Abstract
Background
Vitamin D may modify risk of cardiometabolic outcomes (type 2 diabetes, hypertension, or cardiovascular disease).
Purpose
Examine the association of vitamin D status and the effect of vitamin D supplementation on cardiometabolic outcomes in generally healthy adults.
Data Sources
English-language studies in MEDLINE (inception to 4 November 2009) and the Cochrane Clinical Trials Register (4th quarter of 2009).
Study Selection
Eleven reviewers screened citations to identify longitudinal cohort studies reporting associations of vitamin D status and randomized trials of vitamin D supplementation on cardiometabolic outcomes.
Data Extraction
Five independent reviewers extracted data about study conduct, participant characteristics, outcomes, and quality. Differences were resolved by consensus.
Data Synthesis
Thirteen observational studies (14 cohorts) and 18 trials were eligible. Three of 6 analyses (from 4 different cohorts) reported a lower incident diabetes risk in highest versus lowest vitamin D status groups. Seven trials found no effect of vitamin D supplementation on glycemia or incident diabetes. By meta-analysis of 3 cohorts, lower 25-hydroxyvitamin D concentration was associated with incident hypertension (relative risk 1.8; 95% confidence interval [CI] 1.3, 2.4). By meta-analyses of 10 trials, supplementation nonsignificantly reduced systolic blood pressure (weighted mean difference [WMD] −1.9; 95% CI −4.2, 0.4 mm Hg) and did not affect diastolic blood pressure (WMD −0.1; 95% CI −0.7, 0.5 mm Hg). Lower 25-hydroxyvitamin D concentration was associated with incident cardiovascular disease in 5 of 7 analyses (6 cohorts). Four trials found no effect of supplementation on cardiovascular outcomes.
Limitations
Studies included primarily whites. Observational studies were heterogeneous. Several trials reported post hoc analyses.
Conclusions
An association between vitamin D status and cardiometabolic outcomes is uncertain. Trials showed no clinically significant effect of vitamin D supplementation at doses given.
Keywords: Vitamin D, diabetes mellitus, hypertension, cardiovascular disease, systematic review, meta-analysis
Increasing evidence suggests that vitamin D may have an important role in modifying risk of cardiometabolic outcomes, including type 2 diabetes, hypertension, and cardiovascular disease (1, 2). Most studies that have shown an association between lower vitamin D status and increased risk of cardiometabolic outcomes, however, are cross-sectional, limiting the strength of their conclusions. Ecological studies have also reported higher rates of diabetes, hypertension and coronary heart disease with increasing distance from the equator, suggesting a possible association with vitamin D insufficiency in regions with less sun exposure (3–5).
Recently, a large number of longitudinal observational studies and trials have been published on the relationship between vitamin D and cardiometabolic outcomes. To determine the potential role of vitamin D in cardiometabolic outcomes, we performed a systematic review of longitudinal observational studies of vitamin D status and randomized controlled trials of vitamin D supplementation on cardiometabolic outcomes.
METHODS
This paper comprises an expansion of an evidence report commissioned by the Agency for Healthcare Research and Quality (AHRQ) for an Institute of Medicine panel that is revisiting the dietary reference intakes for vitamin D and calcium (6). The AHRQ evidence report on vitamin D (and calcium) evaluated 17 clinical outcomes in the general healthy population, including incident hypertension and cardiovascular disease. This report includes an additional focused systematic review to include cardiometabolic outcomes related to type 2 diabetes. A standard protocol was developed and followed for the overall review.
Data Sources and Study Selection
We conducted two independent searches of MEDLINE (inception to 4 November 2009) and the Cochrane Central database (4th quarter of 2009, last searched 4 November 2009). Each search included longitudinal observational studies of vitamin D status and randomized controlled trials of vitamin D supplementation (cholecalciferol [D3] or ergocalciferol [D2] with or without calcium) in adults.
The broad systematic review for the AHRQ evidence report identified longitudinal observational studies where vitamin D status was assessed by plasma or serum 25-hydroxyvitamin D (25(OH)D) concentration. This literature search was last conducted on 30 April 2009. It included the cardiometabolic outcomes: incident hypertension, incident cardiovascular disease, and (in trials only) change in blood pressure. Only generally healthy populations were included (<20% of study participants had major chronic diseases such as diabetes, cancer, or cardiovascular disease at baseline). The search also included studies of calcium intake alone, which are not reported here.
The focused literature search also included studies where vitamin D status was assessed by self-reported vitamin D intake or 25(OH)D score predicted from self-reported data; studies with diabetes-related outcomes: incident type 2 diabetes and (in trials only) change in glycemia (fasting plasma glucose, 2-hour glucose after oral glucose tolerance test or hemoglobin A1c); and all adult populations regardless of baseline disease (exceptions described below). This literature search was last conducted on 4 November 2009
The searches combined terms for vitamin D, hypertension and cardiovascular disease (both searches) and diabetes mellitus (focused search) and were restricted to English language publications. Additional studies were sought in personal reference lists and citation sections of recovered articles. We excluded cross-sectional or retrospective cohort studies, standard case-control studies, and short-term (<1 month) randomized trials. We included nested case-control studies where data on vitamin D status were collected prior to outcome assessment. We excluded studies on type 1 diabetes because of its different pathophysiology, studies in children, pregnant women, and patients with conditions that affect vitamin D metabolism (e.g., chronic kidney disease, hyperparathyroidism) and trials that used a vitamin D preparation other than D3 or D2, or non-oral vitamin D administration.
