Abstract
Despite a vast amount of experimental and clinical data on the underlying ionic, cellular and tissue substrates, the mechanisms of common atrial arrhythmias (such as atrial fibrillation, AF) arising from the functional interactions at the whole atria level remain unclear. Computational modelling provides a quantitative framework for integrating such multi-scale data and understanding the arrhythmogenic behaviour that emerges from the collective spatio-temporal dynamics in all parts of the heart. In this study, we have developed a multi-scale hierarchy of biophysically detailed computational models for the human atria – 3D virtual human atria. Primarily, diffusion tensor MRI reconstruction of the tissue geometry and fibre orientation in the human sinoatrial node (SAN) and surrounding atrial muscle was integrated into the 3D model of the whole atria dissected from the Visible Human dataset. The anatomical models were combined with the heterogeneous atrial action potential (AP) models, and used to simulate the AP conduction in the human atria under various conditions: SAN pacemaking and atrial activation in the normal rhythm, break-down of regular AP wave-fronts during rapid atrial pacing, and the genesis of multiple re-entrant wavelets characteristic of AF. Contributions of different properties of the tissue to the mechanisms of the normal rhythm and AF arrhythmogenesis are investigated and discussed. The 3D model of the atria itself was incorporated into the torso model to simulate the body surface ECG patterns in the normal and arrhythmic conditions. Therefore, a state-of-the-art computational platform has been developed, which can be used for studying multi-scale electrical phenomena during atrial conduction and arrhythmogenesis. Results of such simulations can be directly compared with experimental electrophysiological and endocardial mapping data, as well as clinical ECG recordings. More importantly, the virtual human atria can provide validated means for directly dissecting 3D excitation propagation processes within the atrial walls from an in vivo whole heart, which are beyond the current technical capabilities of experimental or clinical set-ups.
Keywords: virtual human atria, sinoatrial node, atrial fibrillation, body surface ECG
1. Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia (Nattel et al., 2005). It annually affects a total of ~500,000 individuals in the UK alone, and the rate of hospitalization for AF and the cost of its treatment are increasing in epidemic proportions (Stewart et al., 2004; Anter et al., 2009). AF is considered to be a major cause of morbidity and mortality: it can cause a reduction in cardiac output, predispose to heart failure and stroke, and increase sudden death rates (Benjamin et al., 1998; Anter et al., 2009). Although AF is a very costly public health problem, mechanisms underlying its genesis and control are incompletely understood yet, and its clinical treatments all have significant intrinsic limitations (Ehrlich and Nattel, 2009). Experimental animal studies and clinical endocardial mapping have suggested that high-frequency irregular electrical activity in AF may be sustained by re-entrant wavelets propagating in an abnormal atrial tissue substrate (Harada et al., 1996; Jalife et al., 2002; Nattel et al., 2005). However, mechanisms underlying initiation of a re-entry initiation and the nature of the re-entrant substrate remain unclear.
It is believed that atrial tissues with substantial regional differences in intrinsic electrical properties are more susceptible to re-entry: the latter may result in conduction slowing and block in atrial tissue regions with longer refractoriness (Spach et al., 1989; Jalife et al., 2002; Nattel et al., 2005). Experimental data from animal cell and tissues indicate that both atria are characterized by significant regional differences in the action potential morphology and duration (APD), which are attributed to intrinsic variations in the ionic channel currents through the atria (Feng et al., 1998; Li et al., 2001; Sarmast et al., 2003). Similar heterogeneities in the APD have also been seen in human studies (Chen et al., 1999), although little data on regional heterogeneity of underlying ionic currents are available. Besides, clinical endocardial mapping studies and ECG data have shown heterogeneities in spatial distributions of the electrical excitation frequencies within the atria during AF (Haissaguerre et al., 1998; Sanders et al., 2005). However, an integrative approach to simultaneous studies of the ionic channel, action potential and conduction heterogeneities in the whole atria, and the resultant ECGs, is extremely difficult (if not impossible) to implement in an experimental or clinical set-up.
Thus, despite a considerable amount of experimental and clinical data on the heterogeneous ionic, cellular and tissue levels are available, the mechanisms of AF arising from the multi-scale functional interactions at the whole atria level remain unclear. Computational modelling provides a quantitative framework for integrating such multi-scale data and understanding the arrhythmogenic behaviour that emerges from the complex and collective spatio-temporal dynamics in all parts of the heart, across all scales and in various conditions (Rudy, 2000; Noble, 2005; Clayton et al., 2011). However, due to the lack of complete experimental datasets from human (as well as for the reasons of computational efficiency), existing models of the atria use either simplistic descriptions of the electrical properties or idealized tissue structure and anisotropy (Harrild and Henriquez, 2000; Seemann et al., 2006; Jacquemet et al., 2006; Ridler et al., 2006). Therefore, there is a need for efficient and quantitative atrial models that integrate data at all of ionic channel, cellular, anisotropic tissue and whole atria levels, which are validated against clinical data from humans.
In this study, we have constructed a hierarchy of models for human atrial cells and tissues, based on previously existing models (Courtemanche et al., 1998; Seemann et al., 2006; Kharche and Zhang, 2008), but substantially expanded to include (i) diffusion tensor MRI reconstruction of the human sinoatrial node (SAN) and right atrial (RA) tissue, (ii) anatomically detailed model of the entire heterogeneous atria and (iii) a human torso model simulating body surface ECGs. We demonstrate cross-scale relations between these models and the underlying experimental data. The developed 3D computational platform is used to study patterns of atrial conduction and mechanisms underlying the genesis of re-entrant AF associated with atrial spatial heterogeneity and anisotropy.
2. Methods
Station-temporal dynamics of electrical excitations in cardiac tissues can be described by the nonlinear partial differential equation (PDE) (Rudy, 2000; Clayton et al., 2011):
| (1) |
Here V (mV) is the membrane potential, 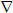 is a spatial gradient operator defined within the tissue geometry, and t is time (s). is a tensor of diffusion coefficients (mm2 ms−1) that characterise electrotonic spread of voltage via gap junctional coupling, Cm (pF) is the cell membrane capacitance, and Iion is the total membrane ionic current (pA). Note that when the diffusion term is neglected, Equations (1) becomes an ordinary differential equation describing excitation of a single cell.
is a spatial gradient operator defined within the tissue geometry, and t is time (s). is a tensor of diffusion coefficients (mm2 ms−1) that characterise electrotonic spread of voltage via gap junctional coupling, Cm (pF) is the cell membrane capacitance, and Iion is the total membrane ionic current (pA). Note that when the diffusion term is neglected, Equations (1) becomes an ordinary differential equation describing excitation of a single cell.
2.1. Heterogeneous human atrial and sinoatrial node cell models
Families of electrophysiologically detailed cell models have been developed to describe the voltage- and time-dependent current, Iion, in human atrial cells (Courtemanche et al., 1998; Feng et al., 1998) and in human SAN cells (Chandler et al., 2011). The atrial models are based on voltage-clamp and mRNA expression datasets experimentally measured for individual ionic currents in various parts of the atria, and allow accurate simulations of the heterogeneous action potential (AP) morphologies.
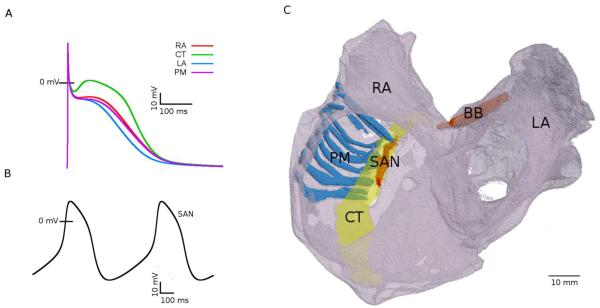
In this study the human atrial AP model developed by Courtemanche et al. (1998) was modified based on voltage-clamp data showing large electrophysiological heterogeneity of the canine atria (Feng et al., 1998; Li et al., 2001). Primarily, conductances of the L-type Ca2+ current (ICaL), transient outward K+ current (Ito) and rapidly activated potassium current (IKr) were rescaled based on experimental data from dog (as described before by Seemann et al., 2006) to reproduce AP morphologies in various parts of the atria. Validated values of the conductances in the right (RA) and left (LA) atria, and bundles of the crista terminalis (CT) and pectinate muscles (PM) are summarized in Table 1. Note that due to lack of experimental data, the Bachmann’s bundle (BB) is assumed to have same electrophysiology as the thick CT bundle. Table 1 also summarized the resultant values of APD90 (AP duration at 90% repolarisation) measured in simulations of steady-state pacing of the RA, LA, CT and PM cells at the physiological cycle length of 700 ms. APs in these four cell types exhibit heterogeneous morphologies with significant differences in the plateau potential and APD, the latter ranging from 235 ms in the LA to 278 ms in the CT (Figure 1A).
