Abstract
A general status of oxidative stress in plants caused by exposure to elevated metal concentrations in the environment coincides with a constraint on mitochondrial electron transport, which enhances ROS accumulation at the mitochondrial level. As mitochondria are suggested to be involved in redox signaling under environmental stress conditions, mitochondrial ROS can initiate a signaling cascade mediating the overall stress response, i.e., damage versus adaptation. This review highlights our current understanding of metal-induced responses in plants, with focus on the production and detoxification of mitochondrial ROS. In addition, the potential involvement of retrograde signaling in these processes will be discussed.
Keywords: toxic metals, oxidative stress, reactive oxygen species (ROS), plant mitochondria, oxidative damage, signaling
1. Introduction
Worldwide, metal industry and agricultural use of metal-containing fertilizers and pesticides have contributed significantly to metal pollution. Resulting concentrations of toxic metals in the environment often exceed those from natural sources [1]. Metals such as copper (Cu), iron (Fe), nickel (Ni) and zinc (Zn) are essential for functioning of physiological and biochemical processes and, consequently, for normal growth and development of organisms [2,3]. However, elements such as aluminium (Al), cadmium (Cd) and lead (Pb) are considered to be non-essential and generate toxic responses even at low exposure concentrations. An excess of toxic metals commonly has a negative impact on physiological and biochemical processes in organisms, resulting in major risks to the environment and also for human health. Numerous in vitro and in vivo population-based studies have demonstrated the risks of enhanced metal exposure for human health [4–7]. If toxic metals accumulate in crop plants, the uptake of potentially hazardous elements via the food chain tremendously increases in humans [8]. A better understanding of metal-induced molecular, cellular and physiological responses in plants will therefore contribute to the development or adjustment of strategies to alleviate metal-associated risks for human health.
Plants growing on metal-enriched soils suffer from decreased growth and performance, both restricting crop yield. At the molecular level, oxidative stress is widely studied as a key sign of plant stress. This process is commonly described as an imbalance between reactive oxygen species (ROS) and antioxidants in favor of the former. Sharma and Dietz [9] recently summarized the intense relationship between metal toxicity, redox homeostasis and antioxidant capacity in plants. Depending on the chemical properties and behavior of metals in biological systems, their toxicity is attributed to either one of the following mechanisms: (1) interference with functional sites in proteins; (2) displacement of essential elements, thereby disturbing enzymatic functions; or (3) enhanced ROS production [9]. Redox-active metals such as Cu and Fe directly induce ROS production through Fenton and Haber-Weiss reactions [10,11]. Non-redox-active metals (Cd, Pb and Zn) however only enhance ROS production via indirect mechanisms such as inhibiting enzymes functioning in the cellular antioxidative defense network [10]. While an enzymatic oxidative burst mediated by NADPH oxidases is well studied under conditions of pathogen attack, a clear role for these ROS producing enzymes after metal application is demonstrated in various studies [12,13]. In addition to enzymatic pathways, excess metals increase ROS production in subcellular organelles such as peroxisomes, chloroplasts and mitochondria, which together constitute the predominant sources of ROS production in plants. Their highly oxidizing nature and the presence of electron transport chains in chloroplasts and mitochondria makes both organelles a preferential site for metal-induced ROS production [11,14]. Chloroplasts are intensively studied in the light of photosynthesis and its accompanying ROS production under metal stress conditions [15]. However, research has extended to plant mitochondria in recent years [9,16]. A clear relationship was demonstrated between metal stress, redox homeostasis and antioxidant metabolism at the cytosolar and organellar level [9]. As mitochondria are key players in cellular redox homeostasis and signaling [16], this review focuses on ROS production and antioxidative defense mechanisms in mitochondria and how these processes are affected by metal exposure. The resulting oxidative challenge in mitochondria further implicates downstream signaling responses via ROS and/or other signaling intermediates, which will be discussed in the light of metal stress in this review.
2. Metal-Induced ROS Production: Interplay between Cytosol and Mitochondria
Mitochondria are the principal organelles performing plant aerobic respiration. In this process, organic acids are oxidized to CO2 and H2O in the tricarboxylic acid (TCA) cycle in the mitochondrial matrix, thereby fuelling electrons from reducing NADH (and FADH2) equivalents to O2 via the respiratory electron transport chain (ETC) present in the inner mitochondrial membrane (Figure 1). This electron transfer process is coupled to the synthesis of energy in the form of ATP in a process termed oxidative phosphorylation [17]. When plants are exposed to toxic metals, apoplastic transport followed by cytosolar uptake and distribution of metals to organelles causes ROS generation due to their redox-active nature or the effects on subcellular metabolism [9]. Van Belleghem et al. [18] have shown both cytosolar and vacuolar sequestration of Cd using energy-dispersive X-ray microanalysis (EDXMA) on high-pressure frozen and freeze-substituted tissues of Arabidopsis seedlings chronically exposed to 0.1, 1, 5 or 50 μM Cd via the roots. However, no Cd could be detected in mitochondria [18]. As recently reviewed by Nouet et al. [19], the proteins involved in metal import into plant mitochondria remain mostly unknown. Therefore, it is important to keep in mind that mitochondrial responses during metal exposure are not necessarily evoked by metals reaching and accumulating in these organelles per se. Nevertheless, it is crucial to study the potential interplay between cytosolar and mitochondrial responses to metal stress in plants in order to obtain further insights into a broader cellular picture (Figure 2). To unravel metal-induced mitochondrial responses, these organelles should be isolated from different organs of plants exposed to excess metals to obtain full and organelle-specific transcriptome, proteome and/or enzyme activity spectra. In this regard, care has to be taken as the experimental setup will definitely influence the outcome. Metal speciation needs to be considered as it modulates the results when exposing either plants or cell suspension cultures. Moreover, exposing whole plants or cell cultures to metals prior to isolating mitochondria will lead to other—potentially conflicting—results as compared to exposing isolated mitochondria to excess metals. Which setup to choose ultimately depends on the research question, but it is key to interpret all results in a broader context.
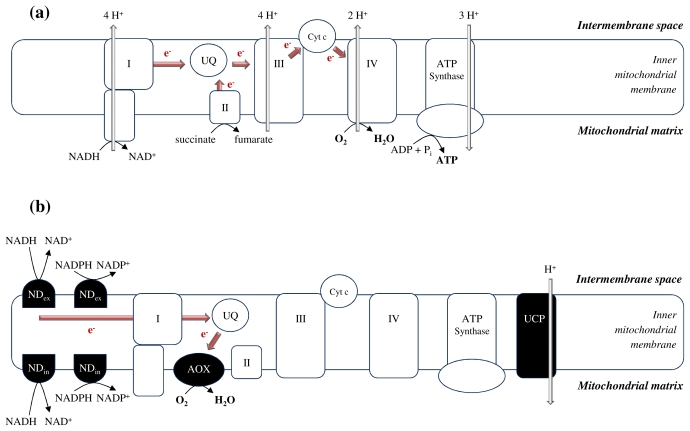
Figure 1.
Simplified overview of the components involved in conventional and alternative reactions of mitochondrial electron transport and oxidative phosphorylation in plants. (a) In the “standard” cytochrome pathway, electrons pass from respiratory complexes I (NADH dehydrogenase) and II (succinate dehydrogenase) to the electron carrier ubiquinone (UQ). Via complex III (ubiquinol-cytochrome bc1 reductase) and cytochrome c (cyt c), O2 is ultimately reduced to H2O at the level of complex IV (cytochrome c oxidase). The ATP synthase complex catalyzes the formation of ATP in the mitochondrial matrix driven by the proton gradient resulting from electron transfer; (b) In addition, plant mitochondria contain an alternative pathway consisting of a non-proton-pumping alternative oxidase (AOX) as well as alternative NAD(P)H dehydrogenases (NDs) on either the external (NDex) or internal (NDin) side of the inner mitochondrial membrane. Electrons are passed from the alternative NDs to ubiquinone and directly to AOX reducing O2 to H2O. Uncoupling proteins (UCPs) are able to dissipate the proton electrochemical gradient over the inner membrane created by the transfer of electrons, thereby acting as an alternative path to mitochondrial oxidative phosphorylation.
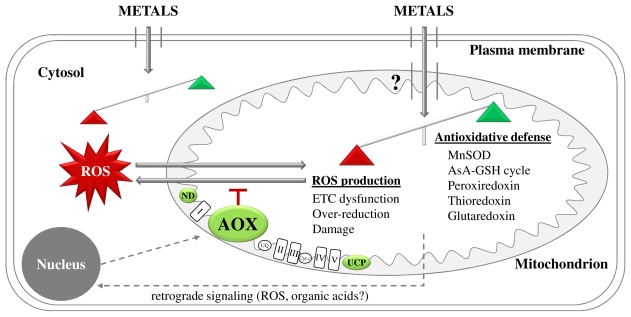
Figure 2.
