Abstract
Context
Cardiovascular (CV) risk markers, including high-sensitivity C-reactive protein (hsCRP), are increasingly important in predicting cardiac events. A favorable CV risk profile might be expected in anorexia nervosa (AN) due to low body weight and dietary fat intake. However, women with AN have decreased IGF-I levels reflecting decreased GH action, and IGF-I deficiency is associated with elevated hsCRP. Moreover, oral estrogens, known to increase hsCRP in other populations, are commonly prescribed in AN. To date, hsCRP levels and their physiological determinants have not been reported in women with AN.
Objective
We examined the relationship between CV risk markers, undernutrition, IGF-I, and oral estrogens, specifically hypothesizing that in the setting of undernutrition, AN would be associated with low hsCRP despite low IGF-I levels and that those women taking oral contraceptive pills (OCPs) would have higher hsCRP and lower IGF-I levels.
Design and Setting
We conducted a cross-sectional study at a clinical research center.
Study Participants
Subjects included 181 women: 140 women with AN [85 not receiving OCPs (AN-E) and 55 receiving OCPs (AN+E)] and 41 healthy controls [28 not receiving OCPs (HC-E) and 13 receiving OCPs (HC+E)].
Main Outcome Measures
We assessed hsCRP, IL-6, IGF-I, low-density lipoprotein (LDL), and high-density lipoprotein (HDL).
Results
Despite low weight, more than 20% of AN+E had high-risk hsCRP levels. AN+E had higher hsCRP than AN-E. AN-E had lower mean hsCRP levels than healthy controls (HC+E and HC-E). IL-6 levels were higher in AN+E with elevated hsCRP (>3 mg/liter) than in AN+E with normal hsCRP levels. IGF-I was inversely associated with hsCRP in healthy women, suggesting a protective effect of GH on CV risk. However, this was not seen in AN. Few patients in any group had high-risk LDL or HDL levels.
Conclusions
Although hsCRP levels are lower in AN than healthy controls, OCP use puts such women at a greater than 20% chance of having hsCRP in the high-CV-risk (>3 mg/liter) category. The elevated mean IL-6 in women with AN and high-risk hsCRP levels suggests that increased systemic inflammation may underlie the hsCRP elevation in these patients. Although OCP use in AN was associated with slightly lower mean LDL and higher mean HDL, means were within the normal range, and few patients in any group had high-risk LDL or HDL levels. IGF-I levels appear to be important determinants of hsCRP in healthy young women. In contrast, IGF-I does not appear to mediate hsCRP levels in AN.
Serum predictors of cardiovascular (CV) risk are increasingly recognized for their importance in guiding targeted preventive care, even in young, healthy populations. Atherosclerotic plaque formation begins early in life, long before the appearance of clinical sequelae (1). Exogenous oral estrogens have been shown to have positive effects on high-density lipoprotein (HDL) and low-density lipoprotein (LDL), although adversely altering levels of high-sensitivity C-reactive protein (hsCRP), a key CV risk marker (2, 3). A role for the GH axis in regulating hsCRP has been postulated (4). We have previously demonstrated that treatment of GH deficiency, a condition associated with low IGF-I, high hsCRP, and increased CV risk, results in lowering of hsCRP levels (4) and that normalizing IGF-I levels in patients with GH hypersecretion increases CRP levels (5). In healthy populations, hsCRP has been found to be inversely proportional to IGF-I (6, 7). Estrogen also affects this axis. Oral estrogens are known to lower IGF-I levels and promote GH resistance (3).
Women with anorexia nervosa (AN), a psychiatric disease characterized by self-imposed starvation, medical complications, and premature mortality, have elevated GH and low IGF-I levels, thought to represent a state of acquired GH resistance and decreased GH action (8, 9). HsCRP levels, which increase in obesity, decrease with weight loss, and increase with oral estrogen use in healthy women (10–13), have not been reported in women with AN. Reports of lipoprotein levels in AN are inconsistent, demonstrating normal or elevated mean LDL and HDL levels (14–21), and the effect of oral contraceptives, commonly prescribed in AN on CV risk markers in such women is unknown. A favorable CV risk profile might be expected in AN due to low body weight and dietary fat intake. However, low IGF-I and oral contraceptive pill (OCP) use in this population could result in an elevation of hsCRP, the best-validated marker of CV disease. To investigate the hormonal and nutritionally dependent determinants of hsCRP in this population, we examined the relationship between CV risk markers, undernutrition, IGF-I, and use of oral estrogens in women with AN compared with healthy women.
Subjects and Methods
We studied 181 women: 140 women with AN [55 receiving OCPs (AN+E) and 85 not receiving OCPs (AN-E)] and 41 healthy controls [13 receiving OCPs (HC+E) and 28 not receiving OCPs (HC-E)]. Subjects with AN met Diagnostic and Statistical Manual of Mental Disorders IV criteria, including percent ideal body weight less than 85 (22). Healthy controls had regular menses and no medical condition, including diabetes mellitus, CV disease, major depression, or disordered eating. The study was approved by the Partners Institutional Review Board, and written informed consent was obtained from all subjects. Clinical characteristics, including medical history, vital signs, laboratory abnormalities, and body composition have been previously reported in subsets of these patients (23–27).