The two literature searches were screened independently. For the broad systematic review, 10 investigators performed single screening of search results for outcomes of interest (see acknowledgments). For the focused systematic review, one additional investigator screened all abstracts. Results from the two independent searches were combined if studies met the specific criteria for the focused systematic review on cardiometabolic outcomes (type 2 diabetes, hypertension, and cardiovascular disease). Discrepancies were resolved by consensus in group conference.
Data Extraction and Quality Assessment
Data from each study were extracted independently by one of five investigators and confirmed by at least one other. The extracted data included: study design; participant characteristics; longest reported follow-up period; method of assessing vitamin D status or details of vitamin D supplementation; association between vitamin D status or supplementation and outcome; potential confounding variables adjusted for, with particular emphasis on age, race, weight, and variables related to sun exposure (e.g. season, location); method of ascertaining cardiometabolic outcome, and statistical analyses.
We assessed the methodological quality of each study based on predefined criteria, in accordance with AHRQ’s suggested methods for systematic reviews (7). Study quality was determined by the primary data extractor and confirmed by at least one other reviewer. Good quality studies adhere most closely to the commonly held concepts of high quality including clear descriptions of the population and setting; unbiased assessments of vitamin D status and outcomes; appropriate statistical analysis including multivariable analysis adjusting for age, race, weight, and sun exposure; no obvious reporting omissions or errors; and <20% dropouts. Fair quality studies have some deficiencies in the above criteria unlikely to cause major bias. Poor quality studies have major deficiencies such that major bias could not be excluded. We considered factors in the STROBE statement for observational studies (8), nutrition-specific items from a critical appraisal of micronutrient systematic reviews (9), and the CONSORT statement for reporting clinical trials (10).
Data Synthesis and Analysis
We performed random effects model meta-analyses when similar data from three or more observational cohorts or trials were available (11). For observational studies, we synthesized relative risks (RR) (or hazard or odds ratios) comparing the extreme categories of vitamin D status (highest vs. lowest, as defined within each study) provided that the categories corresponded to similar levels of vitamin D intake or 25(OH)D concentration across studies. For randomized trials, we combined net differences for continuous outcomes and RR for dichotomous outcomes. We tested between-study heterogeneity with the Q statistic (significant when p<0.10) and quantified its extent with I2 (12).
Role of the Funding Source
The funding sources (National Institute of Diabetes and Digestive and Kidney Disease, the NIH Office of Dietary Supplements, Food and Drug Administration, AHRQ, and Public Health Agency of Canada) had no role in the design, conduct, or reporting of the study or in the decision to submit the manuscript for publication, with the exception that AHRQ participated in formulating the study questions for the evidence report (6).
RESULTS
Search Results
The two independent searches identified 5,739 and 2,087 abstracts. For the broad search 106 articles were retrieved for full-text review and for the focused search 127 articles were retrieved. From both searches combined, 32 studies qualified. Reasons for exclusion are shown in the Appendix Figure available at www.annals.org.
Vitamin D and Type 2 Diabetes
Longitudinal observational cohort studies
Three studies with four cohorts reported the association between vitamin D status and risk of type 2 diabetes (13–15) (Appendix Table 1 available at www.annals.org). The studies included 95,243 participants (98% white) who were followed from 9 to 20 years. Two studies assessed vitamin D status by self-reported total vitamin D intake (13, 14) and were rated fair quality; the other study measured serum 25(OH)D concentration (15) and was rated good quality. Ascertainment of type 2 diabetes was by validated self-report in two studies (13, 14) and by national registry-based data in the third (15). Two studies reported multivariable adjusted results; one adjusted only for age (13).
In two analyzed groups of men in one article (15), the association between higher vitamin D status and lower risk of incident type 2 diabetes was statistically significant in one (Mini-Finland Health Survey cohort) and nearly statistically significant in the other (Finnish Mobile Clinic Health Examination Survey). In three of four analyzed groups of women there was no association (14, 15). Only the Women’s Health Study cohort found an association between higher vitamin D status and lower risk of incident type 2 diabetes (13). The variable predictors analyzed (type of assessment and definitions of risk categories) precluded meta-analyses.
Randomized trials
Seven trials reported the effect of vitamin Dsupplementation on glycemia (fasting plasma glucose or hemoglobin A1c) (16–23) or incident diabetes by self-report (19) (Appendix Table 2 available at www.annals.org). Three of the trials were designed for non-glycemic outcomes (16, 18, 19). Study duration varied from 2 months to 7 years and doses ranged from 400 to 5714 IU/day. Two studies gave vitamin D3 in combination with calcium (18, 19). Only two trials were rated good quality (19, 20, 23). Among five trials of participants with normal glucose tolerance at baseline, vitamin D supplementation had no effect on fasting plasma glucose (weighted mean difference −0.002 mmol/L; 95% CI −0.055, 0.050 for vitamin D supplementation versus placebo) (16, 18–21, 23) or incident diabetes (19). In a subgroup analysis of participants with impaired fasting glucose at baseline, combined vitamin D3 (700 IU/day) and calcium carbonate (500 mg/day) supplementation attenuated the increase in fasting glycemia that occurs over time in this population (Pittas et al. (18)). In two trials of participants with stable type 2 diabetes, glycemic measures did not change after 8 or 24 weeks of vitamin D supplementation (17, 22).