Schematic overview of metal-induced responses in plant cells focusing on mitochondrial effects. Metal exposure has shown to cause mitochondrial electron transport chain (ETC) dysfunction and over-reduction, thereby increasing mitochondrial ROS production. However, more research is needed to determine whether this is the direct consequence of metals entering the mitochondria, since cytosolar ROS production cannot be excluded in the light of metal stress and can influence mitochondrial responses. As they are able to cross cellular membranes, ROS serve signaling functions outside the mitochondria (dashed line) and can induce retrograde signaling to the nucleus, which could also be regulated via organic acids. As AOX is able to reduce mitochondrial ROS production, modulate programmed cell death (PCD) and tricarboxylic acid (TCA) cycle activity, this enzyme is suggested to play a key role in metal-induced responses in plant mitochondria.
2.1. The Unique Demands Placed on Plant Mitochondria
In addition to respiration, plant mitochondria play a role in the metabolism of diverse amino acids, vitamins and lipids essential for organellar biogenesis and maintenance. The relative importance of these processes depends on the cell type and developmental stage. Studying the responses of plant mitochondria to metal stress implies the need to fractionate the responses in different plant organs. In leaves, the cellular environment of plant mitochondria is rather distinctive as compared to animal cells due to the presence of photosynthesis-derived O2 and carbohydrates. In plant roots however, a different cellular environment as compared to the leaves will definitely influence the response of mitochondria to excess metals [16,17]. It is noteworthy to mention that plant mitochondria differ from their animal counterparts in that the former function in distinct processes, such as photorespiration, and that they possess unique components (alternative oxidase (AOX), alternative NAD(P)H dehydrogenases (NDs) and uncoupling proteins (UCPs) (Figure 1b) [16,17,20]. Electrons can pass through either the “standard” cytochrome pathway to complex IV (cytochrome c oxidase) (Figure 1a) or through the alternative pathway to the cyanide-insensitive AOX (Figure 1b). Although this alternative route does not contribute to ATP synthesis, AOX is currently considered both a target and regulator of stress responses in plants (Sections 3.1 and 4.3) [21,22].
2.2. Metal Exposure Increases ROS Generation in Plant Mitochondria
Under normal conditions, the mitochondrial ETC and ATP synthesis are tightly coupled. However, plants suffering from biotic or abiotic stress often show an over-reduction of electron carriers such as ubiquinone, causing electron leakage from the system. These electrons possess a sufficient amount of free energy to directly reduce molecular O2, with increased production of ROS such as superoxide (O2°−) and hydrogen peroxide (H2O2) as unavoidable byproducts of aerobic metabolism [17,21,23–26].
The known sites of ROS production in the mitochondrial ETC are complexes I and III (for reviews, see [21,23]), where O2°− is formed and subsequently dismutated to H2O2. The uncharged H2O2 molecule is able to penetrate membranes and has a longer half-life as compared to O2°−. Both properties make H2O2 an ideal candidate for ROS signaling from mitochondria to other organelles [17]. However, H2O2 can also react with reduced Fe2+ and Cu+ in the mitochondrion itself to produce highly toxic hydroxyl radicals and cause oxidative damage to mitochondrial proteins, lipids and DNA [21,25,27].
In mitochondria, transition metals such as Cu, Fe and Zn are crucial for a proper functioning of several enzymes involved in the TCA cycle, electron transport, synthesis of ATP and antioxidative defense [19,27]. However, the presence of free (redox-active) metal cations can initiate or propagate oxidative stress (Table 1). Results of numerous studies investigating plant metal stress responses point toward the generation of oxidative stress and mitochondrial dysfunction as determinants in metal-induced cytotoxicity. In several plant species, metal stress enhances mitochondrial ROS generation mainly by affecting respiratory gas exchange rates [28]. An excess of redox-active metals such as Fe and Cu influences mitochondrial respiration activity (Table 1) [29,30], which could be related to their direct potential to increase mitochondrial ROS production via Fenton and Haber-Weiss reactions. However, also non-redox-active metals negatively affect respiration processes (Table 1). Dixit et al. [31] studied the in vivo effects of chromium (Cr) in pea root mitochondria and observed an inactivated electron transport and enhanced generation of O2° −. The inhibition of root elongation after exposing pea to Al was attributed to metal-induced ROS production, inhibition of respiration and ATP depletion [32]. However, as adenylates show a drastic decrease in response to phosphate deficiency [33], which commonly coincides with Al exposure due to restricted phosphorus uptake [34], the indicative value of the observed ATP depletion is rather low. Instead, one should determine the ATP/ADP ratio to adequately rationalize the effects of metals on the efficiency of the mitochondrial respiratory chain.
Table 1.
Exposure to excess metals has consequences for plant mitochondria at different levels. The effects of excess Al, Cd, Cr, Cu, Fe, Pb and Zn are shown and categorized based upon the experimental setup used (isolated mitochondria (A), cell cultures/protoplasts (B) or intact plants (C)). Metals impose a mitochondrial oxidative challenge characterized by an increased ROS production and altered antioxidative defense. This oxidative challenge is most likely the result of metal-induced ETC dysfunction at the level of the cytochrome pathway. Several metals activate the alternative respiratory pathway at different levels, but also induce mitochondrial damage versus signaling and defense (e.g., programmed cell death) responses. Metal-induced responses related to plant mitochondria are described schematically in the column “Observations”.
| A. METAL-INDUCED RESPONSES IN ISOLATED MITOCHONDRIA | ||||||
|---|---|---|---|---|---|---|
| Metal | Concentration | Exposure Time | Setup | Species | Observations | Ref. |
| Al | 50 μM | 18 h | Isolation after exposing cells | N. tabacum | ↑ ROS production (O2°− and H2O2) ↓ O2 consumption ↓ ATP content ↓ cytochrome capacity ↓ AOX capacity opening of mitochondrial permeability transition pore cytochrome c release and nuclear fragmentation ~ PCD distorted mitochondrial membrane architecture |
[43] |
| 0.1–0.5–1 mM | 60 min | Exposure after isolation out of mesophyll protoplasts | A. thaliana | ↑ ROS production (O2°− and H2O2) ↓ complex I and III activity |
[44] | |
| Cd | 10–30 μM | 30 min | Exposure after isolation out of tubers | S. tuberosum | ↑ ROS production (O2°− and H2O2) | [36] |
| 5 mM | 12 to 120 h | Isolation after exposing germinating seeds | P. sativum | ↓ glutaredoxin, GR, GSH | [39] | |
| Cr | 20 or 200 μM | 7 days | Isolation out of roots after exposing plants | P. sativum | ↑ O2°− lipid peroxidation of mitochondrial membranes altered SOD activity ↓ respiratory complex activity (IV most sensitive) |
[31] |
| Cu | 2–20–50 μM | 6 days | Isolation after exposing cells | A. pseudoplatanus | ↑ alternative respiratory pathway (KCN-resistant) ↑ AOX protein content |
[30] |
| Pb | 0.1–0.5 mM | Up to 3 days | Isolation out of roots after exposing plants | P. sativum | ↑ H2O2 (mitochondria main site) ↑ AOX transcription and protein content |
[45] |
| 0.5–1 mM | 2 to 96 h | Isolation out of roots after exposing plants | P. sativum | ↑ MnSOD activity ↑ alternative respiratory pathway (KCN-resistant) ↑ AOX protein content ↓ number of mitochondrial cristae |
[46] | |
| Al | 25–50–75–100 μM | 6 to 24 h | Cell culture | N. tabacum | ↑ ROS production (O2°− and H2O2) ↓ mitochondrial activity ↓ respiration (O2 uptake) ↓ ATP content |
[32] |
| 0.5 mM | 60 to 100 min | Protoplasts | A. thaliana | ↑ ROS production (O2°− and H2O2) ↑ AOX1a transcription ↓ mitochondrial transmembrane potential ↑ caspase-3-like protease activity ~ PCD disrupted mitochondrial ultrastructure |
[44] | |
| Cd | 20 μM | 5 h | Protoplasts | A. thaliana | ↑ H2O2 in mitochondria prior to chloroplasts mitochondrial clustering and restricted movement | [37] |
| 0.5–2–5–20– 50–200 μM | 24 h | Cell culture | A. thaliana | ↑ MDHAR, peroxiredoxin | [47] | |
| 100 or 150 μM | 3 days | Cell culture | A. thaliana | ↑ PCD | [41] | |
| 3 mM | 1 h | Cell culture | N. tabacum | ↑ O2°− | [48] | |
| Cu | 2–20–50 μM | Up to 6 days | Cell culture | A. pseudoplatanus | ↓ respiration (O2 uptake) ↑ alternative respiratory pathway (KCN-resistant) ↑ AOX1 transcription |
[30] |
| Al | 5–10–15– 20 μM | 4 to 24 h | Root apices | P. sativum | ↓ respiration (O2 uptake) ↓ ATP content |
[32] |
| 100 μM | 1 to 48 h | Root tips | T. aestivum | ↑ MSD transcription | [49] | |
| Cd | 30–60–100 μM | Up to 10 days | Roots and leaves | H. distichum | ↓ respiration (O2 uptake) ↑ alternative respiratory pathway (SHAM) |
[50] |
| Cr | 2–5–10 mg/L | 6 days | Leaves | S. minima | ↑ AOX capacity (SHAM) | [51] |
| Cu | 2 or 5 μM | 24 h | Roots | A. thaliana | ↓ MSD1 transcription | [52] |
| Fe | 100 μM | 12 h | Root cutting exposure | N. plumbaginifolia | ↑ respiration in leaves before (O2 uptake) | [29] |
| Zn | 1–5–10– 25 mM | 10 min to 9 h | Roots | O. sativa | mitochondrial ROS potentially involved in cell death | [53] |
Abbreviations: AOX, alternative oxidase; GR, glutathione reductase; GSH, reduced glutathione; H2O2, hydrogen peroxide; KCN, potassium cyanide; MDHAR, monodehydroascorbate reductase; MnSOD, manganese superoxide dismutase; MSD1, manganese superoxide dismutase isoform 1; O2°−, superoxide; PCD, programmed cell death; SHAM, salicylhydroxamic acid; SOD, superoxide dismutase.
