Abstract
Microfluidic devices allow for the controlled perfusion of human or mouse blood over defined prothrombotic surfaces at venous and arterial shear rates. To mimic in vivo injuries such a plaque rupture, the need exists to link lipidated tissue factor (TF) to surface bound collagen fibers. Recombinant TF was relipidated in liposomes of phosphatidylserine/phosphatidylcholine/biotin-linked phosphatidylethanolamine (20:79:1 PS:PC:bPE molar ratio). Collagen was patterned in a 250-micron wide stripe and labeled with biotinylated anti-collagen antibody which was then bound with streptavidin, allowing the subsequent capture of the TF liposomes. To verify and detect the TF liposome-collagen assembly, individual molecular complexes of TF-factor VIIa on collagen were visualized using the Proximity Ligation Assay (PLA) to produce discretely localized fluorescent events that were strictly dependent on the presence of factor VIIa and primary antibodies against TF or factor VIIa. Perfusion for 450 sec (wall shear rate, 200 s−1) of corn trypsin inhibitor (CTI, a factor XIIa inhibitor) treated whole blood over the stripe of TF-collagen enhanced platelet adhesion by 30 ± 8% (p < 0.001) and produced measurable fibrin (>50-fold increase) as compared to surfaces lacking TF. PS:PC:bPE liposomes lacking TF resulted in no enhancement of platelet deposition. Essentially no fibrin was formed during perfusion over collagen surfaces or collagen surfaces with liposomes lacking TF despite the robust platelet deposition, indicating a lack of kinetically significant platelet-borne tissue factor in healthy donor blood. This study demonstrates a reliable approach to link functionally-active TF to collagen for microfluidic thrombosis studies.
Keywords: Shear stress, hemodynamics, coagulation, tissue factor, Factor VIIa
Introduction
Tissue factor (TF) in the vessel wall is exposed during injury or atherosclerotic plaque rupture and binds circulating factor VIIa. As a cofactor in this complex, TF dramatically enhances the catalytic activity of factor VIIa toward factor X and factor IX. Generation of factor Xa results in formation of the prothrombinase complex (Xa/Va) to generate thrombin. Thrombin, a potent activator of platelets, is necessary for the production of clot stabilizing fibrin.
The incorporation of TF into growing thrombi has been suggested to occur through a variety of mechanisms. While the presence of TF in platelets and other non-activated blood cells in healthy blood has been an ongoing debate,1-3 several studies have shown the localization of TF-bearing microparticles to sites of injury via the P-selectin/PSGL1 interaction,4 and its synthesis by a variety of extra-cellular matrix cells in normal and atherosclerotic vessels.5 For decades, various TF preparations have been used as a clinical laboratory reagent in the study of prothrombin times, but TF has rarely been deployed in microfluidic injury models. For microfluidic thrombosis models, it is critical to present both collagen to platelets as well as lipidated TF to the plasma. By simultaneously activating and capturing platelets with surface bound collagen along with triggering of the coagulation protease cascade with surface-bound TF, the central biology of thrombosis and hemostasis is recreated. In contrast, addition of exogenous TF into blood absolutely fails to recreate the dynamical aspects of thrombosis reliant on the surface presentation of TF to flowing blood.