Vitamin D and Hypertension
Longitudinal observational cohort studies
Three studies reported data from four cohorts on the association between vitamin D status and risk of incident hypertension (24–26) (Appendix Table 1). The studies included 32,181 participants (98% white) with follow-up between 7 and 10 years. One study assessed vitamin D status by self-reported vitamin D intake (26); the other two measured 25(OH)D concentration (24, 25). In all studies, ascertainment of hypertension was by validated self-report, without actual measurement of blood pressure; therefore all were graded fair quality. All studies reported multivariable adjusted results.
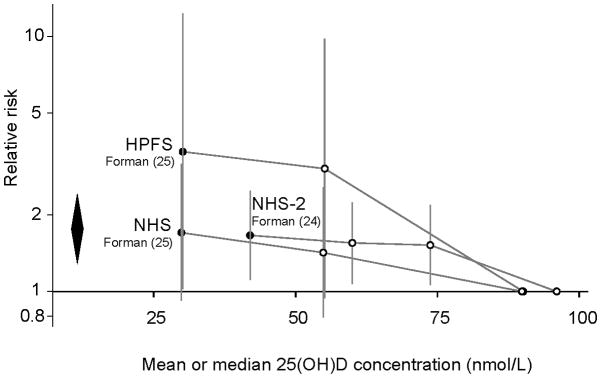
Among three cohorts, two (one of men (25), one of women (24)) found a statistically significant association between lower 25(OH)D concentration and higher risk of incident hypertension after 7 or 8 years, while the third (25) reported an association in the same direction in women, which was not statistically significant at 8 years. In one study, the association was reported to be stronger for men and women after 4 compared to 8 years of follow-up (RR 6.13; 95% CI 1.00, 37.8 (4 y); 3.53; 95% CI 1.02, 12.3 (8 y) in men; RR 2.67; 95% CI 1.05, 6.79 (4 y); 1.70; 95% CI 0.92, 3.16 (8 y) in women) (25). Meta-analyses of these three cohorts (24, 25) found a statistically significant association comparing the lowest (<37–51 nmol/L [<15–21 ng/mL]) versus the highest (>75–81 nmol/L [>30–32 ng/mL]) category of 25(OH)D and incident hypertension after 7 to 8 years (RR=1.76; 95% CI 1.27, 2.44; Figure 1A) without heterogeneity among studies (I2=0%).
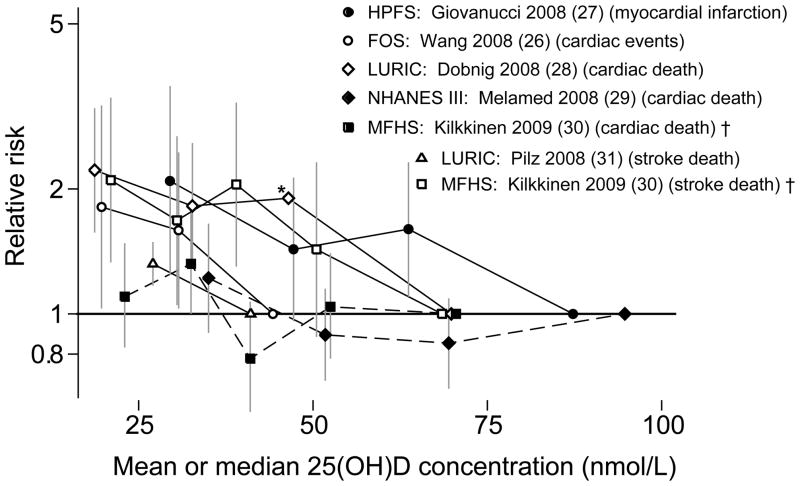
Figure 1.
Association between vitamin D status and incident hypertension (Figure 1A) and cardiovascular disease (Figure 1B) in longitudinal observational cohorts.
25(OH)D, 25-hydroxyvitamin D; FOS, Framingham Follow-up Study; HPFS, Health Professionals Follow-up Study; LURIC, Ludwigshafen Risk and Cardiovascular Health; MFHS, Mini-Finland Health Survey; NHANES III, Third National Health and Nutrition Examination Survey; NHS, Nurses Health Study; NHS-2, Nurses Health Study-2.
To convert 25(OH)D concentration from nmol/L to ng/mL divide by 2.459.
