1 is essential for Drosophila development, the establishment of pericentric heterochromatin, and a normal polytene chromosome structure. Genes Dev. 2009;23:452–465. doi: 10.1101/gad.1749309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gunjan A, Paik J, Verreault A. Regulation of histone synthesis and nucleosome assembly. Biochimie. 2005;87:625–635. doi: 10.1016/j.biochi.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 87.Henikoff S, Ahmad K. Assembly of variant histones into chromatin. Annu. Rev. Cell Dev. Biol. 2005;21:133–153. doi: 10.1146/annurev.cellbio.21.012704.133518. [DOI] [PubMed] [Google Scholar]
- 88.Pina B, Suau P. Changes in histones H2A and H3 variant composition in differentiating and mature rat brain cortical neurons. Dev. Biol. 1987;123:51–58. doi: 10.1016/0012-1606(87)90426-x. [DOI] [PubMed] [Google Scholar]
- 89.Ahmad K, Henikoff S. Histone H3 variants specify modes of chromatin assembly. Proc. Natl. Acad. Sci. USA. 2002;99:16477–16484. doi: 10.1073/pnas.172403699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmad K, Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell. 2002;9:1191–1200. doi: 10.1016/s1097-2765(02)00542-7. [DOI] [PubMed] [Google Scholar]
- 91.Bernstein E, Hake SB. The nucleosome: a little variation goes a long way. Biochem. Cell Biol. 2006;84:505–517. doi: 10.1139/o06-085. [DOI] [PubMed] [Google Scholar]
- 92.Talbert PB, Henikoff S. Histone variants–ancient wrap artists of the epigenome. Nat. Rev. Mol. Cell Biol. 2010;11:264–275. doi: 10.1038/nrm2861. [DOI] [PubMed] [Google Scholar]
- 93.Wiedemann SM, Mildner SN, Bonisch C, Israel L, Maiser A, Matheisl S, Straub T, Merkl R, Leonhardt H, Kremmer E, et al. Identification and characterization of two novel primate-specific histone H3 variants, H3.X and H3.Y. J. Cell Biol. 2010;190:777–791. doi: 10.1083/jcb.201002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haushalter KA, Kadonaga JT. Chromatin assembly by DNA-translocating motors. Nat. Rev. Mol. Cell Biol. 2003;4:613–620. doi: 10.1038/nrm1177. [DOI] [PubMed] [Google Scholar]
- 95.Mellone BG, Zhang W, Karpen GH. Frodos found: Behold the CENP-a “Ring” bearers. Cell. 2009;137:409–412. doi: 10.1016/j.cell.2009.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Polo SE, Almouzni G. Chromatin assembly: a basic recipe with various flavours. Curr. Opin. Genet. Dev. 2006;16:104–111. doi: 10.1016/j.gde.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 97.Loyola A, Almouzni G. Marking histone H3 variants: how, when and why? Trends Biochem. Sci. 2007;32:425–433. doi: 10.1016/j.tibs.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 98.Tachiwana H, Osakabe A, Shiga T, Miya Y, Kimura H, Kagawa W, Kurumizaka H. Structures of human nucleosomes containing major histone H3 variants. Acta Crystallogr. D Biol.Crystallogr. 2011;67:578–583. doi: 10.1107/S0907444911014818. [DOI] [PubMed] [Google Scholar]
- 99.Jin C, Felsenfeld G. Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev. 2007;21:1519–1529. doi: 10.1101/gad.1547707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goldberg AD, Banaszynski LA, Noh KM, Lewis PW, Elsaesser SJ, Stadler S, Dewell S, Law M, Guo X, Li X, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140:678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jin C, Zang C, Wei G, Cui K, Peng W, Zhao K, Felsenfeld G. H3.3/H2A.Z double variant-containing nucleosomes mark “nucleosome-free regions” of active promoters and other regulatory regions. Nat. Genet. 2009;41:941–945. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mito Y, Henikoff JG, Henikoff S. Genome-scale profiling of histone H3.3 replacement patterns. Nat. Genet. 2005;37:1090–1097. doi: 10.1038/ng1637. [DOI] [PubMed] [Google Scholar]