Table 1.
Current densities and APD (at 700 ms) in various regions of the human atria.
| RA | LA | CT/BB | PMs | |
|---|---|---|---|---|
| ICaL, nS/pF | 0.1294 | 0.1294 | 0.2067 | 0.1238 |
| Ito, nS/pF | 0.1650 | 0.1650 | 0.2115 | 0.1650 |
| IKr, nS/pF | 0.0294 | 0.0470 | 0.0294 | 0.0294 |
| APD90, ms | 266 | 235 | 278 | 262 |
Figure 1.
Electrophysiologically and anatomically heterogeneous model of the human atria. A: AP profiles in the RA, LA, PM and CT cells at the cycle length of 700 ms. B: spontaneous APs in the SAN; C: 3D anatomical model of the human atria showing the main conductive bundles. Bulk atrial tissue in the LA and RA is anatomically homogeneous (translucent grey). The SAN and the major conductive bundles (CT, PM and BB) are coloured and marked by the respective abbreviations.
There is no biophysically-detailed model for the human SAN, which is due to the lack of relevant electrophysiological data. Therefore, for the human SAN, conductances of ionic currents in the Courtemanche et al. model were modified based on experimentally measured differences in ion channel mRNA expression between the RA and SAN in human as described previously (Chandler et al., 2011), resulting in feasible morphologies of spontaneous “pacemaker” APs (Figure 1B).
2.2. Heterogeneous 3D anatomical model of human atria
The spatially heterogeneous 3D anatomical model of the atria (Figure 1C) has been reconstructed by segmenting the Visible Female dataset (National Library of Medicine of the National Institute of Health, Bethesda, USA) in our previous study (Seemann et al., 2006). The 3D geometry has a spatial resolution of 0.33 × 0.33 × 0.33 mm3. Primarily, specific atrial regions were segmented manually from the reconstructed atrial geometry based on well-established anatomic features. The RA and LA were separated, and the distinctive bundles of the CT, PMs and BB were identified (Figure 1C). The SAN was reconstructed separately and merged with the atria (see below). Single cell models were mapped onto the respective regions of the 3D atrial tissue model.
Within the atria fibre orientation was introduced manually: the fibres were assumed aligned along the major atrial bundles – CT, PMs and BB. Values of the diffusion coefficients along and transverse to the fibers, D|| = 20 mm2 ms−1 and D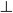 = 2 mm2 ms−1 (which correspond to integral gap junctional conductances of 2000 nS and 200 nS), were chosen to reproduce experimentally observed anisotropic AP conduction in the RA. Primarily, simulations with such diffusion coefficients resulted in anisotropic conduction along the CT and in the RA wall with the velocities ν|| = 1.3 m s−1 and ν
= 2 mm2 ms−1 (which correspond to integral gap junctional conductances of 2000 nS and 200 nS), were chosen to reproduce experimentally observed anisotropic AP conduction in the RA. Primarily, simulations with such diffusion coefficients resulted in anisotropic conduction along the CT and in the RA wall with the velocities ν|| = 1.3 m s−1 and ν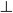 = 0.7 m s−1, respectively. Thus, the simulated anisotropy ratio was v||/ν
= 0.7 m s−1, respectively. Thus, the simulated anisotropy ratio was v||/ν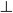 ≈ 1.9, in agreement with the respective experimental measurements of ~1.4 from the rabbit RA (Yamamoto et al., 1998) and 1.1-3.8 from human patients during atrial flutter (Liu et al., 2004). Note that the simulated values of the longitudinal and transverse conduction velocities, ν|| and ν
≈ 1.9, in agreement with the respective experimental measurements of ~1.4 from the rabbit RA (Yamamoto et al., 1998) and 1.1-3.8 from human patients during atrial flutter (Liu et al., 2004). Note that the simulated values of the longitudinal and transverse conduction velocities, ν|| and ν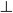 , are also in agreement with experimental data from humans (Bouneau et al., 1985).
, are also in agreement with experimental data from humans (Bouneau et al., 1985).
Thus, an anatomically and biophysically detailed, heterogeneous and anisotropic 3D model of the human atria (including the RA, LA, CT, PMs, BB and SAN) was constructed (Figure 1).
2.3. DT-MRI reconstruction of the human SAN anatomy
Details of fibre architecture in cardiac tissue can be determined using a non-destructive method of diffusion tensor magnetic resonance imaging (DT-MRI). The method calculates the diffusion tensor for protons in the tissue from the signal attenuation and the intensity of magnetic gradient applied during a diffusion-weighted spin echo experiment (Basser et al., 1994). At each point within the tissue three principal directions of diffusion – along the fibre axis, perpendicular to the fibre axis in the sheet plane, and normal to the sheet plane – are given by the orthogonal eigenvectors f, s and n of the diffusion tensor D. The primary eigenvector, f, has been validated as a measure of cardiac fibre orientation in the case of ventricular tissues (Hsu et al., 1998; Holmes et al., 2000).
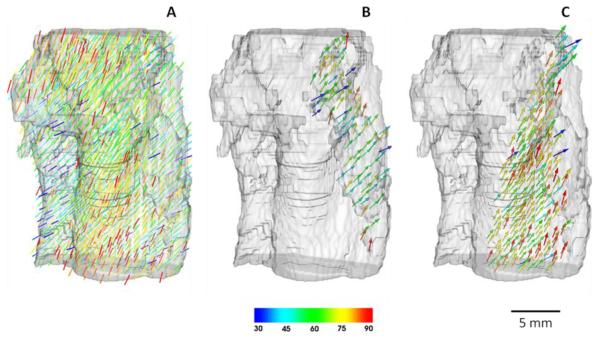
We have used DT-MRI in order to reconstruct the tissue geometry and fibre orientation of the human SAN, and the surrounding RA tissue; details of these experiments have been published elsewhere (Chandler et al., 2011). As a result, 60 contiguous DT-MRI images were acquired with 0.5 mm resolution between tissue slices and 0.25 × 0.25 mm2 resolution of each slice. In order to make resolution uniform in all three directions, a new slice was interpolated between each two adjacent slices, giving a dataset with 0.25 × 0.25 × 0.25 mm3 voxel resolution. Image analysis of the dataset using DTI-Studio 2.4 (Laboratory of Brain Anatomical MRI and Center for Imaging Science at Johns Hopkins University, USA) allowed calculation of the diffusion tensor, D, and its primary eigenvector, f, for each voxel. Visualisation of the latter (Figure 2) revealed a clear arrangement of fibres aligned along the CT and PM bundles of the RA, and a previously unknown loose pattern of fibres within the SAN. These distinct patterns of fibre orientations in the tissue have also been validated by histological experiments (Chandler et al., 2011). Resultant DT-MRI based tissue geometry and fibre orientation datasets was used to construct a detailed 3D computational model for the electrical pacemaking and AP conduction in the human SAN and the surrounding atrial tissue.
Figure 2.
Anatomical model of the human SAN with fibre orientations. Fibre directions are indicated by the direction as well as the colour of the arrows; the palette shows values of the fibre angle in respect to the horizontal plane (such that 900 corresponds to vertical fibres) A: The entire tissue comprising the SAN and surrounding atrium. B: SAN formed by loosely packed nodal cells. C: Atial tissue in the area of CT - atrial cells are largely aligned along the CT and towards the SAN.
