Abstract
Background
Recent studies have suggested that microvascular and macrovascular diseases are associated with coronary events.
Objective
To test the hypothesis that asymptomatic coronary heart disease (CHD) may be present in many patients with diabetes with vascular complications.
Design
From April 2009 to August 2010, the authors conducted a cross-sectional study to assess the prevalence of asymptomatic CHD among patients with type 2 diabetes with vascular complications at a national diabetes centre in Japan. Eligibility criteria included patients with type 2 diabetes with no known CHD and one or more of the following four criteria: (1) proliferative diabetic retinopathy or after photocoagulation; (2) estimated glomerular filtration rate <30 ml/min/1.73 m2 or an estimated glomerular filtration rate <45 ml/min/1.73 m2 plus albuminuria; (3) peripheral arterial disease; and (4) cerebrovascular disease. Each patient underwent a stress single-photon emission computed tomography; patients with myocardial perfusion abnormalities then underwent coronary angiography.
Results
A total of 1008 patients with type 2 diabetes were screened, and 122 eligible patients consented to participate. Stress single-photon emission computed tomography revealed myocardial perfusion abnormalities in 96 (79%) patients. Of the 112 patients who completed the study protocol, 59 (53%) had asymptomatic CHD with ≥50% diameter stenosis. Additionally, 35 (31%) patients had multivessel disease or left main disease, and 42 (38%) had a coronary artery with ≥75% diameter stenosis. In the multivariate logistic-regression analysis to identify coronary risk factors associated with asymptomatic CHD, the only significant predictor was male sex (OR 6.18; 95% CI 2.30 to 16.64; p<0.001).
Conclusions
Asymptomatic CHD with ≥50% diameter stenosis and myocardial perfusion abnormalities was detected in more than half of the patients with type 2 diabetes with vascular complications.
Article summary
Article focus
Many past studies have reported that some patients with diabetes have asymptomatic coronary heart disease (CHD).
No definite markers for effectively identifying the presence of asymptomatic CHD in patients with diabetes presently exist.
In recent studies, microvascular and macrovascular diseases were associated with the subsequent coronary events.
Key messages
Asymptomatic CHD with ≥75% diameter stenosis and myocardial perfusion abnormalities was detected in around 40% of the patients with type 2 diabetes with vascular complications.
Traditional coronary risk factors might not be effective in screening for asymptomatic CHD among patients with type 2 diabetes.
Strengths and limitations of this study
This study revealed that many patients with type 2 diabetes with vascular complications have asymptomatic CHD with severe multivessel stenosis, as well as myocardial ischaemia on stress single-photon emission computed tomography.
We demonstrate that patients with type 2 diabetes with advanced microvascular or macrovascular diseases had a far greater prevalence of myocardial perfusion abnormalities and asymptomatic CHDs, compared with previous data.
This study was performed at a single centre and was limited to a specific geographical area.
Introduction
Diabetes is a risk factor of coronary heart disease (CHD) which is a leading cause of mortality.1 Many studies have revealed that some patients with diabetes may have asymptomatic CHD, and a retrospective study and a small randomised trial suggested a possible benefit from CHD screening.2 3 In a large randomised controlled trial, however, routine screening for asymptomatic CHD among patients with type 2 diabetes was of no benefit to the cardiac outcome.4 In addition, traditional coronary risk factors such as hypertension and dyslipidaemia were not associated with silent ischaemia and asymptomatic CHD.5 6 Therefore, aggressive routine screening for asymptomatic CHD among all patients with diabetes with or without these risk factors is not recommended at present. No definite markers for effectively identifying the presence of asymptomatic CHD in patients with diabetes presently exist, and further investigations are needed.
In recent studies, microvascular and macrovascular diseases were associated with the subsequent coronary events. Diabetic retinopathy was associated with the onset of CHD and cardiovascular disease.7–10 Patients with proliferative diabetic retinopathy (PDR) and after photocoagulation had a particularly high risk of cardiovascular disease.11 An independent association was also observed between renal dysfunction and cardiovascular events,12–16 and the risk of cardiovascular disease was increased when proteinuria developed.17 18 Moreover, many studies suggested that macrovascular diseases such as peripheral arterial disease (PAD) and cerebrovascular disease were strongly associated with CHD.19–24
Thus, we hypothesised that asymptomatic CHD may be present in many patients with type 2 diabetes with vascular complications such as advanced diabetic retinopathy, renal dysfunction, PAD or cerebrovascular disease, although each complication is regarded as aetiologically non-identical to others, especially between micro- and macrovascular complications for which hyperglycaemia and hypercholesterolaemia play an important role, respectively.10 24 The aim of this study was to assess the prevalence of asymptomatic CHD among patients with type 2 diabetes with vascular complications and no known CHD.