Relative risks (and 95% confidence intervals) of each quantile of 25(OH)D concentration compared to the highest concentration quantile for studies with incident hypertension (1A) and cardiovascular events (1B) outcomes. In figure 1A, the diamond represents the meta-analysis summary relative risk and 95% confidence interval for the lowest quantiles (black circles) compared to the highest quantiles (on the reference line); Relative risk=1.76; 95% CI 1.27, 2.44; I2=0%. Figure 1B does not include data from Marniemi et al. (32) because the quantiles were not defined. Solid lines in Figure 1B indicate that trends were statistically significant; dashed lines that trends were not statistically significant.
* Estimate from reported data; no confidence interval data available.
† To make studies graphically comparable, data were converted to provide estimates of relative risks if the lowest quintile were the reference group. The original study (Kilkkinen et al. (30)) used the highest quintile as the reference group.
One study evaluated the association between vitamin D intake and incident hypertension (26). A statistically significant trend across quintiles of dietary vitamin D intake was reported (P=0.02); however, there was no consistency in the direction of the adjusted RRs across quintiles and all were close to 1.0 (0.95 to 1.04). No association was found with supplemental vitamin D.
Randomized trials
Nine trials reported the effect of vitamin D supplementation on blood pressure (17, 20, 22, 33–38) or incident hypertension (38) (Appendix Table 2). Vitamin D was given either alone (17, 20, 22, 33–38) or in combination with calcium (36–38) at doses equivalent to 400 to 8571 IU/day. Eight studies used D3 (33–38) and one D2 (17). Another trial compared UV-B (which increases cutaneous synthesis of vitamin D) with UV-A exposure (which does not) (39). Follow-up varied from 5 to 52 weeks in most studies and was 7 years in the Women’s Health Initiative trial (38). The total number of participants was 37,162, with the Women’s Health Initiative trial contributing 36,282 participants. The study populations were heterogeneous, including healthy participants (38), participants with established hypertension (39), heart failure (34), type 2 diabetes (17, 22) or overweight/obesity (20, 35). Three trials were rated good (20, 33, 38), five fair (17, 22, 34–36) and two poor quality (37, 39).
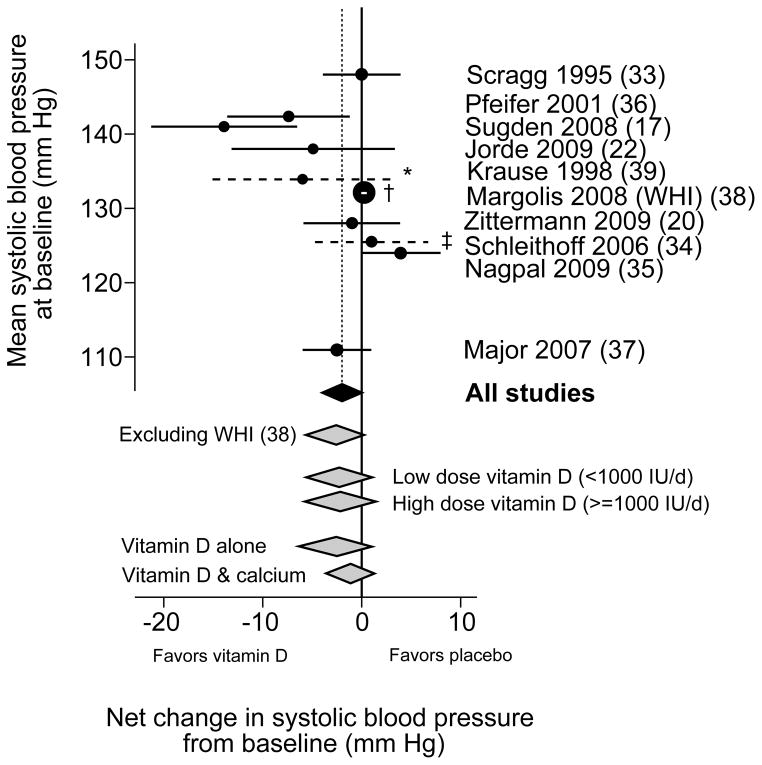
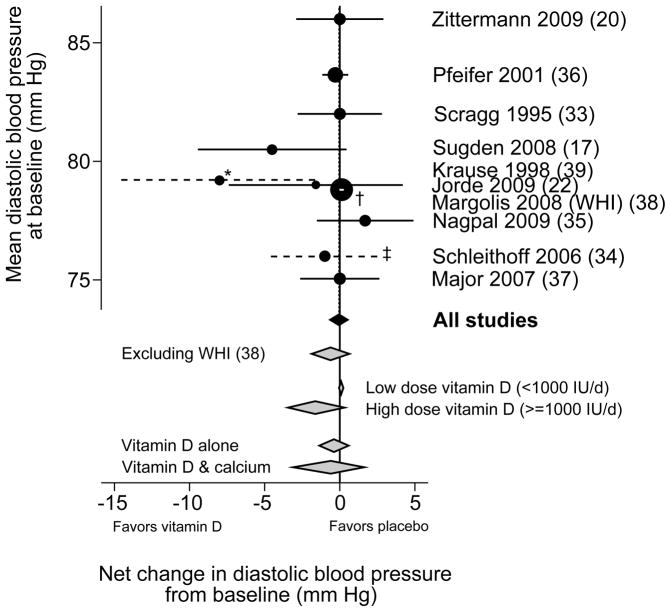
Most trials found no statistically significant effects on either systolic or diastolic blood pressure. Two trials reported relatively large net effects of vitamin D supplementation on systolic blood pressure, −7 mm Hg (Pfeifer et al (36)) and −14 mm Hg (Sugden et al (17)). The trial that compared UV-B with UV-A exposure also reported a large net effect on systolic blood pressure favoring UV-B (−6 mm Hg), but it was not clear whether the net effect was statistically significant (Krause et al (39)). The latter study was the only one that found a large net difference in diastolic blood pressure with UV-B exposure (39). In the largest and longest duration trial, the Women’s Health Initiative, combined low-dose vitamin D3 (400 IU/day) and calcium carbonate supplementation (1000 mg/day) had no effect on self-reported incident hypertension after 7 years of follow-up (38). In subgroup analyses from this trial, supplementation increased the risk of incident hypertension among blacks (RR 1.2; 95% CI 1.0, 1.4) (38). In meta-analysis of all trials, there was no statistically significant effect of vitamin D supplementation versus placebo on systolic blood pressure (weighted mean difference −1.9 mm Hg; 95% CI −4.2, 0.4; Figure 2A), but with significant heterogeneity (I2 = 69%) or on diastolic blood pressure (weighted mean difference −0.1 mm Hg; 95% CI −0.7, 0.5; I2 = 23%; Figure 2B). Excluding the large, long-term Women’s Health Initiative trial, the weighted mean differences for systolic and diastolic blood pressures were similar. There was no difference in systolic or diastolic blood pressure change in trials that provided vitamin D alone or in combination with calcium, or in changes in systolic blood pressure in trials that provided higher (≥1000 IU/day) versus lower (<1000 IU/day) dose of vitamin D. Studies using higher dose vitamin D had a statistically significant different effect on diastolic blood pressure (weighted mean difference −1.0 mm Hg) than studies using lower dose (1.0 mm Hg; P=0.039)