Single cell models for the SAN and RA were mapped onto the respective regions of the 3D SAN tissue model. Values of the diffusion coefficients along and transverse to the fibers were same as in the atrial model (section 2.2), D|| = 20 mm2 ms−1 and D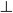 = 2 mm2 ms−1. However, both values were decreased 10-fold in the SAN in order to account for experimentally observed differences in Cx43 expression between the SAN and atrial tissues (Chandler et al., 2011). Combination of the coefficients, D|| and D
= 2 mm2 ms−1. However, both values were decreased 10-fold in the SAN in order to account for experimentally observed differences in Cx43 expression between the SAN and atrial tissues (Chandler et al., 2011). Combination of the coefficients, D|| and D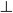 , with the primary eigenvector f give the components of the tensor, , in Equation 1 for each voxel (Benson et al., 2008; Clayton et al., 2011).
, with the primary eigenvector f give the components of the tensor, , in Equation 1 for each voxel (Benson et al., 2008; Clayton et al., 2011).
The reconstructed 3D human SAN model with the detailed fibre orientations was integrated into the 3D model of the whole human atria (see below).
2.4. Torso model and body surface potentials
The ECG is the most common non-invasive method of monitoring the activity of the heart in a clinical environment by measuring the body surface potential (BSP) distribution. The latter results from electrical current flows inside the torso and the heart associated with a propagating AP. The BSP can be simulated by solving the forward problem: mapping simulated voltage distributions on the heart surface into a torso model, and then simulating propagation of the electrical activity from the surface of the heart to the surface of the body (Barr et al., 1966). The latter involves solving Poisson’s equation within the body using the boundary element method (Clayton and Holden, 2002).
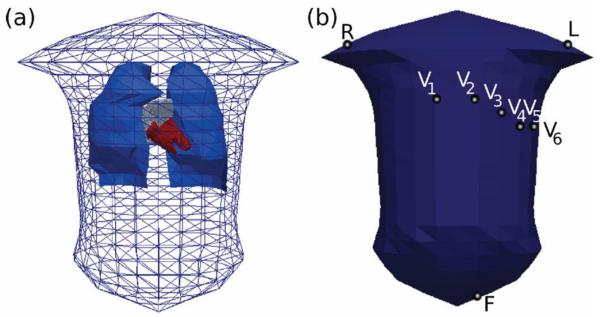
The torso geometry (Figure 3A) has been created previously (Weixue and Ling, 1996): it was derived from CT images of the human torso, and includes geometric representations for the lungs and the blood masses of the atria and ventricles. The 3D atrial model (see section 2.1) was positioned within the torso using a recently published method (Ho and Sanchez-Quintana, 2009). First, the model was centred in the atrial co-ordinate system. Using the z-x-z co-ordinate convention for Euler angles acting on the heart (rather than the co-ordinate system of the heart), the atrium was rotated by (315, 135, 310). Following the rotation, a translation of (−0.003, −0.075, −0.217) was applied to the atrium.
Figure 3.
Model of the human torso. A: The torso geometry used in the body surface potential simulations, shown here with the lungs (blue) and heart blood masses (red) within the surface mesh. B: Triangular elements selected as the positions of the electrodes used for deriving 12-lead ECGs. Lead potentials are defined as super-positions of the body surface potentials under the electrodes shown in (B): lead II = F - R, lead III = F - L, lead aVL = L - (R + F)/2, lead aVF = F - (R + L)/2.
With the integrated 3D atria and torso model (see section 2.5 below), BSP distribution on the torso surface was reconstructed using the boundary element method (Clayton and Holden, 2008). Several of the triangular elements on the torso surface were picked as the sites of the surface potential “electrodes”, with their locations shown in Figure 3B. A standard 12-lead ECG – in case of the atria, twelve profiles of the P-wave corresponding to the atrial activation – was derived from the BSP time series. Correspondence between the locations of electrodes on the torso surface and the definitions of ECG leads used throughout the study is described in Figure 3.
2.5. Integrating SAN, atrium and torso models
The 3D model of the human atria (section 2.2) was completed by incorporation of the SAN model with the DT-MRI fibre reconstruction (section 2.3), and placed within the torso model (section 2.4).Note that the original SAN and atrial geometries had different resolutions (0.5 × 0.25 × 0.25 mm3 and 0.33 × 0.33 × 0.33 mm3, respectively). To merge the two geometries together, resolution of the SAN dataset was rescaled to match that of the atrial dataset: 1) resolution of the SAN dataset was reduced to 0.5 × 0.5 × 0.5 mm3 by neglecting every second grid node within every 0.25 × 0.25 mm2 slice; 2) two equidistant new nodes were interpolated between every two existing adjacent nodes, which resulted in a new geometry grid with resolution of 0.166 × 0.166 × 0.166 mm3; 3) resolution was reduced to 0.33 × 0.33 × 0.33 mm3 by neglecting every second node within the new grid.
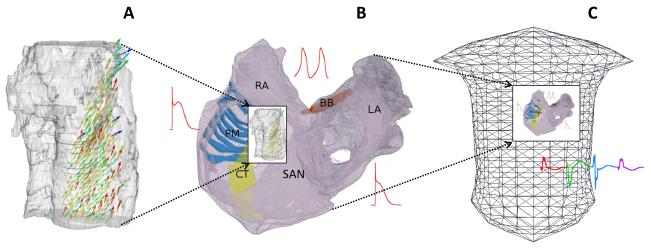
Alignment of the CT and PM bundles between the atrial and SAN geometries (by coordinate rotation and translation of the latter) ensured correct positioning of the SAN within the RA. The fibre orientations in the SAN were then put through the same rotation and translation as the geometry itself and merged with the fibres introduced in the 3D atrial tissue bundles (section 2.2).Primarily, DT-MRI based fibre orientations in the SAN and adjacent parts of the CT and PM replaced manually introduced fibre orientations in the respective parts of the atrial dataset; manual fibre orientations in the rest of the atial dataset (for example, the entire BB) remained unchanged.Thus, an integrative model incorporating detailed electrical heterogeneity, tissue geometry and fibre orientation within the human atrium and SAN has been constructed and placed inside a torso model (Figure 4). Each of the models within this multi-scale hierarchy was validated against available experimental data and used to study various aspects of electrical conduction in the human atria.
Figure 4.
Multi-scale interactions of the multi-scale models of the human atria. A: DT-MRI reconstruction of the geometry and fibre orientation the human SAN and surrounding RA can be integrated into B: 3D model of the whole atria, which can be incorporated into C: the torso model.
2.6. Numerical and computational methods
Equation 1 was solved numerically on the geometry grid using a finite-difference PDE solver based on the explicit Euler method with time step Δt = 0.005 ms, and the space steps Δx corresponding to the spatial resolutions of the tissue models used (Δx = 0.33 mm in the whole atria model and Δx = 0.25 mm in the standalone SAN model). We also ran test simulations with the space step in the atrial model scaled down to 0.25 mm (which corresponded to “shrinking” of the atria by ~25%). Note that such a scaling down made the resolution of the atrial geometry similar to that of the SAN geometry, thus making the procedure of merging these two geometries (section 2.5) more straightforward. The test simulations produced results qualitatively similar to those obtained with the space step of 0.33 mm, demonstrating stability of the model. Computer code implementing the PDE solver was parallelized under OpenMP and run on a 12-core Dell Precision T7500 desktop machine: simulations of 1 second of activity in the whole 3D heterogeneous atria took ~2 hours.
2.7. Initiation of AP propagation and re-entry
In the standalone 3D model of the atria (section 2.2), several pacing protocols were applied to initiate AP propagation: (i) the tissue was stimulated in the presumed SAN area (superior part of the CT) at cycle length of 700 ms to simulate the normal rhythm (Protocol 1); (ii) the tissue was stimulated in the RA between the CT and PMs at cycle length of 200 ms to simulate a rapid atrial focus and the initiation of re-entry and AF (Protocol 2) - a similar protocol has been used previously to initiate re-entry in the rabbit RA model (Aslanidi et al., 2009); (iii) pacing Protocols 1 and 2 were applied simultaneously to simulate interactions between the normal rhythm and AF (Protocol 3).