Methods
Study design and participants
From April 2009 to August 2010, we conducted a cross-sectional study, and patients were enrolled at the National Center for Global Health and Medicine in Tokyo, Japan. The institutional review boards approved this study, and all the patients provided their written informed consent. Eligibility criteria included patients with type 2 diabetes without suggestive symptoms of CHD between the ages of 40 and 75 years. Additionally, all the patients had one or more of the following four criteria: (1) PDR or after photocoagulation; (2) renal dysfunction; (3) PAD; and (4) cerebrovascular disease. An ophthalmologist diagnosed the PDR or after photocoagulation. Renal dysfunction was defined as an estimated glomerular filtration rate (GFR) of <30 ml/min/1.73 m2 or an estimated GFR of <45 ml/min/1.73 m2 plus albuminuria, corresponding to ≥30 mg/day or ≥30 mg/g of creatinine. The estimated GFR was calculated using the following formula,25 as recommended by the Japanese Society of Nephrology: estimated GFR (ml/min/1.73 m2)=194× Cre−1.094× Age−0.287 (×0.739 if the patient is a woman). PAD was defined as an ankle–brachial index (ABI) <0.9, confirmed peripheral artery stenosis based on radiological images, or after surgical treatment. Cerebrovascular disease was defined as stroke or transient ischaemic attack. The exclusion criteria included (1) known CHD or suspected CHD; (2) the presence of antibodies to glutamic acid decarboxylase; (3) acute kidney injury; and (4) a very poor prognosis and inappropriate conditions for testing. The inclusion criteria and exclusion criteria were confirmed using clinical records, laboratory data, questionnaires and questioning by the physician.
Diagnostic approach and evaluation of outcomes
Stress single-photon emission computed tomography (SPECT) has a high negative predictive value to rule out CHD and does not require the use of contrast medium. To avoid unnecessary tests, we first performed stress SPECT in all the patients who met the study criteria; patients who exhibited myocardial perfusion abnormalities then underwent conventional coronary angiography (CAG), 64-slice multidetector-row computed tomography (MDCT) coronary angiography, or both examinations. We referred to the conventional CAG findings when the patients underwent both imaging procedures. The left main coronary artery, the left anterior descending coronary artery, the left circumflex coronary artery and the right coronary artery were each assessed using the American Heart Association classification.26 We diagnosed asymptomatic CHD when a coronary artery with ≥50% diameter stenosis was confirmed using CAG.19 The primary end point of this study was the prevalence of asymptomatic CHD among patients with type 2 diabetes with vascular complications.
Stress SPECT
ECG-gated stress SPECT imaging was performed in all the patients using a dual-headed γ camera (E.cam; Siemens, Munich, Germany). Patients underwent exercise stress (n=75) according to the Bruce protocol. Exercise testing was terminated when the patients achieved a heart rate of 85% or more of the predicted maximal heart rate, a sufficient blood pressure response (such as a systolic blood pressure ≥250 mm Hg), a feeling that further exercise was impossible or significant severe ischaemic changes on an ECG recording. Patients who were unable to perform the exercise (n=47) underwent a pharmacological stress test comprised of a 6 min adenosine infusion protocol, as recommended by The Japanese Society of Nuclear Cardiology. Technetium-99m tetrofosmin SPECT imaging was performed using a 1-day protocol in 108 patients, and thallium-201 was used in 14 patients. Two experienced doctors of nuclear cardiology independently evaluated the images without knowing the details of the clinical information. We diagnosed myocardial perfusion abnormalities when one or more of the doctors pointed out the presence of abnormal myocardial perfusion on the stress SPECT images. For the image analysis, quantitative gated SPECT data were also used. Prior to interpreting the images, the data were reviewed for artefacts. A 20-segment model was used for reference, and the images were comprehensively interpreted. Ischaemia was diagnosed when one or more of the doctors pointed out a reversible defect. On the other hand, a scar was diagnosed when the defect was not reversible.
Conventional CAG
When patients had severe coronary calcifications with coronary artery calcium (CAC) scores ≥400 Agatston units, an irregular heart rhythm, advanced renal dysfunction or severe stenosis on MDCT coronary angiography, we aggressively conducted conventional CAG to assess the coronary arteries. Conventional CAG images were interpreted by two experienced cardiologists blinded to the detailed patient characteristics in a usual clinical setting.
Sixty-four-slice MDCT
In the absence of contraindications, the patients who had myocardial perfusion abnormalities underwent MDCT tests to determine their CAC scores followed by CAG. The imaging was performed using 64-slice MDCT with a slice thickness of 0.5 mm (Aquilion64; Toshiba Medical Systems, Otawara, Japan). If necessary and tolerated, oral beta-blockers (metoprolol 40–100 mg) were provided before the scan to achieve a heart rate <60 beats/min. We performed a non-enhanced prospective electrocardiographically gated scan to measure the CAC scores, which were calculated using the Agatston method.27 The MDCT coronary angiography was performed using 64×0.5 mm collimation and retrospective electrocardiographic gating. MDCT images were interpreted by an experienced cardiologist in cooperation with radiologists who were unaware of the detailed clinical backgrounds in a usual clinical setting. All the coronary arteries and side branches with a luminal diameter ≥ 2.0 mm were assessed.
Other measurements
The rest and exercise stress ECG tests were assessed by a trained cardiologist who had no knowledge of the clinical findings of the patients. We diagnosed an abnormality on the rest ECG findings if ST segment abnormalities, T wave abnormalities, an abnormal Q wave, or a complete left bundle branch block were observed; ischaemia was diagnosed on an exercise stress ECG if the exercise induced any ischaemic changes.
We measured the ABI to evaluate PAD and the brachial–ankle pulse wave velocity to assess the presence of arteriosclerosis in the peripheral arteries (Form PWV/ABI; Colin Company, Komaki, Japan).28 The lowest ABI and the highest brachial–ankle pulse wave velocity for the left and right sides were used in subsequent analyses.
