Abstract
Background
A polyphenol constituent of green tea, epigallocatechin gallate (EGCG), has anti-carcinogenic properties. A growing number of studies document EGCG-mediated induction of apoptotic pathways and inhibition of pro-survival factors when combined with chemotherapy or radiation. We evaluated the efficacy of EGCG in modulating Photofrin (PH) mediated Photodynamic Therapy (PDT) responses.
Methods
Mouse mammary carcinoma (BA) cells and transplanted BA tumors growing in C3H mice were treated with PH-mediated PDT. Select groups of treated cells and mice also received EGCG and then cytotoxicity, tumor response, and expression of survival molecules were evaluated in all experimental groups.
Results
EGCG increased apoptosis and cytotoxicity in BA cells exposed to PH-mediated PDT. The initial pro-survival phase of the unfolded protein response (UPR), characterized by increased expression of the 78 kDa glucose regulated protein (GRP-78), was induced by PDT. The second pro-apoptotic phase of the UPR, characterized by phospho-c-Jun N-terminal kinase (p-JNK) expression, activation of caspases-3 and 7, poly ADP ribose polymerase (PARP) cleavage, and expression of C/EBP homologous protein (CHOP) was observed when PDT was combined with EGCG. EGCG also decreased the expression of the pro-survival proteins GRP-78 and survivin, and attenuated PDT-induced prostaglandin E2 (PGE2) expression in PDT treated cells. Comparable responses also were observed when BA tumors were treated with PDT and EGCG. In addition, PDT-induced expression of metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF) was down-regulated in treated tumor tissue by EGCG.
Conclusions
The polyphenol EGCG improves PDT efficacy by increasing tumor apoptosis and decreasing expression of pro-survival and angiogenic molecules within the tumor microenvironment.
Keywords: apoptosis, COX-2, EGCG, GRP-78, PDT, survivin
INTRODUCTION
Tumor treatment with PDT involves the administration of a photosensitizer or prodrug followed by exposure of the target lesion to visible light (1, 2). Photochemical generation of reactive oxygen species leads to cell death and acute changes within the tumor microenvironment (1). Cell killing involves activation of apoptotic, necrotic, and/or autophagic pathways (3-5). Treatment responses include inflammation, vascular injury, and hypoxia within PDT irradiated tumor tissue. These reactions activate transcription factors, cytokines, growth factors and proteinases and can lead to the over-expression of pro-survival and pro-angiogenic factors (6). Targeting these molecular responses may improve overall PDT efficacy and an increasing number of laboratories are addressing this issue using a variety of combined modality approaches (7-11).
The primary polyphenol component of green tea, EGCG, possesses anti-carcinogenic and anti-tumor properties and this compound is being investigated as a cancer treatment agent when combined with chemotherapy or radiation (12). Signaling pathways involved with tumor growth are blocked by EGCG as are angiogenic and inflammatory responses (13,14). Targets of EGCG include a number of PDT inducible molecules. EGCG binds to the ATPase site of the pro-survival chaperone GRP-78 which inhibits the binding of this stress protein to caspase-7 and increases apoptosis (15,16). EGCG also decreases the activity of MMPs, COX-2, HIF-1α and VEGF (17-19). A previous study demonstrated that cellular and tumor photosensitization is increased and COX-2 levels are decreased when EGCG is included in a PDT regimen involving the chlorin photosensitizer radachlorin (20). In the current study we examined cellular and tumor responses when PH mediated PDT was combined with EGCG. Our data indicate that EGCG ameliorates PDT by enhancing apoptosis and by targeting mediators of an induced inflammatory/angiogenic response.
MATERIALS AND METHODS
Drugs
Photofrin porfimer sodium (PH), provided by Axcan Pharma, Inc. (Mont-Saint Hilaire, QC, Canada), was dissolved in 5% dextrose in water to make a 2.5 mg/ml stock solution and frozen at –20 °C until used. Stock solutions were diluted to 25 μg/ml in culture medium prior to in vitro experiments or to a concentration of 0.5 mg/ml in saline prior to in vivo experiments. EGCG, purchased from Sigma (St. Louis, MO), was dissolved in ddH2O to make a stock concentration of 1 mM for in vitro experiments. Stock solutions were diluted to reach working concentrations with medium immediately before in vitro experiments. For in vivo studies EGCG was diluted in saline to obtain a working concentration of 4 mg/ml.