In the integrated whole SAN-atria model (section 2.5), APs were generated spontaneously due to the presence of the SAN pacemaker. Protocol 2 was applied to study interactions of the normal pacemaking and AF. In all protocols, each stimulus had the amplitude of 2 nA and duration of 2 ms.
3. Results
2.8. Simulated normal activation in the 3D atrial models
The 3D human atrial models without (section 2.2) and with (section 2.5) the integrated SAN are used to simulate the AP propagation through the atria. The resultant atrial activation sequences are 1) validated against results of detailed endocardial mapping in human patients (Lemery et al., 2007), 2) compared to each other in order to evaluate the effects of the SAN inclusion into the integrated model; 3) mapped inside the human torso model to simulate the BSPs and P-waves of the ECG, which were also compared between the two models. These simulations are illustrated in Figure 5.
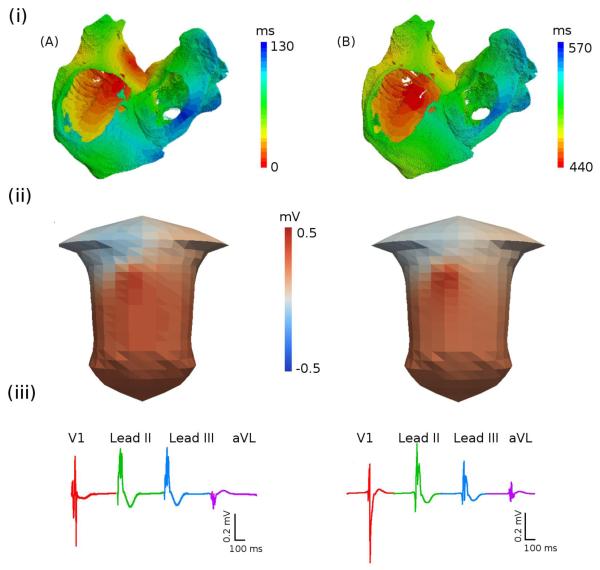
Figure 5.
Activation sequence of the atria during the sinus rhythm. A: Paced atrial model without the SAN. B: Atrial model with the pacemaking SAN. (i) Atrial activation times (measured as the time for a cell to reach the membrane potential of −60 mV); (ii) snapshots of the BSP distributions on the surface of the torso; (iii) P-waves in four representative ECG leads.
Activation pattern simulated using the 3D models without and with the SAN are shown in Figures 5A(i) and 5B(i), respectively. In Figure 5A(i), AP is initiated pacing in the SAN area (Protocol 1, section 2.7); in Figure 5B(i), AP is generated spontaneously due to pacemeking SAN. In both cases, the AP is conducted rapidly down the CT and along the PM: the effect of the fibre orientation in these regions can be clearly seen in the width of the isochrones in Figures 5A(i) and B(i). The AP reaches the BB after ~30 ms and rapidly spreads into the LA; activation of the RA is completed after ~95 ms and activation of the LA (and hence, both atria) is completed in ~120 ms. The values are in a good agreement with the respective clinical mapping data (Lemery et al., 2007).
Table 2 compares activation times calculated for both models with the experimental data of Lemery et al. (2007): the comparison shows that integration of the SAN improves agreement between the simulation data and experiment data for activation time sequence of both atria. This may be explained by the fact that the SAN model includes detailed fibre orientations (primarily for parts of the RA), and hence, can simulate the activation sequence more accurately. Note also that in the model without the SAN the activation times are calculated from the moment of stimulation, whereas in the model with integrated SAN the activation times are calculated from the moment when the AP exits from the SAN. The calculated activation time of the SAN (from the spontaneous AP initiation to its slow exit into the RA) is ~110 ms, which is close to experimental measurements of 103 ms (Juillard et al., 1983).
Table 2.
Activations times in various regions of the atria in two models and experiment. SVC and IVC, superior and inferior vena cava; BB, Bachmann's bundle; RUPV and LIPV, right upper and left inferior pulmonary vein; RA and LA, right and left atrium.
| Atrial model without SAN |
Atrial model with SAN |
Experiment (Lemery et al.) |
|
|---|---|---|---|
| First SVC | 18 ms | 23 ms | 26±22 ms |
| First BB | 26 ms | 29 ms | 31±13 ms |
| First RUPV | 72 ms | 76 ms | 75±24 ms |
| First IVC | 92 ms | 86 ms | 88±33 ms |
| Latest RA | 95 ms | 92 ms | 93±17 ms |
| First LIPV | 99 ms | 102 ms | 101±23 ms |
| Latest LA | 111 ms | 118 ms | 116±18 ms |
Figure 5 also shows BSPs corresponding to the AP propagation in the human atrial models without (Figure 5A(ii)) and with (Figure 5B(ii)) the SAN. The BSP distributions in both cases are qualitatively similar. AP conduction from the superior to the inferior CT results in a BSP wave which emerges over the lower right lung and propagates down towards the left leg, leaving a large positive region in the lower left torso and a negative region over the right shoulder (Figures 5A(ii) and B(ii)). However, P-waves calculated from the BSPs for several ECG leads (Figures 5A(iii) and B(iii)) exhibit small differences between the two models. Primarily, the P-wave in lead aVL is downward in the model without the SAN (Figure 5A(iii)) and upward in the model with the SAN (Figure 5B(iii)). Note that a variety of P-waves morphologies has been observed in clinical ECG recordings (Mirvis, 1980): simulated P-wave morphologies are qualitatively similar to these data.
The simulation presented above demonstrate that 1) the 3D human atrial model reproduces activation patterns of the atria (as observed clinically during the normal rhythm by Lemery et al., 2007) with good accuracy, 2) pacing of the standalone atrial model at a physiological rate provides a good approximation for the normal rhythm, 3) integration of the SAN model into the atrial model enables spontaneous pacemaking and improves agreement between the modelling and clinical data.
3.2. Transition from the normal rhythm to re-entry – initiation of AF
Figure 6A(i) shows a regular electrical excitation wave on the epicardial surface of the atria during the normal rhythm with a period of ~700 ms. The excitation wave is first generated by the pacemaking SAN and then propagates through the RA (and later LA) maintaining a regular wave-front. Corresponding BSP and P-waves are shown in Figures 6A(ii) and 6A(iii), respectively. A similar normal excitation pattern (“normal rhythm”) is observed in the case when the superior part of the CT is paced at 700 ms (Figure 5A), with the wave propagation pattern on the epicardial surface, BSP and P-waves qualitatively similar to those shown in Figure 6A.
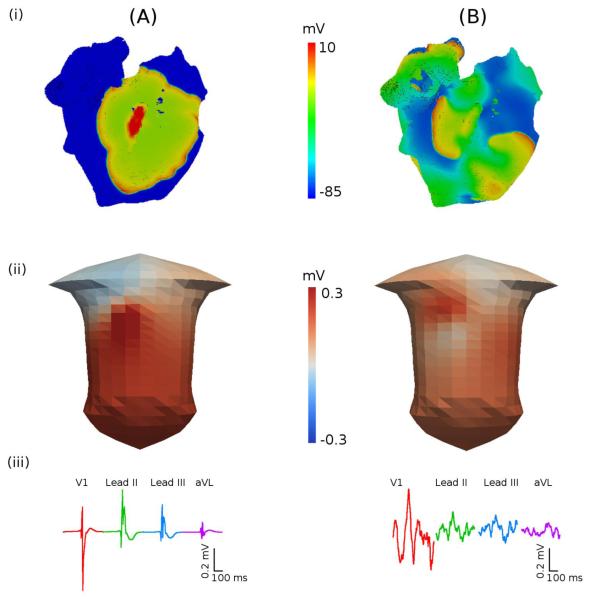
Figure 6.