Figure 2.
Meta-analyses of the effect of vitamin D supplementation on net change in systolic (2A) and diastolic (2B) blood pressure.
WHI, Women’s Health Initiative trial.
Weighted mean difference and 95% confidence intervals in change in systolic (Figure 2A) and diastolic (Figure 2B) blood pressure for vitamin D supplementation versus placebo. The studies are arranged along the vertical axis according to the baseline blood pressure. The circle sizes are proportional to the study size. The black diamond represents the primary meta-analyses. Grey diamonds represent sensitivity and subgroup analyses. Dashed lines indicate studies for which standard errors were not reported; see footnotes.
* (Krause (39)) An estimate for the 95% confidence interval was derived from the reported full ranges of changes in blood pressure.
† (Margolis (WHI) (38)) The 95% confidence interval is within the circle.
‡ (Schleithoff (34)) An estimate for the 95% confidence interval was derived from the reported interquartile ranges of changes in blood pressure.
Vitamin D and Cardiovascular Disease
Longitudinal observational cohort studies
Seven studies analyzed vitamin D status and cardiovascular endpoints in nine different analyses from six cohorts (27–32, 40) (Appendix Table 1). The studies included 43,527 participants (89% white) who were followed from 5 to 27 years for incident cardiovascular disease. Cardiovascular endpoints included myocardial infarction (27, 32), cardiovascular-related mortality (28–30), a composite cardiovascular endpoint (40), and stroke (30–32). All studies measured 25(OH)D concentration and all reported multivariable adjusted results. Five studies were rated as good (27, 28, 30, 31, 40) and 2 poor quality (29, 32).
Overall, five of the nine analyses found that lower 25(OH)D concentration was associated with increased risk of incident cardiovascular disease (27, 28, 30, 31, 40) Figure 1B, Appendix Table 1). The Framingham Offspring Study found an association between lower 25(OH)D concentration and increased risk of overall cardiovascular events (40); however the association appeared to be nonlinear (somewhat U-shaped) and in subgroups analyses (not shown here) the association was statistically significant only among participants with hypertension at baseline. Among the studies that evaluated fatal cardiovascular events, two of three (28–30) found statistically significant associations for all fatal cardiovascular events (cardiac or stroke), favoring higher vitamin D status; two (30, 31) found similar significant associations with fatal stroke; and one (30) found no significant association with fatal cardiac events. Among the two studies that evaluated myocardial infarction (27, 32), only the analysis in the Health Professionals Follow-up Study (men only) (27) found a significant association between lower 25(OH)D concentration and increased risk. Because of the heterogeneity of outcomes, meta-analyses were not performed.
Randomized trials
Four trials (in 5 articles) reported the effect of vitamin D supplementation on incident cardiovascular disease (Appendix Table 2) (23, 41, 42, 43, 44). Three trials were rated fair quality (41, 42, 44) and one good quality (23, 43). None reported a statistically significant effect of vitamin D supplementation (with or without calcium) on various cardiovascular outcomes, including myocardial infarction, stroke, and other cardiac and cerebrovascular outcomes. Study participants were followed for 1, 5, or 7 years. The Women’s Health Initiative trial performed 12 analyses of different cardiovascular outcomes (23, 43), and reported a near statistically significant harmful effect with combined vitamin D and calcium supplementation on one composite cardiac outcome that included non-fatal myocardial infarction, coronary heart disease death or need for revascularization (RR 1.08; 95% CI 0.99, 1.19). The interventions and outcomes were too heterogeneous to perform meta-analysis.