Cell Culture and Tumor Models
Mouse mammary carcinoma (BA) cells were grown as monolayer cultures in RPMI 1640 supplemented with 10% FCS and antibiotics. BA tumors were generated by subcutaneous trochar injection of 1-mm3 pieces of tumor to the right hind flank of 8- to 12-week-old female C3H/HeJ mice (10). Treatments were started when tumors reached 6 mm in largest diameter. Our Institutional Animal Care and Use Committee approved all in vivo experiments.
In vitro and In vivo Treatment Protocols
BA cells were seeded onto plastic cell culture dishes and incubated for 24 hours in complete growth medium to allow for attachment (10). Prior to light exposure, cells were incubated for 16 hr in the dark with PH (25 μg/ml) in medium containing 1% serum. Cells were then incubated for an additional 30 minutes in fresh medium containing 5% FCS and rinsed in medium without serum prior to exposure to varying doses of broad spectrum (570-650 nm) red light generated by a parallel series of red Mylar-filtered 30 W fluorescent bulbs using a dose rate of 0.35 mW/cm2. For combination experiments, PDT treated cells were incubated with medium in the presence or absence of EGCG (50 μM) for the remainder of the experiment prior to analysis of clonogenic survival, protein expression and levels of apoptosis (10). Levels of MMPs secreted into serum free media of control and treated cells were analyzed by zymography.
PDT treatment of BA tumors included an i.v. injection of PH (5 mg/kg) followed 24 hours later with irradiation of the tumor using a solid-state diode laser from Diomed (Andover, MA) emitting red light at 630 nm (11, 13). Light was delivered through a quartz fiber fitted with a microlens on its distal tip and light irradiance were measured with a power meter. A delivered light dose rate of 75 mW/cm2 and a total light dose of 200 J/cm2 were used for all in vivo PDT treatments. EGCG was administered via ip injection at a dose of 40 mg/kg starting immediately after light exposure (time 0) and then once a day for 10 days. Treated mice were monitored 3 times per week for tumor recurrence and a treatment cure was defined as being tumor free for 90 days post PDT.
Apoptosis Assay
Apoptosis was assayed using the Cell Death Detection ELISA Plus kit from Roche Diagnostic (Mannheim, Germany). This kit quantifies mono- and oligonucleosomes from cell lysates using mouse monoclonal antibodies against DNA and histones in a quantitative photometric sandwich enzyme immunoassay (11). Cells plated in 100-mm dishes were treated as described above. Cells were lysed and analyzed for apoptosis 72 after light treatment. Absorbance at 450 nm was documented for treated and control samples to determine apoptotic enrichment factors. Results were normalized for protein concentrations.
Western Immunoblot Analysis
Protein expression profiles were documented by Western immunoblot analysis as previously described (10,11). Cells were collected at specified times following treatment and sonicated in 1X lysis buffer from Cell Signaling Technology (Danvers, MA) containing phenylmethanesulfonyl fluoride. Tumors were collected 24 hr following treatment, homogenized with a polytron in 1 X reporter lysis buffer from Promega (Madison, WI). Samples were size-separated on discontinuous polyacrylamide gels (10-16%) and transferred overnight to nitrocellulose membranes. Filters were blocked for 2 hr with 5% nonfat milk and then incubated overnight with either rabbit polyclonal antibody to GRP 78 from Santa Cruz Biotech (Santa Cruz, CA), mouse monoclonal antibody to PARP from Calbiochem/EMD Biosciences (La Jolla, CA), mouse monoclonal anti-CHOP from Santa Cruz Biotech, rabbit polyclonal antibody to survivin from Chemicon International (Temecula, CA), rabbit polyclonal antibody to caspase-3 from BD Biosciences Pharmingen (San Diego, CA), rabbit polyclonal antibody to cleaved caspase-7 and rabbit polyclonal antibody to phospho -SAPK/JNK from Cell Signaling Technology. Filters were then incubated with either an anti-mouse or an anti-rabbit peroxidase conjugate from Sigma (St. Louis, MO), and the resulting complexes were visualized by enhanced chemiluminescence autoradiography from Amersham Life Science (Chicago, IL). Protein loading was evaluated by incubating the same filters with a mouse monoclonal anti-actin antibody from MP Biochemicals (Aurora, OH). Autoradiographs were quantified by scanning densitometry. Experiments were repeated 2-4 times.