The activity of tissue factor relies on its insertion into a phospholipid membrane containing approximately 20% negatively charged phosphatidylserine.6 Reliable techniques using readily available reagents have been developed to “relipidate” TF into phospholipid vesicles or membranes.7 In vitro flow studies which include these designs are rare, however, and mainly focus on the dynamics of thrombin or fibrin production.8 In traditional microfluidic studies, the roles of platelet adhesion and coagulation are commonly separated through the use of heparin or Phe-Pro-Arg-chloromethylketone (PPACK, an irreversible thrombin inhibitor) or through the perfusion of platelet free plasma over a previously formed platelet surface.9,10 When studied in vivo in the mouse laser injury model, however, platelet deposition and thrombin production occur simultaneously.11 These models have been helpful in defining a role for thrombin in platelet adhesion and fibrin generation in platelet plug stabilization, however they lack characterization and control of the local hemodynamic conditions.12
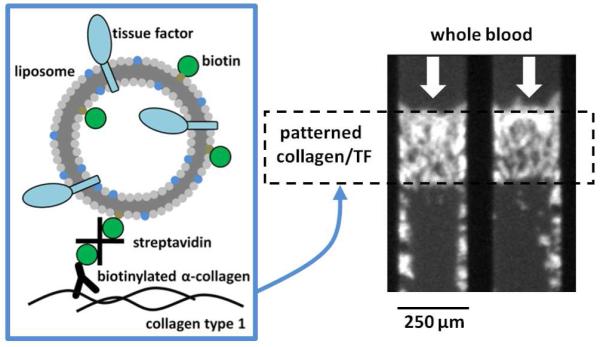
In this study, we describe a method for generating reproducible surfaces of collagen and active tissue factor for use in microfluidic analyses of platelet function. We have modified the TF-bearing liposomes described by Smith et al.7 to include a biotinylated lipid which we use to bind the vesicles to a collagen surface via a biotinylated anti-collagen antibody and streptavidin (Fig. 1). We have verified that these thrombogenic surfaces are functionally active leading to enhanced platelet deposition and measurable fibrin formation. Furthermore, we have characterized the surface through the use of the Proximity Ligation Assay (PLA), a dual antibody immunodetection technique capable of visualizing individual molecule complexes based on molecular-scale proximity.
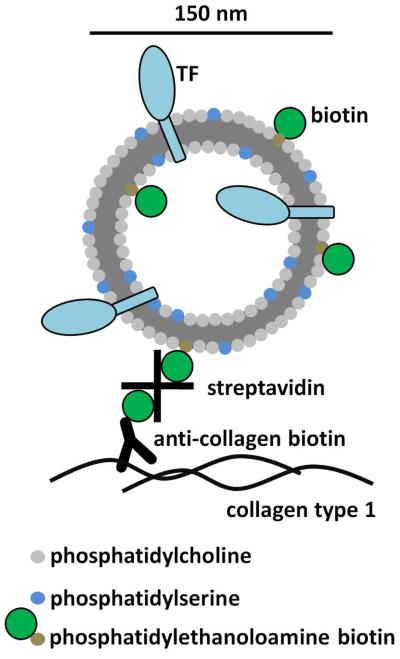
Figure 1.
Tissue factor was relipidated in liposomes consisting of a molar ratio of 79:20:1 phosphatidylcholine to phosphatidylserine to biotinylated phosphatidylethanolamine in a lipid to TF ratio of 10,000:1. Dynamic light scattering indicated that the average vesicle radius was approximately 74.4 ± 28.0 nm. The liposomes were physically linked to collagen type 1 surface treated with a goat polyclonal antibody conjugated to biotin through a streptavidin linker. 82×139mm (300 × 300 DPI)
Materials and Methods
Materials
Lipids, L-α-phosphatidylcholine (PC), L-α-phosphatidylserine (PS), and biotinylated phosphatidylethanolamine (bPE) were from Avanti Polar Lipids (Alabaster, AL, USA). Recombinant human tissue factor was from Haematologic Technologies Inc. (Essex Junction, VT, USA). Bio-Beads SM-2 (BioRad Laboratories, Hercules, CA, USA) were cleaned in methanol and stored under HEPES Buffered Saline (HBS, 20 mM HEPES, 150 mM NaCl, pH 7.4 [NaOH]) at 4 °C. The detergent, Triton X-100, was from Fisher Scientific (Fair Lawn, NJ, USA.) Duolink proximity ligation assay (PLA) amplification and detection reagents were from O-Link Bioscience through distributor Axxora (San Diego, CA, USA). The primary monoclonal PLA antibody against tissue factor was from Haematologic Technologies and polyclonal primary against factor VIIa was from Abcam (Cambridge, MA, USA), as well as the biotinylated goat polyclonal anti collagen type 1 antibody. Streptavidin was from Sigma (St. Louis, MO, USA). Acid-insoluble collagen type 1 from equine tendon was from Chronolog Corp. (Havertown, PA, USA) and acid-soluble human collagen type 1 was from Advanced Biomatrix (San Diego, CA, USA). Poly(dimethylsiloxane), PDMS, for the microfluidic devices (Sylgard 184) was from Ellsworth Adhesives (Germantown, WI, USA) and Sigmacote used for hydrophobic treatment of glass slides was from Sigma. Finally, the anticoagulants corn trypsin inhibitor (CTI) and Pro-Arg-chloromethylketone (PPACK) were from Haematologic Technologies, and platelet labeling anti-CD41 antibody was from AbDSerotec (Raleigh, NC, USA). The fibrin-specific fluorescent monoclonal antibody was a generous gift from the Mortimer Poncz lab at the Children’s Hospital of Philadelphia.
