Abstract
The cucurbits translocate the galactosyl-sucrose oligosaccharides raffinose and stachyose, therefore, α-galactosidase (α-d-galactoside galactohydrolase, EC 3.2.1.22) is expected to function as the initial enzyme of photoassimilate catabolism. However, the previously described alkaline α-galactosidase is specific for the tetrasaccharide stachyose, leaving raffinose catabolism in these tissues as an enigma. In this paper we report the partial purification and characterization of three α-galactosidases, including a novel alkaline α-galactosidase (form I) from melon (Cucumis melo) fruit tissue. The form I enzyme showed preferred activity with raffinose and significant activity with stachyose. Other unique characteristics of this enzyme, such as weak product inhibition by galactose (in contrast to the other α-galactosidases, which show stronger product inhibition), also impart physiological significance. Using raffinose and stachyose as substrates in the assays, the activities of the three α-galactosidases (alkaline form I, alkaline form II, and the acid form) were measured at different stages of fruit development. The form I enzyme activity increased during the early stages of ovary development and fruit set, in contrast to the other α-galactosidase enzymes, both of which declined in activity during this period. In the mature, sucrose-accumulating mesocarp, the alkaline form I enzyme was the major α-galactosidase present. We also observed hydrolysis of raffinose at alkaline conditions in enzyme extracts from other cucurbit sink tissues, as well as from young Coleus blumei leaves. Our results suggest different physiological roles for the α-galactosidase forms in the developing cucurbit fruit, and show that the newly discovered enzyme plays a physiologically significant role in photoassimilate partitioning in cucurbit sink tissue.
The galactosyl-Suc sugars stachyose and raffinose, together with Suc, are the primary translocated sugars in the phloem of cucurbits (Gross and Pharr, 1982; Richardson et al., 1982; Schaffer et al., 1996), including melon (Cucumis melo) (Mitchell et al., 1992; Chrost and Schmitz, 1997). The very low concentrations of raffinose and stachyose in fruit tissues of C. melo (Hubbard et al., 1989; Chrost and Schmitz, 1997) suggest that galactosyl-Suc unloaded from phloem is rapidly metabolized, with the initial hydrolysis by αgalactosidase.
The enzyme α-galactosidase (EC 3.2.1.22, α-d-galactoside galactohydrolase) catalyzes the hydrolytic cleavage of the terminal-linked α-Gal moiety from Gal-containing oligosaccharides. It is likely that α-galactosidase, as the initial enzyme in the metabolic pathway of stachyose and raffinose catabolism (Keller and Pharr, 1996), plays an important role in the carbohydrate partitioning in the cucurbits.
Plant α-galactosidases from numerous sources have been studied, and multiple forms of the enzyme have been described (for review, see Keller and Pharr, 1996). These can be divided into two groups, acid and alkaline, based on their activity response to pH. Most studies have dealt with the acid forms of the enzyme, which play important roles in seed development and germination (Keller and Pharr, 1996). In the cucurbits Gaudreault and Webb (1982, 1983, 1986) described an alkaline α-galactosidase from young leaves of Cucurbita pepo, in addition to multiple acid forms of the enzyme. The alkaline form was unique in that it showed a high affinity for stachyose and little activity toward raffinose compared with the acid forms, for which raffinose was found to be the preferred substrate.
Recently, the study of α-galactosidase activities in cucurbit fruit has attracted attention. Irving et al. (1997) reported the developmental changes in α-galactosidase activities measured at acid and alkaline pH in Cucurbita maxima fruit. They found that at anthesis alkaline activity was higher than acid and that both activities declined during fruit development. Chrost and Schmitz (1997) reported approximately similar activities of α-galactosidase at acid and alkaline pH in C. melo fruit at the anthesis stage. They observed a transient burst of α-galactosidase activity before (and apparently unrelated to) the onset of Suc accumulation. Pharr and Hubbard (1994) correlated the activity of alkaline α-galactosidase with stachyose levels in portions of the Cucumis sativus pedicel and concluded that the alkaline activity, rather than the acid activity, was responsible for stachyose hydrolysis. All of these studies were carried out using the synthetic substrate pNPG rather than the natural substrates raffinose and stachyose.