EIA Assay
An enzyme immunoassay kit from Cayman Chemical Co. (Ann Arbor, MI) was used to quantify PGE2 levels from culture media and from tumor homogenates (10). Results obtained from tumor homogenates were normalized for total protein concentrations.
SDS-PAGE Zymography
Aliquots from tumor lysates and conditioned media from control and treated cells were denatured at room temperature in Laemli Tris-Glycine SDS-PAGE denaturing buffer, run on 10% gelatin gels from Invitrogen Life Technologies, Inc. (Carlsbad, CA), and renatured by soaking the gels for 30 min in renaturing buffer containing a nonionic detergent from Invitrogen Life Technologies, Inc. Gels were subsequently incubated overnight with gentle agitation at 37 °C in developing buffer from Invitrogen Life Technologies, Inc. Resulting gels were stained with 0.5% Coomassie Blue R-250, destained in a solution containing 10% methanol and 7.5% acetic acid, and dried. Proteins having gelatinolytic activity were visualized as white bands on blue gel.
Statistics
The Wilcoxon log-rank test was used to determine statistical significance between treatment groups for tumor cure analysis. A two-tailed t-test analysis was used to determine statistical differences within survival, apoptotic, EIA, and densitometric assays. Differences with p < 0.05 were considered significant.
RESULTS
EGCG enhances PDT-mediated photosensitization and apoptosis
Cytotoxicity of BA cells exposed to PDT, EGCG, or PDT combined with EGCG was determined using a clonogenic survival assay (Fig. 1 A). As expected, EGCG and PDT exhibited measurable cytotoxicity as single treatments and combining the two treatments enhanced the level of toxicity. Cells exposed to PH photosensitization showed minimal apoptosis while apoptotic levels were significantly increased when the treatment regimen included both agents (Fig. 1B). These data demonstrate a concomitant increase in cellular cytotoxicity and apoptosis when EGCG is combined with PH-photosensitization.
Fig. 1.
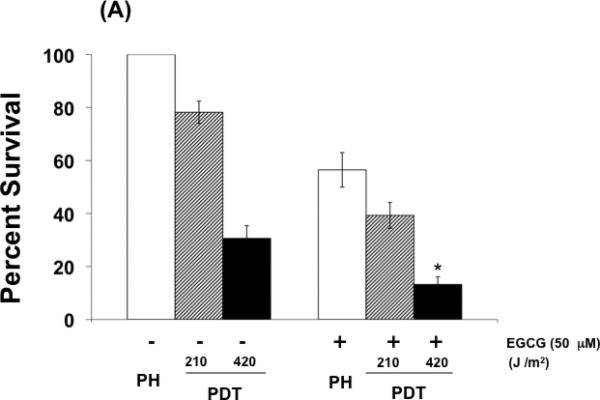
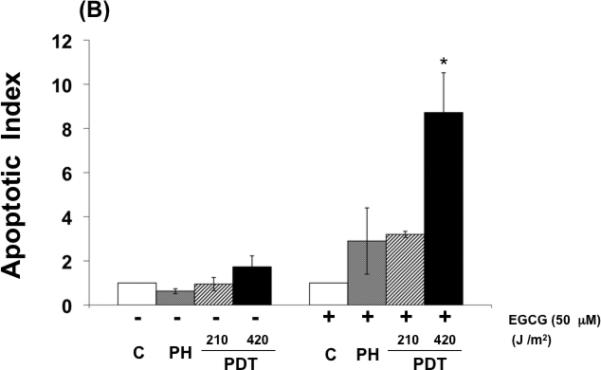
EGCG increases phototoxicity and apoptosis in PDT-treated BA mammary cells. (A) Clonogenic survival of cells exposed to PDT, EGCG, or the combination of PDT and EGCG. Cells were incubated with PH (25 ug/ml) in the dark for 16 hr and exposed to red light at the doses of 210 and 420 J/cm2. EGCG was added to the culture medium immediately after PDT and remained in the medium for the duration of the experiment. (B) Apoptosis enhancement factors for BA cells exposed to PDT, EGCG, or PDT combined with EGCG. Cells were analyzed for apoptosis using the Cell Death Detection ELISA Plus kit 72 hrs after light treatment. Results were normalized to protein concentrations. Columns, mean; bars, ± SE of four-six separate experiments; *, p< 0.05 (PDT versus PDT + EGCG).