Blood collection and preparation
Blood was collected via venipuncture from healthy donors who were self-reported as free of medication for at least 10 days into CTI (40 μg/mL) for microfluidic assays or PPACK (100 μM) for TF detection assays. All volunteers provided informed consent in accordance with IRB approval and the Declaration of Helsinki. Whole blood was treated with alexafluor 647-conjugated anti CD-41 monoclonal antibody for platelet detection in microfluidic assays 5 min prior to perfusion. PFP was generated for TF/VIIa complex detection assays by centrifugation of whole blood at 1000g for 10 min. All blood samples were used within 1 hr of the draw.
Preparation of biotinylated TF liposomes
Relipidation of recombinant tissue factor into liposomes was performed according to a previously described technique.7 Briefly, PC, PS, and b-PE stored in chloroform were dried under vacuum in a 79:20:1 molar ratio, respectively. The dried film was resuspended in 1 mL of 4 mM Triton X-100 in HBS and allowed to hydrate for 30 min. Recombinant tissue factor was added and incubated for 10 min (10,000:1 lipid to TF). To this 50 mg of Bio-Bead slurry was added and was gently agitated for 90 min and then 350 mg more of Bio-Bead slurry was added and gently agitated for the same amount of time. The beads were allowed to settle and the supernatant was collected. The hydrodynamic radius of the resulting liposomes was determined by dynamic light scattering (Protein Solutions DynaPro, Wyatt Technology, Santa Barbara, CA, USA) to be 74.4 ± 28.0 nm.
Preparation of collagen-liposome surfaces for detection
Acid-soluble collagen was first neutralized to a pH of 7.4 with 1M NaOH and then incubated on Sigmacote treated glass slides for 2.5 hrs (300 μg/mL). The collagen surfaces were rinsed in HBS and treated with goat polyclonal anti-collagen biotin (10 μg/mL) for 10 minutes and rinsed again. The droplet area was then treated with streptavidin (10 μg/mL) for another 10 minutes before rinsing. 10 μL of the relipidated tissue factor liposome solution was added to the surface and allowed to rest for 1 hour. A representation of the liposomes/collagen surface is presented in fig. 1. The relipidated tissue factor surfaces were finally treated with PFP (100 μM PPACK) for 30 min in order to generate TF/VII complexes.13 When these surfaces were prepared for microfluidic assays the steps were performed in a microfluidic patterning device which consisted of a single channel 250 μm wide as described in.14 Acid-insoluble collagen was used for these studies, as it better supports platelet adhesion. The final result was a strip of protein rather than a droplet. These surfaces were not treated with plasma as they would be introduced to whole blood in the flow experiment.
Detection of TF/VIIa complexes
Single tissue factor/factor VIIa complexes were detected on our thrombogenic surfaces using the Duolink PLA.15 Briefly, a mouse monoclonal antibody to tissue factor and a rabbit polyclonal antibody to factor VIIa were selected as primary antibodies. The binding of these antibodies to the TF/VIIa complex served as a localization site for two species specific antibodies conjugated to short oligonucleotides (PLA probes). When the probes are in close proximity (i.e. bound to their antigens which are bound to a protein complex) they may hybridize with two other oligonucleotides in solution forming a loop. This loop is then ligated to create a closed circle which is an active site for rolling circle DNA amplification. Finally, fluorescently labeled oligonucleotides hybridize with the replication product to produce a detection signal. Fig. 2 is an illustration of this technique.