Protocol
Study subjects were recruited through collaborating physicians, advertisements in community newspapers, and online referral sites. History, physical exam, and serum samples were obtained. Level of activity was assessed using the Paffenbarger scale (28). Research dieticians measured metabolic weight and height and calculated body mass index (BMI) (kg/m2). Body composition was determined by dual-energy x-ray absorptiometry (Hologic 4500; Waltham, MA). HC+E subjects provided historical information and serum samples.
Biochemical analysis
HsCRP was determined using an Hitachi 917 analyzer (Roche Diagnostics, Indianapolis, IN) with reagents and calibrators from Equal Diagnostics (Exton, PA). The intraassay coefficient of variation was less than or equal to 10% for hsCRP, and the sensitivity was 0.1 mg/liter. IGF-I was determined by immunoradiometric assay from Diagnostic Systems Laboratories (Webster, TX). The intraassay coefficient of variation was 3.9–7.0% for IGF-I, and the sensitivity was 4.15 ng/ml. Serum IL-6 was measured using a high-sensitivity sandwich enzyme immunoassay (R&D Systems, Inc., Minneapolis, MN), with a sensitivity of 0.039 pg/ml and intraassay coefficient of variation of 6.9–7.5%. Total cholesterol, HDL cholesterol, and triglycerides were determined using the Hitachi 917 analyzer with reagents from Roche Diagnostics and ChemTrak calibrators from MAS Inc. (Camarillo, CA). The intraassay coefficient of variation was less than or equal to 2.0% for total cholesterol, less than or equal to 3.3% for HDL, and less than or equal to 2.5% for triglycerides. LDL cholesterol was calculated using the Friedewald formula (29).
Data analysis
JMP Statistical Discoveries (version IV; SAS Institute, Inc., Cary, NC) was used for statistical analysis. Variables were tested for normality using the Shapiro-Wilk test. Because the majority of variables were not normally distributed, clinical characteristics were compared with non-parametric Wilcoxon testing. Multiple comparisons were controlled for using the Tukey-Kramer test for log-transformed CV risk markers, which were significantly different between groups. Because age was higher in the HC+E group compared with the other three groups, age was controlled for in all comparisons of CV risk markers involving the HC+E group, using analysis of covariance of log-transformed variables. Univariate regression analysis was used to investigate possible associations between clinical characteristics and CV risk markers. The χ2 test was used to compare proportions between groups. Statistical significance was defined as a two-tailed P value < 0.05. Data are reported as mean ± SEM.
Results
Patient characteristics
Clinical characteristics of AN-E, AN+E, HC-E, and HC+E were compared and are shown in Table 1. Demographic variables were well matched between the groups, including AN-E vs. AN+E, except for a few variables including a higher mean age of HC+E and a larger number of smokers in the AN than HC groups. Therefore, these variables were controlled for in all comparisons of CV risk markers involving these groups. As expected, AN had lower BMI and percent fat mass than HC.
TABLE 1.
Clinical and biochemical characteristics
| AN-E (n = 85) | AN+E (n = 55) | HC-E (n = 28) | HC+E (n = 13) | AN-E vs. AN+E P value |
AN-E vs. HC-E P value |
AN-E vs. HC+E P value |
AN+E vs. HC-E P value |
AN+E vs. HC+E P value |
HC-E vs. HC+E P value |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | 25.6 ± 0.8 | 24.2 ± 0.7 | 24.2 ± 0.6 | 31.6 ± 1.7 | NS | NS | 0.003 | NS | 0.0005 | 0.0004 |
| BMI (kg/m2) | 16.9 ± 0.2 | 17.4 ± 0.2 | 21.0 ± 0.3 | 21.9 ± 0.6 | NS | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS |
| Fat mass (%) | 17.2 ± 0.6 | 18.1 ± 0.7 | 27.2 ± 0.7 | NA | NS | <0.0001 | NA | <0.0001 | NA | NA |
| Smoking (no., %) | 10 (12%) | 7 (13%) | 0 (0%) | 0 (0%) | NS | 0.01 | 0.08 | 0.01 | NS | NS |
| Activity (h/wk) | 5.8 ± 0.8 | 4.4 ± 0.9 | 6.3 ± 0.8 | NA | NS | NS | NA | 0.03 | NA | NA |
| Alcohol (no./wk) | 1.4 ± 0.3 | 1.8 ± 0.3 | 1.7 ± 0.4 | 1.8 ± 0.6 | 0.04 | NS | NS | NS | NS | NS |
| HsCRP (mg/liter) | 0.54 ± 0.09 | 2.21 ± 0.47 | 0.89 ± 0.18 | 3.32 ± 0.67 | <0.0001 | <0.0001 | <0.0001 | NS | 0.003 | <0.0001 |
| LDL (mg/dl) | 95 ± 3 | 85 ± 3 | 98 ± 4 | 94 ± 7 | 0.04 | NS | NS | 0.01 | NS | NS |
| HDL (mg/dl) | 68 ± 2 | 73 ± 2 | 54 ± 2 | 65 ± 4 | NS | 0.0002 | NS | <0.0001 | NS | 0.008 |
| IGF-I (ng/ml) | 328 ± 17 | 322 ± 19 | 392 ± 27 | 278 ± 23 | NS | 0.03 | NS | 0.009 | NS | 0.006 |
Data are mean ± SEM. NS, Not significant; NA, data not available.