These developmental changes in α-galactosidase activities, in addition to analogous changes during the sink-to-source transition in cucurbit leaves, suggested that the alkaline α-galactosidase plays a role in phloem unloading and catabolism of transported stachyose in this sink tissue (Pharr and Sox, 1984; Gaudreault and Webb, 1986). However, Madore (1995) has recently pointed to the dilemma of stachyose and raffinose metabolism in the cucurbits in light of only the stachyose-specific alkaline α-galactosidase discovered by Gaudreault and Webb (1983). It is unlikely that stachyose and raffinose catabolism would take place via a two-step process in which one enzyme has an alkaline pH optimum and the other has an acid pH optimum, as this would imply separate compartments for the two linked steps.
In the present study we identified two alkaline and one acid α-galactosidase in C. melo fruit, including a novel alkaline form with activity toward a broader spectrum of galactosyl saccharides, particularly raffinose. In addition, we measured the activities of the three α-galactosidases from before anthesis until maturity to shed light on the role of α-galactosidase hydrolysis in photoassimilate metabolism in the melon fruit sink
MATERIALS AND METHODS
Fruit Materials and Chemicals
Melon (Cucumis melo L. cv C-8) plants were grown under standard conditions in a greenhouse in Bet Dagan, Israel. Female flowers were hand-pollinated and tagged at anthesis, and fruit load was limited to 1 fruit per plant after 10 DAA. Primary fruits were harvested from 3 d prior to anthesis and throughout fruit development. For fruits younger than 6 DAA the whole fruit was sampled, whereas for fruit after 10 DAA the inner mesocarp was sampled. Tissue was thinly sliced and immediately frozen in liquid N2 prior to storage at −80°C. Unless otherwise specified, we purchased chemicals and enzymes from Sigma or Boehringer Mannheim.
Assays for α-Galactosidase
For routine analysis and monitoring of activity in the purification steps, α-galactosidase was assayed as described by Smart and Pharr (1980) using pNPG as a substrate. We initiated the reaction by adding a 50-μL aliquot of pNPG either to 200 μL of 100 mm McIlvaine buffer, pH 5.5, or to 100 mm Hepes buffer, pH 7.5, containing 5 mm pNPG, and incubated at 35°C. The reaction was terminated after 10 min by adding 1 mL of 5% (w/v) Na2CO3. Activity was expressed as nanomoles of nitrophenol per minute as measured at 410 nm. The hydrolysis of the natural substrates stachyose, raffinose, and melibiose by α-galactosidases was measured with 10 mm substrate at pH 5.5 or 7.5, as in the assay with pNPG. We started the assay by adding 25 to 50 μL of enzyme preparation at 35°C, and terminated it after 20 min by 2 min of boiling. We estimated enzyme activities by determining the amounts of Gal released, as described by Smart and Pharr (1980), using an enzyme-coupled reaction with NAD and β-Gal dehydrogenase (EC 1.1.1.48).
Purification of α-Galactosidases
We performed an initial partial purification to separate and characterize the various α-galactosidases present in C. melo fruit tissue. Mesocarp tissue (200 g fresh weight) from 10-DAA fruit was homogenized in 200 mL of chilled extraction buffer containing 50 mm Hepes-NaOH, pH 7.5, 2 mm MgCl2, 2 mm EDTA, and 5 mm DTT. The homogenate was filtered through four layers of cheesecloth and centrifuged at 18,000g for 30 min. We used PEG-6000 to precipitate proteins from crude extract because there was a significantly irreversible loss of activity when we used (NH4)2SO4. Precipitated proteins were collected from the 5% to 50% (w/v) PEG-6000 fraction, suspended in 50 mL of buffer, pH 7.5, containing 25 mm Hepes and 1 mm DTT (buffer A), and applied to an ion-exchange column (1.2 × 25 cm; DEAE-Sepharose CL-6B, Pharmacia) previously equilibrated with buffer A. Unbound protein was eluted with buffer A, and the bound protein was eluted at flow rate of 1 mL min−1 with a linear gradient of 0 to 0.45 m NaCl in buffer A. Fractions (3.5 mL fraction−1) were collected and assayed for α-galactosidase activity at pH 5.5 or 7.5 with pNPG as a substrate.