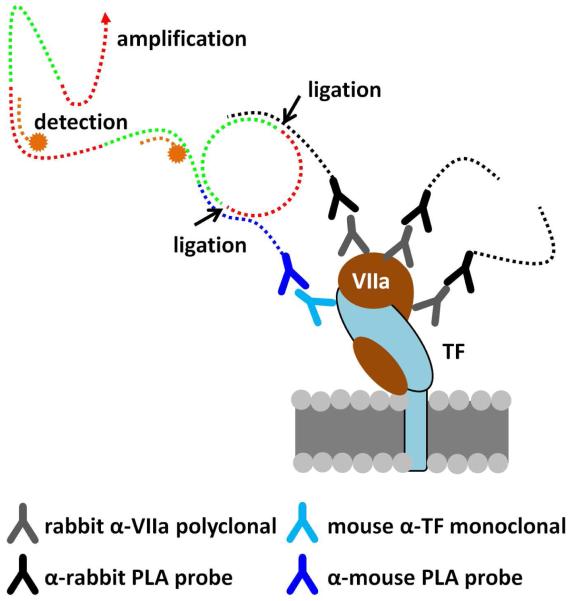
Figure 2.
The Duolink proximity ligation assay is capable of detecting single molecule complexes. Primary antibodies directed against the components of the complex act to localize two “PLA probes”, which are species specific antibodies conjugated to short oligonucleotides. When the probes are brought together they form a closed loop when complimentary oligonucleotides are added. This loop is enzymatically ligated which provides a template for rolling circle amplification. Small fluorescently labeled oligonucleotides hybridize with the amplified product producing a detection signal. 127×139mm (300 × 300 DPI)
Microfluidic platelet adhesion model
Two sets of microchannels were designed in PDMS according to a previously described technique.14,16 First a patterning device consisting of a single channel 250 μm wide and 5 cm long was used to generate strips of thrombotic protein (collagen and tissue factor) according to the procedure above. This device was then removed and a flow device placed over the protein strip with perpendicular 250 μm channels resulting in a platelet/protein interaction zone measuring 250×250 μm in each channel. The channels were blocked with 0.5% bovine serum albumin in HBS for 30 min prior to perfusion with whole blood. Blood was perfused through the channels at a wall shear rate of 200 s−1 via syringe pump (Harvard Apparatus PHD 2000, Holliston, MA, USA) and the interaction zone monitored via fluorescence microscopy (IX81, Olympus America Inc., Center Valley, PA, USA) in 15 second intervals. Images were captured using a CCD camera (ORCA-ER, Hamamatsu, Bridgewater, NJ, USA) illuminated with a mercury lamp (100W, 620 nm Ex / 700 nm Ex). Fluorescent intensity was evaluated using the freely available ImageJ software (NIH).
Results
Detection of single TF/VIIa complexes
Tissue factor VIIa complexes were generated on a polymerized human collagen type 1 surface by linking relipidated tissue factor liposomes with 1% biotinylated-PE to a biotinylated anti collagen antibody via streptavidin. We chose to use soluble collagen for this application as it creates a more uniform layer with less background fluorescence than fibrillar collagen surfaces. All surfaces were blocked in solutions of 0.5% BSA for 30 min prior to detection. The primary antibodies were used at a final concentration of 1 μg/mL and were incubated in open droplet reactions for 1 hr at 37 °C. Following this the PLA reaction was run according to the manufacturer’s instructions.