Similar to animals [35], the plant mitochondrial ETC is also considered to be an important target of Cd toxicity [36–39]. Heyno et al. [36] demonstrated a fast Cd-induced stimulation of ROS generation inside root cells, mainly originating from the mitochondrial ETC. Exposure to Cd impairs proper mitochondrial functioning partly by affecting the organellar redox balance as shown by Smiri et al. [39]. Bi et al. [37] confirmed ROS production in mitochondria prior to chloroplasts in combination with altered mitochondrial distribution and mobility patterns in Cd-exposed Arabidopsis thaliana protoplasts (Table 1).
In addition to ROS, plant mitochondria are also able to produce nitric oxide (NO) under low oxygen levels that affects the activity of mitochondrial ETC and matrix enzymes and transcriptionally upregulates AOX. The role NO plays during oxidative signaling falls out of the scope of this review, but was reviewed in detail elsewhere [25,40]. A potential role for NO during metal stress responses in plants is suggested by the results of De Michele et al. [41], who demonstrated the involvement of NO in Cd-induced programmed cell death of Arabidopsis thaliana suspension cultures. Arasimowicz-Jelonek et al. [42] recently discussed the mode of action of NO during Cd stress in plants, with a potential intense relationship between NO and ROS signaling during metal stress as was also shown to occur in plant responses to biotic stress.
3. Mechanisms to Control Mitochondrial ROS Production under Metal Stress
Plants contain a dynamic antioxidative defense network to counterbalance the accumulation of ROS, thereby limiting their detrimental effects while still allowing redox signaling throughout the plant [21,25]. During environmental stress conditions such as metal exposure, an integrated antioxidative response in and between different cellular organelles and compartments is required to locally act against ROS production [25,54]. Sweetlove et al. [55] studied the impact of oxidative stress induced by H2O2, menadione (an intracellular O2°− generator) or antimycin A (an inhibitor of respiratory complex III) in Arabidopsis cells and provided direct evidence for plant mitochondria using an array of enzymes to detoxify ROS (thioredoxin-based redox pathway) or repair oxidative stress-induced damage (protein disulphide isomerase) [55] (Figure 2). Based on the fact that metals induce mitochondrial ROS production and thus oxidative stress, it could be rationalized that similar mechanisms may be involved to counterbalance the accumulation of ROS. In the following paragraphs, the potential mechanisms exploited by plant mitochondria to avoid or detoxify metal-induced ROS will be discussed.
3.1. Avoidance of Mitochondrial ROS Production at the ETC Level as a First Line of Defense
Stress-induced over-reduction of ETC components and resulting electron leakage from the system is a principal cause of mitochondrial ROS production. Plant mitochondria contain energy-dissipating systems able to regulate the mitochondrial membrane potential, thereby decreasing mitochondrial ROS production due to ETC over-reduction. However, one must keep in mind that these systems are not able to prevent damage by cytosolar ROS diffusing into the mitochondria [56]. Van Dongen et al. [57] recently reviewed the important role of alternative pathways regulating plant respiration. These pathways ensure metabolic adaptation to hypoxia or altered O2 availability, which could also indicate their importance during metal stress conditions in plants.
Plant mitochondria contain several proteins present in the vicinity of the “classical” ETC components, which can alleviate the degree of coupling between electron transport and ATP synthesis. They bypass ETC complexes by diverting electrons from the primary cytochrome c pathway, while energy is dissipated as heat. The first enzyme to be discussed in the context of this alternative pathway is AOX, a terminal oxidase accepting electrons directly from ubiquinone and thereby bypassing complexes III and IV (Figure 1b). Since it was first suggested by Purvis and Shewfelt [58], several studies confirmed that AOX prevents mitochondrial oxidative stress (Figure 2). Maxwell et al. [59] demonstrated a direct link between functional AOX levels and mitochondrial ROS production in Arabidopsis cells, thereby confirming the ability of AOX to reduce mitochondrial ROS levels. Exposure to several metals has been shown to affect the alternative respiratory pathway mediated by AOX at different levels (Table 1). Excess Cu induced AOX at transcriptional and protein levels in sycamore cells [30]. In the protist Euglena gracilis, Cd stress led to an increased AOX content and capacity [60]. This was also demonstrated in barley plants, where Cd exposure altered the contribution of the alternative respiratory pathway to total respiration as measured by O2 uptake in the presence of the AOX inhibitor salicylhydroxamic acid (SHAM). The authors have shown strong effects of high Cd concentrations on total and alternative respiratory rate and suggested the activated alternative respiration to act as a homeostasis mechanism in Cd-stressed root cells [50]. In addition, Cr exposure enhanced the alternative respiratory rate in Salvinia leaves [51]. Pea root tissues exposed to Pb showed a dose-dependent increase in the expression of genes coding for AOX, which was immunologically confirmed [45]. Also, the transcript level of the major AOX isoform in Arabidopsis (AOX1a) increased in Al-stressed protoplasts in a time-dependent way [44]. From these observations, it is clear that AOX activation could be involved in avoiding metal-induced oxidative stress in plants.
In addition to AOX, plant mitochondria contain alternative NDs able to oxidize cytosolic or matrix NADH/NADPH, thereby bypassing complex I and reducing ubiquinone without pumping protons across the inner membrane (Figure 1b) [61]. Although co-regulated expression patterns for several members of the AOX and alternative ND families were detected under multiple stress conditions affecting mitochondrial respiration [62,63], more research is needed to explore the potential role of alternative NDs during metal stress in plants. Results of our work indicate a possible co-regulation of AOX and alternative ND transcription in Cd-exposed Arabidopsis roots and leaves [64]. This suggests the formation of an abridged but functional alternative respiratory chain with electrons from the alternative NDs passing to AOX via ubiquinone (Figure 1b) under conditions comprising the mitochondrial ETC such as Cd exposure.
Further, mitochondrial UCPs catalyze a proton leak that dissipates the proton electrochemical gradient over the inner mitochondrial membrane, thereby shortcutting the ATP synthase complex and thus oxidative phosphorylation (Figure 1b) [65]. A number of observations suggest a role for UCPs mediating cellular tolerance to oxidative stress [66]. Superoxide [67] and lipid peroxidation products [68] stimulate UCP activity in plant mitochondria. The expression of genes coding for UCP is induced by low temperature conditions [69] and treatment with H2O2 [70,71]. Overexpressing a gene coding for UCP conferred tolerance to oxidative stress in rice [72] and lack of UCP induced localized oxidative stress in Arabidopsis [73]. In durum wheat mitochondria, it was shown that drought stress induced ROS production, which further promoted UCP activity. Thereby, the authors validated a feedback link between stress-induced mitochondrial ROS production and UCP-mediated inhibition of further ROS accumulation [25,74]. As metals induce mitochondrial oxidative stress and ROS production (Section 2.2), a role for UCP is plausible and further research should be conducted in this area. Promising results were published by Yin et al. [75], who demonstrated increased lipid peroxidation products shown to activate UCP [68] in Al-stressed tobacco roots. Although both AOX and UCP activity confer dissipation of energy as heat, they respond to different environmental stimuli. Rasmusson et al. [76] suggested AOX activity to be involved in the acute response to ETC over-reduction, while UCP could become important during prolonged mitochondrial oxidative stress based on their direct versus indirect effect on the transfer of electrons. In addition, the AOX enzyme can be inhibited by lipid peroxidation products, while UCP is stimulated by O2°− [63,77], thereby signifying the role both enzymes can play in metal-induced oxidative stress as mentioned above.