- 103.Sakai A, Schwartz BE, Goldstein S, Ahmad K. Transcriptional and developmental functions of the H3.3 histone variant in Drosophila. Curr. Biol. 2009;19:1816–1820. doi: 10.1016/j.cub.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hodl M, Basler K. Transcription in the absence of histone H3.3. Curr. Biol. 2009;19:1221–1226. doi: 10.1016/j.cub.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 105.Loppin B, Bonnefoy E, Anselme C, Laurencon A, Karr TL, Couble P. The histone H3.3 chaperone HIRA is essential for chromatin assembly in the male pronucleus. Nature. 2005;437:1386–1390. doi: 10.1038/nature04059. [DOI] [PubMed] [Google Scholar]
- 106.van der Heijden GW, Dieker JW, Derijck AA, Muller S, Berden JH, Braat DD, van der Vlag J, de Boer P. Asymmetry in histone H3 variants and lysine methylation between paternal and maternal chromatin of the early mouse zygote. Mech. Dev. 2005;122:1008–1022. doi: 10.1016/j.mod.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 107.Bonnefoy E, Orsi GA, Couble P, Loppin B. The essential role of Drosophila HIRA for de novo assembly of paternal chromatin at fertilization. PLoS Genet. 2007;3:1991–2006. doi: 10.1371/journal.pgen.0030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Konev AY, Tribus M, Park SY, Podhraski V, Lim CY, Emelyanov AV, Vershilova E, Pirrotta V, Kadonaga JT, Lusser A, et al. CHD1 motor protein is required for deposition of histone variant H3.3 into chromatin in vivo. Science. 2007;317:1087–1090. doi: 10.1126/science.1145339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gaspar-Maia A, Alajem A, Polesso F, Sridharan R, Mason MJ, Heidersbach A, Ramalho-Santos J, McManus MT, Plath K, Meshorer E, et al. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature. 2009;460:863–868. doi: 10.1038/nature08212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wong LH, McGhie JD, Sim M, Anderson MA, Ahn S, Hannan RD, George AJ, Morgan KA, Mann JR, Choo KH. ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res. 2010;20:351–360. doi: 10.1101/gr.101477.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc. Natl. Acad. Sci. USA. 2010;107:14075–14080. doi: 10.1073/pnas.1008850107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Drane P, Ouararhni K, Depaux A, Shuaib M, Hamiche A. The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes Dev. 2010;24:1253–1265. doi: 10.1101/gad.566910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Torras-Llort M, Moreno-Moreno O, Azorin F. Focus on the centre: the role of chromatin on the regulation of centromere identity and function. EMBO J. 2009;28:2337–2348. doi: 10.1038/emboj.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dalal Y, Furuyama T, Vermaak D, Henikoff S. Structure, dynamics, and evolution of centromeric nucleosomes. Proc. Natl. Acad. Sci. USA. 2007;104:15974–15981. doi: 10.1073/pnas.0707648104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sullivan BA, Blower MD, Karpen GH. Determining centromere identity: cyclical stories and forking paths. Nat. Rev. Genet. 2001;2:584–596. doi: 10.1038/35084512. [DOI] [PubMed] [Google Scholar]