DISCUSSION
Cross-sectional studies have reported consistent associations between lower vitamin D status and prevalent cardiometabolic outcomes (1, 45). In longitudinal observational studies, reviewed here, lower vitamin D status was associated with increased risk of incident hypertension and possibly cardiovascular disease, but the strengths of associations were attenuated compared to cross-sectional studies. The evidence from longitudinal studies of type 2 diabetes was sparse. In trials, there was no statistically significant effect of vitamin D supplementation on diastolic or systolic blood pressure, glycemic, or cardiovascular outcomes. However, there was a suggestion of lower systolic blood pressure (by 2 mm Hg, statistically nonsignificant) with vitamin D supplement use. Further data are necessary to adjudicate this observation.
There are several plausible mechanisms of how vitamin D may modify risk of cardiometabolic outcomes. Vitamin D may have direct (via vitamin D receptor activation) and indirect (via calcium homeostasis regulation) effects on various mechanisms related to type 2 diabetes pathophysiology, including impaired pancreatic beta cell function and insulin resistance (1). Regarding cardiovascular outcomes, vitamin D regulates the renin-angiotensin system (46), suppresses proliferation of vascular cell smooth muscle (47), ameliorates insulin resistance (18) improves endothelial cell-dependent vasodilation (48, 49), inhibits anticoagulant activity (50) and myocardial cell hypertrophy (51–53), and may modulate macrophage activity (54) and cytokine generation (34, 55).
Several possible reasons may explain the lack of apparent concordance between the cross-sectional, longitudinal observational, and randomized studies. The inverse association between vitamin D status and cardiometabolic outcomes may be confounded by a variety of factors. First, vitamin D status is an excellent marker of good health, including positive associations with young age, normal body weight, and a healthy lifestyle (56) and negative associations with smoking, parental history of myocardial infarction, and alcohol intake (2, 14, 27). Second, lower vitamin D status may reflect chronic non-specific illness. Therefore, the inverse association seen in cross-sectional studies may be due to reverse causation. Third, additional components in foods rich in vitamin D (e.g., fish or fortified dairy products) may have direct effects on cardiometabolic disease or, alternatively, foods rich in vitamin D may replace other foods that increase risk of cardiometabolic disease (e.g., fortified milk replacing sweetened drinks) (57). Finally, observational studies have used single measurements of serum or plasma 25(OH)D as a proxy of vitamin D status, which may not reflect long-term vitamin D status.
The Women’s Health Initiative, which is the largest trial on vitamin D and calcium supplementation to-date, reported no statistically significant effects for all cardiometabolic outcomes examined. However, this trial used a relatively small dose of vitamin D (400 IU/day), had difficulties with compliance over 7 years, and allowed participants in both intervention groups to take supplemental vitamin D. Based on dose and compliance, the effect of supplementation on 25(OH)D concentration in the Women’s Health Initiative trial has been estimated it to be only 5 nmol/L (58), an increment very unlikely to be associated with any change in risk of cardiometabolic outcomes based on data from the observational studies.
The optimal 25(OH)D concentration is currently under review by the Institute of Medicine. For a variety of skeletal and non-skeletal outcomes, it has been proposed that 25(OH)D concentration <25 nmol/L defines vitamin D deficiency, while a level >75 nmol/L is associated with improved bone and non-bone related outcomes (59). In the longitudinal observational studies, any decreased risk of cardiometabolic outcomes was seen only among those with a moderate 25(OH)D concentration (62–87 nmol/L) compared to those with relatively low levels (25–37 nmol/L). In several studies, an apparent threshold was seen where risk of cardiovascular disease did not decrease further with 25(OH)D concentration >50 nmol/L (24, 27–29, 40), indicating that vitamin D deficiency may increase risk but higher 25(OH)D concentration may not necessarily lower risk proportionately. These data suggest that clinically significant effects of improving vitamin D status on cardiometabolic outcomes may be seen only among those with vitamin D deficiency. Higher levels of 25(OH)D concentration (>250 nmol/L) have been recommended by some to optimize outcomes; however, there is a complete lack of longitudinal data (observational or trials) to support such a recommendation.
Our study has certain limitations based on the quality of the published studies included in the review. In the observational studies, the outcome was ascertained by self-report or from national registry data. A positive self-report is generally quite accurate in epidemiologic studies (60) and most of the included studies validated the outcome. However, early-stage cardiometabolic outcomes may have been undiagnosed and therefore missed and not included in the analyses. There was also substantial heterogeneity among studies, especially in vitamin D thresholds or doses analyzed, specific outcomes, and confounders adjusted for. Importantly, not all studies adjusted for sun exposure. Finally, study participants were mostly whites aged approximately 40 to 70 years old, thus limiting the applicability to other racial groups and life stages.