HsCRP
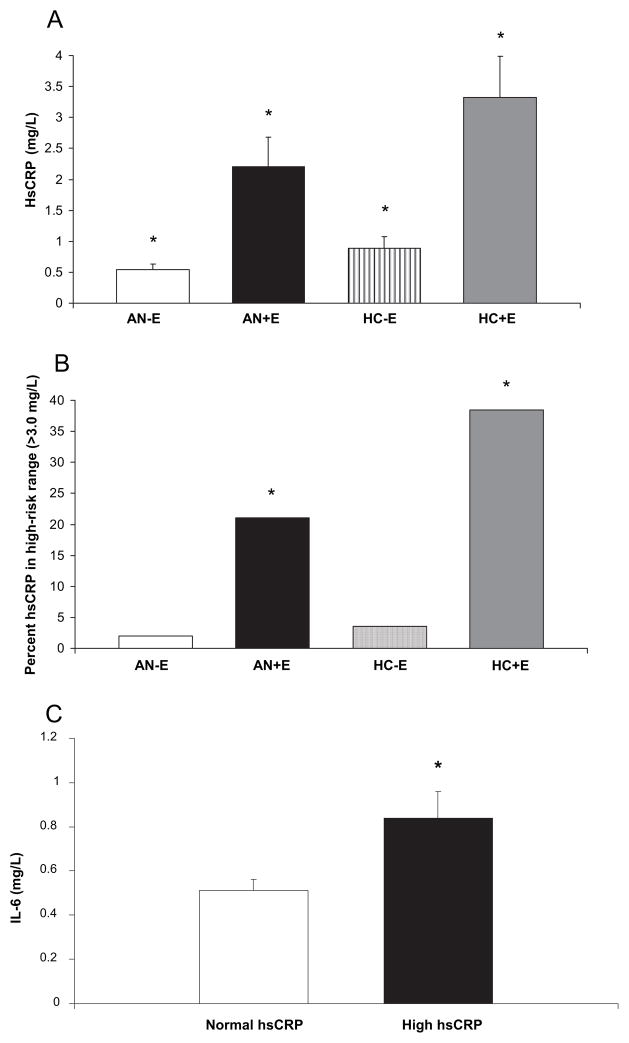
AN+E had higher mean hsCRP levels than AN-E (P < 0.0001). HC+E had higher mean hsCRP than HC-E (P < 0.0001). AN-E had lower mean hsCRP levels than both HC+E and HC-E (P < 0.0001; Fig. 1A). All significant differences in mean hsCRP levels delineated in Table 1 remained significant after controlling for multiple comparisons. Mean hsCRP levels remained higher in AN+E compared with AN-E after controlling for smoking, and in a subset analysis of nonsmokers only, mean hsCRP levels in AN+E were higher than AN-E (P < 0.0001). All comparisons with HC+E remained significant after controlling for age. More than 20% of AN+E had high-risk [>3.0 mg/liter (30)] hsCRP levels, and this was higher than in AN-E (21.4 vs. 2.4%, P = 0.0002) and than HC-E (P = 0.02; Fig. 1B), even after controlling for age, smoking, alcohol intake, activity, and BMI (P < 0.006); 38.5% of HC+E had high-risk hsCRP levels compared with 3.6% of HC-E (P = 0.004).
Fig. 1.
HsCRP and IL-6. A, Mean hsCRP in AN-E (white; 0.54 ± 0.09 mg/liter), AN+E (black; 2.21 ± 0.47 mg/liter), HC-E (striped; 0.89 ± 0.18 mg/liter), and HC+E (gray; 3.32 ± 0.67). *, P < 0.01 for all intragroup comparisons, except AN+E vs. HC-E. B, Percent with high-risk hsCRP levels (>3.0 mg/liter) in AN-E (white; 2.4%), AN+E (black; 21.4%), HC-E (striped; 3.6%), and HC+E (gray; 38.5%). *, P < 0.05 compared with AN-E and HC-E. C, Mean IL-6 levels in AN+E with hsCRP levels in the high-risk range (black; 0.84 ± 0.12 mg/liter) and not in the high-risk range (white; 0.51 ± 0.05 mg/liter). High-risk hsCRP: 3.0 mg/liter. *, P = 0.005.
IL-6
Mean IL-6 levels were higher in study subjects (AN and HC combined) with elevated hsCRP levels (>3 mg/liter) compared with those of subjects with normal hsCRP levels (0.86 ± 0.08 vs. 0.59 ± 0.03 mg/liter, P = 0.003). Mean IL-6 levels were higher in women with AN who had elevated hsCRP levels than in women with AN with normal hsCRP levels (0.84 ± 0.37 vs. 0.59 ± 0.04 mg/liter, P = 0.04). In AN+E, mean IL-6 levels were higher in those with elevated compared with those with normal hsCRP (0.84 ± 0.12 mg/liter vs. 0.51 ± 0.05 mg/liter, P = 0.005; Fig. 1C).
HDL
AN-E and AN+E had higher mean HDL than HC-E (P = 0.0002, and P < 0.0001, respectively; Table 1), even after controlling for smoking and multiple comparisons. Mean HDL was higher in HC+E than HC-E (P = 0.008), but this difference was no longer significant after controlling for age or multiple comparisons. One subject in each of the following groups, AN-E, HC-E, and HC+E, had an HDL level in the high-risk range [<40 mg/dl (31)]. HDL was negatively associated with percent fat mass (r = −0.23; P = 0.002) and BMI (r = −0.17; P = 0.02) and positively associated with alcohol consumption (r = 0.33; P < 0.0001).