EGCG activates the unfolded protein response (UPR) and a pro-apoptotic signaling pathway during PH mediated photosensitization
The UPR is an endoplasmic reticulum (ER) stress reaction. The initial UPR phase increases expression of ER chaperones like GRP-78 to assist in proper protein folding (21). Under severe and prolonged ER stress the UPR triggers apoptosis by JNK phosphorylation and initiation of a signaling pathway involving caspase activation (22). Figure 2 (A) shows that PH mediated photosensitization induced GRP-78 expression and activated the initial UPR phase in treated cells. The addition of EGCG to the treatment protocol induced the pro-apoptotic UPR phase with decreased GRP-78 and survivin expression, increased phosphorylation/activation of JNK, activation of caspase-3 and caspase-7, and induced PARP cleavage as shown in Fig 2 (A). PGE2 is a pro-survival molecule with angiogenic and anti-apoptotic properties (10). PH mediated photosensitization increases expression of this prostanoid while the addition of EGCG decreased induced levels in treated cells (Fig. 2 B). These results indicate that the increased cytotoxicity observed when EGCG is combined with PH mediated photosensitization involves an activated UPR and ER stress-associated apoptosis as well as down regulation of pro-survival molecules.
Fig. 2.
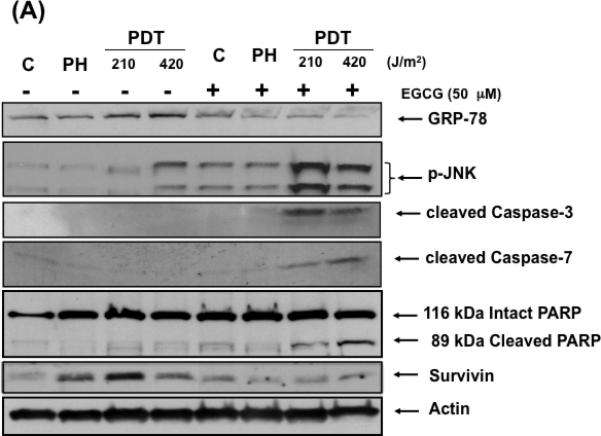
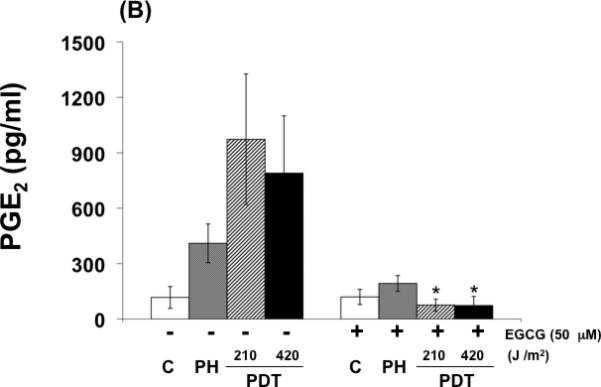
EGCG induces a pro-apoptotic response in PDT treated cells. (A) Western immunoblot analysis documenting levels of GRP-78, phosphorylated JNK, cleaved caspase-3, caspase-7 and PARP, and survivin expression in control and treated cells. Cell lysates were collected and analyzed for protein expression by Western immunoblot analysis using commercially available antibodies. (B) EGCG blocks expression of PGE2 in BA cells following PDT. Readings obtained from an EIA kit for PGE2. Columns, mean; bars, ± SE; of three separate experiments *, p< 0.05 (PDT versus PDT + EGCG).
EGCG enhances PDT responsiveness, decreases GRP-78 levels, and increases expression of UPR pro-apoptotic molecules in treated tumors
We next examined the tumoricidal effectiveness of PDT when combined with EGCG. Figure 3 (A) shows the percentage of mice without tumor recurrences as a function of days after treatment. PDT, using a single 200 J/cm2 light dose, resulted in a 50% tumor cure rate while the same PDT protocol combined with EGCG resulted in an 80% tumor cure rate and this difference was found to be statistically significant (p < 0.05). The identical EGCG treatment regimen, without PDT, did not produce any cures but did prolong tumor regrowth kinetics (time for tumors to grow to 250 mm3) from 11.3 days to 16.3 days (data not shown). Normal skin reactions above and around the treated tumors were comparable for all experimental groups. Protein expression patterns of lysates from treated tumors in Fig 3 (B) show that EGCG inhibited PDT-induced GRP-78, increased CHOP expression, increased JNK phosphorylation, activated caspase-3 and caspase-7, produced PARP cleavage and decreased survivin levels. These results suggest that EGCG improves PDT tumoricidal effectiveness and produces an UPR-associated pro-apoptotic reaction comparable to what is observed in PDT treated cells.