Partial Purification of the Acid Form of α-Galactosidase
The fractions active at pH 5.5 were pooled and concentrated by reverse dialysis against solid Suc. We chromatographed the concentrated fractions on a gel-filtration column (4.5 × 120 cm; Sephacryl-S 200, Pharmacia), previously equilibrated with buffer A and containing 150 mm NaCl, at a flow rate of 0.5 mL min−1. We collected and assayed fractions of 3.5 mL for α-galactosidase activity at pH 5.5, using pNPG as a substrate. The active fractions were pooled and NaCl was added to 0.5 m before loading onto a lectin-affinity column (1 × 5 cm; Con A-Sepharose 4B, Pharmacia) previously equilibrated with buffer A containing 0.5 m NaCl. Unbound proteins were eluted with the same buffer, and bound proteins were eluted with the same buffer containing 50 mm methyl α-d-glucopyranoside. The active fractions were then desalted by dialysis against buffer A for 12 h with two changes of the buffer. We used this enzyme fraction for the characterization of the acid form of α-galactosidase, which was not further purified.
Alkaline α-Galactosidase Purification
We pooled and dialyzed the fractions from the DEAE-Sepharose chromatography that were active at pH 7.5 against buffer A for 12 h before loading onto a Mono-Q HR 5/5 column (Pharmacia), previously equilibrated with buffer A. Bound proteins were eluted with a linear gradient of 0.1 to 0.45 m NaCl, and the active fractions were detected using pNPG as a substrate at pH 7.5, as described above. Two peaks of alkaline α-galactosidase, labeled I and II according to elution, were separated by Mono-Q chromatography.
For the further purification of form II, the active fraction of the peak II was chromatographed on hydrophobic interaction chromatography. The fractions were pooled, brought to 1 m (NH4)2SO4, and loaded on to a Phenyl-Sepharose 6 fast-flow column (0.5 × 12 cm, Pharmacia) previously equilibrated with buffer A containing 1 m (NH4)2SO4. The protein was eluted with a reverse-stepwise gradient from 1 to 0 m (NH4)2SO4 at 50 mm intervals in buffer A. We pooled and dialyzed the active fractions for 12 h against buffer A and concentrated the dialysate by reverse dialysis against solid Suc. We used the enzyme after hydrophobic-interaction chromatography for characterization of the form II. In addition, the active fractions from the hydrophobic interaction column were further purified. We separated the active fractions electrophoretically using a Mini Prep Cell (Bio-Rad) for discontinuous native-PAGE with 7% polyacrylamide according to the manufacturer's instructions. Fractions (0.25 mL fraction−1) were assayed at pH 7.5 with pNPG as a substrate for the activity. The active fractions were pooled, concentrated (Vivaspin Concentrator, Vivascience, Lincoln, UK), and electrophoresed in 8% SDS-PAGE, as described below.
We did not apply hydrophobic-interaction chromatography to the fractions of peak I because there was a great loss of the activity in the (NH4)2SO4 solution. Therefore, for the characterization of form I, we used the active fractions after Mono-Q chromatography. In addition, we carried out further purification of alkaline α-galactosidase form I. The fractions of peak I obtained after Mono-Q chromatography were chromatographed on a hydroxylapatite column (0.5 × 12 cm; BioGel BTP, Bio-Rad) previously equilibrated with 10 mm NaPi buffer, pH 7.0, containing 0.5 mm DTT. The enzyme was eluted with a 60-mL, 10 to 100 mm NaPi linear gradient. The active fractions were pooled and concentrated. The concentrated protein was separated electrophoretically on nondenaturing PAGE with a Mini-Protean II apparatus (Bio-Rad) using 1-mm-thick slab gels containing 10% acrylamide, according to the procedure of Laemmli (1970). We identified the active band as a yellowish band in an activity stain containing 50 mm Hepes, pH 7.5, and 2 mm pNPG incubated at 35°C. Following native electrophoresis, the active band was excised and the protein was eluted with water overnight and electrophoresed in 8% SDS-PAGE.
SDS-PAGE
SDS-PAGE was carried out with a Mini-Protean II apparatus (Bio-Rad) using 1-mm-thick slab gels containing 8% acrylamide according to the procedure of Laemmli (1970). Gels were stained with Coomassie brilliant blue R-250 and destained in a methanol:acetic acid:water solution. Molecular-mass standards (Pharmacia) were phosphorylase b (94 kD), albumin (67 kD), ovalbumin (43 kD), carbonic anhydrase (30 kD), trypsin inhibitor (20.1 kD), and α-lactalbumin (14.4 kD).