In conclusion, a lower vitamin D status was possibly associated with higher risk of incident hypertension and cardiovascular disease, but the association with diabetes-related outcomes remains unclear. As a whole, trials showed no statistically significant effect of vitamin D supplementation on cardiometabolic outcomes. The available data are inadequate to support the contention that cardiometabolic outcomes can be improved by raising vitamin D intake or serum or plasma 25(OH)D concentrations. It is therefore imperative that adequate randomized trials are conducted in well-defined populations (e.g. pre-diabetes, pre-hypertension, whites vs. non-whites) to test the potential role of vitamin D in primary prevention or therapy. Vitamin D remains a promising, though unproven, new element in the prevention and management of cardiometabolic disease.
Supplementary Material
Literature search and selection of studies for review.
* A total of 16,733 citations were screened for a wide array of clinical outcomes. The 5,739 citations refer to those specifically from the cardiovascular outcomes search, but potentially relevant citations from searches for other outcomes were also screened for cardiovascular outcomes.
† A total of 584 full text articles were retrieved for review for a wide array of clinical outcomes. The 106 articles refer to those specifically marked as having a cardiovascular outcome, but potentially relevant citations from searches for other outcomes were also screened for cardiovascular outcomes.
Acknowledgments
Funding: This review was funded under Contract No. HHSA 290-2007-10055-I from the Agency for Healthcare Research and Quality, NIH Research grants R01DK76092 and R01DK79003 (funded by the National Institute of Diabetes and Digestive and Kidney Disease and the NIH Office of Dietary Supplements), R21DK78867 (funded by the National Institute of Diabetes and Digestive and Kidney Disease), U.S. Department of Health and Human Services; and the Public Health Agency of Canada.
Primary Funding Sources: National Institute of Diabetes and Digestive and Kidney Disease, the National Institutes of Health Office of Dietary Supplements, Food and Drug Administration, Agency for Healthcare Research and Quality, and Public Health Agency of Canada.
We would like to acknowledge five additional investigators who participated in the design, implementation, and writing of the Tufts Evidence-based Practice Center evidence report on vitamin D and calcium, including abstract screening (6), but they were not primarily responsible for cardiometabolic outcomes and did not participate in writing this manuscript: Stanley Ip, MD; Jounghee Lee, PhD; Gowri Raman, MD; Athina Tatsioni, MD, PhD; and Teruhiko Terasawa, MD.
Footnotes
Disclaimer: The opinions expressed in this document are those of the authors and do not reflect the official position of the Agency for Healthcare Research and Quality, the National Institutes of Health, the U.S. Department of Health and Human Services, the Public Health Agency of Canada, or Health Canada.
Potential Financial Conflicts of Interest: None disclosed.
“This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.”
References
- 1.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2008;205(1):255–60. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Fleck A. Latitude and ischaemic heart disease. Lancet. 1989;1(8638):613. doi: 10.1016/s0140-6736(89)91634-6. [DOI] [PubMed] [Google Scholar]
- 4.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30(2 Pt 1):150–6. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 5.Voors AW, Johnson WD. Altitude and arteriosclerotic heart disease mortality in white residents of 99 of the 100 largest cities in the United States. J Chronic Dis. 1979;32(1–2):157–62. doi: 10.1016/0021-9681(79)90044-4. [DOI] [PubMed] [Google Scholar]
- 6.Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, et al. Evidence Report No. 183. (Prepared by the Tufts Evidence-based Practice Center under Contract No. HHSA 290-2007-10055-I) AHRQ Publication No. 09-E105. Rockville, MD: Agency for Healthcare Research and Quality; [Accessed 12 November, 2009]. Vitamin D and Calcium: A Systematic Review of Health Outcomes. Available at http://www.ahrq.gov/downloads/pub/evidence/pdf/vitadcal/vitadcal.pdf. [Google Scholar]
- 7.Agency for Healthcare Research and Quality. Methods Reference Guide for Effectiveness and Comparative Effectiveness Reviews, version 1.0. Rockville, MD: Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services; 2007. [Assessed 12 November, 2009]. Available at http://effectivehealthcare.ahrq.gov/repFiles/2007_10DraftMethodsGuide.pdf. [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition. J Nutr. 2008;138(12):2297–306. doi: 10.3945/jn.108.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Jama. 2001;285(15):1987–91. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28(12):2926–32. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 14.Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29(3):650–6. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 15.Knekt P, Laaksonen M, Mattila C, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19(5):666–71. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 16.Nilas L, Christiansen C. Treatment with vitamin D or its analogues does not change body weight or blood glucose level in postmenopausal women. Int J Obes. 1984;8(5):407–11. [PubMed] [Google Scholar]
- 17.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25(3):320–5. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 18.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30(4):980–6. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 19.de Boer IH, Tinker LF, Connelly S, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;31(4):701–7. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zittermann A, Frisch S, Berthold HK, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89(5):1321–7. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 21.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2009:1–7. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 22.Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr. 2009;48(6):349–54. doi: 10.1007/s00394-009-0020-3. [DOI] [PubMed] [Google Scholar]