AP propagation in the 3D model of the human atria. A: Spontaneous normal rhythm. B: Pacing-induced AF. (i) snapshots of the membrane potential distribution on the epicardial surface of the atria; (ii) snapshots of the BSP in the torso; (iii) P-waves in four representative ECG leads.
However, rapid pacing of the 3D atrial model at the cycle length of 200 ms near the CT can result in a completely different pattern of wave propagation (Figure 6B): after only three cycles of pacing, the wave-fronts are broken down, leading to multiple re-entrant wavelets propagating through the atria (Figure 6B(i)). This is characteristic of electrical activation patterns observed in the atria during AF (Harada et al., 1996; Nattel et al., 2005). The BSP corresponding to the multiple re-entrant wavelets (Figure 6B(ii)) is different from the one in the normal rhythm (Figure 6A(ii)). The difference is clearly evident from the P-waves that show irregular “saw-tooth” morphologies (Figure 6B(iii)) characteristic of AF (Fuster et al., 2006). Only P-waves in lead V1 look similar to tachycardic “flutter waves”, which is also commonly seen in ECG recording from AF patients (Fuster et al., 2006). Note that AF patterns (as seen in Figure 6B) are simulated by both models with and without the SAN: in the case when the SAN is integrated into the atria, the rapidly (~4 Hz) rotating re-entrant wavelets suppress the relatively slow (~1.5 Hz) pacemaking by the SAN.
Under normal conditions, AF in these simulations is not sustained as re-entrant wavelets are self-terminated after ~1 s of rotation, after which the SAN restores its normal pacemaking rhythm. However, 2-fold reduction of the cell-to-cell coupling (i.e., the diffusion coefficients) in the model results in sustained AF: the irregular pattern of multiple re-entrant wavelets is maintained for the entire duration of simulations (10 s). This can be explained by the fact that the coupling reduction results in a decrease of the conduction velocity of wavelets, and hence, effectively increases the tissue substrate available for their rotation. Note that decreases of the atrial conduction velocity have been observed in AF patients (Kojodjojo et al., 2007), and the underlying coupling reduction can be due to structural remodelling of atrial tissue associated with AF (Everett et al., 2006).
AF in our simulations was initiated by rapid pacing in the RA close to the CT, which is in agreement with several experimental observations: transition from periodic waves to AF during rapid pacing of the sheep RA (Mandapati et al., 2000), clinically observed re-entrant circuits at the CT (Cox et al., 1991), and high incidence of dominant frequencies in the RA in patients with persistent AF (Sanders et al., 2005). Below we study the mechanisms of AF generation in the RA, primarily the role of RA tissue heterogeneity and anisotropy in the genesis of AF.
3.3. Mechanisms of AF initiation and development
The developed 3D human atrial model is used to dissect the mechanisms underlying the transition from rapid focal waves to re-entry and AF in the RA (Figure 7). In simulation illustrated in Figure 7, the RA is paced at the border between the CT and PM (Protocol 2, section 2.7), where both the intrinsic APD difference and anisotropy of the bundles are pronounced. Primarily, the APD in the CT cells is longer (Table 1) and electrotonic coupling transverse to fibres of the CT is weak. Therefore, rapid pacing by three focal stimuli applied at the cycle length of 200 ms to the border between the CT and PM regions results in a conduction block of the third focal wave towards the CT (Figure 7A). The broken wave spreads into the PMs and then the rest of RA (including the now-recovered CT), and after ~200 ms re-enters the initial pacing site from the superior and inferior CT directions. Hence, after ~600 ms of rapid pacing an almost-symmetric pair of re-entrant scroll waves are generated, which rotate in the RA and evade into the LA (Figure 7A).
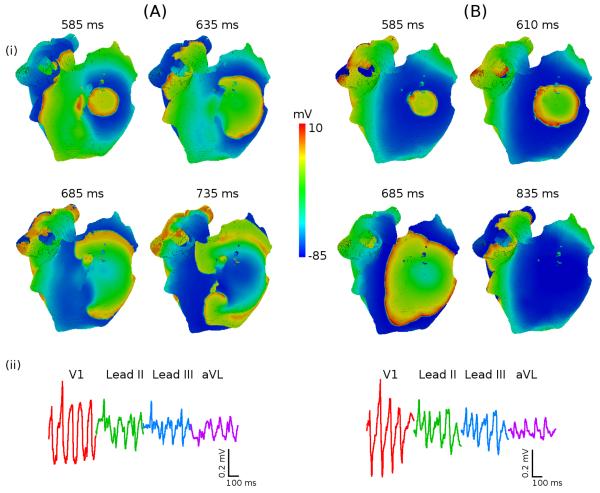
Figure 7.
Role of atrial heterogeneity in the initiation of re-entry. A: Re-entry in heterogeneous atria. B: Focal activity in homogeneous atria. In both A and B, the RA is paced at the cycle length of 200 ms between the CT and PMs. (i) snapshots of the potential distribution on the epicardial surface of the atria for successive moments of time; (ii) P-waves in four representative ECG leads.
Note that the short coupling (200 ms) between the successive focal waves is important for the re-entry initiation, as shown before for the rabbit RA (Aslanidi et al., 2009). Primarily, it 1) allows to maintain the high APD gradient between the CT and PM, and 2) results in conduction slowing of short-coupled waves, which gives sufficient time for the development of re-entry.
Further simulations suggest that both heterogeneity and anisotropy are also important for the re-entry initiation and development into the multi-wavelet state of AF. Figure 7B illustrates that rapid pacing at any rate cannot generate re-entry in the homogeneous anisotropic RA (where all cell are assumed to have same electrophysiology as the bulk RA cells). As there is no APD gradient between the CT and PM in this case, even short-coupled focal waves propagate in all directions without breaking their wave-fronts (Figure 7B). The contribution of anisotropy to the initiation of re-entrant waves seems less important, as re-entry can still be initiated by rapid pacing in the heterogeneous isotropic RA (where the diffusion coefficient is assumed the same in all directions).
However, anisotropy contributes to breaking down of the initial re-entrant waves (Figure 7A) into multiple wavelets characteristic of AF (Figure 8). Figure 8A(i) shows the pattern of electrical excitation on the epicardial surface of the 3D atrial model after 3 s of simulation. The pattern is very irregular, as several wavelets meanders in different directions through changing re-entrant circuits. As before, P-waves show characteristic “saw-tooth” morphologies and “flutter waves” in lead V1 (Figure 8A(ii)). A similar simulation with heterogeneous isotropic RA model shows a much more regular excitation pattern (Figure 8B(i)): the initial pair of re-entrant waves (seen in Figure 7A) is preserved, although it is not as symmetric as before due to irregularities of the tissue structure. Morphologies of P-waves in this case are also more regular, with clear peaks (Figure 8B(ii)). Therefore, anisotropy of the RA bundles (such as the CT and PM) is important in the break-down of the initial pair of re-entrant waves, leading into multiple meandering wavelets characteristic of AF.
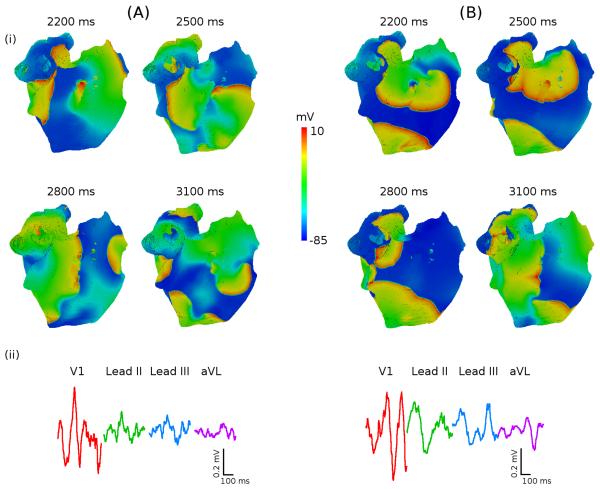
Figure 8.