Fig. 3.
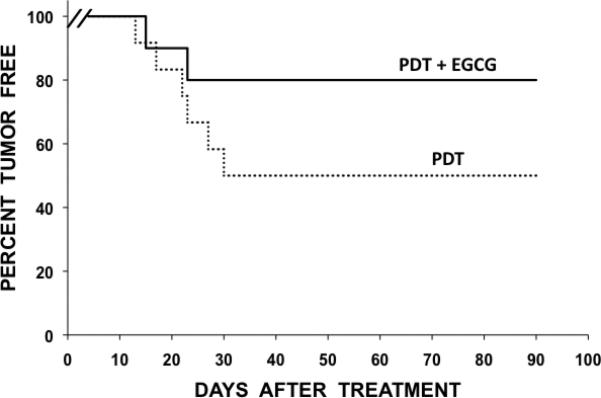

EGCG enhances the tumoricidal action of PDT and increases in-vivo expression of pro-apoptotic molecules in BA tumors. (A) Percentage of tumor-free mice as a function of time after either PDT alone (n=12) and PDT combined with EGCG (n=12). Percent cures are plotted as a function of time after treatment. A statistically significant difference (p< 0.05) was observed for PDT plus EGCG versus PDT alone. (B) Tumor lysates were collected 24 hours after PDT and assayed for GRP-78, phosphorylated JNK, cleaved caspase-3, cleaved caspase-7, PARP, survivin and actin by Western blot analysis using commercially available antibodies.
EGCG targets tumor microenvironment
The effects of EGCG on the expression of inflammatory and angiogenic related proteins induced by PDT in tumors are shown in Fig. 4 (A-C) . In agreement with previous findings, PGE2, VEGF and MMP expression in tumors, examined by ELISA (Fig. 4 A,B) or by zymography (Fig. 4 C), increased after PDT (9,10,23). Combination treatments involving PDT and EGCG inhibited PDT-mediated induction of PGE2, MMP-2 and MMP-9, and partially reduced PDT induced VEGF levels. These results suggest that EGCG may increase tumoricidal activity in part by decreasing expression of PDT-induced inflammatory and pro-angiogenic molecules.
Fig. 4.
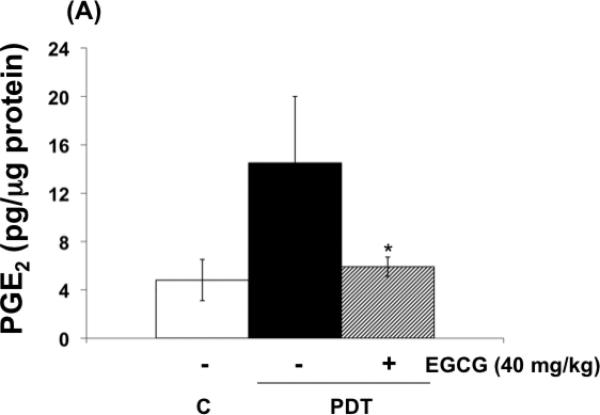
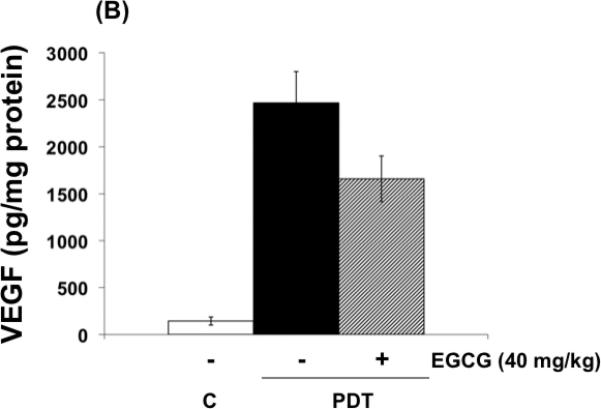
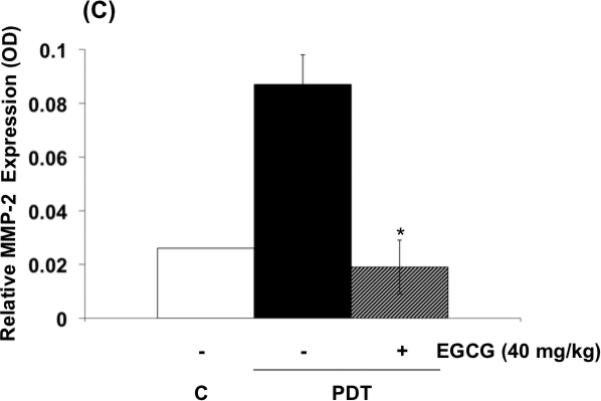
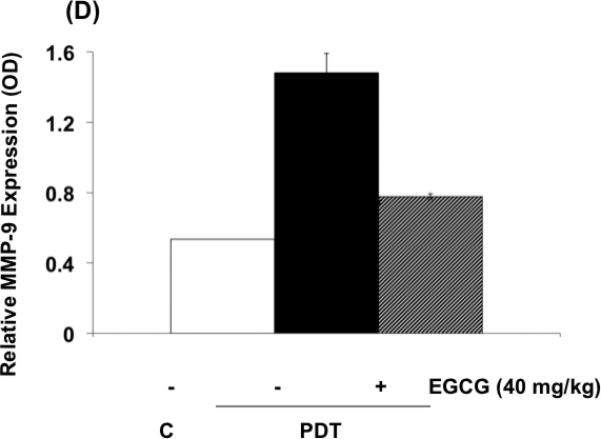
EGCG decreases the expression of inflammatory and pro-angiogenic factors in PDT-treated BA tumors. Tumor lysates were collected 24 hours after PDT and levels of PGE2 and VEGF (A, B) were determined using EIA kits. MMP enzymatic activity was visualized via gel zymography. Levels of MMP-2 and MMP-9 were determined by densitometry from gelatinolytic bands (C, D). Columns, mean; bars, ± SE; of two separate experiments *, p< 0.05 (PDT versus PDT + EGCG).