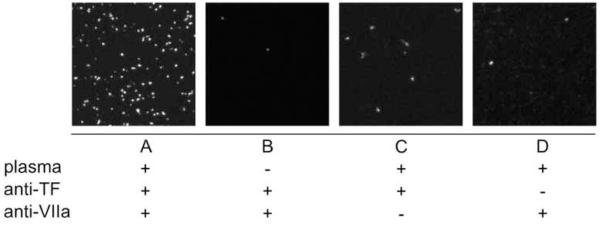
The amplification product was observed as an approximately 1 μm sized fluorescent dot (Fig. 3) that was at least 3-fold brighter than background. In order to confirm that we were detecting the desired complexes and not non-specific antibody binding, two sets of controls were run. First, samples with linked tissue factor liposomes were either treated or untreated with plasma (+/− factor VIIa). Each sample was treated with both primary and secondary antibodies as well as all Duolink amplification and detection reagents. The images presented are representative of several 20× fields of view (Fig. 3a and b). Identical samples with linked TF liposomes were incubated with human plasma and were treated with just the TF primary antibody or just the factor VIIa primary antibody (Fig. 3c and 3d). These samples were treated according the unmodified Duolink procedure. The results demonstrate that the complete TF/VIIa complex must be present and that it must be treated with both primary antibodies for detection by PLA. Fluorescent staining observed under the negative control conditions is believed to be the result of non-specific binding of the secondary PLA probes.
Figure 3.
The PLA signal appears as 1 μm fluorescent dots (A). We have demonstrated that in the absence of complexes (no factor VII from plasma [B]) or of either primary antibody (VIIa [C] or TF [D]), detection does not occur. 66×25mm (300 × 300 DPI)
Effect of TF liposomes under flow conditions
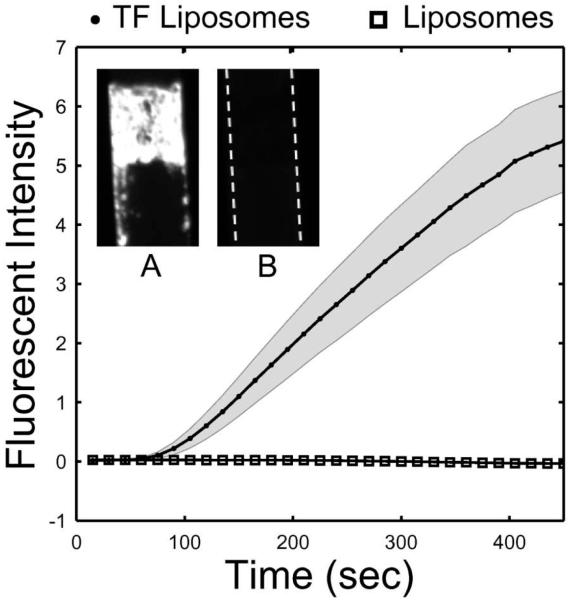
Fibrillar collagen surfaces with linked tissue factor liposomes were generated in a microfluidic model of thrombosis as well as surfaces with liposomes lacking tissue factor. Whole blood anticoagulated with CTI (40 μg/mL), to inhibit the contact activation pathway, was perfused over the surfaces at an inlet wall shear rate of 200 s−1. The experiment was performed in multi-channel microfluidic device that allowed for 8 separate (but equal) adhesion events to be monitored simultaneously.16 The data represented in fig. 4 is the fluorescent intensity of the surfaces captured in 15 sec intervals over a 450 sec period. The intensity represents the average mass of platelets aggregating on the surface. A significant increase (30 ± 8%, p<.0001) was observed in platelet deposition when the surface contained TF positive liposomes after 450 sec of perfusion. Platelets also adhered downstream from the collagen patch, possibly on fibrin deposits (Fig. 4a). Platelet adhesion to the liposome surface lacking tissue factor was undistinguishable from a control sample untreated with antibody, streptavidin, or liposomes after 450 sec.
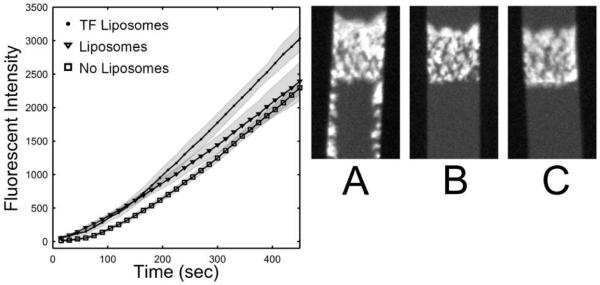
Figure 4.
