Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that affects both upper and lower motor neurons (MN) resulting in weakness, paralysis and subsequent death. Insulin-like growth factor-I (IGF-I) is a potent neurotrophic factor that has neuroprotective properties in the central and peripheral nervous systems. Due to the efficacy of IGF-I in the treatment of other diseases and its ability to promote neuronal survival, IGF-I is being extensively studied in ALS therapeutic trials. This review covers in vitro and in vivo studies examining the efficacy of IGF-I in ALS model systems and also addresses the mechanisms by which IGF-I asserts its effects in these models, the status of the IGF-I system in ALS patients, results of clinical trials, and the need for the development of better delivery mechanisms to maximize IGF-I efficacy. The knowledge obtained from these studies suggests that IGF-I has the potential to be a safe and efficacious therapy for ALS.
Keywords: Amyotrophic lateral sclerosis, insulin-like growth factor-I, treatment
Introduction
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS), commonly referred to at Lou Gehrig’s disease, is the most common adult-onset motor neuron (MN) disease with a lifetime risk of approximately 1 in 800 (1). ALS is characterized by a selective and progressive degeneration of both upper and lower MNs in the spinal cord, brainstem and cortex. Clinical symptoms include muscle atrophy, weakness, paralysis, and ultimately death due to respiratory failure, which typically occurs within three to five years after diagnosis. The mechanisms of MN degeneration in ALS are undetermined; however, approximately 10% of cases are familial (FALS) and exhibit a disease course and symptoms clinically and pathologically indistinguishable from sporadic ALS (SALS) (2).
Approximately 20–25% of all FALS cases result from mutations of Cu2+/Zn2+ superoxide dismutase (SOD1), an antioxidant enzyme that catalyzes the conversion of the superoxide radical into hydrogen peroxide (3,4). There are over 100 known SOD1 mutations that cause ALS (2). The mutation sites are distributed throughout the structure and involve the metal binding site, adjacent electrostatic and zinc loops, disulfide bond locations, and the dimer interface. The mutations are generally classified into two groups: 1) metal-binding region (MBR) mutations involving residues that coordinate the metal binding site; and 2) wild-type-like (WTL) mutations, including all the remaining mutations. MBR mutations cause significant changes in the biophysical properties of SOD1, whereas WTL mutations do not alter normal SOD1 function (5,6). Despite distinctions seen in biophysical properties of varying SOD1 mutations, there is no correlation between type of mutation and survival (7). This suggests the possibility of a common mechanism for all types of ALS, both sporadic and inherited, and further suggests that studies of SOD1-related ALS may be applicable to all forms of the disease.
Transgenic mouse models demonstrate that over-expression of human G93A-SOD1 and wild-type SOD1 result in increased SOD1 activity, but only expression of the mutant results in ALS (8). Similar results demonstrate that decreased expression levels of either the mutant or wild-type SOD1 proteins results in decreased SOD1 function, but again, only the mutant results in ALS (9). In fact, a complete SOD1 knockout model does not produce ALS (10). Given that the majority of SOD1 mutations do not result in a loss of SOD1 catalytic activity and that complete loss of SOD1 activity does not result in ALS, the mutated enzyme is proposed to acquire an unknown toxic property. In addition to this toxic gain-of-function mechanism associated with SOD1 mutations, additional gene mutations have also been identified, and numerous other mechanisms are proposed to explain the loss of MN in ALS (Table I). One popular idea centers on glutamate-induced neurotoxicity. Increased concentrations of glutamate in cerebrospinal fluid of ALS patients (11) are associated with loss of spinal MN (12–14), and glutamate treatment is linked to MN cell death in vitro (15,16). The only drug currently available for the treatment of ALS is riluzole, a compound that blocks the release of glutamate from glial cells (17). Riluzole treatment, however, only extends survival in ALS patients for a few months at best. Another proposed mechanism is altered neurotrophism, which has prompted a wide range of studies on whether growth factors such as vascular endothelial growth factor (VEGF) and insulin-like growth factor-I (IGF-I) could be potential treatments for ALS. IGF-I offers much promise: it is neuroprotective, blocks oxidative stress and programmed cell death, and has proven safe in clinical trials (18,19).
Table I.
Proposed ALS disease mechanisms.
| Mechanisms | Reference |
|---|---|
| Altered neurotrophism (growth factors) | (80) |
| Glutamate toxicity | (81,82) |
| Autoimmune response/inflammation | (83,84) |
| Neurofilament aggregation | (85,86) |
| Mitochondrial impairment | (87,88) |
| Oxidative stress/free radicals | (89,90) |
| Genetic mutations: | (91393) |
| Cu/Zn superoxide dismutase | (94) |
| Alsin | (95,96) |
| Dynactin | (97,98) |
| Angiogenin | (99) |
| Senataxin | (100) |
| VAPB | (101) |
Insulin-like growth factor-I
IGF-I is a neurotrophic factor that exerts multiple actions within the peripheral and central nervous systems (20). Numerous in vitro and in vivo studies (discussed in further detail in the upcoming sections of this review) demonstrate that IGF-I promotes MN survival and has the potential to become a valuable therapy for ALS. IGF-I is used therapeutically in disorders ranging from growth deficiency to diabetes due to its well-established role in growth and metabolism, and interest in its potential use in neurodegenerative disorders like ALS continues to grow. An understanding of the IGF-I system in neurons, including the regulation of IGF-I availability and signaling mechanisms initiated by IGF-I activation of the IGF-I receptor (IGF-IR), is an important starting point for discussing the therapeutic potential of IGF-I in ALS. The IGF-I system includes three structurally related ligands (IGF-I, IGF-II and insulin), their respective receptors, and at least six IGF-I binding proteins (IGFBP) (21). The majority of circulating IGF-I is bound and sequestered by IGFBPs, thereby extending the half-life of IGF-I, regulating its distribution, and controlling its bioavailability in various tissues.
IGF-I functions are mediated via the IGF-IR. The IGF-IR is a hetero-tetramer consisting of two extracellular α-subunits which contain the ligand binding site and two transmembrane β-subunits which have tyrosine kinase activity upon ligand binding and catalyze the auto-phosphorylation of tyrosine residues on the intracellular domain of the β-subunit (22). Auto-phosphorylation of the receptor causes recruitment of the adaptor molecules insulin receptor substrate 1 and 2 (IRS1 and IRS2) (23). Upon phosphorylation, the IRS proteins activate signaling pathways including the PI3K/Akt and p44/42 MAPK pathways (23,24). Additional pathways linked to IGF-I signaling include the JNK, p38 MAPK, and mTOR signaling pathways (25–28). Signaling pathways activated by IGF-I result in a wide range of cellular effects including cellular proliferation, differentiation and inhibition of apoptosis (29). More details on the IGF-I system are available in additional review articles (30–32).