DISCUSSION
Photochemical reactions associated with PDT generate extensive oxidative stress in the form of singlet oxygen within exposed cells and tissues (1,3). One of the responses to oxidative stress is induction of a multi phase UPR within the endoplasmic reticulum (ER) (22). A major component of the initial prosurvival phase of the UPR involves increased expression of the ER molecular chaperone GRP-78 (24). This stress protein is actively involved in proper protein folding and assembly, the degradation of misfolded proteins, suppression of caspase-7 activation, and calcium homeostasis within the ER. A growing number of studies document that increased GRP-78 expression is correlated with tumor recurrence and decreased survival, making this ER chaperone a useful biomarker of poor overall treatment response (25). A variety of preclinical pharmacologic and genetic approaches are used to down regulate GRP-78 with the goal of increasing tumor responsiveness and treatment outcome. One such strategy involves EGCG, a major constituent of green tea, to directly inhibit GRP-78 activity (16). The ATP binding site of GRP-78 is targeted by EGCG and effectively blocks the chaperone and prosurvival actions of this protein (15). Our current study confirms that PDT induces significant GRP-78 expression (26) and that combining EGCG with PDT decreases GRP-78 and activates the pro-apoptotic phase of UPR as characterized by JNK phosphorylation, caspase 7 and caspase 3 induction together with PARP cleavage, CHOP expression, and increased levels of apoptosis. EGCG is also reported to down regulate survivin in leukemia cells and xenografts and this response is associated with mitochondria damage and release of the caspase activator cytochrome c into the cytoplasm (27). We documented that EGCG down regulated PDT induced expression of survivin and this also would contribute to the pro-apoptotic phenotype exhibited by our combined treatment approach (28).