- 116.Allshire RC, Karpen GH. Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat. Rev. Genet. 2008;9:923–937. doi: 10.1038/nrg2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gkikopoulos T, Singh V, Tsui K, Awad S, Renshaw MJ, Scholfield P, Barton GJ, Nislow C, Tanaka TU, Owen-Hughes T. The SWI/SNF complex acts to constrain distribution of the centromeric histone variant Cse4. EMBO J. 2011;30:1919–1927. doi: 10.1038/emboj.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dunleavy EM, Roche D, Tagami H, Lacoste N, Ray-Gallet D, Nakamura Y, Daigo Y, Nakatani Y, Almouzni-Pettinotti G. HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell. 2009;137:485–497. doi: 10.1016/j.cell.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 119.Foltz DR, Jansen LE, Bailey AO, Yates JR, 3rd, Bassett EA, Wood S, Black BE, Cleveland DW. Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell. 2009;137:472–484. doi: 10.1016/j.cell.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams JS, Hayashi T, Yanagida M, Russell P. Fission yeast Scm3 mediates stable assembly of Cnp1/CENP-A into centromeric chromatin. Mol. Cell. 2009;33:287–298. doi: 10.1016/j.molcel.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pidoux AL, Choi ES, Abbott JK, Liu X, Kagansky A, Castillo AG, Hamilton GL, Richardson W, Rappsilber J, He X, et al. Fission yeast Scm3: A CENP-A receptor required for integrity of subkinetochore chromatin. Mol. Cell. 2009;33:299–311. doi: 10.1016/j.molcel.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sanchez-Pulido L, Pidoux AL, Ponting CP, Allshire RC. Common ancestry of the CENP-A chaperones Scm3 and HJURP. Cell. 2009;137:1173–1174. doi: 10.1016/j.cell.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perpelescu M, Nozaki N, Obuse C, Yang H, Yoda K. Active establishment of centromeric CENP-A chromatin by RSF complex. J. Cell Biol. 2009;185:397–407. doi: 10.1083/jcb.200903088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Okada M, Okawa K, Isobe T, Fukagawa T. CENP-H-containing complex facilitates centromere deposition of CENP-A in cooperation with FACT and CHD1. Mol. Biol. Cell. 2009;20:3986–3995. doi: 10.1091/mbc.E09-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Walfridsson J, Bjerling P, Thalen M, Yoo EJ, Park SD, Ekwall K. The CHD remodeling factor Hrp1 stimulates CENP-A loading to centromeres. Nucleic Acids Res. 2005;33:2868–2879. doi: 10.1093/nar/gki579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Podhraski V, Campo-Fernandez B, Worle H, Piatti P, Niederegger H, Bock G, Fyodorov DV, Lusser A. CenH3/CID incorporation is not dependent on the chromatin assembly factor CHD1 in Drosophila. PLoS One. 2010;5:e10120. doi: 10.1371/journal.pone.0010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hanai K, Furuhashi H, Yamamoto T, Akasaka K, Hirose S. RSF governs silent chromatin formation via histone H2Av replacement. PLoS Genet. 2008;4:e1000011. doi: 10.1371/journal.pgen.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Erhardt S, Mellone BG, Betts CM, Zhang W, Karpen GH, Straight AF. Genome-wide analysis reveals a cell cycle-dependent mechanism controlling centromere propagation. J. Cell Biol. 2008;183:805–818. doi: 10.1083/jcb.200806038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu X, Bowen J, Gorovsky MA. Either of the major H2A genes but not an evolutionarily conserved H2A.F/Z variant of Tetrahymena thermophila can function as the sole H2A gene in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 1996;16:2878–2887. doi: 10.1128/mcb.16.6.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Faast R, Thonglairoam V, Schulz TC, Beall J, Wells JR, Taylor H, Matthaei K, Rathjen PD, Tremethick DJ, Lyons I. Histone variant H2A.Z is required for early mammalian development. Curr. Biol. 2001;11:1183–1187. doi: 10.1016/s0960-9822(01)00329-3. [DOI] [PubMed] [Google Scholar]