Insulin-like growth factor-I in ALS
The neuroprotective properties of IGF-I have been addressed in many models of neuronal degeneration. Studies from our laboratory have repeatedly exhibited beneficial effects of IGF-I in neuronal cell types including human neuroblastoma cells, dorsal root ganglion cells and MN (16,32–35). IGF-I has been studied in numerous contexts of ALS (Figure 1). Studies in cell lines and animal models offer a great deal of insight into the potential of IGF-I as a treatment for ALS. Furthermore, understanding the status of the endogenous IGF-I system in ALS patients is important to predicting the therapeutic value of IGF-I in ALS. Clinical trials, although few and with conflicting results, also provide evidence towards IGF-I efficacy. The results and interpretations of these studies and approaches are discussed below.
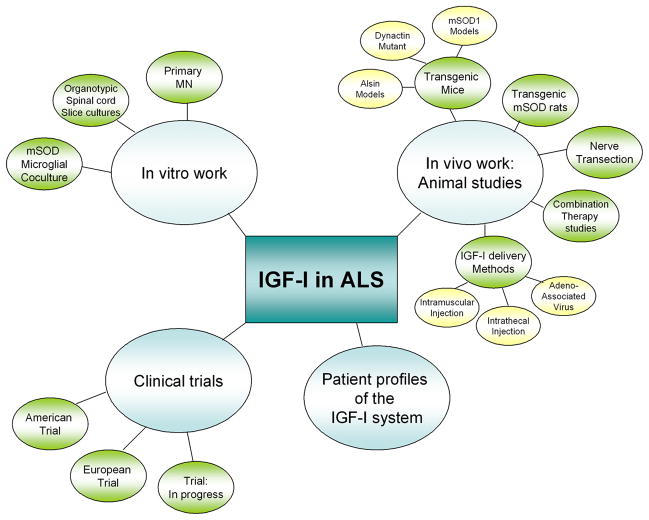
Figure 1.
Mutliple facets of research on IGF-I efficacy for ALS. Numerous approaches have been taken in an attempt to understand the mechanisms and therapeutic efficacy of IGF-I in ALS. Studies range from in vitro and in vivo studies to patient and clinical trials have all contributed to our understanding of how IGF-I functions in the cells affected by ALS and have offered insight into the clinical potential of IGF-I for ALS therapy. Results at these multiple levels will be discussed in this review.
In vitro studies
In vitro studies have characterized the neuroprotective properties of IGF-I in models of ALS. One such model system is primary embryonic rat spinal MN, which express IGF-IR (Figure 2) and respond to exogenous IGF-I treatment. Studies using primary MN cultures demonstrate that IGF-I prevents glutamate-induced caspase-3 cleavage, DNA fragmentation and cell death (16). The window for the protective effects of IGF-I in these studies was small following exposure to glutamate and implies that the effects of IGF-I might impact early events in the activation of cell death in this model system (16). These studies further demonstrate that activation of the PI3K/Akt and p44/42 MAPK signaling pathways are instrumental in the protective effects of IGF-I, as determined utilizing pathway-specific small molecule inhibitors (16). These signaling pathways are also correlated with enhanced axon outgrowth in postnatal corticospinal MN (CSMN), which are subject to degeneration in ALS (36). These studies confirm that IGF-I leads to activation of the PI3K/Akt and p44/42 MAPK signaling pathways in CSMN and indicate that blockade of these pathways results in defects in axonal outgrowth. Adenoviral-associated viral (AAV)-mediated expression of IGF-I in SH-SY5Y neurons and primary MN also causes significant protection against glutamate-induced toxicity (37). Strikingly, neighboring neurons without AAV-IGF-I expression were also protected against glutamate-induced toxicity, indicating that this delivery method produces biologically active IGF-I which is released from transfected cells. Taken together, these data indicate that IGF-I is protective to MN, activates signaling pathways associated with cell survival, and promotes axonal outgrowth.
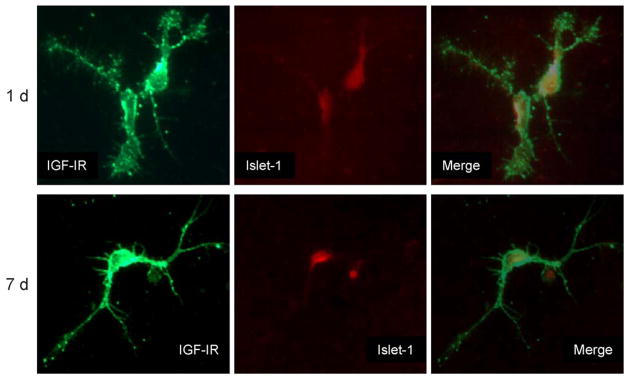
Figure 2.
IGF-I receptor expression in primary motor neurons. Purified MN in culture express IGF-IR. After one or seven days in culture, MN were fixed and double-stained for IGF-IR and the MN markers SMI-32 or islet-1. The figure shows representative MN identified by the islet-1 marker. The axons appear shorter and less mature at one day than seven days and there was no change in IGF-IR expression or islet-1 over time. MN were double-labeled for islet-1 in the cell body and IGF-IR throughout the cell and notably in punctate sites on the axons. After seven days, most of the punctate localizations of IGF-IR are at the ends of the axons. Using two MN markers (SMI-32 and islet-1) also demonstrated colocalization in the same cells (not shown). (Reproduced with permission from (16)).
Research utilizing organotypic spinal cord cultures also demonstrates the impact of growth factors, including IGF-I, on MN viability. These cultures are taken from postnatal rats and more closely mimic in vivo environments as they contain both MN and glial components. Thus, the contributions of glia to glutamate metabolism and excitotoxicity can be studied in organotypic spinal cord cultures taken from animal models of ALS. Measurement of choline acetyltransferase (ChAT) enzyme activity in these cultures demonstrates a neurotrophic effect for IGF-I (38,39). ChAT activity reflects MN viability and neurotrophism, specifically representing the degree of muscle innervation and acetylcholine neurotransmitter formation in MN. In these organotypic spinal cord cultures, IGF-I is capable of promoting neuroprotection against glutamate toxicity induced by treatment with a glutamate transport inhibitor. Studies utilizing cocultures of both MN and glia expressing wild-type or G93A-SOD1 addressed the contribution of glia to ALS in further detail and offer further support for the therapeutic potential of IGF-I for ALS (40,41). Activation of microglia expressing G93A-SOD1 with lipopolysaccharide leads to increased production of reactive oxygen species including nitric oxide and super-oxide, as well as decreased production of IGF-I from microglia (40,41). Coculturing MN with G93A-SOD1 microglia results in abnormal MN morphology and increased MN death compared to MN cocultured with wild-type microglia. Further studies showed that the increased production of reactive species by G93A-SOD1 microglia is suppressed by treatment with interleukin-4, which increases free IGF-I production from microglia in the absence of lipopolysaccharide (40). These in vitro studies strongly support IGF-I as a potentially efficacious therapy for ALS.