LDL
Mean LDL was lower in AN+E compared with HC-E (P = 0.01; Table 1) and remained significant after controlling for smoking but not after adjusting for multiple comparisons. AN+E had lower LDL than AN-E (P = 0.04), but this was no longer significant after controlling for multiple comparisons. Two AN+E, one AN-E, and no healthy controls had high-risk LDL levels (>159 mg/dl) (31). There was a weak association between LDL and percent fat mass (r = 0.17; P = 0.03)
IGF-I
Mean IGF-I levels were lower in AN and HC+E compared with HC-E (P ≤ 0.03; Table 1). There were no differences in IGF-I between AN+E, AN-E, or HC+E. IGF-I was negatively associated with age (r = −0.33; P < 0.01) and positively associated with percent fat mass (r = 0.24; P < 0.01).
Associations between hsCRP, IGF-I, LDL, and HDL
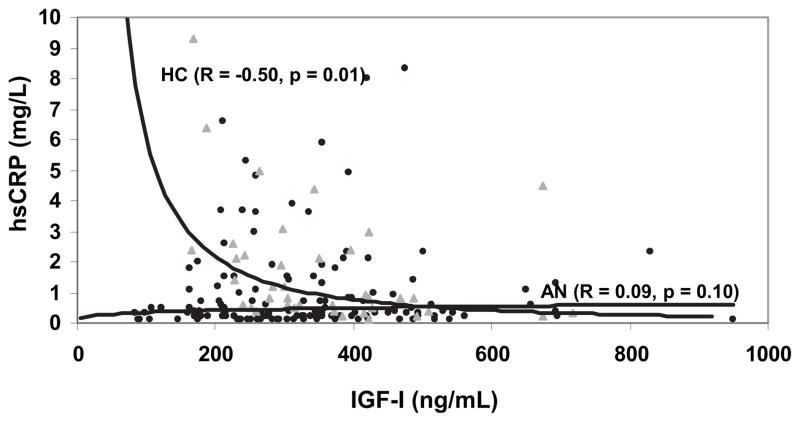
IGF-I was inversely associated with hsCRP in healthy women (r = −0.50; P = 0.001), but there was no association between IGF-I and hsCRP in women with AN (Fig. 2). There was an inverse association between IGF-I and HDL (r = −0.21; P = 0.004). This relationship was present in HC and in AN (r = −0.43, P = 0.006; r = −0.18, P = 0.04, respectively). IGF-I and LDL were not significantly associated.
Fig. 2.
Association between hsCRP and IGF-I. IGF-I levels inversely predicted hsCRP levels in HC (triangles) but not in AN (circles.)
Discussion
Although hsCRP levels in women with AN are lower than in healthy women, more than 20% of women with AN on OCPs fell into the high-risk (hsCRP > 3 mg/liter) category, despite profound undernutrition. Women with AN who were receiving OCPs had a higher mean level of hsCRP than women with AN or HC not receiving oral estrogens/progestins. A relationship was clearly seen between IGF-I and hsCRP levels in healthy women, although this relationship was not observed in AN, likely due to underlying suppression of IGF-I in these women by undernutrition.
In a survey of Society for Adolescent Medicine physicians by Robinson et al. (32), 78% of respondents said they prescribed hormone replacement therapy to their patients with AN. Although bone loss is a major complication of AN (24), prospective trials have failed to show efficacy of OCP therapy alone in preventing or treating AN-induced bone loss in adults or adolescents (33–36). Therefore, unlike other hypoestrogenemic states associated with bone loss, such as menopause or possibly functional normal-weight hypothalamic amenorrhea, there is no known therapeutic role for estrogen in AN. Contraception remains an indication for oral contraceptive use in this population. However, Robinson et al. (32) found that fewer than 10% of prescribers were influenced by whether or not the patient was sexually active. Despite the lack of clear benefit for the majority of patients with AN receiving OCPs, they continue to be widely prescribed for this population. Therefore, determination of the risks vs. benefits of OCP use in AN is important.
Whether OCP use in healthy young women is associated with increased CV risk is controversial. However, a recent meta-analysis of 14 studies showed that current use of low-dose OCPs increased the risk for myocardial infarction by 84% (37). More data are available regarding CV risk associated with estrogen/progestin use in older women. The Heart and Estrogen/Progestin Replacement Study showed an early increase in events and no benefit overall in women with known CV disease, and the Women’s Health Initiative (WHI) trial demonstrated an increase in CV events in healthy women (38, 39). One proposed mechanism for this effect is an increased production of inflammatory cytokines, because elevated hsCRP is a consistent finding in women taking oral estrogens with or without progestins (10, 11, 40, 41).