Determination of the Native Molecular Mass and pI
The partially purified enzymes were chromatographed on a gel-filtration column (Superdex 200H 10/30, Pharmacia) equilibrated with 50 mm Na-phosphate buffer, pH 7.0, containing 0.15 m NaCl and 1 mm DTT. Retention time was compared with that of gel-filtration markers run simultaneously with the α-galactosidase proteins. The markers used were β-amylase (200 kD), alcohol dehydrogenase (150 kD), BSA (66 kD), carbonic anhydrase (29 kD), and Cyt c (12.4 kD). The estimation of pI was carried out using IEF (PhastGel and the PhastSystem, Pharmacia), pH 4.0 to 6.5. We loaded the proteins to duplicate gels and focused them according to the manufacturer's instructions (Pharmacia). One of the gels was stained for protein using Coomassie blue. The duplicate gel was sliced into a 1-mm segment and assayed for enzyme activity using pNPG at pH 7.5. We used standards with pIs of 4.55, 5.2, and 5.85 (Sigma) for comparison, and estimated the pIs of the enzymes from the calibration curve and the distance of the active band from the anode.
Enzyme Properties
We determined the optimum pH for each partially purified enzyme using 5 mm pNPG, 10 mm stachyose, or 10 mm raffinose as a substrate in 100 mm McIlvaine buffer over a pH range of 4.0 to 7.0, 100 mm Hepes buffer at a pH range of 7.0 to 8.0, or 50 mm Tris buffer at a pH range of 8.0 to 8.7, all at 35°C. The substrate specificity of the α-galactosidases was tested with pNPG, stachyose, raffinose, and melibiose. Km and Vmax values for pNPG, stachyose, raffinose, and melibiose were determined by Lineweaver-Burk plots, as were Ki (inhibition) values for d-Gal inhibition.
Activities of α-Galactosidases in Developing Fruits
We estimated the activities of α-galactosidases in the developing fruits in crude extracts with either raffinose or stachyose as the substrate at both pH 5.5 or 7.5. Ovaries and inner mesocarps of 10- and 45-DAA fruits were homogenized in a chilled mortar with 4 volumes of chilled extraction buffer containing 50 mm Hepes-NaOH, pH 7.5, 2 mm MgCl2, 2 mm EDTA, and 5 mm DTT. After centrifugation at 18,000g for 30 min, the supernatant was desalted with a 5-mL Sephadex G-25 column and used as the crude enzyme extract.
Enzyme extracts from 10 g of 0- and 10-DAA fruits were also characterized after separation on a Mono-Q column. The 3% to 50% PEG-6000 (w/v) fraction from the above supernatant was separated on a Mono-Q HR 5/5 column previously equilibrated with buffer A with a linear gradient of 0 to 0.45 m NaCl, as described above. Active fractions were detected with the assays using pNPG, stachyose, and raffinose as substrates at pH 5.5 and 7.5.
α-Galactosidases from Tissues of Other Species
We carried out a survey of α-galactosidase activity from the tissues of other galactosyl-Suc-translocating species using raffinose or stachyose as the substrate at acid (pH 5.5) and alkaline (pH 7.5) conditions. Young leaves of the cucurbits C. melo, Cucurbita maxima, Lagenaria ciceravia, and Coleus blumei (Madore, 1990) of the Lamiaceae, roots of C. melo, and pre-anthesis ovaries of C. maxima were homogenized as described above. We also sampled young leaves of Suc-translocating pepper (Capsicum annuum) plants for comparison. We used crude enzyme extracts without (NH4)2SO4 precipitation for the enzyme assays, as described above.
Protein Estimation
We used the Bio-Rad protein assay and BSA as a standard to estimate the protein according to the method of Bradford (1976).
RESULTS
Purification of α-Galactosidases
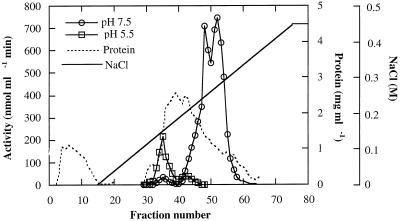
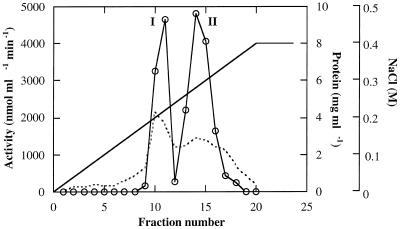
Three forms of α-galactosidase were resolved from young (10 DAA) C. melo fruit mesocarp by DEAE-Sepharose ion-exchange chromatography, in conjunction with Mono-Q chromatography, using pNPG as a substrate (Figs. 1 and 2). The first peak (Fig. 1) showed higher activity at pH 5.5 than at pH 7.5, whereas the latter two peaks showed activity at pH 7.5 and little activity at pH 5.5. Accordingly, we refer to the first peak as the acid form of α-galactosidase and the other two peaks as alkaline α-galactosidases I and II, respectively. The three enzyme forms were partially purified for the purposes of characterization (Table I).