In vivo studies
IGF-I in models of MN injury and disease
IGF-I is important for the maintenance and survival of MN in the spinal cord, and in vivo studies in various MN disease and injury models suggest it has therapeutic efficacy. The mutant wobbler mouse is an established model of lower MN degeneration. Daily subcutaneous IGF-I administration in these mice shows benefits in functional behavioral tests such as grip strength and skeletal muscle fiber diameter; however, no changes were seen in MN number or ChAT activity (42). The efficacy of IGF-I therapy was studied in rats following sciatic nerve transection (43). This model results in reductions in spinal cord MN numbers, possibly due to loss of neurotrophic support and/or overactivity of excitatory amino acids like glutamate. Intraperitoneal administration of both IGF-I and riluzole following nerve transection results in increased MN survival over that of either compound alone (43). These studies all demonstrate positive therapeutic effects of IGF-I in models of MN disease and injury; however, further studies are required to understand the mechanisms underlying these observations.
Studies of the IGF-I system in transgenic animal models that express G93A-SOD1 also provide convincing support for the utilization of IGF-I as a therapeutic factor for ALS. Studies of human patients demonstrate alterations in serum IGF-I levels (44), whereas levels in the spinal cord appeared to be normal (45); these observations led to the question of whether alterations might exist in the levels of IGF-I signaling system components in the CNS, a question addressed in transgenic animals. Immunohistochemical studies in G93A-SOD1 transgenic mice demonstrate increased levels of IGF-IR in reactive astrocytes and this up-regulation of receptor levels in the spinal cords could reflect a compensatory mechanism in response to reduced trophic support (46). Decreased levels of free IGF-I caused by up-regulation of IGFBPs in mouse spinal cord further support the premise of a compensatory up-regulation of IGF-IR levels (47); similarly, alterations in IGFBP levels are reported in ALS patients (48). These studies in transgenic mice reveal the importance of understanding alterations in IGF-IR and IGFBP levels as they have the potential to regulate the availability and action of both endogenous and therapeutically administered IGF-I.
The status of the IGF-I system is important to consider in the interpretation of some in vivo studies using transgenic G93A-SOD1 models. One group crossed transgenic mice overexpressing human IGF-I with G93A-SOD1 transgenic mice in an effort to see if increased endogenous expression of IGF-I could counter the onset and progression of ALS. No differences were observed in disease symptoms, MN number, or survival in the double transgenic mice (49). The lack of therapeutic benefit observed in this study may be the result of the multiple effects of systemic IGF-I production on other tissues, regulation of IGF-I expression during development, or ultimately changes in bioavailability caused by alterations in the expression of IGFBPs and IGF-IR levels resulting from chronic overexpression of IGF-I. Others demonstrate that IGF-I may play a role in MN survival. Two studies using neural stem cells demonstrate positive effects on MN survival, disease progression and lifespan (50,51). Upon implantation, the stem cells differentiated into MN and provided increased levels of VEGF and IGF-I, as determined by ELISA of both the cell culture media prior to implantation and in the spinal cords after integration (50). Transgenic animals that received stem cells also showed decreases in compensatory increased levels of IGF-IR and IGFBP-5 compared to untreated transgenic animals, implying an increase in free IGF-I derived from the transplanted cells (50). While the double-transgenic mice overexpressing IGF-I did not show a benefit for IGF-I on MN or mouse survival, the grafting of neural stem cells into the spinal cords of adult G93A-SOD1 transgenic mice and the associated effects on the IGF-I system did have a significant impact on survival (50). This again emphasizes the importance of understanding the IGF-I system as a whole, in both animal models and ultimately human studies, since regulation of IGF-I bioavailability will impact its ability to offer neurotrophic support.
IGF-I delivery mechanisms
The delivery mechanism of IGF-I is another important factor. As mentioned earlier, subcutaneous administration of IGF-I to the wobbler mouse resulted in modest beneficial effects (52) and our laboratory demonstrates no significant effects of subcutaneous IGF-I injection on G93A-SOD1 transgenic mouse survival (unpublished observations). Additional approaches for delivering IGF-I to MN in G93A-SOD1 mouse models, however, have also been examined and show promising results. Intrathecal injection of IGF-I increases motor performance, delays onset of disease, and increases survival of G93A-SOD1 mice (53,54). Additionally, intrathecal IGF-I increases the levels of phosphorylated Akt and 44/42 MAPK in the MN of these animals (53). These findings are associated with increased MN survival and are consistent with in vitro studies mentioned earlier linking activation of the PI3K/Akt and p44/42 MAPK signaling pathways by IGF-I to MN survival. Continuous intrathecal administration of IGF-I also decreased levels of IGF-IR and IRS-1, which are up-regulated in the transgenic mice compared to control mice (54). These two reports on intrathecal administration of IGF-I demonstrate activation of survival-related PI3K/Akt and p44/42 MAPK signaling pathways and decreased IGF-IR and IRS-1, which are all correlated with MN survival (53,54).
Another potential delivery mechanism for IGF-I studied in transgenic animals is the use of gene therapy and AAV vectors. These vectors are injected into skeletal muscle and taken to the spinal cord MN via retrograde axonal transport, and afford sustained gene delivery (55,56). Production of IGF-I in the MN cell bodies following AAV-IGF-I transport results in increased survival when injected either before disease onset or at the time of symptom onset. This result is promising given that there is no way to identify ALS patients prior to symptom presentation, and any ALS therapy needs to be efficacious following disease onset (57,58). Distal delivery of an IGF-I vector, transport to the MN cell bodies and ultimately production of IGF-I may offer neurotrophic support to the MN and surrounding cells in the spinal cord, and possibly alter the production of toxic compounds from surrounding cells, such as inflammatory cytokines and glutamate. As mentioned earlier, in vitro studies on primary MN demonstrate significant protection against glutamate-induced toxicity in non-transfected neighboring cells, confirming the potential of AAV-IGF-I constructs to produce secretable, biologically active IGF-I (37). Thus, AAV delivery of IGF-I is a promising avenue for future ALS therapeutic studies.
Positive results are reported in G93A-SOD1 mice with muscle-specific expression of an IGF-I isoform (59). While these studies were performed in transgenic mice, they have the potential for translation into clinically relevant delivery through the utilization of AAV vectors and/or IGF-I splice variants. IGF-I undergoes tissue-specific regulation of its expression and processing in vivo (60–62) and the transgenic mice used in these studies express an alternatively spliced isoform of IGF-I (mIGF-I) that has lower affinity for IGFBPs and does not enter the circulation (63). mIGF-I expression is driven by skeletal muscle-specific myosin-light chain elements (MLC/mIGF-I), resulting in muscle-specific IGF-I overexpression (63,64). In double transgenic MLC/mIGF-I/G93A-SOD1 mice, expression stabilized neuromuscular junctions, decreased muscular atrophy and astrocytosis in the spinal cord, and extended MN survival compared to G93A-SOD1 littermates (59). Thus, these effects of IGF-I in muscle are linked with delaying the onset and progression of the disease in the mice and demonstrate the potential therapeutic efficacy of IGF-I alternative isoforms. Overall, the majority of in vivo studies in models of MN injury using different delivery mechanisms of IGF-I therapy give credence to the therapeutic potential of IGF-I in the treatment of ALS.