In both diseased and apparently healthy populations, hsCRP is a hepatic marker of systemic inflammation and indicator of CV risk (42, 43). The Women’s Health Study prospectively evaluated more than 20,000 healthy postmenopausal women and found that of 12 lipid or inflammatory indicators, baseline levels of hsCRP provided the strongest predictor of subsequent CV disease (44). Follow-up analysis after a mean of 8 yr confirmed that hsCRP exceeds LDL in predictive value (45). It is important to note that because the mean age of participants in the WHI was 63 yr old, the generalizability of these findings to other groups of women, particularly younger women, remains to be determined. A meta-analysis including 14 studies and more than 2500 subjects estimated that those with baseline hsCRP levels in the highest third of the distribution had twice the risk of CV events than those in the lowest (46). In the Physicians’ Health Study, hsCRP levels predicted sudden cardiac death in healthy men and suggested that chronic cardiac exposure to inflammatory mediators like hsCRP may directly promote arrhythmias (47). The finding of an elevation of hsCRP in AN, a disease associated with structural cardiac abnormalities, electrocardiogram changes, and metabolic alterations and sometimes treated with medications that may predispose to rhythm disturbances, may be of potential concern over a period of prolonged exposure. Because the rate of CV events is low in young women, elevated hsCRP in women with AN receiving oral contraceptives predicts a small absolute increase in CV events in those women in whom AN is limited to a short period of time in their 20s. This is consistent with the preponderance of published data demonstrating elevated mortality rates for young women with AN attributable to suicide, complications of alcohol use, and arrhythmias (8, 48–51). A large proportion of deaths reported in this population are from undetermined etiologies, and an elevated incidence of CV events as a cause of death has not been demonstrated. The standardized mortality rate has been shown to increase with age for women with AN, although the specific cause or causes for this trend have not been identified (52). For the growing number of women for whom AN is chronic and especially for those in whom it persists into menopause, our findings are therefore potentially significant.
Although oral contraceptive use and hormone replacement therapy clearly increase hsCRP, the mechanism is not understood, nor is it known whether the elevation in hsCRP is due to the progestin or estrogen component. However, the clinical significance of elevated hsCRP in this setting is strongly supported by the WHI prospective observational study, which showed that healthy women with comparable hsCRP levels had a similar risk of future CV events, regardless of whether they were receiving estrogens/progestins (53). This suggests that elevated hsCRP is a strong predictor of CV events in women receiving estrogens/progestins as well as in those who are not. Of note, although none of the seminal hsCRP studies included women younger than 45 yr old, they all clearly demonstrated that the predictive value of hsCRP remained significant after controlling for age (44, 54), raising the possibility that elevated hsCRP levels in young women, particularly if sustained chronically, could be predictive of cardiac events later in life. The possibility that progestins, not estrogens, could be the cause of hsCRP elevations was also raised by the WHI, which reported that combined estrogens/progestins led to increased CV risk, whereas estrogen alone did not (38, 55). These data are consistent with a recent analysis from the randomized Post-menopausal Estrogen Progestin Intervention study that found that IL-6, a critical cytokine stimulus of hsCRP production, increased concurrently with hsCRP in women receiving estrogen plus progestins but was inverse to the rise in hsCRP in those receiving estrogen only (56). The authors concluded that although progestins may promote hsCRP via an inflammatory pathway, estrogen likely stimulates hsCRP production independently and thus may not carry the same risk. This is consistent with a number of other studies examining estrogen effects on hsCRP, which have not reported a corresponding rise in IL-6 (41, 57–59). In addition, data in postmenopausal women demonstrated that transdermal and intranasal hormone replacement therapy preparations that avoid first-pass liver metabolism do not affect hsCRP levels, whereas oral estrogens consistently promote a rise in hsCRP (57, 60–62). This has led some to suggest that oral estrogens may directly stimulate hepatic hsCRP production independent of IL-6 or even of inflammatory pathways (38). However, we found IL-6 levels to be elevated in women with AN receiving oral contraceptives who had elevated hsCRP levels. This suggests that in women with AN receiving oral contraceptives, elevated hsCRP may reflect systemic inflammation. Whether the etiology of the IL-6 elevation is the estrogen or progestin component of the OCP, or both, could not be determined by our study. Our data raise the very real possibility that elevated hsCRP in young women with AN could be predictive of future CV events, particularly if the hsCRP elevation is sustained chronically. Additional studies in this area are therefore merited.
The GH axis has been identified as a potential mediator of hsCRP production and may be a mechanism underlying the effect of OCPs to increase hsCRP in healthy women (4, 5). In addition to increasing hsCRP levels, oral but not transdermal estrogens suppress IGF-I production, likely reflecting a first-pass hepatic effect (61). A randomized controlled trial of GH administration on CV risk markers in 40 men with GH deficiency demonstrated a decrease in hsCRP and IL-6 with GH treatment compared with placebo (4). We have also shown that by normalization of IGF-I in acromegalic patients using a GH receptor antagonist, mean hsCRP increased from below normal to a level comparable to baseline healthy controls, independent of IL-6 levels (5). The GH receptor antagonist reduces IGF-I and elevates GH levels. This suggests that IGF-I, rather than GH itself, is the mediator of effects on hsCRP. In our study, the expected drop in IGF-I with OCPs was not seen in women with AN, perhaps because of already suppressed IGF-I in the setting of starvation-induced GH resistance. We also found that although IGF-I was inversely associated with hsCRP levels in healthy women, this IGF-I to hsCRP relationship was not evident in AN, suggesting that a significant effect of OCPs on hsCRP is independent of the GH axis in AN.