3.2. Mitochondrial Enzymes and Metabolites Involved in the Detoxification of Mitochondrial ROS
Once formed, O2°− radicals are rapidly dismutated to H2O2 via the manganese superoxide dismutase (MnSOD) enzyme present in the mitochondrial matrix [56,78]. It was shown that transition metals such as Cu, Fe and Zn do not affect MnSOD activity in tobacco cell cultures [79]. However, MSD (MnSOD) transcript levels were slightly affected by Cd and Cu exposure in Arabidopsis thaliana [52] and the MnSOD activity in pea root mitochondria increased after Pb exposure [46]. These apparent conflicting results (Table 1) can be attributed to the chosen experimental setup. The observed effects can differ when measured on whole plants versus cell cultures and depending on the used metal concentrations, which greatly hinders accurate interpretation of the available experimental data. However, a role for MnSOD in the adaptation to Al stress is suggested by experimental data using transgenic plants overexpressing MnSOD. Aluminium-induced inhibition of root growth, lipid peroxidation and callose accumulation—a marker for Al injury—decreased in homozygous transgenic overexpressor plants as compared to wildtypes. In conclusion, the authors suggested an improved resistance to Al toxicity mediated by MnSOD in Brassica napus [49].
In order to provide optimal defense against O2°−-derived H2O2 production, MnSOD must act in concert with H2O2-scavenging components. An important system removing H2O2 is the ascorbate-glutathione cycle comprised of four enzymes (ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDHAR) and glutathione reductase (GR)) and two metabolites (ascorbate (AsA) and glutathione (GSH)) that are regenerated using NADPH equivalents [80]. Jiménez et al. [81] demonstrated the presence of this cycle in plant mitochondria and several cycle enzymes were shown to be dually targeted to mitochondria and chloroplasts [82]. In general, several reports demonstrated that metal stress affects the plant AsA-GSH cycle at both enzyme and metabolite levels (Table 1; Seth et al. [14] and references therein) and mitochondria show an interesting link to this cycle as they harbor the last enzyme in the AsA biosynthesis pathway. In this final step, l-galactono-γ-lactone (GL) is converted to AsA by the membrane-bound l-galactono-γ-lactone dehydrogenase (GLDH), which uses cytochrome c as an electron acceptor and thereby donates electrons to the ETC [25,83]. Zhao et al. [84] have demonstrated a protective role for GL during Cd stress in winter wheat. Application of this AsA precursor lowered Cd-induced H2O2 production and increased peroxidase activities [84]. In addition to AsA biosynthesis, it was recently shown that AsA regeneration from dehydroascorbate is also coupled to the plant mitochondrial ETC at the level of complex II, i.e., succinate dehydrogenase [25,85]. As metals strongly affect this complex [50,86], they can also influence the rate of AsA reduction under stress conditions when AsA functions as an important antioxidant.
Plant mitochondria also contain peroxiredoxin, thioredoxin and glutaredoxin enzyme systems capable of scavenging H2O2. These enzymes became a topic of great interest over the recent years (for a review see [56]). In Arabidopsis thaliana cell cultures, Cd application increased the mitochondrial peroxiredoxin content (Table 1) [47]. Finkemeier et al. [87] demonstrated a principal role for the mitochondrial peroxiredoxin isoform F (PrxII F) in antioxidant defense and potential redox signaling in plant cells using knockout (KO)-AtPrxII F Arabidopsis seedlings. Under CdCl2 exposure and after SHAM-administration, the root growth of KO seedlings was more compromised as compared to wildtype seedlings, thereby signifying the involvement of this mitochondrial peroxiredoxin in Cd detoxification [87]. Gelhaye et al. [88] have demonstrated the presence of a mitochondrial thioredoxin isoform in plant mitochondria capable of AOX regulation. This suggests its involvement in metal-induced mitochondrial responses via AOX-mediated modulation of ROS formation. Lastly, Smiri et al. [39] observed a Cd-evoked decrease in glutaredoxin activity measured in burst mitochondria extracts from germinating pea seeds. Although these results point towards the possible involvement of peroxiredoxin, thioredoxin and glutaredoxin enzyme systems in mitochondrial stress responses, the exact mechanisms under metal exposure need to be resolved.
4. Cellular Acclimation to Metal Exposure in Relation to Plant Mitochondria
Changes in mitochondrial electron transport and/or ROS production can have consequences for all other organelles in the plant cell. Indeed, plant mitochondria have a central position in the cellular carbon and nitrogen metabolism via the TCA cycle and their role in photorespiration [17]. Dutilleul et al. [20] have demonstrated this using a Nicotiana sylvestris mutant (CMSII) lacking functional complex I. This induces signaling throughout the cell to reset its antioxidative capacity completely, thereby coping with the loss of a major NADH sink and enhancing resistance to ozone and Tobacco mosaic virus [20]. Schwarzländer et al. [89] studied the importance of mitochondria in oxidative stress and redox signaling by assessing the in vivo oxidation state of a redox-sensitive GFP targeted to Arabidopsis mitochondria. They demonstrated that mitochondria are highly sensitive to redox perturbation evoked by Cd, with their redox state recovering slower from an oxidative insult as compared to the cytosol or chloroplasts [89]. In addition, the mitochondrial ETC is also required to process excess reductants originating from the photosynthetic light reactions [21,33,77,90]. Overall, this suggests that mitochondria play a central role in perception as well as response signaling during the oxidative challenge in metal-exposed plants [17,89]. However, next to metal-induced ROS production and the thereby imposed (cellular) oxidative challenge, a direct link between metals and plant mitochondria is mediated by organic acids produced in the mitochondrial matrix [33]. These may be directly involved in the acclimation of plant cells to enhanced metal concentrations and will be discussed in the light of mitochondrial alternative respiration (Section 4.4).
4.1. Mitochondrial ROS-Induced Damage
Once ROS are formed in mitochondria of metal-stressed plants, they can either diffuse out of the mitochondria to mediate signaling functions or induce protein, lipid and DNA damage in the organelle itself [21] (Figure 2). Compared to nuclear DNA, the sensitivity of mitochondrial DNA to oxidative stress-induced damage is much higher due to the lack of chromatin organization and lower DNA repair activity [91]. Lipid peroxidation mediated by the interaction between ROS and membrane lipids can distort mitochondrial membrane integrity as shown by Panda et al. [43] in Al-stressed tobacco cells, thereby restricting ETC function. To detoxify lipid peroxidation products, plant mitochondria contain amongst other mechanisms a glutathione-S-transferase (GST) that was strongly upregulated in Cd-exposed plant cells, further supporting its critical role during metal stress (Table 1) [47].
Furthermore, plant mitochondrial proteins are susceptible to metal-catalyzed oxidation, leading to the irreversible formation of reactive carbonyl groups on amino acid side chains and hence reduced protein function [27]. Substantial evidence of both TCA cycle and photorespiration pathway proteins as important targets for ROS or lipid peroxidation products produced under environmental stresses such as drought, high light and metal exposure was summarized by Taylor et al. [92]. Bartoli et al. [93] have demonstrated a higher carbonyl accumulation in the wheat mitochondrial proteome as compared to other ROS-producing organelles such as chloroplasts and peroxisomes during well-irrigated and drought stress conditions. Mitochondrial protein carbonylation is rather selective, with not all proteins evenly susceptible to this process. This was demonstrated by Kristensen et al. [94], who have shown distinct subpopulations of the mitochondrial matrix proteome to be carbonylated after Cu and H2O2 treatment. Mitochondrial aconitase seems particularly prone to oxidative damage [63] as it is either carbonylated after in vitro oxidation with Cu and H2O2 [94] or fragmented after oxidative stress [55]. In addition, its abundance was decreased in Cd-exposed Arabidopsis thaliana cells [47].