ALS patient studies
A number of studies have examined potential changes in the components of the IGF-I system in ALS patients, often with conflicting results. Studies report normal circulating IGF-I levels (65–67), decreased circulating IGF-I levels (44), or increased epidermal and circulating IGF-I expression in ALS patients (68,69). Interestingly, Hosback et al. (69) found that longer-surviving ALS patients had a 36% increase in peripheral IGF-I compared to healthy matched controls. A few studies have examined circulating IGFBP levels in ALS patients as well. Hosback et al. (69) reported a 34% decrease in IGFBP-1 that drops to 58% in long-surviving patients. On the other hand, another study found significant increases in three of the circulating IGFBPs – IGFBP-2, 5 and 6 (48). These inconsistent results demonstrate the difficulty in definitively characterizing the peripheral status of the IGF-I signaling system in ALS patients.
Analyses of IGF-I signaling components in the central nervous system present a more consistent picture. Several studies report increased IGF-IR expression in the ventral horn of the spinal cord, along with increased ability to bind I125-IGF-I (70–72). IGF-I immunoreactivity was not found to be different in the spinal cords of ALS patients compared to healthy controls (45), nor was total IGF-I altered in ventral horn homogenates from ALS patients. However, free IGF-I was 53% lower in ALS patients compared to healthy controls. This coincided with profound increases in IGFBP-1, -3, and -4 levels (64%, 46%, and 33%, respectively.) While IGF-I immunoreactivity may not be altered in ALS patients, IGF-I, insulin, and to a lesser extent growth hormone levels were reduced in the cerebrospinal fluid of ALS patients. Thus, in the ALS spinal cord it appears that there is a deficit in bioavailable IGF-I (potentially through excess IGFBP production) that may be driving compensatory up-regulation of the IGF-IR. A recent study found that some ALS patients have reactive microglia positive for IGF-II staining, suggesting that glia may further attempt to compensate for this neurotrophically deficient environment (73). While it is unknown how these changes in the IGF system relate to the pathophysiology of ALS, these findings do suggest a significant lack of available neurotrophic IGF-I to MN.
Clinical trials
The small ALS patient population and the limited resources available at specialized medical centers that care for ALS patients severely limit the number of new therapies that may be evaluated in the clinic (74). Despite this, and based on the strength of the pre-clinical evidence, two randomized double-blind placebo-controlled phase III trials examining the efficacy of subcutaneous recombinant human IGF-I (rhIGF-I) in the treatment of ALS have been completed (75,76); however, the results of these two trials conflict. A study of 266 patients treated in the United States found that a nine-month course of 0.10 mg/kg/day rhIGF-I led to a 26% deceleration in functional impairment compared to placebo-treated controls (p=0.01) (76). A similar study in 183 European patients found no significant difference among treatment groups (75). Both studies used similar endpoints (disease progression assessed by the Appel ALS rating scale) and found that rhIGF-I was safe and well-tolerated. A meta-analysis combining data from both studies found a significant benefit to high-dose rhIGF-I treatment in the primary outcome measurement (Appel ALS rating), but only trends towards therapeutic benefit in other secondary measurements (AALSRS scores, quality of life, survival and adverse events) (77). No study to date assessed survival as a primary endpoint. Given apparent safety and trends towards therapeutic efficacy, a third trial is underway to hopefully clarify the utility of rhIGF-I therapy in ALS.
As mentioned previously, subcutaneous administration of IGF-I to wobbler mice and transgenic ALS mice failed to demonstrate convincing effects on survival ((52) and unpublished results); therefore, alternative delivery mechanisms may enhance the efficacy of IGF-I. IGF-I is a peptide and presents obstacles in terms of delivery convenience, duration of effect, and access to tissues beyond the blood-brain barrier. Inactivation of IGF-I by sequestration with IGFBPs and limited delivery directly to MNs are also confounding factors in IGF-I efficacy. A small study in Japan examined the effects of intrathecal IGF-I injection on nine ALS patients (78). Patients received either high (3 μg/kg) or low (0.5 μg/kg) IGF-I every two weeks for 40 weeks. High-dose intrathecal IGF-I significantly decreased total deterioration and limb motor function as measured by the Norris scale, but did not affect bulbar motor function or vital capacity. This low-powered study suggests that IGF-I has therapeutic benefits in ALS when administered intrathecally, and further suggests these effects are apparent even when high doses of IGF-I are administered relatively infrequently.
The use of IGF-I variants with lower affinity for IGFBPs and/or gene therapy has the potential to build upon the moderate effects of subcutaneous and intrathecal IGF-I administration by increasing the concentration of bioavailable IGF-I or providing targeted delivery directly to MNs (55,56,60–62,79). Animal studies show that adenoviral administration of IGF-I is efficacious. Ideally, the practical difficulties associated with administration of recombinant IGF-I protein would be overcome if AAV-IGF-I had the same effects in humans as it does in animals. Injection of the construct distally into muscle could lead to its retrograde transport to the spinal cord, where infected MN could produce a paracrine IGF-I supply that activates signaling in neighboring uninfected cells. This could then result in comparatively long-lasting and continuous IGF-I-driven neuro-trophism. AAV-delivered factor IX has proven safe in hemophiliacs; however, the safety, durability, and efficacy of AAV-IGF-I has not been tested in humans, although trials are in development for ALS (74).
Conclusions and future directions
Despite the fact that the exact mechanism(s) causing ALS remain unknown, data support the therapeutic use of IGF-I. In vitro studies link IGF-I-induced signaling to cell survival and axonal outgrowth in MN, and demonstrate the ability of IGF-I to protect against glutamate-induced caspase-3 cleavage and cell death. In vitro research also reveals the contribution of glia and other supporting cells in the pathogenesis of ALS and the effects of IGF-I on these cells. Animal studies in models of MN injury and disease have shown that IGF-I has positive effects on nerve function, MN survival, disease onset and progression, and lifespan. In vivo studies are instrumental in comparing various delivery mechanisms and understanding the factors that impact IGF-I bioavailability in the spinal cord. Alterations in IGFBP expression in the spinal cords of MN disease models and patients may diminish the bioavailability of both endogenous and therapeutic IGF-I and explain altered expression of IGF-I signaling components in the glia of ALS spinal cords. AAV-mediated delivery offers the greatest potential for directed administration and production of IGF-I at the level of the MN. Secreted IGF-I affects MN and surrounding glia in the spinal cord, and further increases neuromuscular junction stability. ALS patient studies of the profile of the IGF-I signaling system also substantiate the use of IGF-I for intervention. Clinical trials have had mixed success and highlight the need to consider innovative delivery mechanisms. Results suggest that intrathecal administration of IGF-I has clinical significance, and with the development of better delivery, the therapeutic efficacy of IGF-I may be further enhanced. Overall, the studies presented here establish that IGF-I impacts all components of ALS from MN to supporting glia to muscular targets (Figure 3). Further clinical exploration of delivery mechanisms that have been successful in animal studies will be important for validating the utility of IGF-I as a therapy for ALS.