To estimate cardiac function such as the ejection fraction and the wall motion, we conducted an echocardiography examination (Aplio 80; Toshiba Medical Systems, Otawara, Japan). The examinations were performed by a trained ultrasonographer and cardiologist who had no knowledge of the patients' clinical information. Doppler echocardiography was also performed to assess the peak Doppler velocities of the early (E) and late diastolic flows (A), the deceleration time (DT) and the E/A ratio for the mitral inflow. Moreover, tissue Doppler imaging of the mitral annulus was measured from the apical four-chamber view. A sample volume was placed at the septal mitral annulus, and the early (E′) diastolic velocity was measured. We analysed the E/E′ ratio to assess the cardiac function.29
The carotid intima-media thickness (IMT) was evaluated using high-resolution B-mode ultrasound with a 10 MHz linear transducer (Aplio XG; Toshiba Medical Systems, Otawara, Japan).30 A trained ultrasonographer and a doctor or a skilled doctor alone who were unaware of the patient' characteristics assessed the maximum carotid IMT. The maximum carotid IMT was defined as the thickest IMT for the left and right sides from the common carotid artery to the internal carotid artery.
Statistical methods
Data are presented as the number (%), mean with SD, and median with lower and upper ends of the IQR. Continuous variables were compared using t tests and Wilcoxon rank sum tests. Categorical variables were compared using χ2 tests. A multivariate logistic-regression analysis of coronary risk factors, such as age, sex, overweight or obesity, current smoking habit, hypertension and dyslipidaemia, was performed to identify coronary risk factors associated with asymptomatic CHD. p values <0.05 according to a two-sided test were considered statistically significant for all the tests. All analyses were performed using Stata software, V.11.1.
Results
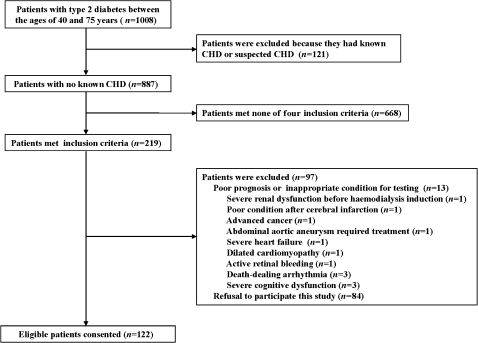
A total of 1008 patients with type 2 diabetes between the ages of 40 and 75 years were screened, and 219 met the inclusion criteria for this study. Of the 206 eligible patients without any exclusion criteria, 122 consented to participate (figure 1). The clinical characteristics of this study population are presented in table 1. The mean age was 64.1±8.3 years, 84 (68%) were men, and the mean A1C was 7.7±1.5%. The numbers of patients with PDR or after photocoagulation, renal dysfunction, PAD and cerebrovascular disease were 76 (62%), 25 (20%), 21 (17%) and 46 (37%), respectively.
Figure 1.
Flow chart of study participants. CHD, coronary heart disease.
Table 1.
Characteristics of study population*
| N | All | Men | Women | |
| 122 | 84 (69%) | 38 (31%) | ||
| Age (years) | 122 | 64.1 (8.3) | 63.9 (8.3) | 64.6 (8.5) |
| Weight (kg) | 122 | 64.6 (12.4) | 66.7 (12.1) | 60.1 (12.2) |
| Body-mass index (kg/m2)† | 122 | 24.5 (4.0) | 24.3 (3.7) | 25.1 (4.5) |
| Waist (cm) | 112 | 90.3 (11.2) | 89.8 (10.7) | 91.5 (12.5) |
| Smoking | 122 | |||
| Non | 47 (39%) | 16 (18%) | 31 (82%) | |
| Former | 37 (30%) | 34 (41%) | 3 (8%) | |
| Current | 38 (31%) | 34 (41%) | 4 (10%) | |
| History of cerebrovascular disease‡ | 122 | 46 (37%) | 32 (38%) | 14 (36%) |
| Duration of diabetes (years) | 122 | 16.0 (10.2) | 16.5 (9.8) | 15.2 (11.1) |
| Age (years) at the time of diabetes diagnosis | 122 | 49.0 (12.2) | 48.4 (11.8) | 50.4 (13.1) |
| Any diabetic retinopathy | 122 | 97 (79%) | 67 (79%) | 30 (79%) |
| Proliferative diabetic retinopathy or after photocoagulation | 122 | 76 (62%) | 50 (59%) | 26 (68%) |
| A1C (%) | 122 | 7.7 (1.5) | 7.8 (1.7) | 7.5 (1.2) |
| Treatment for diabetes | 122 | |||
| Diet | 7 (6%) | 7 (8%) | 0 (0%) | |
| Oral agents | 59 (48%) | 37 (44%) | 22 (58%) | |
| Insulin | 15 (12%) | 13 (16%) | 2 (5%) | |