Role of atrial anisotropy in the genesis of AF. A: Multiple meandering wavelents in anisotropic atria. B: Pair of re-entrant waves in isotropic atria. In both A and B, re-entry is initiated as shown in Figure 7A. RA (i) snapshots of the potential distribution on the epicardial surface of the atria for successive moments of time; (ii) P-waves in four representative ECG leads. ECG in the anisotropic case A(ii) shows “flutter wave” in lead V1 and “saw-tooth” morphologies in other leads, which are features of clinical AF. ECG in the isotropic case B(ii) shows P-waves with clear peaks.
Thus, our simulations with the 3D human atrial model show that APD heterogeneity is important for the initial conduction block and generation of a pair of re-entrant waves (Figure 7), whereas anisotropy of the RA bundles contributes to the symmetry breaking of the pair and genesis of multiple re-entrant wavelets and resultant “saw-tooth” P-waves (Figure 8) seen in clinical AF.
4. Discussion
In this study, we have developed a multi-scale hierarchy of biophysically detailed computational models for the human atria, which is summarised in Figure 4. Primarily, DT-MRI reconstruction of the tissue geometry and fibre orientation of the human SAN and surrounding RA (Figure 4A) can be integrated into the 3D model of the whole atria based on the Visible Female dataset (Figure 4B). The integrated anatomical model can be combined with the heterogeneous AP models (Figure 4B), and used to simulate the AP conduction in the human atria in various conditions: SAN pacemaking and atrial activation in the normal rhythm (Figure 5), the genesis of multiple re-entrant wavelets and AF by rapid pacing (Figure 6), and the effects of atrial heterogeneity and anisotropy (Figures 7, 8) on the development of AF. The 3D model of the atria itself can be incorporated into the torso model (Figure 4C), and used to simulate the body surface ECG patterns in the normal and arrhythmic conditions (Figures 5-7). Primarily, the torso model allows simulations of “saw-tooth” P-wave morphologies characteristic of clinical AF. Thus, a powerful computational platform for studying atrial conduction and arrhythmogenesis – the virtual human atria – has been developed. Below we discuss various components of the 3D virtual atria, their limitations, and interactions between them.
4.1. Model of the human SAN
Although several single cell and tissue models have been developed for the SAN in many animal species (Wilders et al., 2007), a model for the human SAN have been created only recently in our group (Chandler et al., 2011). This allowed us for the first time to incorporate the SAN model into the 3D model of the human atria and study the atrial activation due to the SAN pacemaking.
The SAN model based on DT-MRI reconstruction of the tissue geometry and detailed fibre orientation (Chandlier et al., 2011) has several limitations. Thus, although the primary eigenvector of the diffusion tensor has been validated as a measure of the fibre orientation in tissue with a clear arrangement of densely packed long thin cells (such as atrial myocytes), there is no sufficient histological data to make such validation for areas formed by small spherical cells (SAN). However, our data provides evidence that diffusion is anisotropic not only in the atrium, but also in the SAN. First, as three eigenvectors of the diffusion tensor are statistically different throughout the entire tissue (Chandler et al., 2011), the primary eigenvector can be used as a reliable measure of fiber orientation tissue. Second, DT-MRI shows a clear arrangement of fibers in the SAN (Figure 2B), and immunohistochemistry reveals a network of cells expressing gap-junction protein Cx43 (Chandler et al., 2011). Hence, the SAN contains clusters that can maintain diffusion between cells.
Although there are very limited data on AP properties of the SAN, APs generated by the model developed have realistic morphology and pacemaking rate (Figure 1B), which is sufficient for simulating periodic AP generation and conduction into the RA (Figure 5B): simulated epicardial activation sequence is similar to experimental data (Boineau et al., 1988; Lemery et al., 2007).
Besides, spatial heterogeneity within the SAN is important for the mechanisms of pacemaking (Zhang et al., 2000), but again there are no data from human SAN cells, primarily on 1) proportion and spatial distribution of central and peripheral cells, 2) differences in their electrophysiological properties, 3) shape and extent of electrical gradients in the ionic currents. Importantly, the lack of heterogeneity within the SAN does not affect its ability to drive the atrium – which is the primary subject of our study. Our preliminary simulations show that electrical gradients between the SAN centre and periphery can be introduced into the human SAN model based on the data from rabbit (Zhang et al., 2000); however, details of these of simulations are beyond the scope of this study.
4.2. Model of the human atria
We have developed a detailed 3D model of the human atria, which incorporates anatomical and electrophysiological heterogeneities (Figure 4B). The atrial model can incorporate a smaller-scale model of the human SAN (Figure 4A), and can be itself incorporated in a larger-scale model of the human torso (Figure 4C). The resultant multi-scale model accurately simulates atrial activation patterns (Figure 5), which are in good agreement with clinical mapping data by Lemery et al. (see in Table 2). Integration of the SAN model into the atrial model not only enables spontaneous pacemaking, but improves agreement between the model and clinical data (Table 2).
The model has been used for studying the mechanisms of AF arrhythmogenesis. Results of these simulations are discussed in detail below (section 4.3). In simulations, rapid atrial pacing at the CT produced patterns of multiple reentant wavelets characteristic of AF (Figures 6-8), which is in agreements with experimental observations (Cox et al., 1991; Mandapati et al., 2000). Morphologies of the corresponding ECG P-waves (Figure 6B(iii)) showed irregular “saw-tooth” baseline and “flutter waves” in lead V1, which both are features of clinical AF (Fuster et al., 2006).
Limitations of the 3D atrial model are largely inherited from the earlier models on which it was based. The Courtemanche et al. model was used here to simulate cellular APs of human atrial myocytes; the limitations of this model have been discussed elsewhere (Courtemanche et al., 1998;Nygren et al., 2001; Cherry et al., 2008). In the tissue model, intrinsic electrical heterogeneity and anisotropic intercellular coupling were introduced for several atrial regions phenomenologically (Seemann et al., 2005), due to the lack of detailed experimental data from human atria. For the electrical heterogeneity, the model used experimental data from dog (Feng et al., 1998; Li et al., 2001), whereas anisotropy parameters were introduced based on measurements from rabbit (Yamamoto et al., 1998). However, as mentioned above, the atrial activation sequence simulated with the resultant model was in quantitative agreement with human data (Lemery et al., 2007).
4.3. Comparison to other models
Several 3D models of the human atria have been constructed before (Harrild and Henriquez, 2000; Seemann et al., 2006; Jacquemet et al., 2006; Cherry and Evans, 2008); most of them were based on the single cell description of Courtemanche et al. (1998) and the Visible Female geometry dataset. While these models included various details of atrial anatomy and electrophysiology, each of these studies had certain limitations. Anisotropic 3D model by Harrild and Henriquez (2000) did not include electrophysiological heterogeneties of the human atria, and only studied normal atrial activation. Seemann et al. (2006) included such heterogeneities, but their model of the SAN was idealised, and the simulated atrial activation sequence showed limited agreement with experimental data; besides, they did not consider AF. Jacquemet et al. (2006) considered a detailed 3D model with MRI-based atrial geometry, and studied initiation of AF-like state by rapid atrial pacing, similar to our study. However, in order to obtain such AF, Jacquemet et al. introduced patchy non-physiological heterogeneities throughout the atria, which had no relation to naturally existing electrophysiological heterogeneities in human atria. Finally, Cherry and Evans (2008) used the model by Harrild and Henriquez to simulate re-entrant scroll waves, and observed transient break-ups of the scrolls into multiple wavelets. However, their models did not consider electrical heterogeneity in the atria, and the original scroll wave was initiated by an appropriately timed cross-field stimulation. We believe our 3D model of the human atria overcomes many limitations of those earlier models by considering not only detailed atrial anatomy and electrophysiology, but also accurate regional heterogeneity and anisotropy. Primarily the latter two features enable simulations of AF arrhythmogenesis without using any idealised heterogeneities or stimulation protocols.