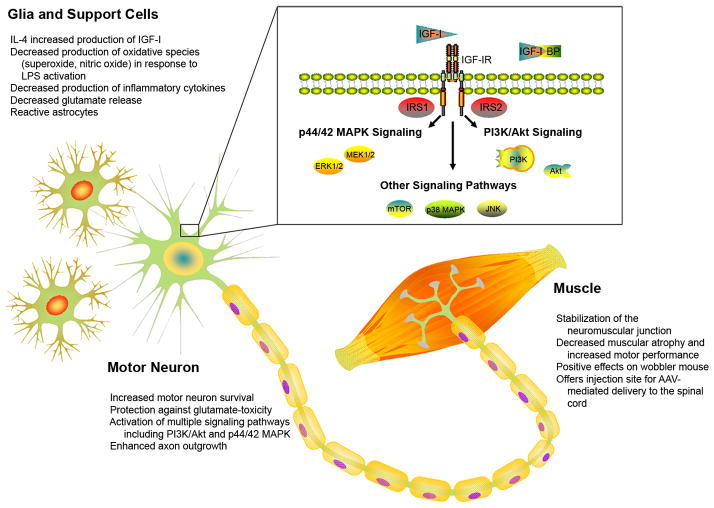
Figure 3.
Sites and mechanisms of IGF-I action in ALS. IGF-I can impact various aspects of MN degeneration and survival in ALS. IGF-I effects are mediated in MN, muscle and glia via activation of the IGF-IR, subsequent activation of IRS, and downstream activation of major survival-related pathways including PI3K/Akt and p44/42 MAPK signaling pathways (as represented in the MN cell body). Activation of these pathways can function in an autocrine manner directly promoting the survival of the MN themselves, or act in a paracrine fashion through its effects on support cells by mediating the release of toxic or inflammatory molecules, or on muscle fibers functioning to aid in maintenance of neuromuscular junctions and ultimately MN survival. One, or likely all, of these effects could be key players in the role of IGF-I as an efficacious therapy for ALS.
Acknowledgments
The authors would like to thank the ALS Association, the A. Alfred Taubman Medical Research Institute, and the Program for Neurology Research and Discovery for supporting our research in ALS. SS is supported by NIH T32 NS007222-26. The authors would also like to thank Ms. Julie Erwin for excellent secretarial support during the preparation of this manuscript.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Dunckley T, Huentelman MJ, Craig DW, Pearson JV, Szelinger S, Joshipura K, et al. Whole-genome analysis of sporadic amyotrophic lateral sclerosis. The New England Journal of Medicine. 2007;357:775–88. doi: 10.1056/NEJMoa070174. [DOI] [PubMed] [Google Scholar]
- 2.Valentine JS, Doucette PA, Potter SZ. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annual Review of Biochemistry. 2005;74:563–93. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 3.Valentine JS, Hart PJ. Misfolded Cu/Zn SOD and amyotrophic lateral sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3617–22. doi: 10.1073/pnas.0730423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 5.Ratovitski T, Corson LB, Strain J, Wong P, Cleveland DW, Culotta VC, et al. Variation in the biochemical/biophysical properties of mutant superoxide dismutase 1 enzymes and the rate of disease progression in familial amyotrophic lateral sclerosis kindreds. Human Molecular Genetics. 1999;8:1451–60. doi: 10.1093/hmg/8.8.1451. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, et al. Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Human Molecular Genetics. 2003;12:2753–64. doi: 10.1093/hmg/ddg312. [DOI] [PubMed] [Google Scholar]
- 7.Cudkowicz ME, McKenna-Yasek D, Sapp PE, Chin W, Geller B, Hayden DL, et al. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Annals of Neurology. 1997;41:210–21. doi: 10.1002/ana.410410212. [DOI] [PubMed] [Google Scholar]
- 8.Gurney ME, Pu H, Chiu AY, Canto MCD, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu/Zn superoxide dismutase mutation. SCIENCE. 1994;264:1772–5. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 9.Dal Canto MC, Gurney ME. A low expressor line of transgenic mice carrying a mutant human Cu/Zn superoxide dismutase (SOD1) gene develops pathological changes that most closely resemble those in human amyotrophic lateral sclerosis. Acta Neuropathologica. 1997;93:537–50. doi: 10.1007/s004010050650. [DOI] [PubMed] [Google Scholar]
- 10.Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nature Genetics. 1996;13:43–7. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 11.Spreux-Varoquaux O, Bensimon G, Lacomblez L, Salachas F, Pradat PF, Le Forestier N, et al. Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. J Neurol Sci JID-0375403. 2002;193:73–8. doi: 10.1016/s0022-510x(01)00661-x. [DOI] [PubMed] [Google Scholar]
- 12.Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci JID-8102140. 1996;16:4069–79. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schubert D, Piasecki D. Oxidative glutamate toxicity can be a component of the excitotoxicity cascade. Journal of Neuroscience. 2001;21:7455–62. doi: 10.1523/JNEUROSCI.21-19-07455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarabal O, Caldero J, Llado J, Oppenheim RW, Esquerda JE. Long-lasting aberrant tubulovesicular membrane inclusions accumulate in developing motor neurons after a sublethal excitotoxic insult: a possible model for neuronal pathology in neurodegenerative disease. Journal of Neuroscience. 2001;21:8072–81. doi: 10.1523/JNEUROSCI.21-20-08072.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilak MM, Kuncl RW. Delayed application of IGF-I and GDNF can rescue already injured postnatal motor neurons. Neuroreport. 2001;12:2531–5. doi: 10.1097/00001756-200108080-00048. [DOI] [PubMed] [Google Scholar]
- 16.Vincent AM, Mobley BC, Hiller A, Feldman EL. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiology of Disease. 2004;16:407–16. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Bensimon G, Lacomblez L, Meininger V, Group ALRS. A controlled trial of riluzole in amyotrophic lateral sclerosis. New England Journal of Medicine. 1994;330:585–91. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 18.Feldman EL, Sullivan KA, Kim B, Russell JW. Insulin-like growth factors regulate neuronal differentiation and survival. Neurobiology of Disease. 1997;4:201–14. doi: 10.1006/nbdi.1997.0156. [DOI] [PubMed] [Google Scholar]
- 19.Vincent AM, Feldman EL, Song DK, Jung V, Schild A, Zhang W, et al. Adeno-associated viral-mediated insulin-like growth factor delivery protects motor neurons in vitro. Neuro Molecular Medicine. 2004;6:79–85. doi: 10.1385/NMM:6:2-3:079. [DOI] [PubMed] [Google Scholar]
- 20.Dore S, Kar S, Quirion R. Rediscovering an old friend, IGF-I: potential use in the treatment of neurodegenerative diseases. Trends in Neurosciences. 1997;20:326–31. doi: 10.1016/s0166-2236(96)01036-3. [DOI] [PubMed] [Google Scholar]
- 21.Duan C. Specifying the cellular responses to IGF signals: roles of IGF-binding proteins. The Journal of Endocrinology. 2002;175:41–54. doi: 10.1677/joe.0.1750041. [DOI] [PubMed] [Google Scholar]
- 22.LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocrine Reviews. 1995;16:143–63. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 23.De Meyts P, Wallach B, Christoffersen CT, Urso B, Gronskov K, Latus LJ, et al. The insulin-like growth factor-I receptor. Structure, ligand-binding mechanism and signal transduction. Hormone Research. 1994;42:152–69. doi: 10.1159/000184188. [DOI] [PubMed] [Google Scholar]