The tumor microenvironment represents an additional treatment target when EGCG is used with PDT. Along with the pro-apoptotic UPR reaction activated when EGCG and PDT are combined, we also detected decreased expression of inflammatory and angiogenic molecules in tumor tissue including MMPs, COX-2, and VEGF. We previously demonstrated PDT induces increased expression of MMP-2 and MMP-9 within treated tumor tissue and a broad spectrum MMP inhibitor, Prinomastat, effectively enhanced PDT responsiveness by decreasing MMP activity (23). EGCG is a potent inhibitor of MMPs (17) and we observed significant attenuation of PDT induced MMPs when EGCG was added to the PDT treatment regimen. These effects are not observed in BA cells treated in culture following single or combined procedures (data not shown) and this indicates that non-malignant cells within the tumor microenvironment are actively involved in the response to our combined modality protocol. Inflammatory reactions within a tumor are associated with poor treatment outcomes including increased angiogenesis (6). PDT is a potent inducer of COX-2 and this leads to increased expression of PGE2 within treated tumors (10). Inhibition of COX-2 is one of the mechanisms identified for green tea mediated anti-proliferation (18). We observed a significant decrease in PGE2 when PDT was combined with EGCG. This agrees with results from a recent study using mouse TC-1 lung cancer cells and tumors to examine the combination of radachlorin mediated PDT and EGCG (20). EGCG also exerts anti-angiogenic activity in part by disturbing VEGFR-2 activation in endothelial cells (29) and inhibiting tube formation by endothelial cells (30). In the current study we confirm increased VEGF expression within PDT treated tumor tissue and the ability of the EGCG to modestly decrease the induced VEGF levels. This reduction in VEGF may also contribute to decreases in VEGFR-2 activity and angiogenesis (31).
In summary, EGCG continues to be evaluated in clinical trials as an adjunctive therapy for oncologic and non-oncologic disorders. We show EGCG effectively improves PDT responsiveness both in-vitro and in-vivo through multiple mechanisms. EGCG enhanced PDT-mediated cytotoxicity by inducing pro-apoptotic pathways and by inhibiting the expression of pro-angiogenic molecules. These results support additional preclinical studies designed to examine EGCG optimization during PDT.
Acknowledgments
Contract grant sponsor: National Institutes of Health, RO1 CA31230, Contract grant sponsor: Las Madrinas Endowment for Experimental Therapeutics
REFERENCES
- 1.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic Therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dougherty TJ. An update on photodynamic therapy applications. J Clin Laser Med Surg. 2002;20:3–7. doi: 10.1089/104454702753474931. [DOI] [PubMed] [Google Scholar]
- 3.Oleinick NL, Evans HH. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res. 1998;150:s146–s156. [PubMed] [Google Scholar]
- 4.Kessel D, Reiners JJ. Apoptosis and autophagy after mitochondrial or endoplasmic reticulum photodamage. Photochem Photobiol. 2007;83:1024–1028. doi: 10.1111/j.1751-1097.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta. 2007;1776:86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Gomer CJ, Ferrario A, Luna M, Rucker N, Wong S. Photodynamic therapy: combined modality approaches targeting the tumor microenvironment. Lasers Surg Med. 2006;38:516–521. doi: 10.1002/lsm.20339. [DOI] [PubMed] [Google Scholar]
- 7.Moor AC. Signaling pathways in cell death and survival after photodynamic therapy. J Photochem Photobiol B-Biology. 2000;57:1–13. doi: 10.1016/s1011-1344(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 8.Gollnick SO, Evans SS, Baumann H, Owczarczak B, Maier P, Vaughan L, Wang WC, Unger E, Henderson BW. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br J Cancer. 2003;88:1772–1779. doi: 10.1038/sj.bjc.6600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrario A, Gomer CJ. Avastin enhances photodynamic therapy treatment of Kaposi's sarcoma in a mouse tumor model. J Environ Pathol Oncol. 2006;25:251–259. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.160. [DOI] [PubMed] [Google Scholar]
- 10.Ferrario A, Fisher A, Rucker N, Gomer CJ. Celecoxib and NS-398 enhance photodynamic therapy by increasing in vitro apoptosis and decreasing in vivo inflammatory and angiogenic factors. Cancer Res. 2005;65:9473–9478. doi: 10.1158/0008-5472.CAN-05-1659. [DOI] [PubMed] [Google Scholar]