| Insulin+oral agents§ | 41 (34%) | 21 (32%) | 14 (37%) | |
| Hypertension¶ | 122 | 95 (77%) | 66 (78%) | 29 (76%) |
| Treatment | ||||
| Angiotensin II receptor blockers/angiotensin-converting enzyme inhibitors | 88 (72%) | 61 (72%) | 27 (71%) | |
| Calcium-channel blockers | 55 (45%) | 37 (44%) | 18 (47%) | |
| Diuretics | 16 (13%) | 9 (10%) | 7 (18%) | |
| Others | 4 (3%) | 3 (3%) | 1 (2%) | |
| Dyslipidaemia** | 122 | 90 (73%) | 60 (71%) | 30 (79%) |
| Total cholesterol (mg/dl) | 192.7 (39.0) | 194.7 (42.4) | 188.3 (30.9) | |
| LDL cholesterol (mg/dl) | 115.6 (40.5) | 119.6 (43.8) | 106.5 (30.4) | |
| HDL cholesterol (mg/dl) | 52.4 (14.8) | 51.1 (13.5) | 55.3 (17.2) | |
| Triglyceride (mg/dl) | 143.3 (85.9) | 142.7 (86.1) | 144.6 (86.7) | |
| LDL/HDL ratio | 2.4 (1.1) | 2.5 (1.1) | 2.2 (0.9) | |
| Treatment | ||||
| Statin | 47 (38%) | 25 (29%) | 22 (57%) | |
| Others | 9 (7%) | 7 (8%) | 2 (5%) | |
| Use of aspirin | 122 | 33 (27%) | 23 (27%) | 10 (26%) |
| Creatinine (mg/dl) | 120 | 1.04 (0.60) | 1.08 (0.60) | 0.94 (0.60) |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | 120 | 62.1 (22.0) | 63.3 (20.4) | 59.7 (25.4) |
| <45 | 28 (23%) | 17 (20%) | 11 (28%) | |
| <30 | 10 (8%) | 5 (6%) | 5 (13%) | |
| Use of haemodialysis | 2 | 2 (1%) | 2 (2%) | 0 (0%) |
| Albuminuria (mg/day or mg/g of creatinine) | 117 | |||
| <30 | 32 (27%) | 16 (20%) | 16 (45%) | |
| 30–299 | 40 (34%) | 28 (34%) | 12 (33%) | |
| ≥300 | 45 (39%) | 37 (46%) | 8 (22%) | |
| Renal dysfunction†† | 122 | 25 (20%) | 17 (20%) | 8 (21%) |
| Brain natriuretic peptide (pg/ml) | 113 | 37.2 (26.1 to 48.2) | 19.4 (7.5 to 47.5) | 24.8 (11 to 39.8) |
| Rest ECG | ||||
| Any abnormal findings | 122 | 27 (22%) | 19 (22%) | 8 (21%) |
| Abnormal Q wave changes | 3 (2%) | 2 (2%) | 1 (2%) | |
| Ischaemic findings on exercise stress ECG | 64 | 25 (39%) | 15 (36%) | 10 (43%) |
| Cardiothoracic ratio on chest x-ray (%) | 113 | 48.0 (4.4) | 47.6 (4.5) | 48.9 (4.0) |
| Ankle–brachial index | 122 | 1.07 (0.17) | 1.06 (0.18) | 1.09 (0.17) |
| Brachial–ankle pulse wave velocity (cm/s) | 108 | 2012.2 (377.3) | 1940.5 (300.3) | 2168.3 (474.8) |
| Peripheral arterial disease | 122 | 21 (17%) | 17 (20%) | 4 (10%) |
| Echocardiography‡‡ | ||||
| Ejection fraction (%) | 108 | 67.3 (8.6) | 66.4 (9.5) | 69.4 (6.0) |
| Septal+posterior wall thickness (mm) | 108 | 21.2 (3.4) | 21.3 (3.4) | 20.9 (3.4) |
| >25 | 10 (9%) | 6 (8%) | 4 (11%) | |
| E/A ratio | 107 | 0.76 (0.21) | 0.77 (0.17) | 0.75 (0.28) |
| Deceleration time (ms) | 107 | 236.4 (59.1) | 232.6 (60.5) | 244.6 (56.0) |
| E/E′ ratio | 96 | 11.3 (3.4) | 11.0 (2.86) | 12.1 (4.35) |
| Abnormal wall motion | 108 | 6 (5%) | 6 (8%) | 0 (0%) |
| Ultrasound of carotid artery | 81 | |||
| Maximum carotid intima-media thickness (mm) | 1.78 (1.07) | 1.97 (1.13) | 1.39 (0.86) | |
| ≥1.1 | 53 (67%) | 41 (77%) | 12 (46%) | |
| Coronary artery calcium scores (Agatston units) | 83 | 216.8 (52.0 to 602.7) | 300.4 (65.6 to 1201.2) | 110.9 (0 to 275.8) |
To convert the values for cholesterol and triglycerides to millimoles per litre (mmol/l), multiply by 0.02586 and 0.01129, respectively.
Data are number, number (%), mean (SD) or median (IQR).
Body-mass index was calculated as the weight in kilograms divided by the square of height in metres.
Cerebrovascular disease was defined as stroke or transient ischaemic attack.
Oral agents for the treatment of diabetes included metformin, sulfonylureas, thiazolidinediones, meglitinides and alpha-glucosidase inhibitors.
Hypertension was defined as a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or the use of antihypertensive medication.
Dyslipidaemia was defined as low-density lipoprotein (LDL) ≥140 mg/dl, high-density lipoprotein (HDL) <40 mg/dl, or the use of lipid-lowering drug.