This study is the first to report increased hsCRP in women with AN taking OCPs and lower hsCRP levels in those women with AN who are not receiving OCPs. Although, as anticipated, IGF-I levels were lower in healthy women receiving OCPs than those who were not, there was no difference in mean IGF-I levels between the AN groups, perhaps because of GH resistance. The inverse relationship between IGF-I and hsCRP observed in healthy young women in our study was not found in AN, suggesting that the OCP-induced elevation in hsCRP is not mediated by the GH axis. Lipoprotein levels in both groups of women with AN were well within the normal range likely due to low weight, the effect of OCP use on these levels were modest, and few patients had high-risk levels. In contrast, the elevation of hsCRP in women with AN receiving OCPs was substantial and resulted in a significant proportion of women with hsCRP above 3.0, a level associated with twice the risk of a future CV event compared with women with hsCRP levels in the lowest tertile (30). IL-6 levels were elevated in women with AN receiving OCPs with elevated hsCRPs, suggesting that the elevated hsCRPs in this population reflect increased systemic inflammation. Additional research will be important to determine whether elevated hsCRP associated with OCP use in AN results in increased risk of CV events. Because no beneficial effect of oral contraceptives on bone density or other endpoints has been demonstrated, and our data demonstrate possible deleterious effects on CV risk, women with AN who are not sexually active should not be offered oral contraceptive treatment. In addition, alternatives to oral contraceptives, such as barrier methods, should be discussed with those who are at risk of becoming pregnant. However, because the consequences of pregnancy during AN can be serious, oral contraceptives should not be withheld from women with AN who are sexually active and unlikely to use alternative birth control methods, pending additional research in this area.
Acknowledgments
We thank the nurses and bionutritionists at the Massachusetts General Hospital Clinical Research Center and the patients who participated in the study.
This work was supported in part by National Institutes of Health Grants R01-DK52625 and MO1 RR01066.
Abbreviations
- AN
Anorexia nervosa
- BMI
body mass index
- CV
cardiovascular
- −E
not receiving oral contraceptives
- +E
receiving oral contraceptives
- HC
healthy controls
- HDL
high-density lipoprotein
- hsCRP
high-sensitivity C-reactive protein
- LDL
low-density lipoprotein
- OCP
oral contraceptive pill
- WHI
Women’s Health Initiative
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP, III, Herderick EE, Cornhill JF for the Pathobiological Determinants of Atherosclerosis in Youth Research Group. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999;281:727–735. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- 2.Lobo R, Skinner J, Lippman J, Cirrillo S. Plasma lipids and desogestrel and ethinyl estradiol: a meta-analysis. Fertil Steril. 1996;65:1100–1109. [PubMed] [Google Scholar]
- 3.Leung KC, Johannsson G, Leong GM, Ho KK. Estrogen regulation of growth hormone action. Endocr Rev. 2004;25:693–721. doi: 10.1210/er.2003-0035. [DOI] [PubMed] [Google Scholar]
- 4.Sesmilo G, Biller BMK, Llevadot J, Hayden D, Hanson G, Rifai N, Klibanski A. Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. Ann Intern Med. 2000;133:111–122. doi: 10.7326/0003-4819-133-2-200007180-00010. [DOI] [PubMed] [Google Scholar]
- 5.Sesmilo G, Fairfield WP, Katznelson L, Pulaski K, Freda PU, Bonert V, Dimaraki E, Stavrou S, Vance ML, Hayden D, Klibanski A. Cardiovascular risk factors in acromegaly before and after normalization of serum IGF-I levels with the GH antagonist pegvisomant. J Clin Endocrinol Metab. 2002;87:1692–1699. doi: 10.1210/jcem.87.4.8364. [DOI] [PubMed] [Google Scholar]
- 6.Sesmilo G, Miller KK, Hayden D, Klibanski A. Inflammatory cardiovascular risk markers in women with hypopituitarism. J Clin Endocrinol Metab. 2001;86:5774–5781. doi: 10.1210/jcem.86.12.8087. [DOI] [PubMed] [Google Scholar]
- 7.Heald AH, Anderson SG, Ivison F, Laing I, Gibson JM, Cruickshank K. C-reactive protein and the insulin-like growth factor (IGF)-system in relation to risk of cardiovascular disease in different ethnic groups. Atherosclerosis. 2003;170:79–86. doi: 10.1016/s0021-9150(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 8.Keel P, Dorer D, Eddy K, Franko D, Charatan D, Herzog D. Predictors of mortality in eating disorders. Arch Gen Psychiatry. 2003;60:179–183. doi: 10.1001/archpsyc.60.2.179. [DOI] [PubMed] [Google Scholar]
- 9.Counts D, Gwirtsman H, Carlsson L, Lesem M, Cutler G., Jr The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75:762–767. doi: 10.1210/jcem.75.3.1381372. [DOI] [PubMed] [Google Scholar]
- 10.Raitakari M, Mansikkaniemi K, Marniemi J, Viikari JS, Raitakari OT. Distribution and determinants of serum high-sensitivity C-reactive protein in a population of young adults: the Cardiovascular Risk in Young Finns Study. J Intern Med. 2005;258:428–434. doi: 10.1111/j.1365-2796.2005.01563.x. [DOI] [PubMed] [Google Scholar]