Tissue factor liposomes linked to collagen increased platelet deposition in a microfluidic injury model 30 ± 8.0% as compared to a similar surface with liposomes lacking TF or a control collagen surface. Whole blood was perfused over these surfaces at 200 s−1 in the presence of CTI (40 μg/mL). Each condition was performed in 8 separate channels and monitored simultaneously. The data represent the mass of platelets adhering at the collagen patch (in arbitrary units) and are presented ± STD. Representative images of platelet adhesion at 450 sec are presented for each case ([A] TF liposomes, [B] liposomes, [C] control surface). Flow is from top to bottom. Platelet accumulation for the TF surface appears brighter, denser, and protrudes upstream. Platelets also adhere downstream of the main platelet mass when TF is present on the liposome/collagen surface, possibly as a result of fibrin deposition. 84×41mm (300 × 300 DPI)
In a parallel experiment unlabeled platelets in whole blood (40 μg/mL CTI) were perfused over a collagen surface treated with TF positive or negative liposomes in the presence of a fluorescently labeled fibrin-specific antibody (3 μg/mL). The data reveal that liposomes containing tissue factor are required for fibrin formation in the platelet mass during the 450 sec perfusion. Detectable fibrin appears between 15 and 30 sec into the TF liposome perfusion. Fig. 5a shows the deposition of fibrin downstream from the collagen strip. When PS-PC-bPE liposomes lacking TF were attached to collagen, there was no increase in platelet deposition and no production of fibrin, consistent with the fact that PSPC liposomes do not trigger thrombin production in CTI-treated plasma.17
Figure 5.
Whole blood treated with CTI (40 μg/mL) was perfused over a TF liposome/collagen surface or liposome/collagen surface at 200 s−1 in the presence of a fluorescently labeled fibrin specific monoclonal antibody. Each condition was performed and monitored simultaneously in 8 separate channels. Fluorescent intensity (in arbitrary units) of the fibrin mass accruing in the platelet mass is presented ± STD. The inset illustrates the two conditions after 450 sec of perfusion (flow is from top to bottom). No fibrin deposition appears with the TF negative liposomes (B) while massive deposition appears for the positive liposomes (A). Fibrin deposition is detected downstream of the main thrombus as well. 79×83mm (300 × 300 DPI)
Discussion
Utilizing the tight binding between biotin and streptavidin, we have created a thrombogenic surface that consists of relipidated TF liposomes linked to an immobilized collagen surface. This construct produces a physiologically relevant hemostatic response which includes platelet activation, platelet adhesion and coagulation, as is observed in vivo. The advantage of our ex vivo system, however, is that it allows for robust and reproducible control of hemodynamics, surface composition, and blood pharmacology. Furthermore, it represents a higher throughput and lower cost approach than animal models.
The results presented as part of this work were intended to verify the new TF liposome/collagen injury model against previous findings. Prior work in our lab conducted by Okorie et al., who spotted TF liposomes onto collagen features, demonstrated a 25% increase in platelet adhesion when the highest concentration of printed liposomes was used (25 molecules/μm2).18 In the new model we observe a similar response (30% increase ± 8%) and capture the robust increase in fibrin formation reported in that study, as well. While we estimate a slightly lower TF concentration on our surface (see below) [0.1 to 1 molecules/μm2], we may explain the increased potency by the physical linking of liposomes to the collagen surface. The observation that platelet adhesion does not increase on liposome surfaces containing TF until ~200 sec is supported by other groups who observed lag times in thrombin generation of >60 sec,19,20 as well as the lag time observed in fibrin generation in Fig. 5. We also suggest that thrombin generation during primary adhesion has a less noticeable effect due to strong GPVI signaling generated by fibrillar collagen at early times.