4.4. Mechanisms of AF
Experimental animal studies and clinical mapping data have suggested that high-frequency irregular electrical activity in AF may be sustained by re-entrant wavelets propagating in an abnormal atrial tissue substrate (Harada et al., 1996; Jalife et al., 2002; Nattel et al., 2005). Atrial tissues with large regional differences in electrical properties are more susceptible to re-entry, which may result from conduction slowing in atrial tissue regions with long refractoriness or high conduction anisotropy (Allessie et al., 1976; Spach et al., 1989; Aslanidi et al., 2009). Multiple experimental and clinical results have also suggested that “driver” regions acting as high-frequency sources during AF may be located in the LA, whereas the RA is characterised by heterogeneous electrical activity and multiple wavelets (Haissaguerre et al., 1998; Chen et al., 1999; Sanders et al., 2005). The origin of such multiple wavelets is unclear; our simulations of the break-down of excitation wave-fronts in the heterogeneous RA bundles may provided new insights into the mechanisms of their generation.
The basis for LA predominance of AF is also unknown. Myocardial sleeves of the pulmonary veins (PVs) in the LA are recognized as important sources of ectopic electrical activity - ablation of the PV-LA junctions to electrically isolate PVs from the LA can terminate AF (Haissaguerre et al., 1998; Chen et al., 1999). Knowledge of the arrhythmogenic substrate in the PVs is limited. Hence, the 3D atrial model can benefit from incorporation of the PV model, which is subject to availability of experimental data from human or electrophysiologically similar large animal species. As junctions between the LA and PV are characterized by high electrical heterogeneity and anisotropy (Chen et al., 1999; Arora et al., 2003), they may provide a substrate for the generation of re-entry from ectopic beats - via mechanisms similar to those reported in this study for the CT-PM junctions.
Rapid AF is also often progressive, going from paroxysmal to persistent to permanent, with electrical and structural remodelling of the atria leading to a substrate that facilitates self-perpetuation of the arrhythmia (Nattel et al., 2005; Anter et al., 2009). Hence, the effects of remodelling should be incorporated into the model, primarily changes of the ionic channel current densities (e.g., Ehrlich and Nattel, 2009) and in atrial tissue structure (such as atrial fiber disarray, fibrosis and hypertrophy, Everett et al., 2006). Remodelling due to rapid electrical activity is not the only mechanism of AF self-perpetuation: under AF conditions, the acetylcholine-activated current, IK,ACh, can become constitutively active even in the absence of vagal tone - and hence, when enhanced by vagal stimulation, can further promote AF (Sarmast et al., 2003; Katsouras et al, 2009). A wide variety of mechanisms that may be involved in the genesis of AF require further studies.
4.4. Model of the human torso
The 3D atrial model was incorporated into the torso model (Figure 4C), and used to simulate the body surface ECGs in the normal (Figure 5) and abnormal (Figure 6) conditions. This allowed us to simulate “saw-tooth” P-wave morphologies consistent with clinical AF data (Fuster et al., 2006).
Note that P-waves in some leads exhibit ‘notched’ morphologies even in normal conditions, as opposed to smoother P-waves seen in clinical recordings. This may be due to the simplistic torso geometry and omission of several inhomogeneities within the torso. Inclusion of such structures as the skeletal muscle (which is electrically excitable) may contribute to a smoothing of the P-waves. However, this would require the use of the finite element method, which is more computationally costly than the boundary element method (Clayton and Holden, 2008). Note also that even clinically recorded P-wave morphologies are not necessarily smooth (Mirvis, 1980).
4.5. Conclusions
Understanding the spatio-temporal patterns of propagation in the atria during normal sinus rhythm, how it changes with rate, and the genesis of atrial arrhythmias (such as AF) requires full in depth access to cells and tissue, and the possibility to control individual variables. This is extremely difficult (if not impossible) to implement in an experimental or clinical set-up. The 3D virtual human atria, based on detailed ionic channel and cell electrophysiology, and histology and DT-MRI based anatomy, gives a validated means for dissecting and explaining propagation in the normal and arrhythmic atria. Results of the model simulations can be compared with the clinical mapping data and ECG recordings, providing additional validation. Although, due to the lack of experimental detail from human, the model currently lacks some components (such as description of the PV), these can be incorporated in the already built-up platform when more data become available.
In summary, our virtual atria is a state-of-the-art complementary tool for investigating the multi-scale dynamical behaviour in the human atria during the normal rhythm and AF.
Acknowledgements
This work was supported by grants from the Engineering and Physical Sciences Research Council (EP/I029664/1), British Heart Foundation (PG/10/69/28524) and Wellcome Trust (WT/081809/Z/06/Z).
References
- Allessie MA, Bonke FI, Schopmann FTG. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. II. The role of nonuniform recovery of excitability in the occurrence of unidirectional block studied with multiple microelectrodes. Circ Res. 1976;39:168–77. doi: 10.1161/01.res.39.2.168. [DOI] [PubMed] [Google Scholar]
- Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–25. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- Aslanidi OV, Boyett MR, Dobrzynski H, Li J, Zhang H. Mechanisms of transition from normal to re-entrant electrical activity in a model of rabbit atrial tissue: interaction of tissue heterogeneity and anisotropy. Biophys J. 2009;96:798–817. doi: 10.1016/j.bpj.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R, Verheule S, Scott L, et al. Arrhythmogenic substrate of the pulmonary veins assessed by high-resolution optical mapping. Circulation. 2003;107:1816–21. doi: 10.1161/01.CIR.0000058461.86339.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magnet Res Med. 1994;30:201–206. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Barr RC, Pilkington TC, Boineau JP, Spach MS. Determining surface potentials from current cipoles with application to electrocardiography. IEEE Trans Biomed Eng. 1966;13:88–92. doi: 10.1109/tbme.1966.4502411. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- Benson AP, Aslanidi OV, Zhang H, Holden AV. The canine virtual ventricular wall: a platform for dissecting pharmacological effects on propagation and arrhythmogenesis. Prog Biophys Mol Biol. 2008;96:187–208. doi: 10.1016/j.pbiomolbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Boineau JP. Atrial flutter: a synthesis of concepts. Circulation. 1985;72:249–57. doi: 10.1161/01.cir.72.2.249. [DOI] [PubMed] [Google Scholar]
- Boineau JP, Canavan TE, Schuessler RB, Cain ME, Corr PB, Cox JL. Demonstration of a widely distributed atrial pacemaker complex in the human heart. Circulation. 1988;77:1221–37. doi: 10.1161/01.cir.77.6.1221. [DOI] [PubMed] [Google Scholar]
- Chandler NJ, Aslanidi OV, Buckley DL, et al. Computer three-dimensional anatomical reconstruction of the human sinus node and a novel paranodal area. Anat Record. 2011 doi: 10.1002/ar.21379. in press. [DOI] [PubMed] [Google Scholar]
- Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–86. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- Cherry EM, Evans SJ. Properties of two human atrial cell models in tissue: restitution, memory, propagation, and reentry. J Theor Biol. 2008;254:674–90. doi: 10.1016/j.jtbi.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry EM, Hastings HM, Evans SJ. Dynamics of human atrial cell models: restitution, memory, and intracellular calcium dynamics in single cells. Prog Biophys Mol Biol. 2008;98:24–37. doi: 10.1016/j.pbiomolbio.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton RH, Holden AV. Computational framework for simulating the mechanisms and ECG of re-entrant ventricular fibrillation. Physiol Meas. 2002;23:707–26. doi: 10.1088/0967-3334/23/4/310. [DOI] [PubMed] [Google Scholar]