- 11.Williams MJ, Williams SM, Milne BJ, Hancox RJ, Poulton R. Association between C-reactive protein, metabolic cardiovascular risk factors, obesity and oral contraceptive use in young adults. Int J Obes Relat Metab Disord. 2004;28:998–1003. doi: 10.1038/sj.ijo.0802713. [DOI] [PubMed] [Google Scholar]
- 12.Heilbronn LK, Noakes M, Clifton PM. Energy restriction and weight loss on very-low-fat diets reduce C-reactive protein concentrations in obese, healthy women. Arterioscler Thromb Vasc Biol. 2001;21:968–970. doi: 10.1161/01.atv.21.6.968. [DOI] [PubMed] [Google Scholar]
- 13.Tchernof A, Nolan A, Sites CK, Ades PA, Poehlman ET. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564–569. doi: 10.1161/hc0502.103331. [DOI] [PubMed] [Google Scholar]
- 14.Weinbrenner T, Zuger M, Jacoby G, Herpertz S, Liedtke R, Sudhop T, Gouni-Berthold I, Axelson M, Berthold H. Lipoprotein metabolism in patients with anorexia nervosa: a case-control study investigating the mechanisms leading to hypercholesterolaemia. Br J Nutr. 2004;91:959–969. doi: 10.1079/BJN20041151. [DOI] [PubMed] [Google Scholar]
- 15.Case T, Lemieux S, Kennedy S, Lewis G. Elevated plasma lipids in patients with binge eating disorders are found only in those who are anorexic. Int J Eat Disord. 1999;25:187–193. doi: 10.1002/(sici)1098-108x(199903)25:2<187::aid-eat8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Mehler P, Lezotte D, Eckel R. Lipid levels in anorexia nervosa. Int J Eat Disord. 1998;24:217–221. doi: 10.1002/(sici)1098-108x(199809)24:2<217::aid-eat11>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 17.Arden M, Weiselberg E, Nussbaum M, Shenker I, Jacobson M. Effect of weight restoration on the dyslipoproteinemia of anorexia nervosa. J Adolesc Health Care. 1990;11:199–202. doi: 10.1016/0197-0070(90)90348-6. [DOI] [PubMed] [Google Scholar]
- 18.Matzkin V, Geissler C, Coniglio R, Selles J, Bello M. Cholesterol concentrations in patients with anorexia nervosa and in healthy controls. Int J Psychiatr Nurs Res. 2006;11:1283–1293. [PubMed] [Google Scholar]
- 19.Ohwada R, Hotta M, Oikawa S, Takano K. Etiology of hypercholesterolemia in patients with anorexia nervosa. Int J Eat Disord. 2006;39:598–601. doi: 10.1002/eat.20298. [DOI] [PubMed] [Google Scholar]
- 20.Delporte M, Brichard S, Hermans M, Beguin C, Lambert M. Hyperadiponectinaemia in anorexia nervosa. Clin Endocrinol (Oxf) 2003;58:22–29. doi: 10.1046/j.1365-2265.2003.01702.x. [DOI] [PubMed] [Google Scholar]
- 21.Feillet F, Feillet-Coudray C, Bard J, Parra H, Favre E, Kabuth B, Fruchart J, Vidailhet M. Plasma cholesterol and endogenous cholesterol synthesis during refeeding in anorexia nervosa. Clin Chim Acta. 2000;294:45–56. doi: 10.1016/s0009-8981(99)00256-9. [DOI] [PubMed] [Google Scholar]
- 22.Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 23.Grinspoon S, Miller K, Coyle C, Krempin J, Armstrong C, Pitts S, Herzog D, Klibanski A. Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab. 1999;84:2049–2055. doi: 10.1210/jcem.84.6.5792. [DOI] [PubMed] [Google Scholar]
- 24.Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133:790–794. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165:561–566. doi: 10.1001/archinte.165.5.561. [DOI] [PubMed] [Google Scholar]
- 26.Miller K, Grinspoon S, Gleysteen S, Grieco K, Ciampa J, Breu J, Herzog D, Klibanski A. Preservation of neuroendocrine control of reproductive function despite severe undernutrition. J Clin Endocrinol Metab. 2004;89:4434–4438. doi: 10.1210/jc.2004-0720. [DOI] [PubMed] [Google Scholar]
- 27.Miller KK, Parulekar MS, Schoenfeld E, Anderson E, Hubbard J, Klibanski A, Grinspoon SK. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: the effects of body composition and nutritional intake. J Clin Endocrinol Metab. 1998;83:2309–2312. doi: 10.1210/jcem.83.7.4975. [DOI] [PubMed] [Google Scholar]
- 28.Kohl H, Blair S, Paffenbarger R, Macera C, Kronenfeld J. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127:1228–1239. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 30.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 31.Expert Panel of the National Cholesterol Education Program. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Bethesda, MD: National Heart, Lung, and Blood Institute, National Institutes of Health. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 32.Robinson E, Bachrach LK, Katzman DK. Use of hormone replacement therapy to reduce the risk of osteopenia in adolescent girls with anorexia nervosa. J Adolesc Health. 2000;26:343–348. doi: 10.1016/s1054-139x(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 33.Klibanski A, Biller BMK, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898–904. doi: 10.1210/jcem.80.3.7883849. [DOI] [PubMed] [Google Scholar]
- 34.Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87:2883–2891. doi: 10.1210/jcem.87.6.8574. [DOI] [PubMed] [Google Scholar]
- 35.Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15:135–143. doi: 10.1016/s1083-3188(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 36.Munoz M, Morande G, Garcia-Centenera J, Hervas F, Pozo J, Argente J. The effects of estrogen administration on bone mineral density in adolescents with anorexia nervosa. Eur J Endocrinol. 2002;146:45–50. doi: 10.1530/eje.0.1460045. [DOI] [PubMed] [Google Scholar]