Renal dysfunction was defined as the estimated glomerular filtration rate <30 ml/min/1.73 m2 or the estimated glomerular filtration rate <45 ml/min/1.73 m2 plus albuminuria, which was ≥30 mg/day or ≥30 mg/g of creatinine.
E/A ratio was the ratio of the peak Doppler velocities of early to late diastolic flow in the mitral inflow. E/E′ ratio was the ratio of the peak Doppler velocities of early in the mitral inflow to the early diastolic velocity at the septal mitral annulus.
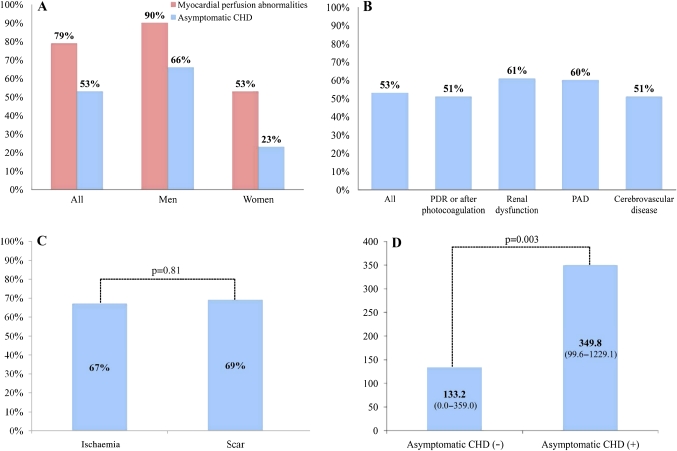
Of the 122 patients, 96 (79%) had myocardial perfusion abnormalities on stress SPECT: 70 (58%) had ischaemia and 26 (21%) had a scar. Of the 96 patients with myocardial perfusion abnormalities, 42 underwent conventional CAG, 83 underwent MDCT coronary angiography, and 39 underwent both examinations. Ten patients did not undergo either MDCT coronary angiography or CAG, and were excluded from the analysis to determine the prevalence of asymptomatic CHD. Of the 112 patients who completed the study protocol, 59 (53%) had asymptomatic CHD (figure 2A). Thus, more than half of the patients who met any one of the four inclusion criteria for vascular complications had asymptomatic CHD (figure 2B). The prevalences of asymptomatic CHD among the patients who met only one criteria and among the patients who met more than one of the four inclusion criteria for vascular complications were not significantly different (p=0.50). The prevalence of asymptomatic CHD in patients with myocardial perfusion abnormalities on stress SPECT is shown in figure 2C. The prevalences of asymptomatic CHD among patients with ischaemia and among those with a scar were not significantly different. The CAC scores for the patients with and for those without asymptomatic CHD were significantly different (figure 2D).
Figure 2.
Prevalence of myocardial perfusion abnormalities and asymptomatic coronary heart disease (CHD) (A). Prevalence of asymptomatic CHD for each of the vascular complications (B). Prevalence of asymptomatic CHD in patients with ischaemia or scar (C). Coronary artery calcium (CAC) scores in patients with or without asymptomatic CHD (D). PAD, peripheral arterial disease; PDR, proliferative diabetic retinopathy.
The majority of patients with asymptomatic CHD had multivessel disease. Fifty-nine patients with asymptomatic CHD had 1.8±0.8 vessels with ≥50% diameter stenosis: 24 (41%) patients had one-vessel disease, 22 (37%) had two-vessel disease, and 13 (22%) had three-vessel disease or left main disease. The maximum percentage diameter stenosis in patients with asymptomatic CHD is also assessed. Forty-two (71%) patients had ≥75% diameter stenosis, and 24 (41%) had ≥90% diameter stenosis. The analyses of the clinical variables among the patients with and those without asymptomatic CHD are shown in table 2. Men, a current smoking habit, age at the time of diabetes diagnosis, the ejection fraction, the E/A ratio, the maximum carotid IMT and the CAC scores differed significantly between the two groups in a univariate analysis. However, abnormal findings on the rest ECG, ischaemic findings on the exercise stress ECG and abnormal wall motion on echocardiography were not significantly different. When a multivariate logistic-regression analysis was performed to identify coronary risk factors independently associated with asymptomatic CHD, the only significant predictor was male sex (OR 6.18; 95% CI 2.30 to 16.64; p<0.001). The result was similar when the duration of diabetes was added to the multivariate analysis.
Table 2.