- 131.van Daal A, Elgin SC. A histone variant, H2AvD, is essential in Drosophila melanogaster. Mol. Biol. Cell. 1992;3:593–602. doi: 10.1091/mbc.3.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Suto RK, Clarkson MJ, Tremethick DJ, Luger K. Crystal structure of a nucleosome core particle containing the variant histone H2A.Z. Nat. Struct. Biol. 2000;7:1121–1124. doi: 10.1038/81971. [DOI] [PubMed] [Google Scholar]
- 133.Fan JY, Rangasamy D, Luger K, Tremethick DJ. H2A.Z alters the nucleosome surface to promote HP1alpha-mediated chromatin fiber folding. Mol. Cell. 2004;16:655–661. doi: 10.1016/j.molcel.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Z, Pugh BF. Genomic Organization of H2Av Containing Nucleosomes in Drosophila Heterochromatin. PLoS One. 2011;6:e20511. doi: 10.1371/journal.pone.0020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hardy S, Jacques PE, Gevry N, Forest A, Fortin ME, Laflamme L, Gaudreau L, Robert F. The euchromatic and heterochromatic landscapes are shaped by antagonizing effects of transcription on H2A.Z deposition. PLoS Genet. 2009;5:e1000687. doi: 10.1371/journal.pgen.1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Henikoff S, Henikoff JG, Sakai A, Loeb GB, Ahmad K. Genome-wide profiling of salt fractions maps physical properties of chromatin. Genome Res. 2009;19:460–469. doi: 10.1101/gr.087619.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 2004;303:343–348. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- 138.Kobor MS, Venkatasubrahmanyam S, Meneghini MD, Gin JW, Jennings JL, Link AJ, Madhani HD, Rine J. A Protein Complex Containing the Conserved Swi2/Snf2-Related ATPase Swr1p Deposits Histone Variant H2A.Z into Euchromatin. PLoS Biol. 2004;2:e131. doi: 10.1371/journal.pbio.0020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Krogan NJ, Keogh MC, Datta N, Sawa C, Ryan OW, Ding H, Haw RA, Pootoolal J, Tong A, Canadien V, et al. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol. Cell. 2003;12:1565–1576. doi: 10.1016/s1097-2765(03)00497-0. [DOI] [PubMed] [Google Scholar]
- 140.Ruhl DD, Jin J, Cai Y, Swanson S, Florens L, Washburn MP, Conaway RC, Conaway JW, Chrivia JC. Purification of a human SRCAP complex that remodels chromatin by incorporating the histone variant H2A.Z into nucleosomes. Biochemistry (Mosc. ) 2006;45:5671–5677. doi: 10.1021/bi060043d. [DOI] [PubMed] [Google Scholar]
- 141.Luk E, Ranjan A, Fitzgerald PC, Mizuguchi G, Huang Y, Wei D, Wu C. Stepwise histone replacement by SWR1 requires dual activation with histone H2A.Z and canonical nucleosome. Cell. 2010;143:725–736. doi: 10.1016/j.cell.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu WH, Alami S, Luk E, Wu CH, Sen S, Mizuguchi G, Wei D, Wu C. Swc2 is a widely conserved H2AZ-binding module essential for ATP-dependent histone exchange. Nat. Struct. Mol. Biol. 2005;12:1064–1071. doi: 10.1038/nsmb1023. [DOI] [PubMed] [Google Scholar]
- 143.Papamichos-Chronakis M, Watanabe S, Rando OJ, Peterson CL. Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell. 2011;144:200–213. doi: 10.1016/j.cell.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park YJ, Chodaparambil JV, Bao Y, McBryant SJ, Luger K. Nucleosome assembly protein 1 exchanges histone H2A-H2B dimers and assists nucleosome sliding. J. Biol. Chem. 2005;280:1817–1825. doi: 10.1074/jbc.M411347200. [DOI] [PubMed] [Google Scholar]