- 24.Parrizas M, Saltiel AR, LeRoith D. Insulin-like growth factor-I inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. The Journal of Biological Chemistry. 1997;272:154–61. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 25.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–37. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 26.Floyd S, Favre C, Lasorsa FM, Leahy M, Trigiante G, Stroebel P, et al. The insulin-like growth factor-I-mTOR signaling pathway induces the mitochondrial pyrimidine nucleotide carrier to promote cell growth. Molecular Biology of the Cell. 2007;18:3545–55. doi: 10.1091/mbc.E06-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science (New York, NY) 1994;265:808–11. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 28.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llama-zares A, Zamanillo D, et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–37. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 29.Siddle K, Urso B, Niesler CA, Cope DL, Molina L, Surinya KH, et al. Specificity in ligand binding and intracellular signaling by insulin and insulin-like growth factor receptors. Biochemical Society Transactions. 2001;29:513–25. doi: 10.1042/bst0290513. [DOI] [PubMed] [Google Scholar]
- 30.Kurmasheva RT, Houghton PJ. IGF-I mediated survival pathways in normal and malignant cells. Biochimica et Biophysica Acta. 2006;1766:1–22. doi: 10.1016/j.bbcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des. 2007;13:663–9. doi: 10.2174/138161207780249146. [DOI] [PubMed] [Google Scholar]
- 32.Feldman EL, Sullivan KA, Kim B, Russell JW. Insulin-like growth factors regulate neuronal differentiation and survival. Neurobiology of Disease. 1997;4:201–14. doi: 10.1006/nbdi.1997.0156. [DOI] [PubMed] [Google Scholar]
- 33.Leinninger GM, Backus C, Uhler MD, Lentz SI, Feldman EL. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J. 2004;18:1544–6. doi: 10.1096/fj.04-1581fje. [DOI] [PubMed] [Google Scholar]
- 34.Leinninger GM, Russell JW, van Golen CM, Berent A, Feldman EL. Insulin-like growth factor-I (IGF-I) regulates glucose-induced mitochondrial depolarization and apoptosis in human neuroblastoma. Cell Death and Differentiation. 2004;11:885–96. doi: 10.1038/sj.cdd.4401429. [DOI] [PubMed] [Google Scholar]
- 35.Vincent AM, Feldman EL. Control of cell survival by IGF signaling pathways. Growth Hormone and IGF Research. 2002;12:193–7. doi: 10.1016/s1096-6374(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 36.Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nature Neuroscience. 2006;9:1371–81. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- 37.Vincent AM, Feldman EL, Song DK, Jung V, Schild A, Zhang W, et al. Adeno-associated viral-mediated insulin-like growth factor delivery protects motor neurons in vitro. Neuromolecular Medicine. 2004;6:79–86. doi: 10.1385/NMM:6:2-3:079. [DOI] [PubMed] [Google Scholar]
- 38.Bilak MM, Corse AM, Kuncl RW. Additivity and potentiation of IGF-I and GDNF in the complete rescue of postnatal motor neurons. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2:83–91. doi: 10.1080/146608201316949523. [DOI] [PubMed] [Google Scholar]
- 39.Corse AM, Bilak MM, Bilak SR, Lehar M, Rothstein JD, Kuncl RW. Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiology of Disease. 1999;6:335–46. doi: 10.1006/nbdi.1999.0253. [DOI] [PubMed] [Google Scholar]
- 40.Zhao W, Xie W, Xiao Q, Beers DR, Appel SH. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motor neuron toxicity induced by activated microglia. Journal of Neurochemistry. 2006;99:1176–87. doi: 10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]
- 41.Xiao Q, Zhao W, Beers DR, Yen AA, Xie W, Henkel JS, et al. Mutant SOD1-G93A microglia are more neurotoxic relative to wild-type microglia. Journal of Neurochemistry. 2007;102:2008–19. doi: 10.1111/j.1471-4159.2007.04677.x. [DOI] [PubMed] [Google Scholar]
- 42.Hantai D, Akaaboune M, Lagord C, Murawsky M, Houenou LJ, Festoff BW, et al. Beneficial effects of insulin-like growth factor-I on wobbler mouse motor neuron disease. Journal of the Neurological Sciences. 1995;129:122–6. doi: 10.1016/0022-510x(95)00081-c. [DOI] [PubMed] [Google Scholar]
- 43.Iwasaki Y, Ikeda K. Prevention by insulin-like growth factorI and riluzole in motor neuron death after neonatal axotomy. Journal of the Neurological Sciences. 1999;169:148–55. doi: 10.1016/s0022-510x(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 44.Torres-Aleman I, Barrios V, Berciano J. The peripheral insulin-like growth factor system in amyotrophic lateral sclerosis and in multiple sclerosis. Neurology. 1998;50:772–6. doi: 10.1212/wnl.50.3.772. [DOI] [PubMed] [Google Scholar]
- 45.Kerkhoff H, Hassan SM, Troost D, van Etten RW, Veldman H, Jennekens FGI. Insulin-like and fibroblast growth factors in spinal cords, nerve roots and skeletal muscle of human controls and patients with amyotrophic lateral sclerosis. Acta Neuropathologica. 1994;87:411–21. doi: 10.1007/BF00313611. [DOI] [PubMed] [Google Scholar]
- 46.Chung YH, Joo KM, Shin CM, Lee YJ, Shin DH, Lee KH, et al. Immunohistochemical study on the distribution of insulin-like growth factor-I (IGF-I) receptor in the central nervous system of SOD1(G93A) mutant transgenic mice. Brain Research. 2003;994:253–9. doi: 10.1016/j.brainres.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 47.Arnold PM, Ma JY, Citron BA, Zoubine MN, Festoff BW. Selective developmental regulation of gene expression for insulin-like growth factor binding proteins in mouse spinal cord. Spine. 2000;25:1765–70. doi: 10.1097/00007632-200007150-00005. [DOI] [PubMed] [Google Scholar]
- 48.Wilczak N, de Vos RA, de Keyser J. Free insulin-like growth factor (IGF)-I and IGF binding proteins 2, 5, and 6 in spinal motor neurons in amyotrophic lateral sclerosis. Lancet. 2003;361:1007–11. doi: 10.1016/S0140-6736(03)12828-0. [DOI] [PubMed] [Google Scholar]
- 49.Messi ML, Clark HM, Prevette DM, Oppenheim RW, Delbono O. The lack of effect of specific overexpression of IGF-I in the central nervous system or skeletal muscle on pathophysiology in the G93A SOD-1 mouse model of ALS. Exp Neurol. 2007;207:52–63. doi: 10.1016/j.expneurol.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corti S, Locatelli F, Papadimitriou D, Del Bo R, Nizzardo M, Nardini M, et al. Neural stem cells LewisX+ CXCR4+ modify disease progression in an amyotrophic lateral sclerosis model. Brain. 2007;130:1289–305. doi: 10.1093/brain/awm043. [DOI] [PubMed] [Google Scholar]