4.2. The Role of Plant Mitochondria in Metal-Induced Programmed Cell Death
Although enhanced mitochondrial ROS levels may serve as monitors and signal the extent of environmental stress throughout plant cells, they may also lead to oxidative damage and programmed cell death (PCD) when mitochondrial and/or cellular antioxidative defense and repair systems are overwhelmed. Programmed cell death is an active and genetically controlled process essential for growth and development, as well as for adaptation to altered environmental conditions [95]. In animals, the essential role of mitochondria in the signaling pathway transducing specific signals into the execution of cell death is widely accepted [96]. A similar function was suggested for plant mitochondria [97], with H2O2 and other ROS as signals modulating plant PCD. The main event in plant PCD is the release of mitochondrial cytochrome c [98]. This was shown to occur in Al-stressed tobacco cells by Panda et al. [43]. In addition, ROS potentially derived from or interfering with mitochondrial functioning could be key mediators of metal-induced cell death as discussed in several research papers. Exposure to Zn induced cell death in rice roots, which could be mediated by ROS derived from the mitochondrial ETC [53]. Recently, Li and Xing [44] investigated possible mechanisms underlying Al-induced PCD in Arabidopsis mesophyll protoplasts using fluorescence techniques to monitor the in vivo behavior of plant mitochondria and caspase-3-like activity. After a quick ROS burst, mitochondrial swelling and loss of the transmembrane potential occurred prior to PCD. Application of AsA prior to Al-exposure slowed down but did not prevent these processes [44]. Mitochondrial O2°− production—rather than NADPH oxidase-derived extracellular H2O2—was shown to be a key event in Cd-induced cell death in tobacco cells [48]. In addition, NO and ROS may be co-involved in Cd-mediated PCD as shown by De Michele et al. [41] and recently discussed by Arasimowicz-Jelonek et al. [42]. Although our insights are currently increasing (Table 1), more research is required to fully unravel the importance of plant mitochondria and the specific mediators involved during metal-induced PCD, which could be a regulated defense response to improve plant survival under metal stress conditions.
4.3. Mitochondrial Retrograde Signaling during Metal Stress in Plants
Depending on the intensity of the stressor, metals are able to induce mitochondrial damage and/or signaling outside the mitochondria. An altered organellar redox state generates signals that are transmitted to the nucleus in a process called retrograde signaling. This process occurs between mitochondria, chloroplasts and the nucleus and can be mediated by ROS or oxidative stress-induced secondary signals. Recently, Suzuki et al. [99] reviewed the intense relationship between mitochondria and chloroplasts in stress-induced redox signaling throughout the cell. Galvez-Valdivieso et al. [100] summarized the involvement of ROS in chloroplastic retrograde signaling, for which more data are available as compared to mitochondria. Due to their ability to signal across organellar membranes, ROS are considered as key components transducing signals between organelles. The dynamics and specificity of ROS-induced signaling are still questioned, but were recently reviewed by Mittler et al. [101]. They suggest ROS signaling to be a dynamic process occurring within cells between different organelles, as well as over long distances between different cells. In addition, ROS-induced oxidative damage to mitochondrial components may produce secondary signals. Møller and Kristensen [102] reviewed the potential of ROS-mediated protein oxidation in plant mitochondria as a stress indicator. Oxidatively damaged proteins such as those functioning in the TCA cycle and antioxidative defense can either be degraded by proteases or serve as an alarm signal to initiate plant responses at the cellular level [102,103].
To date, no specific components of any mitochondrial retrograde signaling pathway have been identified [99]. However, a mechanism of retrograde signaling is suggested to be involved in plant stress responses to excess Al as AOX transcription is increased [44,104]. Therefore, the involvement of retrograde mechanisms in oxidative stress and/or signaling induced by other metals is plausible and deserves further attention. Recently, Van Aken et al. [105] demonstrated that plant mitochondria respond to a wide variety of abiotic stresses (e.g., salt and heat), chemical inhibitors (e.g., rotenone) and hormones (e.g., abscisic and salicylic acid). They also offered a range of stress-responsive genes as potential targets to study novel mitochondrial retrograde signaling pathways.
The most intensively studied model for retrograde signaling between the mitochondrion and nucleus resulting in acclimation to stress conditions is AOX (Section 3.1, Figure 2). This enzyme could play a pivotal role during metal-induced signaling in plant mitochondria since AOX transcription, protein content and/or activity/capacity are commonly increased upon metal exposure (Table 1). Induction of AOX correlates with an enhanced tolerance to ozone and Tobacco mosaic virus in tobacco plants [20], potentially by altering organellar and/or cellular ROS levels [16] which could also be important during metal stress. Van Aken et al. [22] suggest that AOX is not only a target, but also a regulator of diverse stress responses in plants, mainly based on the results of studies using transgenic plants either with increased or reduced AOX content. The absence of AOX in Arabidopsis resulted in acute sensitivity to combined drought and light stress, confirming a role for AOX in determining the steady-state cellular redox balance [106]. This further suggests the possible involvement of AOX during acclimation to metal exposure in plants. Arabidopsis protoplasts lacking the gene coding for the major isoform AOX1a showed a dramatically decreased viability during Al exposure as compared to wildtype protoplasts [44]. Conversely, AOX1a overexpression enhanced Al tolerance, confirming the protective role of AOX against Al-mediated PCD [44]. The possible function of AOX as a mitochondrial “survival” protein was suggested by Robson and Vanlerberghe [107] in transgenic tobacco cells lacking AOX exposed to H2O2, salicylic acid and the protein phosphatase inhibitor cantharidin. It is hypothesized that the survival function of AOX is based on its ability to continuously suppress mitochondrial ROS generation. This further prevents oxidative damage that could otherwise evoke disturbed gene expression and favor PCD. In addition, the maintenance of respiration in stressful conditions by the alternative route also contributes to the hypothesis of AOX as a survival protein [107]. Vanlerberghe et al. [108] recently reviewed the postulated metabolic and physiological roles of the alternative respiratory pathway, with AOX possibly maintaining a homeostatic mitochondrial signal during stress conditions. Interestingly, the AsA biosynthesis capacity increased in isolated mitochondria of plants overexpressing AOX [109], suggesting a link between this energy-dissipating enzyme and mitochondrial and cellular antioxidative metabolism. Further research using transgenic plants exposed to different metals will contribute to our insights into the role of AOX in metal-induced (retrograde) signaling. However, other proteins and/or metabolites should also be studied to fully unravel the mechanisms involved in the acclimation of plants growing on metal-contaminated soils.
4.4. Metal Tolerance Mediated by Mitochondrial Organic Acids
In stress conditions compromising the phosphorylating cytochrome respiratory pathway, such as metal exposure, plant mitochondria use their alternative respiratory pathway to maintain the electron flux to O2. As discussed earlier, this may reduce mitochondrial ROS production by electron leakage from the system, thereby decreasing metal-induced oxidative stress at organellar and possibly cellular levels. In addition, the sustained electron flux allows a continuously operating glycolysis and TCA cycle, thereby ensuring a great metabolic flexibility under stress conditions [33]. This provides an alternative for the non-phosphorylating respiratory bypasses to ameliorate metal stress—next to their proposed role in modulating ROS production. Indeed, an increased TCA cycle flux results in the production of organic acids in the mitochondrial matrix. These metabolites form a direct link between plant mitochondria and acclimation to metal stress as they are associated with metal hyperaccumulation and tolerance in several plant species. Citrate, malate and oxalate have been suggested as key cellular ligands for Cd, Ni and Zn, mediating metal transport through the xylem and vacuolar sequestration of metal-ligand complexes (for reviews see [33,110,111] and references therein). In Lycopersicon esulentum, Cd-induced secretion of oxalate from root apices was found to be associated with Cd resistance [112]. In addition, both citrate and malate were shown to be secreted from root apices of plants exposed to Al, thereby establishing an Al tolerance mechanism [113]. Singh and Chauhan [114] also discussed the potential of organic acids to detoxify absorbed Al in the cytosol, followed by vacuolar sequestration as an internal tolerance mechanism exploited by several Al accumulating plant species. Interestingly, Gray et al. [115] have demonstrated an increased AOX1 transcript level in tobacco cells incubated with organic acids such as citrate, malate and 2-oxoglutarate in a physiologically relevant concentration range. The authors postulate a plant mitochondrial retrograde signaling pathway for the regulation of AOX gene expression based on TCA cycle intermediates, which could function concomitantly with ROS signaling to the nucleus (Figure 2) [115].
5. Conclusions
Due to their central position in the cellular metabolism, the close relationship with chloroplasts and the integration of redox signals, plant mitochondria are main players in metal-induced cellular responses. In addition to being a target of cytosolar ROS, they also represent an important source of ROS production in conditions of metal stress. Depending on the intensity of the stressor, ROS are able to induce mitochondrial damage and/or signaling outside the mitochondria. The involvement of (mitochondrial) ROS in metal-induced PCD has been confirmed in several studies. Functioning as both a target and regulator of stress responses in plants, AOX is of major importance in the mitochondrial metabolism. Due to its ability to reduce mitochondrial ROS production, modulate PCD and TCA cycle activity, AOX is suggested to play a key role in metal-induced responses in plant mitochondria (Figure 2). Further research is needed to explore the role of this and other mitochondrial proteins in PCD and/or signaling-induced acclimation in more detail. Unraveling the role of mitochondria in metal-induced oxidative stress will ultimately contribute to the development and/or selection of crops with enhanced yield under suboptimal conditions such as metal exposure.