In addition to platelet adhesion, the kinetics of fibrin deposition in our assay also compare well to other experimental models. In the mouse laser injury model, for example, measurable fibrin appears at 10-15 sec after insult.21 In these studies the clotting response is triggered by activation of the endothelium and is driven by the exposure of tissue factor, not the subendothelium; Platelet adhesion is strongly dependent on PAR4 activation.22 Therefore, a more relevant model may be the FeCl3 injury, in which collagen and thrombin signaling play important roles regarding time to thrombus initiation and likelihood of vessel occlusion.23-25 In these studies FcγRII-/- or PAR4-/- mice, which lack collagen or thrombin signaling, respectively, have some protection from full vessel occlusion. Factor XIIa is not inhibited in the mouse models whereas CTI is used in our in vitro perfusion studies with human blood.
We have demonstrated that our findings with the new TF/collagen assay compare well to other ex vivo and in vivo work. We have also used PLA to detect TF/factor VIIa complexes. However, quantifying fM to pM levels of surface TF is complex and is a drawback to other models as well.8 With reasonable assumptions we can provide an estimate of the [TF] range. Extensive characterization of tissue factor liposomes has been previously performed.7 Using the relipidation technique described above, 80-90% of TF is recovered in liposomes and approximately 50% of this TF is accessible. In our assay these values predict 5 molecules of TF per liposome are available (10,000:1 lipid to TF ratio). If we consider the results from PLA to represent a lower bound on the number of surface captured liposomes (PLA is known to have significantly less than 100% detection) we estimate 0.02 liposomes/μm2. This value corresponds to 0.1 to 1 molecules TF/μm2 for 100% or 10% PLA detection efficiency, respectively. These estimates compare well to our own prior work and relipidated TF patches created by other groups (0.3 molecules TF/μm2).26 Furthermore, we estimate that the random packing limit for spheres in 2 dimensions sets an upper bound of approximately 102 TF molecules/μm2.
In vivo models of thrombosis have demonstrated the importance of coagulation in response to vessel injury. Using TF liposomes linked to a collagen surface we have demonstrated an ex vivo system that is sensitive to both the coagulation and platelet aggregation components of the hemostatic mechanism.
Acknowledgments
The authors would like to thank Dr. M. Poncz for the fibrin-specific monoclonal antibody.
T. Colace was supported by a National Institutes of Health (NIH) Cardiovascular Training Predoctoral Fellowship. The authors acknowledge research support by NIH R01-HL-103419 (S.L.D).
References
- (1).Bouchard BA, Mann KG, Butenas S. No evidence for tissue factor on platelets. Blood. 2010;116:854–5. doi: 10.1182/blood-2010-05-285627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–50. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- (3).Giesen PLA, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Blood-Borne tissue factor: Another view of thrombosis. Proc. Natl. Acad. Sci. U.S.A. 1996;96:2311–5. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombin in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J. Exp. Med. 2003;197:1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2839–43. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bach R, Gentry R, Nemerson Y. Factor VII binding to tissue factor in reconstituted phospholipid vesicles: Induction of cooperativity by phosphatidylserine. Biochemistry. 1986;25:4007–20. doi: 10.1021/bi00362a005. [DOI] [PubMed] [Google Scholar]
- (7).Smith SA, Morrissey JH. Rapid and efficient incorporation of tissue factor into liposomes. J. Thromb. and Haemost. 2004;2:1155–62. doi: 10.1111/j.1538-7836.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- (8).Shen F, Kastrup CJ, Liu Y, Ismagilov RF. Threshold response of initiation of blood coagulation by tissue factor in patterned microfluidic capillaries is controlled by shear rate. Arterioscler. Thromb. Vasc. Biol. 2008;28:2035–41. doi: 10.1161/ATVBAHA.108.173930. [DOI] [PubMed] [Google Scholar]