- 145.Luk E, Vu ND, Patteson K, Mizuguchi G, Wu WH, Ranjan A, Backus J, Sen S, Lewis M, Bai Y, et al. Chz1, a nuclear chaperone for histone H2AZ. Mol. Cell. 2007;25:357–368. doi: 10.1016/j.molcel.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 146.Straube K, Blackwell JS, Jr, Pemberton LF. Nap1 and Chz1 have separate Htz1 nuclear import and assembly functions. Traffic. 2010;11:185–197. doi: 10.1111/j.1600-0854.2009.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reeves R. Nuclear functions of the HMG proteins. Biochim. Biophys. Acta. 2010;1799:3–14. doi: 10.1016/j.bbagrm.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bianchi ME, Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 2005;15:496–506. doi: 10.1016/j.gde.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 149.Rochman M, Malicet C, Bustin M. HMGN5/NSBP1: a new member of the HMGN protein family that affects chromatin structure and function. Biochim. Biophys. Acta. 2010;1799:86–92. doi: 10.1016/j.bbagrm.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Catez F, Yang H, Tracey KJ, Reeves R, Misteli T, Bustin M. Network of dynamic interactions between histone H1 and high-mobility-group proteins in chromatin. Mol. Cell. Biol. 2004;24:4321–4328. doi: 10.1128/MCB.24.10.4321-4328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rochman M, Postnikov Y, Correll S, Malicet C, Wincovitch S, Karpova TS, McNally JG, Wu X, Bubunenko NA, Grigoryev S, et al. The interaction of NSBP1/HMGN5 with nucleosomes in euchromatin counteracts linker histone-mediated chromatin compaction and modulates transcription. Mol. Cell. 2009;35:642–656. doi: 10.1016/j.molcel.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Phair RD, Scaffidi P, Elbi C, Vecerova J, Dey A, Ozato K, Brown DT, Hager G, Bustin M, Misteli T. Global nature of dynamic protein-chromatin interactions in vivo: three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol. Cell. Biol. 2004;24:6393–6402. doi: 10.1128/MCB.24.14.6393-6402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rattner BP, Yusufzai T, Kadonaga JT. HMGN proteins act in opposition to ATP-dependent chromatin remodeling factors to restrict nucleosome mobility. Mol. Cell. 2009;34:620–626. doi: 10.1016/j.molcel.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Berube NG, Smeenk CA, Picketts DJ. Cell cycle-dependent phosphorylation of the ATRX protein correlates with changes in nuclear matrix and chromatin association. Hum. Mol. Genet. 2000;9:539–547. doi: 10.1093/hmg/9.4.539. [DOI] [PubMed] [Google Scholar]
- 155.Lechner MS, Schultz DC, Negorev D, Maul GG, Rauscher FJ., 3rd The mammalian heterochromatin protein 1 binds diverse nuclear proteins through a common motif that targets the chromoshadow domain. Biochem. Biophys. Res. Commun. 2005;331:929–937. doi: 10.1016/j.bbrc.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 156.Kourmouli N, Sun YM, van der Sar S, Singh PB, Brown JP. Epigenetic regulation of mammalian pericentric heterochromatin in vivo by HP1. Biochem. Biophys. Res. Commun. 2005;337:901–907. doi: 10.1016/j.bbrc.2005.09.132. [DOI] [PubMed] [Google Scholar]
- 157.Emelyanov AV, Konev AY, Vershilova E, Fyodorov DV. Protein complex of Drosophila ATRX/XNP and HP1a is required for the formation of pericentric beta-heterochromatin in vivo. J. Biol. Chem. 2010;285:15027–15037. doi: 10.1074/jbc.M109.064790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Eskeland R, Eberharter A, Imhof A. HP1 binding to chromatin methylated at H3K9 is enhanced by auxiliary factors. Mol. Cell. Biol. 2007;27:453–465. doi: 10.1128/MCB.01576-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hake SB, Allis CD. Histone H3 variants and their potential role in indexing mammalian genomes: the “H3 barcode hypothesis”. Proc. Natl. Acad. Sci. USA. 2006;103:6428–6435. doi: 10.1073/pnas.0600803103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Henikoff S, Furuyama T, Ahmad K. Histone variants, nucleosome assembly and epigenetic inheritance. Trends Genet. 2004;20:320–326. doi: 10.1016/j.tig.2004.05.004. [DOI] [PubMed] [Google Scholar]