Analyses of clinical variables for asymptomatic coronary heart disease
| Univariate analysis | |||
| Variable | Asymptomatic coronary heart disease (+) | Asymptomatic coronary heart disease (−) | p Value |
| 59 (53%) | 53 (47%) | ||
| Men | 51 (86%) | 26 (49%) | <0.001 |
| Age (years) | 62.4 (8.8) | 65.3 (7.7) | 0.07 |
| Weight (kg) | 65.3 (12.9) | 64.7 (12.7) | 0.79 |
| Body-mass index (kg/m2) | 24.0 (3.4) | 25.3 (4.6) | 0.08 |
| Waist (cm) | 90.0 (11.4) | 91.1 (11.9) | 0.60 |
| Current smoker | 26 (44%) | 10 (18%) | 0.004 |
| History of cerebrovascular disease | 22 (37%) | 21 (39%) | 0.80 |
| Duration of diabetes (years) | 16.7 (10.5) | 14.9 (10.0) | 0.34 |
| Age (years) at the time of diabetes diagnosis | 46.7 (12.2) | 51.4 (11.9) | 0.04 |
| Any diabetic retinopathy | 46 (78%) | 42 (79%) | 0.86 |
| Proliferative diabetic retinopathy or after photocoagulation | 36 (61%) | 34 (64%) | 0.73 |
| A1C (%) | 7.3 (1.5) | 7.4 (1.6) | 0.76 |
| Use of insulin | 33 (55%) | 21 (39%) | 0.08 |
| Hypertension | 46 (78%) | 40 (75%) | 0.75 |
| Dyslipidaemia | 45 (76%) | 39 (73%) | 0.74 |
| Total cholesterol (mg/dl) | 192.8 (38.4) | 196.0 (39.5) | 0.67 |
| Low-density lipoprotein cholesterol (mg/dl) | 113.0 (36.6) | 120.0 (38.5) | 0.33 |
| High-density lipoprotein cholesterol (mg/dl) | 50.7 (13.7) | 53.5 (15.7) | 0.32 |
| Triglyceride (mg/dl) | 151.5 (89.0) | 136.6 (82.3) | 0.36 |
| Low-density lipoprotein/high-density lipoprotein ratio | 2.4 (1.0) | 2.4 (1.0) | 0.85 |
| Use of statin | 21 (35%) | 22 (41%) | 0.52 |
| Use of aspirin | 16 (27%) | 15 (28%) | 0.88 |
| Creatinine (mg/dl) | 1.03 (0.60) | 0.87 (0.35) | 0.09 |
| Glomerular filtration rate (ml/min/1.73 m2) | 63.9 (20.7) | 65.2 (20.0) | 0.74 |
| <45 | 13 (22%) | 9 (17%) | 0.50 |
| <30 | 2 (3%) | 3 (5%) | 0.56 |
| Albuminuria ≥30 (mg/day or mg/g of creatinine) | 43 (76%) | 32 (62%) | 0.11 |
| Brain natriuretic peptide (pg/ml) | 20.7 (7.6 to 35.4) | 17.4 (9.5 to 42.9) | 0.99 |
| Rest ECG | |||
| Ischaemic findings on exercise stress ECG | 12 (41%) | 10 (35%) | 0.66 |
| Cardio thoracic ratio on chest x-ray (%) | 47.3 (4.4) | 48.8 (4.1) | 0.08 |
| Ankle–brachial index | 1.06 (0.20) | 1.06 (0.15) | 0.98 |
| Brachial–ankle pulse wave velocity (cm/s) | 1955.3 (306.4) | 2067.4 (448.7) | 0.14 |
| Peripheral arterial disease | 12 (22%) | 8 (16%) | 0.42 |
| Echocardiography | |||
| Ejection fraction (%) | 65.6 (9.1) | 69.0 (8.0) | 0.04 |
| Septal wall+posterior wall thickness (mm) | 20.8 (3.7) | 21.2 (3.0) | 0.55 |
| E/A ratio | 0.81 (0.16) | 0.71 (0.24) | 0.02 |
| Deceleration time (ms) | 225.7 (58.2) | 247.5 (61.9) | 0.07 |
| E/E′ ratio | 10.8 (3.3) | 11.8 (3.2) | 0.15 |
| Abnormal wall motion | 4 (7%) | 2 (4%) | 0.47 |
| Ultrasound of carotid artery | |||
| Maximum carotid intima-media thickness (mm) | 2.11 (1.30) | 1.41 (0.70) | 0.005 |
| ≥1.1 | 28 (75%) | 20 (55%) | 0.07 |
| Coronary artery calcium scores (Agatston units) | 349.8 (99.6 to 1229.1) | 133.2 (0.0 to 359.0) | 0.003 |
| Multivariate analysis | ||
| Variable | OR (95% CI) | p Value |
| Men | 6.18 (2.30 to 16.64) | <0.001 |
| Age (years) | 0.94 (0.89 to 1.00) | 0.07 |
| Body-mass index ≥25 | 0.49 (0.20 to 1.23) | 0.13 |
| Current smoking | 2.05 (0.75 to 5.54) | 0.15 |
| Hypertension | 1.26 (0.45 to 3.50) | 0.65 |
| Dyslipidaemia | 2.16 (0.77 to 6.05) | 0.14 |
Data are number (%), mean (SD), median (IQR), OR, or 95% CI. To convert the values for cholesterol and triglycerides to millimoles per litre (mmol/l), multiply by 0.02586 and 0.01129, respectively.