- Clayton RH, Bernus O, Cherry EM, et al. Models of cardiac tissue electrophysiology: progress, challenges and open questions. Prog Biophys Mol Biol. 2011;104:22–48. doi: 10.1016/j.pbiomolbio.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Cox JL, Canavan TE, Schuessler RB, et al. The surgical treatment of atrial fibrillation, II: Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial futter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101:406–26. [PubMed] [Google Scholar]
- Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol. 1998;275:H301–21. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- Ehrlich JR, Nattel S. Novel approaches for pharmacological management of atrial fibrillation. Drugs. 2009;69:757–74. doi: 10.2165/00003495-200969070-00001. [DOI] [PubMed] [Google Scholar]
- Everett TH, Wilson EE, Verheule S, Guerra JM, Foreman S, Olgin JE. Structural atrial remodeling alters the substrate and spatiotemporal organization of atrial fibrillation: a comparison in canine models of structural and electrical atrial remodeling. Am J Physiol. 2006;291:H2911–23. doi: 10.1152/ajpheart.01128.2005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Yue L, Wang Z, Nattel S. Ionic mechanisms of regional action potential heterogeneity in the canine right atrium. Circ Res. 1998;83:541–51. doi: 10.1161/01.res.83.5.541. [DOI] [PubMed] [Google Scholar]
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- Harada A, Sasaki K, Fukushima T, et al. Atrial activation during chronic atrial fibrillation in patients with isolated mitral valve disease. Ann Thorac Surg. 1996;61:104–11. doi: 10.1016/0003-4975(95)00824-1. [DOI] [PubMed] [Google Scholar]
- Harrild D, Henriquez C. A computer model of normal conduction in the human atria. Circ Res. 2000;87:25–36. doi: 10.1161/01.res.87.7.e25. [DOI] [PubMed] [Google Scholar]
- Ho SY, Sanchez-Quintana D. The importance of atrial structure and fibers. Clinical Anatomy. 2009;22:52–63. doi: 10.1002/ca.20634. [DOI] [PubMed] [Google Scholar]
- Holmes AA, Scollan DF, Winslow RL. Direct histological validation of diffusion tensor MRI in formaldehyde-fixed myocardium. J Magnet Res Med. 2000;44:157–61. doi: 10.1002/1522-2594(200007)44:1<157::aid-mrm22>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Hsu EW, Muzikant AL, Matulevicius SA, Penland RC, Henriquez SC. Magnetic resonance myocardial fiber-orienation mapping with direct histological correlation. Am J Physiol. 1998;274:H1627–34. doi: 10.1152/ajpheart.1998.274.5.H1627. [DOI] [PubMed] [Google Scholar]
- Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res. 2002;54:204–16. doi: 10.1016/s0008-6363(02)00223-7. [DOI] [PubMed] [Google Scholar]
- Jacquemet V, van Oosterom A, Vesin J-M, Kappenberger L. Analysis of electrocardiograms during atrial fibrillation. A biophysical model approach. IEEE Eng Med Biol Mag. 2006;25:79–88. doi: 10.1109/emb-m.2006.250511. [DOI] [PubMed] [Google Scholar]
- Juillard A, Guillerm F, Chuong HV, Barrillon A, Gerbaux A. Sinus node electrogram recording in 59 patients. Comparison with simultaneous estimation of sinoatrial conduction using premature atrial stimulation. Br Heart J. 1983;50:75–84. doi: 10.1136/hrt.50.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouras G, Sakabe M, Comtois P, et al. Differences in atrial fibrillation properties under vagal nerve stimulation versus atrial tachycardia remodeling. Heart Rhythm. 2009;6:1465–72. doi: 10.1016/j.hrthm.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Kharche S, Zhang H. Simulating the effects of atrial fibrillation induced electrical remodeling: a comprehensive simulation study. Conf Proc IEEE Eng Med Biol Soc. 2008;1:593–56. doi: 10.1109/IEMBS.2008.4649222. [DOI] [PubMed] [Google Scholar]
- Kojodjojo P, Peters NS, Davies DW, Kanagaratnam P. Characterization of the electroanatomical substrate in human atrial fibrillation: the relationship between changes in atrial volume, refractoriness, wavefront propagation velocities, and AF burden. J Cardiovasc Electrophysiol. 2007;18:269–75. doi: 10.1111/j.1540-8167.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- Lemery R, Birnie D, Tang AS, Green M, Gollob M, Hendry M, Lau E. Normal atrial activation and voltage during sinus rhythm in the human heart: An endocardial and epicardial mapping study in patients with a history of atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:402–08. doi: 10.1111/j.1540-8167.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- Li D, Zhang L, Kneller J, Nattel S. Potential ionic mechanism for repolarization differences between canine right and left atrium. Circ Res. 2001;88:1168–75. doi: 10.1161/hh1101.091266. [DOI] [PubMed] [Google Scholar]
- Liu TY, Tai CT, Huang BH, et al. Functional characterization of the crista terminalis in patients with atrial flutter: implications for radiofrequency ablation. J Am Coll Cardiol. 2004;43:1639–45. doi: 10.1016/j.jacc.2003.11.057. [DOI] [PubMed] [Google Scholar]
- Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–99. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- Mirvis DM. Body surface distribution of electrical potential during atrial depolarization and repolarization. Circulation. 1980;62:167–73. doi: 10.1161/01.cir.62.1.167. [DOI] [PubMed] [Google Scholar]
- Nattel S, Shiroshita-Takeshita A, Brundel BJ, Rivard L. Mechanisms of atrial fibrillation: lessons from animal models. Prog Cardiovasc Dis. 2005;48:9–28. doi: 10.1016/j.pcad.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Noble D. Modeling the heart – from genes to cells to the whole organ. Science. 2002;295:1678–82. doi: 10.1126/science.1069881. [DOI] [PubMed] [Google Scholar]
- Nygren A, Leon LJ, Giles WR. Simulations of the human atrial action potential. Phil Trans Royal Soc A. 2001;359:1111–25. [Google Scholar]
- Ridler M, McQueen DM, Peskin CS, Vigmond E. Action potential duration gradient protects the right atrium from fibrillating. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3978–81. doi: 10.1109/IEMBS.2006.260522. [DOI] [PubMed] [Google Scholar]
- Rudy Y. From genome to physiome: integrative models of cardiac excitation. Ann Biomed Eng. 2000;28:945–50. doi: 10.1114/1.1308484. [DOI] [PubMed] [Google Scholar]
- Sarmast F, Kolli A, Zaitsev A, et al. Cholinergic atrial fibrillation: IK,ACh gradients determine unequal left/right atrial frequencies and rotor dynamics. Cardiovasc Res. 2003;59:863–73. doi: 10.1016/s0008-6363(03)00540-6. [DOI] [PubMed] [Google Scholar]
- Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–97. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- Seemann G, Hoper C, Sachse FB, Dossel O, Holden AV, Zhang H. Heterogeneous 3-dimensional anatomical and electrophysiological model of human atria. Phil Trans A. 2006;364:1465–81. doi: 10.1098/rsta.2006.1781. [DOI] [PubMed] [Google Scholar]
- Spach MS, Dolber PC, Heidlage JF. Interaction of inhomogeneities of repolarization with anisotropic propagation in dog atria: a mechanism for both preventing and initiating reentry. Circ Res. 1989;65:1612–31. doi: 10.1161/01.res.65.6.1612. [DOI] [PubMed] [Google Scholar]
- Stewart S, Murphy N, Walker A, McGuire A, McMurray JJV. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90:286–92. doi: 10.1136/hrt.2002.008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weixue L, Ling X. Computer simulation of epicardial potentials using a heart-torso model with realistic geometry. IEEE Trans Biomed Eng. 1996;43:211–17. doi: 10.1109/10.481990. [DOI] [PubMed] [Google Scholar]
- Wilders R. Computer modelling of the sinoatrial node. Med Biol Eng Comput. 2007;45:189–207. doi: 10.1007/s11517-006-0127-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Honjo H, Niwa R, Kodama I. Low-frequency extracellular potentials recorded from the sinoatrial node. Cardiovasc Res. 1998;39:360–72. doi: 10.1016/s0008-6363(98)00091-1. [DOI] [PubMed] [Google Scholar]
- Zhang H, Holden AV, Kodama I, Honjo H, Lei M, Varghese T, Boyett MR. Mathematical models of action potentials in the periphery and center of the rabbit sinoatrial node. Am J Physiol. 2000;279:H397–421. doi: 10.1152/ajpheart.2000.279.1.H397. [DOI] [PubMed] [Google Scholar]