- (9).Cosemans JM, Schols SE, Stefanini L, de Witt S, Feijge MAH, Hamulyak K, Deckmyn H, Bergmeier W, Heemskerk JWM. Key role of glycoprotein Ib/V/IX and von Willebrand factor in platelet activation-dependent fibrin formation at low shear flow. Blood. 2011;117:651–60. doi: 10.1182/blood-2010-01-262683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Goel MS, Diamond SL. Neutrophil enhancement of fibrin deposition under flow through platelet-dependent and –independent mechanisms. Arterioscler. Thromb. Vasc. Biol. 2001;21:2093–98. doi: 10.1161/hq1201.100255. [DOI] [PubMed] [Google Scholar]
- (11).Falati S, Gross P, Merril-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor, and fibrin during arterial thrombus formation in the mouse. Nature Med. 2002;8:1175–80. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- (12).Rosen ED, Raymond S, Zollman A, Noria F, Sandoval-Cooper M, Shulman A, Merz JL, Castellino FJ. Laser-induced noninvasive vascular injury models in mice generate platelet- and coagulation-dependent thrombi. Am. J. Pathol. 2001;158:1613–22. doi: 10.1016/S0002-9440(10)64117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ruf W, Kalnik MW, Lund-Hansen T, Edgington TS. Characterization of factor VII association with tissue factor in solution. J. Biol. Chem. 1991;266:15719–25. [PubMed] [Google Scholar]
- (14).Neeves KB, Maloney SF, Fong KP, Schmaier AA, Kahn ML, Brass LF, Diamond SL. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. J. Thromb. Haemost. 2008;6:2193–201. doi: 10.1111/j.1538-7836.2008.03188.x. [DOI] [PubMed] [Google Scholar]
- (15).Soderberg O, Leuchowius KJ, Gullberg M, Jarvius M, Weibrecht I, Larsson LG, Landegren U. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods. 2008;45:227–32. doi: 10.1016/j.ymeth.2008.06.014. [DOI] [PubMed] [Google Scholar]
- (16).Maloney SF, Brass LF, Diamond SL. P2Y12 or P2Y1 inhibitors reduce platelet deposition in a microfluidic model of thrombosis while apyrase lacks efficacy under flow conditions. Integr. Biol. 2010;2:183–92. doi: 10.1039/b919728a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Goel MS, Diamond SL. Neutrophil cathepsin G promotes prothrombinase and fibrin formation under flow conditions by activating fibrinogen-adherent platelets. J. Biol. Chem. 2003;278:9458–63. doi: 10.1074/jbc.M211956200. [DOI] [PubMed] [Google Scholar]
- (18).Okorie UM, Denney WS, Chatterjee MS, Neeves KB, Diamond SL. Determination of surface tissue factor thresholds that trigger coagulation at venous and arterial shear rates: amplification of 100 fM circulating tissue factor requires flow. Blood. 2008;111:3507–13. doi: 10.1182/blood-2007-08-106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Fogelson AL, Tania N. Coagulation under flow: The influence of flow-mediated transport on the initiation and inhibition of coagulation. Pathophysiol. Haemost. Thromb. 2005;34:91–108. doi: 10.1159/000089930. [DOI] [PubMed] [Google Scholar]
- (20).Van ‘t Veer C, Hackeng TM, Delahaye C, Sixma JJ, Bouma BN. Activated factor X and thrombin formation triggered by tissue factor on endothelial cell matrix in a flow model: effect of the tissue factor pathway inhibitor. Blood. 84:1132–42. [PubMed] [Google Scholar]
- (21).Furie B, Furie BC. Thrombus formation in vivo. J. Clin. Invest. 2005;115:3355–59. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Vandendries ER, Hamilton JR, Coughlin SR, Furie B, Furie BC. Par4 is required for platelet thrombus propagation but not fibrin generation in a mouse model of thrombosis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:288–92. doi: 10.1073/pnas.0610188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Dubois C, Panicot-Dubois L, Merrill-Skoloff G, Furie B, Furie BC. Glycoprotein VI-dependent and –independent pathways of thrombus formation in vivo. Blood. 2006;107:3902–6. doi: 10.1182/blood-2005-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Wang L, Miller C, Swarthout RF, Rao M, Mackman N, Taubman MB. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009;113:705–13. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signaling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–8. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- (26).Kastrup CJ, Shen F, Runyon MK, Ismagilov RF. Characterization of the threshold response of initiation of blood clotting to stimulus patch size. Biophys. J. 2007;93:2969–77. doi: 10.1529/biophysj.107.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]