Acknowledgments
The authors apologize to any researcher whose work is not cited here due to limitations of space and scope. This work was supported by the Research Foundation—Flanders (FWO) by a PhD grant for Els Keunen and the project G.0807.09. Support was also obtained from the Methusalem project 08G03VGRJ.
References
- 1.Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. 1988;333:134–139. doi: 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- 2.Marschner H. Functions of Mineral Nutrients: Micronutrients. In: Marschner H, editor. Mineral Nutrition of Higher Plants. 2nd ed. Academic Press; London, UK: 1995. pp. 313–404. [Google Scholar]
- 3.Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- 4.Leonard SS, Bower JJ, Shi X. Metal-induced toxicity, carcinogenesis, mechanisms and cellular responses. Mol. Cell. Biochem. 2004;255:3–10. doi: 10.1023/b:mcbi.0000007255.72746.a6. [DOI] [PubMed] [Google Scholar]
- 5.Hogervorst J, Plusquin M, Vangronsveld J, Nawrot T, Cuypers A, Van Hecke E, Roels HA, Carleer R, Staessen JA. House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environ. Res. 2007;103:30–37. doi: 10.1016/j.envres.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Nawrot T, Plusquin M, Hogervorst J, Roels HA, Celis H, Thijs L, Vangronsveld J, Van Hecke E, Staessen JA. Environmental exposure to cadmium and risk of cancer: A prospective population-based study. Lancet Oncol. 2006;7:119–226. doi: 10.1016/S1470-2045(06)70545-9. [DOI] [PubMed] [Google Scholar]
- 7.Thijssen S, Cuypers A, Maringwa J, Smeets K, Horemans N, Lambrichts I, Van Kerkhove E. Low cadmium exposure triggers a biphasic oxidative stress response in mice kidneys. Toxicology. 2007;236:29–41. doi: 10.1016/j.tox.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Chary NS, Kamala CT, Raj DSS. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008;69:513–524. doi: 10.1016/j.ecoenv.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Sharma SS, Dietz KJ. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009;14:43–50. doi: 10.1016/j.tplants.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Schützendübel A, Polle A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002;53:1351–1365. [PubMed] [Google Scholar]
- 11.Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao F, Wang XC, Chen J. Involvement of plasma-membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedlings. Plant Sci. 2006;170:151–158. [Google Scholar]
- 13.Remans T, Opdenakker K, Smeets K, Mathijsen D, Vangronsveld J, Cuypers A. Metal-specific and NADPH oxidase dependent changes in lipoxygenase and NADPH oxidase gene expression in Arabidopsis thaliana exposed to cadmium or excess copper. Funct. Plant Biol. 2010;37:532–544. [Google Scholar]
- 14.Seth CS, Remans T, Keunen E, Jozefczak M, Gielen H, Opdenakker K, Weyens N, Vangronsveld J, Cuypers A. Phytoextraction of toxic metals: A central role for glutathione. Plant Cell Environ. 2011 doi: 10.1111/j.1365-3040.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- 15.Kučera T, Horáková H, Šonská A. Toxic metal ions in photoautotrophic organisms. Photosynthetica. 2008;46:481–489. [Google Scholar]
- 16.Noctor G, De Paepe R, Foyer CH. Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci. 2007;12:125–134. doi: 10.1016/j.tplants.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Sweetlove LJ, Fait A, Nunes-Nesi A, Williams T, Fernie AR. The mitochondrion: An integration point of cellular metabolism and signalling. Crit. Rev. Plant Sci. 2007;26:17–43. [Google Scholar]
- 18.Van Belleghem F, Cuypers A, Semane B, Smeets K, Vangronsveld J, d’Haen J, Valcke R. Subcellular localization of cadmium in roots and leaves of Arabidopsis thaliana. New Phytol. 2007;173:495–508. doi: 10.1111/j.1469-8137.2006.01940.x. [DOI] [PubMed] [Google Scholar]
- 19.Nouet C, Motte P, Hanikenne M. Chloroplastic and mitochondrial metal homeostasis. Trends Plant Sci. 2011;16:395–404. doi: 10.1016/j.tplants.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Dutilleul C, Garmier M, Noctor G, Mathieu C, Chétrit P, Foyer CH, De Paepe R. Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. Plant Cell. 2003;15:1212–1226. doi: 10.1105/tpc.009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006;141:357–366. doi: 10.1104/pp.106.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Aken O, Giraud E, Clifton R, Whelan J. Alternative oxidase: a target and regulator of stress responses. Physiol. Plant. 2009;137:354–361. doi: 10.1111/j.1399-3054.2009.01240.x. [DOI] [PubMed] [Google Scholar]
- 23.Møller IM. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:561–591. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]
- 24.Amirsadeghi S, Robson CA, Vanlerberghe GC. The role of the mitochondrion in plant responses to biotic stress. Physiol. Plant. 2007;129:253–266. [Google Scholar]
- 25.Blokhina O, Fagerstedt KV. Reactive oxygen species and nitric oxide in plant mitochondria: origin and redundant regulatory systems. Physiol. Plant. 2010;138:447–462. doi: 10.1111/j.1399-3054.2009.01340.x. [DOI] [PubMed] [Google Scholar]
- 26.De Gara L, Locato V, Dipierro S, de Pinto MC. Redox homeostasis in plants. The challenge of living with endogenous oxygen production. Respir. Physiol. Neurobiol. 2010;173:S13–S19. doi: 10.1016/j.resp.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Tan YF, O’Toole N, Taylor NL, Millar AH. Divalent metal ions in plant mitochondria and their role in interactions with proteins and oxidative stress-induced damage to respiratory function. Plant Physiol. 2010;152:747–761. doi: 10.1104/pp.109.147942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lösch R. Plant Mitochondrial Respiration under the Influence of Heavy Metals. In: Prasad MNV, editor. Heavy Metal Stress in Plants From Biomolecules to Ecosystems. 2nd ed. Springer-Verlag; Berlin, Germany: 2004. pp. 182–200. [Google Scholar]
- 29.Kampfenkel K, Van Montagu M, Inzé D. Effects of iron excess on Nicotiana plumbaginifolia plants (implications to oxidative stress) Plant Physiol. 1995;107:725–735. doi: 10.1104/pp.107.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pádua M, Aubert S, Casimiro A, Bligny R, Millar AH, Day DA. Induction of alternative oxidase by excess copper in sycamore cell suspensions. Plant Physiol. Biochem. 1999;37:131–137. [Google Scholar]
- 31.Dixit V, Pandey V, Shyam R. Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv. Azad) root mitochondria. Plant Cell Environ. 2002;25:687–693. [Google Scholar]
- 32.Yamamoto Y, Kobayashi Y, Rama Devi S, Rikiishi S, Matsumoto H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002;128:63–72. [PMC free article] [PubMed] [Google Scholar]
- 33.Plaxton WC, Podestá FE. The functional organization and control of plant respiration. Crit. Rev. Plant Sci. 2006;25:159–198. [Google Scholar]
- 34.Clark RB. Effect of aluminum on growth and mineral elements of Al-tolerant and Al-intolerant corn. Plant Soil. 1977;47:653–662. [Google Scholar]
- 35.Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Ravindran Nair A, Munters E, Artois TJ, et al. Cadmium stress: An oxidative challenge. Biometals. 2010;23:927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- 36.Heyno E, Klose C, Krieger-Liszkay A. Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol. 2008;179:687–699. doi: 10.1111/j.1469-8137.2008.02512.x. [DOI] [PubMed] [Google Scholar]
- 37.Bi YH, Chen WL, Zhang WN, Zhou Q, Yun LJ, Xing D. Production of reactive oxygen species, impairment of photosynthetic function and dynamic changes in mitochondria are early events in cadmium-induced cell death in Arabidopsis thaliana. Biol. Cell. 2009;101:629–643. doi: 10.1042/BC20090015. [DOI] [PubMed] [Google Scholar]
- 38.Verbruggen N, Hermans C, Schat H. Mechanisms to cope with arsenic or cadmium excess in plants. Curr. Opin. Plant Biol. 2009;12:364–372. doi: 10.1016/j.pbi.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Smiri M, Chaoui A, Rouhier N, Kamel C, Gelhaye E, Jacquot JP, El Ferjani E. Cadmium induced mitochondrial redox changes in germinating pea seed. Biometals. 2010;23:973–984. doi: 10.1007/s10534-010-9344-y. [DOI] [PubMed] [Google Scholar]