Discussion
This study reports that many patients with type 2 diabetes with vascular complications have asymptomatic CHD with multivessel disease and severe stenosis in addition to myocardial ischaemia on stress SPECT. Moreover, asymptomatic CHD was detected in more than half of the patients who met any one of the four inclusion criteria for vascular complications. The association found between asymptomatic CHD and PAD in patients with type 2 diabetes was similar to the association discovered in the previous study in which myocardial scintigraphy and CAG were used to examine patients with diabetes for the presence of silent coronary stenosis.20 Many past studies have reported that some patients with diabetes have asymptomatic CHD. However, the conditions under which the patients with diabetes were most likely to have asymptomatic CHD was unclear. We demonstrated that patients with type 2 diabetes with advanced microvascular or macrovascular diseases had a far greater prevalence of myocardial perfusion abnormalities and asymptomatic CHDs, compared with previous data.5 6 Because the diabetic complications had progressed, the patients might have had severe autonomic denervation of the heart, accounting for their asymptomatic presentation.31 The statistical analysis revealed that asymptomatic CHD was more common among men than among women. However, other coronary risk factors were not associated with asymptomatic CHD. These results may be similar to those of previous studies.5 6 Thus, traditional coronary risk factors might not be effective in screening for asymptomatic CHD among patients with type 2 diabetes. We could not fully examine the CAC score (n=83) and the maximum IMT (n=81) in 122 patients. However, the results of the supplemental analyses showed that both the CAC score and maximum carotid IMT were significant predictors when each factor was separately included in the multivariate analysis (CAC scores and maximum carotid IMT values for the analyses were available for 83 subjects and 75 subjects, respectively, and the p value was 0.034 and 0.036, respectively). Further research will be required to identify possibly useful markers such as the ejection fraction, E/A ratio, maximum carotid IMT and CAC scores.
Importantly, some patients without significant coronary stenosis exhibited abnormal findings, such as ischaemia on stress ECG, abnormal wall motion on echocardiography or myocardial perfusion abnormalities on stress SPECT. Although these facts might suggest the possibility of false-positive results, coronary microvascular dysfunction may be responsible for cardiac disorders because the patients had many coronary microvascular risk factors, such as diabetes, cigarette smoking, dyslipidaemia and hypertension.32 33 Further investigation is needed for coronary microvascular dysfunction.
Our study had several limitations. First, this study was performed at a single centre and was limited to a specific geographical area. Thus, large-scale studies at multiple centres throughout the world will be necessary to confirm these results. However, we believe that this is an extremely important study that may lead to numerous future trials and that may have a large influence on diabetic management. Second, we did not perform further tests in patients with normal myocardial perfusion on stress SPECT. Therefore, some patients with normal myocardial perfusion might have had asymptomatic CHD. However, stress SPECT has a very high sensitivity for CHD and affects the decisions regarding future treatment.34 Consequently, this diagnostic approach may enable unnecessary angiography to be effectively avoided. Third, the coronary arteries in some of the patients were evaluated using MDCT coronary angiography alone. In large-scale studies, a high rate of agreement between MDCT coronary angiography and conventional CAG has been confirmed.35 Furthermore, conventional CAG was performed in most of the severe calcification cases with CAC scores ≥400 Agatston units, which could be problematic for MDCT coronary angiography.36 The median CAC scores in the 44 patients who underwent MDCT coronary angiography alone was 149.0 (14.1–383.4) Agatston units. Thus, we assumed that the diagnosis of CHD was accurate. Fourth, our approach based on image information representing anatomical rather than functional aspects possibly misses some of the ischaemic changes of CHD, and this may explain the lack between vasculopathy and asymptomatic CHD observed in our current survey. Lastly, we had only 21 subjects with PAD, and that may be because of the relatively low prevalence of isolated PAD (eg, PAD without CHD) in the Japanese population, and in fact a paper from Japan reported that around half of the patients with PAD in their study also had CHD.37 The high mean PWV values possibly reflected advanced arteriosclerosis of the subjects. In addition, it should be mentioned that the renal function did not worsen in any of the patients after CAG and MDCT in this series of observations.
In conclusion, this study revealed that asymptomatic CHD with myocardial perfusion abnormalities was detected in more than half of the patients with type 2 diabetes with vascular complications. A relationship between CHD and sudden cardiac death has been revealed,38 and we expect that the results of this study may contribute greatly to reducing myocardial infarction and sudden cardiac death among patients with type 2 diabetes. However, the best approach to treating asymptomatic CHD remains unknown. Therefore, randomised controlled trials are needed to determine the optimal management.
Supplementary Material
Footnotes
To cite: Tsujimoto T, Kajio H, Takahashi Y, et al. Asymptomatic coronary heart disease in patients with type 2 diabetes with vascular complications: a cross-sectional study. BMJ Open 2011;2:e000139. doi:10.1136/bmjopen-2011-000139
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Ethics approval was provided by the institutional review boards by the National Center for Global Health and Medicine in Tokyo, Japan.