- 51.Yan J, Xu L, Welsh AM, Chen D, Hazel T, Johe K, et al. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells (Dayton, Ohio) 2006;24:1976–85. doi: 10.1634/stemcells.2005-0518. [DOI] [PubMed] [Google Scholar]
- 52.Hantai D, Akaaboune M, Lagord C, Murawsky M, Houenou LJ, Festoff BW, et al. Beneficial effects of insulin-like growth factor-I on wobbler mouse motor neuron disease. J Neurol Sci. 1995;129:122–6. doi: 10.1016/0022-510x(95)00081-c. [DOI] [PubMed] [Google Scholar]
- 53.Nagano I, Ilieva H, Shiote M, Murakami T, Yokoyama M, Shoji M, et al. Therapeutic benefit of intrathecal injection of insulin-like growth factor-I in a mouse model of amyotrophic lateral sclerosis. Journal of the Neurological Sciences. 2005;235:61–8. doi: 10.1016/j.jns.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Narai H, Nagano I, Ilieva H, Shiote M, Nagata T, Hayashi T, et al. Prevention of spinal motor neuron death by insulin-like growth factor-I associating with the signal transduction systems in SODG-93A transgenic mice. Journal of Neuroscience Research. 2005;82:452–7. doi: 10.1002/jnr.20668. [DOI] [PubMed] [Google Scholar]
- 55.Boulis NM, Willmarth NE, Song DK, Feldman EL, Imperiale MJ. Intraneural colchicine inhibition of adenoviral and adeno-associated viral vector remote spinal cord gene delivery. Neurosurgery. 2003;52:381–7. doi: 10.1227/01.neu.0000044459.24519.3e. [DOI] [PubMed] [Google Scholar]
- 56.Kaspar BK, Erickson D, Schaffer D, Hinh L, Gage FH, Peterson DA. Targeted retrograde gene delivery for neuronal protection. Mol Ther. 2002;5:50–6. doi: 10.1006/mthe.2001.0520. [DOI] [PubMed] [Google Scholar]
- 57.Boillee S, Cleveland DW. Gene therapy for ALS delivers. Trends in Neurosciences. 2004;27:235–8. doi: 10.1016/j.tins.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 58.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-I prolongs survival in a mouse ALS model. Science (New York, NY) 2003;301:839–42. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 59.Dobrowolny G, Giacinti C, Pelosi L, Nicoletti C, Winn N, Barberi L, et al. Muscle expression of a local IGF-I isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168:193–9. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gorecki DC, Beresewicz M, Zablocka B. Neuroprotective effects of short peptides derived from the insulin-like growth factor-I. Neurochemistry International. 2007;51:451–8. doi: 10.1016/j.neuint.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 61.Aperghis M, Johnson IP, Cannon J, Yang SY, Goldspink G. Different levels of neuroprotection by two insulin-like growth factor-I splice variants. Brain Res. 2004;29:213–8. doi: 10.1016/j.brainres.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 62.Ates K, Yang SY, Orrell RW, Sinanan AC, Simons P, Solomon A, et al. The IGF-I splice variant MGF increases progenitor cells in ALS, dystrophic, and normal muscle. FEBS Letters. 2007;581:2727–32. doi: 10.1016/j.febslet.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 63.Adamo ML, Neuenschwander S, LeRoith D, Roberts CT., Jr Structure, expression, and regulation of the IGF-I gene. Advances in Experimental Medicine and Biology. 1993;343:1–11. doi: 10.1007/978-1-4615-2988-0_1. [DOI] [PubMed] [Google Scholar]
- 64.Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, et al. Localized IGF-I transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nature Genetics. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 65.Braunstein GD, Reviczky AL. Serum insulin-like growth factor-I levels in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery and Psychiatry. 1987;50:792–4. doi: 10.1136/jnnp.50.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bilic E, Bilic E, Rudan I, Kusec V, Zurak N, Delimar D, et al. Comparison of the growth hormone, IGF-I, and insulin in cerebrospinal fluid and serum between patients with motor neuron disease and healthy controls. Eur J Neurol. 2006;13:1340–5. doi: 10.1111/j.1468-1331.2006.01503.x. [DOI] [PubMed] [Google Scholar]
- 67.Morselli LL, Bongioanni P, Genovesi M, Licitra R, Rossi B, Murri L, et al. Growth hormone secretion is impaired in amyotrophic lateral sclerosis. Clinical Endocrinology. 2006;65:385–8. doi: 10.1111/j.1365-2265.2006.02609.x. [DOI] [PubMed] [Google Scholar]
- 68.Ono S, Hu J, Imai T, Shimizu N, Tsumura M, Nakagawa H. Increased expression of insulin-like growth factor-I in skin in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2000;69:199–203. doi: 10.1136/jnnp.69.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hosback S, Hardiman O, Nolan CM, Doyle MA, Gorman G, Lynch C, et al. Circulating insulin-like growth factors and related binding proteins are selectively altered in amyotrophic lateral sclerosis and multiple sclerosis. Growth Horm IGF Res. 2007;17:472–9. doi: 10.1016/j.ghir.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Adem A, Ekblom J, Gillberg PG. Growth factor receptors in amyotrophic lateral sclerosis. Molecular Neurobiology. 1994;9:225–31. doi: 10.1007/BF02816121. [DOI] [PubMed] [Google Scholar]
- 71.Adem A, Ekblom J, Gillberg PG, Jossan SS, Höög A, Winblad B, et al. Insulin-like growth factor-I receptors in human spinal cord: changes in amyotrophic lateral sclerosis. J Neural Transm. 1994;97:73–84. doi: 10.1007/BF01277964. [DOI] [PubMed] [Google Scholar]
- 72.Dore S, Krieger C, Kar S, Quirion R. Distribution and levels of insulin-like growth factor (IGF-I and IGF-II) and insulin receptor binding sites in the spinal cords of amyotrophic lateral sclerosis (ALS) patients. Brain Res Mol Brain Res. 1996;41:128–33. doi: 10.1016/0169-328x(96)00081-2. [DOI] [PubMed] [Google Scholar]
- 73.Kihira T, Suzuki A, Kubo T, Miwa H, Kondo T. Expression of insulin-like growth factor-II and leukemia inhibitory factor antibody immunostaining on the ionized calcium-binding adaptor molecule 1-positive microglias in the spinal cord of amyotrophic lateral sclerosis patients. Neuropathology. 2007;27:257–68. doi: 10.1111/j.1440-1789.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 74.Traynor BJ, Bruijn L, Conwit R, Beal F, O’Neill G, Fagan SC, et al. Neuroprotective agents for clinical trials in ALS: a systematic assessment. Neurology. 2006;67:20–7. doi: 10.1212/01.wnl.0000223353.34006.54. [DOI] [PubMed] [Google Scholar]