- 23.Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846–54. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 24.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52(5):828–32. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51(4):1073–9. doi: 10.1161/HYPERTENSIONAHA.107.107821. [DOI] [PubMed] [Google Scholar]
- 27.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 29.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilkkinen A, Knekt P, Aro A, et al. Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170(8):1032–9. doi: 10.1093/aje/kwp227. [DOI] [PubMed] [Google Scholar]
- 31.Pilz S, Dobnig H, Fischer JE, et al. Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke. 2008;39(9):2611–3. doi: 10.1161/STROKEAHA.107.513655. [DOI] [PubMed] [Google Scholar]
- 32.Marniemi J, Alanen E, Impivaara O, et al. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005;15(3):188–97. doi: 10.1016/j.numecd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr. 1995;49(9):640–6. [PubMed] [Google Scholar]
- 34.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 35.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26(1):19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 36.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86(4):1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 37.Major GC, Alarie F, Dore J, Phouttama S, Tremblay A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. 2007;85(1):54–9. doi: 10.1093/ajcn/85.1.54. [DOI] [PubMed] [Google Scholar]
- 38.Margolis KL, Ray RM, Van Horn L, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women’s Health Initiative Randomized Trial. Hypertension. 2008;52(5):847–55. doi: 10.1161/HYPERTENSIONAHA.108.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352(9129):709–10. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 40.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. Bmj. 2003;326(7387):469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brazier M, Grados F, Kamel S, et al. Clinical and laboratory safety of one year’s use of a combination calcium + vitamin D tablet in ambulatory elderly women with vitamin D insufficiency: results of a multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. 2005;27(12):1885–93. doi: 10.1016/j.clinthera.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 43.LaCroix AZ, Kotchen J, Anderson G, et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64(5):559–67. doi: 10.1093/gerona/glp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prince RL, Austin N, Devine A, Dick IM, Bruce D, Zhu K. Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch Intern Med. 2008;168(1):103–8. doi: 10.1001/archinternmed.2007.31. [DOI] [PubMed] [Google Scholar]
- 45.Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy. 2009;29(6):691–708. doi: 10.1592/phco.29.6.691. [DOI] [PubMed] [Google Scholar]
- 46.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90(1–5):387–92. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13(6 Pt 2):954–9. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 48.Borges AC, Feres T, Vianna LM, Paiva TB. Effect of cholecalciferol treatment on the relaxant responses of spontaneously hypertensive rat arteries to acetylcholine. Hypertension. 1999;34(4 Pt 2):897–901. doi: 10.1161/01.hyp.34.4.897. [DOI] [PubMed] [Google Scholar]
- 49.Borges AC, Feres T, Vianna LM, Paiva TB. Recovery of impaired K+ channels in mesenteric arteries from spontaneously hypertensive rats by prolonged treatment with cholecalciferol. Br J Pharmacol. 1999;127(3):772–8. doi: 10.1038/sj.bjp.0702581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohsawa M, Koyama T, Yamamoto K, Hirosawa S, Kamei S, Kamiyama R. 1alpha,25-dihydroxyvitamin D(3) and its potent synthetic analogs downregulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation. 2000;102(23):2867–72. doi: 10.1161/01.cir.102.23.2867. [DOI] [PubMed] [Google Scholar]
- 51.O’Connell TD, Berry JE, Jarvis AK, Somerman MJ, Simpson RU. 1,25-Dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol. 1997;272(4 Pt 2):H1751–8. doi: 10.1152/ajpheart.1997.272.4.H1751. [DOI] [PubMed] [Google Scholar]
- 52.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288(1):E125–32. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 53.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103(3–5):521–4. doi: 10.1016/j.jsbmb.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sadeghi K, Wessner B, Laggner U, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36(2):361–70. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 55.Muller K, Haahr PM, Diamant M, Rieneck K, Kharazmi A, Bendtzen K. 1,25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine. 1992;4(6):506–12. doi: 10.1016/1043-4666(92)90012-g. [DOI] [PubMed] [Google Scholar]
- 56.Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr. 2008;88(2):500S–506S. doi: 10.1093/ajcn/88.2.500S. [DOI] [PubMed] [Google Scholar]
- 57.Jacobs DR, Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78(3 Suppl):508S–513S. doi: 10.1093/ajcn/78.3.508S. [DOI] [PubMed] [Google Scholar]
- 58.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. The American Journal of Clinical Nutrition. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 59.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 60.Midthjell K, Holmen J, Bjorndal A, Lund-Larsen G. Is questionnaire information valid in the study of a chronic disease such as diabetes? The Nord-Trondelag diabetes study. J Epidemiol Community Health. 1992;46(5):537–42. doi: 10.1136/jech.46.5.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Literature search and selection of studies for review.
* A total of 16,733 citations were screened for a wide array of clinical outcomes. The 5,739 citations refer to those specifically from the cardiovascular outcomes search, but potentially relevant citations from searches for other outcomes were also screened for cardiovascular outcomes.
† A total of 584 full text articles were retrieved for review for a wide array of clinical outcomes. The 106 articles refer to those specifically marked as having a cardiovascular outcome, but potentially relevant citations from searches for other outcomes were also screened for cardiovascular outcomes.