- 40.Gupta KJ, Fernie AR, Kaiser WM, van Dongen JT. On the origins of nitric oxide. Trends Plant Sci. 2011;16:160–168. doi: 10.1016/j.tplants.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 41.De Michele R, Vurro E, Rigo C, Costa A, Elviri L, Di Valentin M, Careri M, Zottini M, Sanità di Toppi L, Lo Schiavo F. Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol. 2009;150:217–228. doi: 10.1104/pp.108.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arasimowicz-Jelonek M, Floryszak-Wieczorek J, GwóŸdŸ EA. The message of nitric oxide in cadmium challenged plants. Plant Sci. 2011;181:612–620. doi: 10.1016/j.plantsci.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 43.Panda SK, Yamamoto Y, Kondo H, Matsumoto H. Mitochondrial alterations related to programmed cell death in tobacco cells under aluminium stress. C. R. Biol. 2008;331:597–610. doi: 10.1016/j.crvi.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Xing D. Mechanistic study of mitochondria-dependent programmed cell death induced by aluminium phytotoxicity using fluorescence techniques. J. Exp. Bot. 2011;62:331–343. doi: 10.1093/jxb/erq279. [DOI] [PubMed] [Google Scholar]
- 45.Małecka A, Derba-Maceluch M, Kaczorowska K, Piechalak A, Tomaszewska B. Reactive oxygen species production and antioxidative defense system in pea root tissues treated with lead ions: Mitochondrial and peroxisomal level. Acta Physiol. Plant. 2009;31:1065–1075. [Google Scholar]
- 46.Małecka A, Jarmuszkiewicz W, Tomaszewska B. Antioxidative defence to lead stress in subcellular compartments of pea root cells. Acta Biochim. Pol. 2001;48:687–698. [PubMed] [Google Scholar]
- 47.Sarry JE, Kuhn L, Ducruix C, Lafaye A, Junot C, Hugouvieux V, Jourdain A, Bastien O, Fievet JB, Vailhen D, et al. The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics. 2006;6:2180–2198. doi: 10.1002/pmic.200500543. [DOI] [PubMed] [Google Scholar]
- 48.Garnier L, Simon-Plas F, Thuleau P, Agnel JP, Blein JP, Ranjeva R, Montillet JL. Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ. 2006;29:1956–1969. doi: 10.1111/j.1365-3040.2006.01571.x. [DOI] [PubMed] [Google Scholar]
- 49.Basu U, Good AG, Taylor GJ. Transgenic Brassica napus plants overexpressing aluminium-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminium. Plant Cell Environ. 2001;24:1269–1278. [Google Scholar]
- 50.Garmash EV, Golovko TK. Effect of cadmium on growth and respiration of barley plants grown under two temperature regimes. Russ. J. Plant Physiol. 2009;56:343–347. [Google Scholar]
- 51.Prado C, Rodríguez-Montelongo L, González JA, Pagano EA, Hilal M, Prado FE. Uptake of chromium by Salvinia minima: effect on plant growth, leaf respiration and carbohydrate metabolism. J. Hazard. Mater. 2010;177:546–553. doi: 10.1016/j.jhazmat.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 52.Cuypers A, Smeets K, Ruytinx J, Opdenakker K, Keunen E, Remans T, Horemans N, Vanhoudt N, Van Sanden S, Van Belleghem F, et al. The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J. Plant Physiol. 2011;168:309–316. doi: 10.1016/j.jplph.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Chang HB, Lin CW, Huang HJ. Zinc-induced cell death in rice (Oryza sativa L.) roots. Plant Growth Regul. 2005;46:261–266. [Google Scholar]
- 54.Millar H, Considine MJ, Day DA, Whelan J. Unraveling the role of mitochondria during oxidative stress in plants. IUBMB Life. 2001;51:201–205. doi: 10.1080/152165401753311735. [DOI] [PubMed] [Google Scholar]
- 55.Sweetlove LJ, Heazlewood JL, Herald V, Holtzapffel R, Day DA, Leaver CJ, Millar AH. The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 2002;32:891–904. doi: 10.1046/j.1365-313x.2002.01474.x. [DOI] [PubMed] [Google Scholar]
- 56.Navrot N, Rouhier N, Gelhaye E, Jacquot JP. Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol. Plant. 2007;129:185–195. [Google Scholar]
- 57.Van Dongen JT, Gupta KJ, Ramírez-Aguilar SJ, Araújo WL, Nunes-Nesi A, Fernie AR. Regulation of respiration in plants: A role for metabolic pathways. J. Plant Physiol. 2011;168:1434–1443. doi: 10.1016/j.jplph.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Purvis AC, Shewfelt RL. Does the alternative pathway ameliorate chilling injury in sensitive plant tissues? Physiol. Plant. 1993;88:712–718. doi: 10.1111/j.1399-3054.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 59.Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc. Natl. Acad. Sci. USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castro-Guerrero NA, Rodríguez-Zavala JS, Marín-Hernández A, Rodríguez-Enríquez S, Moreno-Sánchez R. Enhanced alternative oxidase and antioxidant enzymes under Cd2+ stress in Euglena. J. Bioenerg. Biomembr. 2008;40:227–235. doi: 10.1007/s10863-007-9098-6. [DOI] [PubMed] [Google Scholar]
- 61.Rasmusson AG, Geisler DA, Møller IM. The multiplicity of dehydrogenases in the electron transport chain of plant mitochondria. Mitochondrion. 2008;8:47–60. doi: 10.1016/j.mito.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Clifton R, Lister R, Parker KL, Sappl PG, Elhafez D, Millar AH, Day DA, Whelan J. Stress-induced co-expression of alternative respiratory chain components in Arabidopsis thaliana. Plant Mol. Biol. 2005;58:193–212. doi: 10.1007/s11103-005-5514-7. [DOI] [PubMed] [Google Scholar]
- 63.Rasmusson AG, Møller IM. Mitochondrial Electron Transport and Plant Stress. In: Kempken F, editor. Plant Mitochondria Advances in Plant Biology. 1st ed. Springer; New York, NY, USA: 2011. pp. 357–381. [Google Scholar]
- 64.Keunen E, Vangronsveld J, Cuypers A. Hasselt University; Diepenbeek, Belgium: 2011. Unpublished work. [Google Scholar]
- 65.Vercesi AE, Borecký J, de Godoy Maia I, Arruda P, Cuccovia IM, Chaimovich H. Plant uncoupling mitochondrial proteins. Annu. Rev. Plant Biol. 2006;57:383–404. doi: 10.1146/annurev.arplant.57.032905.105335. [DOI] [PubMed] [Google Scholar]
- 66.Nogueira FTS, Sassaki FT, de Godoy Maia I. Arabidopsis thaliana Uncoupling Proteins (AtUCPs): Insights into gene expression during development and stress response and epigenetic regulation. J. Bioenerg. Biomembr. 2011;43:71–79. doi: 10.1007/s10863-011-9336-9. [DOI] [PubMed] [Google Scholar]
- 67.Considine MJ, Goodman M, Echtay KS, Laloi M, Whelan J, Brand MD, Sweetlove LJ. Superoxide stimulates a proton leak in potato mitochondria that is related to the activity of uncoupling protein. J. Biol. Chem. 2003;278:22298–22302. doi: 10.1074/jbc.M301075200. [DOI] [PubMed] [Google Scholar]
- 68.Smith AMO, Ratcliffe RG, Sweetlove LJ. Activation and function of mitochondrial uncoupling protein in plants. J. Biol. Chem. 2004;279:51944–51952. doi: 10.1074/jbc.M408920200. [DOI] [PubMed] [Google Scholar]
- 69.Laloi M, Klein M, Riesmeier JW, Müller-Röber B, Fleury C, Bouillaud F, Ricquier D. A plant cold-induced uncoupling protein. Nature. 1997;389:135–136. doi: 10.1038/38156. [DOI] [PubMed] [Google Scholar]
- 70.Desikan R, A-H-Mackerness S, Hancock JT, Neill SJ. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 2001;127:159–172. doi: 10.1104/pp.127.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brandalise M, de Godoy Maia I, Borecký J, Vercesi AE, Arruda P. ZmPUMP encodes a maize mitochondrial uncoupling protein that is induced by oxidative stress. Plant Sci. 2003;165:329–335. [Google Scholar]
- 72.Ozawa K, Murayama S, Kobayashi-Uehara A, Handa H. Overexpression of wheat mitochondrial uncoupling protein in rice plants confers tolerances to oxidative stress promoted by exogenous hydrogen peroxide and low temperature. Mol. Breed. 2006;18:51–56. [Google Scholar]
- 73.Sweetlove LJ, Lytovchenko A, Morgan M, Nunes-Nesi A, Taylor NL, Baxter CJ, Eickmeier I, Fernie AR. Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc. Natl. Acad. Sci. USA. 2006;103:19587–19592. doi: 10.1073/pnas.0607751103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pastore D, Trono D, Laus MN, Di Fonzo N, Flagella Z. Possible plant mitochondria involvement in cell adaptation to drought stress. A case study: durum wheat mitochondria. J. Exp. Bot. 2007;58:195–210. doi: 10.1093/jxb/erl273. [DOI] [PubMed] [Google Scholar]