- 75.Borasio GD, Robberecht W, Leigh PN, Emile J, Guiloff RJ, Jerusalem F, et al. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology. 1998;51:583–6. doi: 10.1212/wnl.51.2.583. [DOI] [PubMed] [Google Scholar]
- 76.Lai EC, Felice KJ, Festoff BW, Gawel MJ, Gelinas DF, Kratz R, et al. Effect of recombinant human insulin-like growth factor-I on progression of ALS. A placebo-controlled study. The North America ALS/IGF-I Study Group. Neurology. 1997;49:1621–30. doi: 10.1212/wnl.49.6.1621. [DOI] [PubMed] [Google Scholar]
- 77.Mitchell JD, Wokke JH, Borasio GD. Recombinant human insulin-like growth factor-I (rhIGF-I) for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2002:CD002064. doi: 10.1002/14651858.CD002064. [DOI] [PubMed] [Google Scholar]
- 78.Nagano I, Shiote M, Murakami T, Kamada H, Hamakawa Y, Matsubara E, et al. Beneficial effects of intrathecal IGF-I administration in patients with amyotrophic lateral sclerosis. Neurological Research. 2005;27:768–72. doi: 10.1179/016164105X39860. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka M, Sawada M, Miura M, Marunouchi T. Insulin-like growth factor-I analogue prevents apoptosis mediated through an interleukin-1 beta converting enzyme (caspase-1)-like protease of cerebellar external granular layer neurons: developmental stage-specific mechanisms of neuronal cell death. Neuroscience. 1998;84:89–100. doi: 10.1016/s0306-4522(97)00518-6. [DOI] [PubMed] [Google Scholar]
- 80.Ekestern E. Neurotrophic factors and amyotrophic lateral sclerosis. Neurodegenerative Diseases. 2004;1:88–100. doi: 10.1159/000080049. [DOI] [PubMed] [Google Scholar]
- 81.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science (New York, NY) 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 82.Rothstein JD, Tsai G, Kuncl RW. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Annals of Neurology. 1990;28:18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- 83.Appel SH, Smith RG, Engelhardt JI, Stefani E. Evidence for autoimmunity in amyotrophic lateral sclerosis. Journal of the Neurological Sciences. 1994;124 (Suppl):14–9. doi: 10.1016/0022-510x(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 84.Appel SH, Engelhardt JI, Garcia J, Stefani E. Autoimmunity and ALS: a comparison of animal models of immune-mediated motor neuron destruction and human ALS. Advances in Neurology. 1991;56:405–12. [PubMed] [Google Scholar]
- 85.Lin H, Zhai J, Canete-Soler R, Schlaepfer WW. 3′ untranslated region in a light neurofilament (NF-L) mRNA triggers aggregation of NF-L and mutant superoxide dismutase-1 proteins in neuronal cells. J Neurosci. 2004;24:2716–26. doi: 10.1523/JNEUROSCI.5689-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ge WW, Wen W, Strong W, Leystra-Lantz C, Strong MJ. Mutant copper-zinc superoxide dismutase binds to and destabilizes human low molecular weight neurofilament mRNA. The Journal of Biological Chemistry. 2005;280:118–24. doi: 10.1074/jbc.M405065200. [DOI] [PubMed] [Google Scholar]
- 87.Dupuis L, Gonzalez de Aguilar JL, Oudart H, de Tapia M, Barbeito L, Loeffler JP. Mitochondria in amyotrophic lateral sclerosis: a trigger and a target. Neurodegenerative Diseases. 2004;1:245–54. doi: 10.1159/000085063. [DOI] [PubMed] [Google Scholar]
- 88.Bacman SR, Bradley WG, Moraes CT. Mitochondrial involvement in amyotrophic lateral sclerosis: trigger or target? Molecular Neurobiology. 2006;33:113–31. doi: 10.1385/MN:33:2:113. [DOI] [PubMed] [Google Scholar]
- 89.Yim MB, Kang JH, Yim HS, Kwak HS, Chock PB, Stadtman ER. A gain-of-function of an amyotrophic lateral sclerosis-associated Cu/Zn-superoxide dismutase mutant: an enhancement of free radical formation due to a decrease in Km for hydrogen peroxide. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5709–14. doi: 10.1073/pnas.93.12.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu D, Bao F, Wen J, Liu J. Mutation of superoxide dismutase elevates reactive species: comparison of nitration and oxidation of proteins in different brain regions of transgenic mice with amyotrophic lateral sclerosis. Neuroscience. 2007;146:255–64. doi: 10.1016/j.neuroscience.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 91.Gonzalez de Aguilar JL, Echaniz-Laguna A, Fergani A, Rene F, Meininger V, Loeffler JP, et al. Amyotrophic lateral sclerosis: all roads lead to Rome. Journal of Neurochemistry. 2007;101:1153–60. doi: 10.1111/j.1471-4159.2006.04408.x. [DOI] [PubMed] [Google Scholar]
- 92.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nature Reviews. 2006;7:710–23. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 93.Valdmanis PN, Rouleau GA. Genetics of familial amyotrophic lateral sclerosis. Neurology. 2008;70:144–52. doi: 10.1212/01.wnl.0000296811.19811.db. [DOI] [PubMed] [Google Scholar]
- 94.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 95.Hadano S, Hand CK, Osuga H, Yanagisawa Y, Otomo A, Devon RS, et al. A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis. Nature Genetics. 2001;29:166–73. doi: 10.1038/ng1001-166. [DOI] [PubMed] [Google Scholar]
- 96.Yang Y, Hentati A, Deng HX, Dabbagh O, Sasaki T, Hirano M, et al. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nature Genetics. 2001;29:160–5. doi: 10.1038/ng1001-160. [DOI] [PubMed] [Google Scholar]
- 97.Munch C, Sedlmeier R, Meyer T, Homberg V, Sperfeld AD, Kurt A, et al. Point mutations of the p150 subunit of dynactin (DCTN1) gene in ALS. Neurology. 2004;63:724–6. doi: 10.1212/01.wnl.0000134608.83927.b1. [DOI] [PubMed] [Google Scholar]
- 98.Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, et al. Mutant dynactin in motor neuron disease. Nature Genetics. 2003;33:455–6. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 99.Greenway MJ, Andersen PM, Russ C, Ennis S, Cashman S, Donaghy C, et al. ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nature Genetics. 2006;38:411–3. doi: 10.1038/ng1742. [DOI] [PubMed] [Google Scholar]
- 100.Chen YZ, Bennett CL, Huynh HM, Blair IP, Puls I, Irobi J, et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4) American Journal of Human Genetics. 2004;74:1128–35. doi: 10.1086/421054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. American Journal of Human Genetics. 2004;75:822–31. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]