- 11.Ferrario A, Rucker N, Wong S, Luna M, Gomer CJ. Survivin, a member of the inhibitor of apoptosis family, is induced by photodynamic therapy and is a target for improving treatment response. Cancer Res. 2007;67:4989–4995. doi: 10.1158/0008-5472.CAN-06-4785. [DOI] [PubMed] [Google Scholar]
- 12.Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxidant and Redox Signaling. 2005;7:1630–1647. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 13.Fassina G, Vene R, Morini M, Minghello S, Benelli R, Noonan DM, Albini A. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin Cancer Res. 2004;10:4865–4873. doi: 10.1158/1078-0432.CCR-03-0672. [DOI] [PubMed] [Google Scholar]
- 14.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenols epigallocatechin-2-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Yin Y, Hua H, Li T, Wang R, Liu D, Zhang Y, Jiang Y. Blockade of GRP78 sensitizes breast cancer cells to microtubules-interfering agents that induce the unfolded protein response. J Cell Mol Med. 2009;13:3888–3897. doi: 10.1111/j.1582-4934.2009.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, Ma W-Y, Bode AN, Dong Z. (-)-epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9269. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 17.Garbisa S, Sartor L, Biggin S, Salvato B, Benelli R, Albini A. Tumor gelatinase and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer. 2001;91:822–832. doi: 10.1002/1097-0142(20010215)91:4<822::aid-cncr1070>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Peng G, Dixon DA, Muga SJ, Smith TJ, Wargovich MJ. Green tea polyphenols epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol Carcinogenesis. 2006;45:309–319. doi: 10.1002/mc.20166. [DOI] [PubMed] [Google Scholar]
- 19.Lin S-K, Shun C-T, Kok S-H, Wang C-C, Hsiao T-Y, Liu C-M. Hypoxia stimulated vascular endothelial growth factor production in human nasal polyp fibroblasts. Arch Otolaryngol Head Neck Surg. 2008;134:522–527. doi: 10.1001/archotol.134.5.522. [DOI] [PubMed] [Google Scholar]
- 20.Raish M, Husain S, Bae SM, Han SJ, Park CH, Shin JC, Ahn WS. Photodynamic therapy in combination with green tea polyphenols EGCG enhances antitumor efficacy in human papillomavirus 16 (E6/E7) immortalized tumor cells. J Appl Res. 2010;10:58–67. [Google Scholar]
- 21.Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors. J Biol Chem. 2003;23:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 22.Strasser A, Puthalakath H. Fold up or perish: unfolded protein response and chemotherapy. Cell Death Differ. 2008;15:223–225. doi: 10.1038/sj.cdd.4402279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrario A, Chantrain CF, von Tiehl K, Buckley S, Rucker N, Shalinsky DR, Shimada H, DeClerck YA, Gomer CJ. The matrix metalloproteinase inhibitor Prinomastat enhances photodynamic therapy responsiveness in a mouse tumor model. Cancer Res. 2004;64:2328–2332. doi: 10.1158/0008-5472.can-04-0071. [DOI] [PubMed] [Google Scholar]
- 24.Pirko P, Schonthal AH, Hofman FM, Chen T, Lee AS. The unfolded protein response regulator GRP/BiP as a novel target for increasing chemosensitivity in malignant cells. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 25.Virrey JJ, Dong D, Stiles P, Patterson JB, Pen L, Ni M, Schonthal AH, Chen TC, Hofman FM, Lee AS. Stress chaperone GRP-78/BiP confers chemoresistance to tumor-associated endothelial cells. Mol Cancer Res. 2008;6:1268–1275. doi: 10.1158/1541-7786.MCR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomer CJ, Ferrario A, Rucker N, Wong S, Lee AS. Glucose regulated protein induction and cellular resistance to oxidative stress mediated by porphyrin photosensitization. Cancer Res. 1991;51:6574–6579. [PubMed] [Google Scholar]
- 27.Nakazato T, Ito K, Miyakawa Y, Kinjo K, Yamada T, Hozumi N, Ikeda Y, Kizaki M. Catechin, a green tea component, rapidly induces apoptosis of myeloid leukemic cells via modulation of reactive oxygen species production in vitro and inhibits tumor growth in vivo. Hematol J. 2005;90:317–325. [PubMed] [Google Scholar]
- 28.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez SK, Guo W, Liu L, Band MA, Paulson EK, Meydani M. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int J Cancer. 2006;118:1635–1644. doi: 10.1002/ijc.21545. [DOI] [PubMed] [Google Scholar]
- 30.Tran F-Y, Nguyen N, Meydani M. Green tea catechins inhibit VEGF-induced angiogenesis in vitro through suppression of VE-cadherin phosphorylation and inactivation of AKT molecule. Int J Cancer. 2003;106:871–878. doi: 10.1002/ijc.11325. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui IA, Malik A, Adhami VM, Asim M, Hafeez BB, Sarfaraz S, Mukhtar H. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;27:2055–2063. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]