- 75.Yin L, Mano J, Wang S, Tsuji W, Tanaka K. The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol. 2010;152:1406–1417. doi: 10.1104/pp.109.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasmusson AG, Fernie AR, van Dongen JT. Alternative oxidase: a defence against metabolic fluctuations? Physiol. Plant. 2009;137:371–382. doi: 10.1111/j.1399-3054.2009.01252.x. [DOI] [PubMed] [Google Scholar]
- 77.Fernie AR, Carrari F, Sweetlove LJ. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004;7:254–261. doi: 10.1016/j.pbi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 78.Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002;53:1331–1341. [PubMed] [Google Scholar]
- 79.Bueno P, Piqueras A. Effect of transition metals on stress, lipid peroxidation and antioxidant enzyme activities in tobacco cell cultures. Plant Growth Regul. 2002;36:161–167. [Google Scholar]
- 80.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 81.Jiménez A, Hernández JA, del Río LA, Sevilla F. Evidence for the presence of the ascorbate glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997;114:275–284. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chew O, Whelan J, Millar AH. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 2003;278:46869–46877. doi: 10.1074/jbc.M307525200. [DOI] [PubMed] [Google Scholar]
- 83.Bartoli CG, Pastori GM, Foyer CH. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol. 2000;123:335–343. doi: 10.1104/pp.123.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao ZQ, Cai YL, Zhu YG, Kneer R. Cadmium-induced oxidative stress and protection by L-galactono-1,4-lactone in winter wheat (Triticum aestivum L.) J. Plant Nutr. Soil Sci. 2005;168:759–763. [Google Scholar]
- 85.Szarka A, Horemans N, Kovács Z, Gróf P, Mayer M, Bánhegyi G. Dehydroascorbate reduction in plant mitochondria is coupled to the respiratory electron transfer chain. Physiol. Plant. 2007;129:225–232. [Google Scholar]
- 86.Reese RN, Roberts LW. Effects of cadmium on whole cell and mitochondrial respiration in tobacco cell suspension cultures (Nicotiana tabacum L. var. xanthi) J. Plant Physiol. 1985;120:123–130. [Google Scholar]
- 87.Finkemeier I, Goodman M, Lamkemeyer P, Kandlbinder A, Sweetlove LJ, Dietz KJ. The mitochondrial type II peroxiredoxin F is essential for redox homeostasis and root growth of Arabidopsis thaliana under stress. J. Biol. Chem. 2005;280:12168–12180. doi: 10.1074/jbc.M413189200. [DOI] [PubMed] [Google Scholar]
- 88.Gelhaye E, Rouhier N, Gérard J, Jolivet Y, Gualberto J, Navrot N, Ohlsson PI, Wingsle G, Hirasawa M, Knaff DB, et al. A specific form of thioredoxin h occurs in plant mitochondria and regulates the alternative oxidase. Proc. Natl. Acad. Sci. USA. 2004;101:14545–14550. doi: 10.1073/pnas.0405282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schwarzländer M, Fricker MD, Sweetlove LJ. Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochim. Biophys. Acta. 2009;1787:468–475. doi: 10.1016/j.bbabio.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 90.Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S. Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J. Exp. Bot. 2005;56:1481–1489. doi: 10.1093/jxb/eri181. [DOI] [PubMed] [Google Scholar]
- 91.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor NL, Day DA, Millar AH. Targets of stress-induced oxidative damage in plant mitochondria and their impact on cell carbon/nitrogen metabolism. J. Exp. Bot. 2004;55:1–10. doi: 10.1093/jxb/erh001. [DOI] [PubMed] [Google Scholar]
- 93.Bartoli CG, Gómez F, Martínez DE, Guiamet JJ. Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.) J. Exp. Bot. 2004;55:1663–1669. doi: 10.1093/jxb/erh199. [DOI] [PubMed] [Google Scholar]
- 94.Kristensen BK, Askerlund P, Bykova NV, Egsgaard H, Møller IM. Identification of oxidised proteins in the matrix of rice leaf mitochondria by immunoprecipitation and two-dimensional liquid chromatography-tandem mass spectrometry. Phytochemistry. 2004;65:1839–1851. doi: 10.1016/j.phytochem.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Gadjev I, Stone JM, Gechev TS. Programmed cell death in plants: New insights into redox regulation and the role of hydrogen peroxide. Int. Rev. Cell Mol. Biol. 2008;270:87–144. doi: 10.1016/S1937-6448(08)01403-2. [DOI] [PubMed] [Google Scholar]
- 96.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 97.Jones A. Does the plant mitochondrion integrate cellular stress and regulate programmed cell death? Trends Plant Sci. 2000;5:225–230. doi: 10.1016/s1360-1385(00)01605-8. [DOI] [PubMed] [Google Scholar]
- 98.Vianello A, Zancani M, Peresson C, Petrussa E, Casolo V, Krajňáková J, Patui S, Braidot E, Macrì F. Plant mitochondrial pathway leading to programmed cell death. Physiol. Plant. 2007;129:242–252. [Google Scholar]
- 99.Suzuki N, Koussevitzky S, Mittler R, Miller G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2011 doi: 10.1111/j.1365-3040.2011.02336.x. [DOI] [PubMed] [Google Scholar]
- 100.Galvez-Valdivieso G, Mullineaux PM. The role of reactive oxygen species in signalling from chloroplasts to the nucleus. Physiol. Plant. 2010;138:430–439. doi: 10.1111/j.1399-3054.2009.01331.x. [DOI] [PubMed] [Google Scholar]
- 101.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 102.Møller IM, Kristensen BK. Protein oxidation in plant mitochondria as a stress indicator. Photochem. Photobiol. Sci. 2004;3:730–735. doi: 10.1039/b315561g. [DOI] [PubMed] [Google Scholar]
- 103.Møller IM, Sweetlove LJ. ROS signalling—specificity is required. Trends Plant Sci. 2010;15:370–374. doi: 10.1016/j.tplants.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 104.Rhoads DM, Subbaiah CC. Mitochondrial retrograde regulation in plants. Mitochondrion. 2007;7:177–194. doi: 10.1016/j.mito.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 105.Van Aken O, Zhang B, Carrie C, Uggalla V, Paynter E, Giraud E, Whelan J. Defining the mitochondrial stress response in Arabidopsis thaliana. Mol. Plant. 2009;2:1310–1324. doi: 10.1093/mp/ssp053. [DOI] [PubMed] [Google Scholar]
- 106.Giraud E, Ho LHM, Clifton R, Carroll A, Estavillo G, Tan YF, Howell KA, Ivanova A, Pogson BJ, Millar AH, Whelan J. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008;147:595–610. doi: 10.1104/pp.107.115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Robson CA, Vanlerberghe GC. Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and -independent pathways of programmed cell death. Plant Physiol. 2002;129:1908–1920. doi: 10.1104/pp.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vanlerberghe GC, Cvetkovska M, Wang J. Is the maintenance of homeostatic mitochondrial signaling during stress a physiological role for alternative oxidase? Phys. Plant. 2009;137:392–406. doi: 10.1111/j.1399-3054.2009.01254.x. [DOI] [PubMed] [Google Scholar]
- 109.Bartoli CG, Yu J, Gómez F, Fernández L, McIntosh L, Foyer CH. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J. Exp. Bot. 2006;57:1621–1631. doi: 10.1093/jxb/erl005. [DOI] [PubMed] [Google Scholar]
- 110.Rauser WE. Structure and function of metal chelators produced by plants—The case for organic acids, amino acids, phytin, and metallothioneins. Cell Biochem. Biophys. 1999;31:19–48. doi: 10.1007/BF02738153. [DOI] [PubMed] [Google Scholar]
- 111.Haydon MJ, Cobbett CS. Transporters of ligands for essential metal ions in plants. New Phytol. 2007;174:499–506. doi: 10.1111/j.1469-8137.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 112.Zhu XF, Zheng C, Hu YT, Jiang T, Liu Y, Dong NY, Yang JL, Zheng SJ. Cadmium-induced oxalate secretion from root apex is associated with cadmium exclusion and resistance in Lycopersicon esulentum. Plant Cell Environ. 2011;34:1055–1064. doi: 10.1111/j.1365-3040.2011.02304.x. [DOI] [PubMed] [Google Scholar]
- 113.Delhaize E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singh D, Chauhan SK. Organic acids of crop plants in aluminium detoxification. Curr. Sci. 2011;100:1509–1515. [Google Scholar]
- 115.Gray GR, Maxwell DP, Villarimo AR, McIntosh L. Mitochondria/nuclear signaling of alternative oxidase gene expression occurs through distinct pathways involving organic acids and reactive oxygen species. Plant Cell Rep. 2004;23:497–503. doi: 10.1007/s00299-004-0848-1. [DOI] [PubMed] [Google Scholar]