- 37.Baillargeon J-P, McClish DK, Essah PA, Nestler JE. Association between the current use of low-dose oral contraceptives and cardiovascular arterial disease: a meta-analysis. J Clin Endocrinol Metab. 2005;90:3863–3870. doi: 10.1210/jc.2004-1958. [DOI] [PubMed] [Google Scholar]
- 38.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 39.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E for the Heart and Estrogen/Progestin Replacement Study Research Group. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 40.Rossi R, Bursi F, Veronesi B, Cagnacci A, Modena MG. Effects of progestins on estrogen-induced increase in C-reactive protein in postmenopausal women. Maturitas. 2004;49:315–320. doi: 10.1016/j.maturitas.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Hu P, Greendale GA, Palla SL, Reboussin BA, Herrington DM, Barrett-Connor E, Reuben DB. The effects of hormone therapy on the markers of inflammation and endothelial function and plasma matrix metalloproteinase-9 level in postmenopausal women: The postmenopausal estrogen progestin intervention (PEPI) trial. Atherosclerosis. 2006;185:347–352. doi: 10.1016/j.atherosclerosis.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 43.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 44.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 45.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 46.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 48.Herzog DB, Greenwood DN, Dorer DJ, Flores AT, Ekeblad ER, Richards A, Blais MA, Keller MB. Mortality in eating disorders: a descriptive study. Int J Eat Disord. 2000;28:20–26. doi: 10.1002/(sici)1098-108x(200007)28:1<20::aid-eat3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 49.Signorini A, De Filippo E, Panico S, De Caprio C, Pasanisi F, Contaldo F. Long-term mortality in anorexia nervosa: a report after an 8-year follow-up and a review of the most recent literature. Eur J Clin Nutr. 2007;61:119–122. doi: 10.1038/sj.ejcn.1602491. [DOI] [PubMed] [Google Scholar]
- 50.Korndorfer SR, Lucas AR, Suman VJ, Crowson CS, Krahn LE, Melton LJ. Long-term survival of patients with anorexia nervosa: a population-based study in Rochester, Minn. Mayo Clin Proc. 2003;78:278–284. doi: 10.4065/78.3.278. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152:1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 52.Nielsen S, Moller-Madsen S, Isager T, Jorgensen J, Pagsberg K, Theander S. Standardized mortality in eating disorders: a quantitative summary of previously published and new evidence. J Psychosom Res. 1998;44:413–434. doi: 10.1016/s0022-3999(97)00267-5. [DOI] [PubMed] [Google Scholar]
- 53.Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis From the Women’s Health Initiative observational study. JAMA. 2002;288:980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 54.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 55.The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 56.Reuben DB, Palla SL, Hu P, Reboussin BA, Crandall C, Herrington DM, Barrett-Connor E, Greendale GA. Progestins affect mechanism of estrogen-induced C-reactive protein stimulation. Am J Med. 2006;119:167.e1–167.e8. doi: 10.1016/j.amjmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Eilertsen AL, Hoibraaten E, Os I, Andersen TO, Sandvik L, Sandset PM. The effects of oral and transdermal hormone replacement therapy on C-reactive protein levels and other inflammatory markers in women with high risk of thrombosis. Maturitas. 2005;52:111–118. doi: 10.1016/j.maturitas.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Lakoski SG, Brosnihan B, Herrington DM. Hormone therapy, C-reactive protein, and progression of atherosclerosis: data from the Estrogen Replacement on Progression of Coronary Artery Atherosclerosis (ERA) trial. Am Heart J. 2005;150:907–911. doi: 10.1016/j.ahj.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 59.Vitale C, Cornoldi A, Gebara O, Silvestri A, Wajngarten M, Cerquetani E, Fini M, Ramires JA, Rosano GM. Interleukin-6 and flow-mediated dilatation as markers of increased vascular inflammation in women receiving hormone therapy. Menopause. 2005;12:552–558. doi: 10.1097/01.gme.0000172267.24949.70. [DOI] [PubMed] [Google Scholar]
- 60.Kiran H, Kiran G, Ekerbicer HC, Guven AM, Kilinc M. Effects of oestrogen replacement therapy on serum C-reactive protein levels in hysterectomised women. Aust NZ J Obstet Gynaecol. 2004;44:131–134. doi: 10.1111/j.1479-828X.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 61.Vongpatanasin W, Tuncel M, Wang Z, Arbique D, Mehrad B, Jialal I. Differential effects of oral versus transdermal estrogen replacement therapy on C-reactive protein in postmenopausal women. J Am Coll Cardiol. 2003;41:1358–1363. doi: 10.1016/s0735-1097(03)00156-6. [DOI] [PubMed] [Google Scholar]
- 62.Frohlich M, Muhlberger N, Hanke H, Imhof A, Doring A, Pepys MB, Koenig W. Markers of inflammation in women on different hormone replacement therapies. Ann Med. 2003;35:353–361. doi: 10.1080/07853890310007090. [DOI] [PubMed] [Google Scholar]