Contributors: TT conceived the study. TT, MH and MN designed the protocol. MH, MM and KK evaluated the SPECT images. MKa and MH evaluated the coronary angiography images. TT, HK, YT, MKi, HN and RY-H contributed to the data collection and the preparation. TT and TS analysed all the data. All authors contributed to the interpretation of the results and approved the final version.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–34 [DOI] [PubMed] [Google Scholar]
- 2.Sorajja P, Chareonthaitawee P, Rajagopalan N, et al. Improved survival in asymptomatic diabetic patients with high-risk SPECT imaging treated with coronary artery bypass grafting. Circulation 2005;112(Suppl 9):I311–16 [DOI] [PubMed] [Google Scholar]
- 3.Faglia E, Manuela M, Antonella Q, et al. Risk reduction of cardiac events by screening of unknown asymptomatic coronary artery disease in subjects with type 2 diabetes mellitus at high cardiovascular risk: an open-label randomized pilot study. Am Heart J 2005;149:e1–6 [DOI] [PubMed] [Google Scholar]
- 4.Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009;301:1547–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wackers FJ, Young LH, Inzucchi SE, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care 2004;27:1954–61 [DOI] [PubMed] [Google Scholar]
- 6.Scognamiglio R, Negut C, Ramondo A, et al. Detection of coronary artery disease in asymptomatic patients with type 2 diabetes mellitus. J Am Coll Cardiol 2006;47:65–71 [DOI] [PubMed] [Google Scholar]
- 7.Rajala U, Pajunpää H, Koskela P, et al. High cardiovascular disease mortality in subjects with visual impairment caused by diabetic retinopathy. Diabetes Care 2000;23:957–61 [DOI] [PubMed] [Google Scholar]
- 8.Chew EY, Ferris FL, 3rd, Csaky KG, et al. The long-term effects of laser photocoagulation treatment in patients with diabetic retinopathy: the early treatment diabetic retinopathy follow-up study. Ophthalmology 2003;110:1683–9 [DOI] [PubMed] [Google Scholar]
- 9.van Hecke MV, Dekker JM, Stehouwer CD, et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care 2005;28:1383–9 [DOI] [PubMed] [Google Scholar]
- 10.Cheung N, Wang JJ, Klein R, et al. Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care 2007;30:1742–6 [DOI] [PubMed] [Google Scholar]
- 11.Targher G, Bertolini L, Zenari L, et al. Diabetic retinopathy is associated with an increased incidence of cardiovascular events in Type 2 diabetic patients. Diabet Med 2008;25:45–50 [DOI] [PubMed] [Google Scholar]
- 12.Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63:225–32 [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305 [DOI] [PubMed] [Google Scholar]
- 14.Patel UD, Young EW, Ojo AO, et al. CKD progression and mortality among older patients with diabetes. Am J Kidney Dis 2005;46:406–14 [DOI] [PubMed] [Google Scholar]
- 15.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 2006;17:2034–47 [DOI] [PubMed] [Google Scholar]
- 16.Sukhija R, Aronow WS, Kakar P, et al. Relation of microalbuminuria and coronary artery disease in patients with and without diabetes mellitus. Am J Cardiol 2006;98:279–81 [DOI] [PubMed] [Google Scholar]
- 17.Tonelli M, Jose P, Curhan G, et al. Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trial. BMJ 2006;332:1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallan S, Astor B, Romundstad S, et al. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med 2007;167:2490–6 [DOI] [PubMed] [Google Scholar]
- 19.Janand-Delenne B, Savin B, Habib G, et al. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999;22:1396–400 [DOI] [PubMed] [Google Scholar]
- 20.Cosson E, Guimfack M, Paries J, et al. Are silent coronary stenoses predictable in diabetic patients and predictive of cardiovascular events? Diabetes Metab 2003;29:470–6 [DOI] [PubMed] [Google Scholar]
- 21.Nesto RW, Watson FS, Kowalchuk GJ, et al. Silent myocardial ischemia and infarction in diabetics with peripheral vascular disease: assessment by dipyridamole thallium-201 scintigraphy. Am Heart J 1990;120:1073–7 [DOI] [PubMed] [Google Scholar]
- 22.Doobay AV, Anand SS. Sensitivity and specificity of the ankle–brachial index to predict future cardiovascular outcomes: a systematic review. Arterioscler Thromb Vasc Biol 2005;25:1463–9 [DOI] [PubMed] [Google Scholar]
- 23.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation 2006;114:688–99 [DOI] [PubMed] [Google Scholar]
- 24.Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006;295:180–9 [DOI] [PubMed] [Google Scholar]
- 25.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–92 [DOI] [PubMed] [Google Scholar]
- 26.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975;51(Suppl 4):5–40 [DOI] [PubMed] [Google Scholar]
- 27.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32 [DOI] [PubMed] [Google Scholar]
- 28.Koji Y, Tomiyama H, Ichihashi H, et al. Comparison of ankle–brachial pressure index and pulse wave velocity as markers of the presence of coronary artery disease in subjects with a high risk of atherosclerotic cardiovascular disease. Am J Cardiol 2004;94:868–72 [DOI] [PubMed] [Google Scholar]
- 29.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 2000;102:1788–94 [DOI] [PubMed] [Google Scholar]
- 30.O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999;340:14–22 [DOI] [PubMed] [Google Scholar]
- 31.Niakan E, Harati Y, Rolak LA, et al. Silent myocardial infarction and diabetic cardiovascular autonomic neuropathy. Arch Intern Med 1986;146:2229–30 [PubMed] [Google Scholar]
- 32.Nitenberg A, Valensi P, Sachs R, et al. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes 1993;42:1017–25 [DOI] [PubMed] [Google Scholar]
- 33.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–40 [DOI] [PubMed] [Google Scholar]
- 34.Hachamovitch R, Hayes SW, Friedman JD, et al. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107:2900–7 [DOI] [PubMed] [Google Scholar]
- 35.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324–36 [DOI] [PubMed] [Google Scholar]
- 36.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–32 [DOI] [PubMed] [Google Scholar]
- 37.Iwashima Y, Horio T, Suzuki Y, et al. Adiponectin and inflammatory markers in peripheral arterial occlusive disease. Atherosclerosis 2006;188:384–90 [DOI] [PubMed] [Google Scholar]
- 38.Zheng ZJ, Croft JB, Giles WH, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation 2001;104:2158–63 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.