Figure 1.
Separation of acid and alkaline α-galactosidases from C. melo fruit (10 DAA) on ion-exchange chromatography. The protein fraction of 5% to 50% (w/v) PEG-6000 was applied to a column of DEAE-Sepharose 4B and eluted with the indicated linear gradient of 0 to 0.45 m NaCl. Activity of each fraction was assayed with pNPG at pH 5.0 and pH 7.5. Zero activity points are not shown.
Figure 2.
Separation of alkaline α-galactosidase forms I and II on Mono-Q chromatography. The fractions active at pH 7.5, represented in Figure 1, were pooled, desalted, applied to the column, and eluted with the indicated linear gradient of 0.1 to 0.4 m NaCl. Activity of each fraction was assayed with pNPG at pH 5.0 and pH 7.5.
Table I.
Purification scheme for the acidic form and alkaline forms I and II α-galactosidases from C. melo fruit
| Purification Step | Enzyme Activity | Protein | Specific Activity | Recovery | Purification |
|---|---|---|---|---|---|
| units | mg | units × 10 mg−1 protein | % | -fold | |
| Acid form | |||||
| Crude extract | 13.0 | 1152 | 0.11 | 100 | – |
| 5% to 50% PEG fraction | 11.4 | 481 | 0.24 | 88 | 2 |
| DEAE-Sepharose | 4.6 | 45 | 1.02 | 35 | 9 |
| Sephadex-200 | 3.0 | 13 | 2.32 | 23 | 21 |
| ConA | 1.7 | 2.6 | 6.27 | 13 | 55 |
| Alkaline form I | |||||
| Crude extract | 27.7 | 1152 | 0.24 | 100 | – |
| 5% to 50% PEG fraction | 20.7 | 481 | 0.43 | 75 | 2 |
| DEAE-Sepharose | 9.5 | 35 | 2.73 | 34 | 11 |
| Mono-Q | 8.0 | 15 | 5.37 | 29 | 22 |
| Bio-gel HTP | 5.1 | 3.7 | 13.82 | 19 | 57 |
| Alkaline form II | |||||
| Crude extract | 18.4 | 1152 | 0.16 | 100 | – |
| 5% to 50% PEG fraction | 16.4 | 481 | 0.34 | 89 | 2 |
| DEAE-Sepharose | 10.9 | 58 | 1.89 | 59 | 12 |
| Mono-Q | 8.8 | 17 | 5.21 | 48 | 33 |
| Phenyl Sepharose 6 fast flow | 5.8 | 2.9 | 19.73 | 31 | 123 |
All specific activities were assayed using raffinose (for acid and alkaline form I) or stachyose (for alkaline form II) as the substrate.
In our preliminary studies we observed that the ability of the enzyme extract to hydrolyze raffinose at alkaline pH was lost after precipitation with (NH4)2SO4, even after its removal through a Sephadex G-50 column, whereas the ability to hydrolyze stachyose was maintained. The alkaline form I was especially sensitive to (NH4)2SO4, and 65% of the activity of the partially purified enzyme was lost upon the addition of a 10% (w/v) concentration. The partially purified form II was relatively insensitive, losing 25% of its activity in 10% (w/v) (NH4)2SO4 (data not shown). Therefore, we used PEG 6000 for the step of protein precipitation. Hydrophobic-interaction chromatography was useful in the purification of alkaline form II, and hydroxyapatite chromatography was a helpful step in the purification of alkaline form I.
The acid α-galactosidase bound to Con A-Sepharose, indicating that it was a glycoprotein, and this was a useful step in its purification (Table I). The alkaline α-galactosidase forms I and II did not bind to Con A, suggesting that neither was a glycoprotein. The partially purified enzymes were stable for at least 2 months when stored at −80°C.
Properties of α-Galactosidases
The calculated Km and Vmax values of the three α-galactosidases are summarized in Table II. The three enzymes are distinct with respect to their substrate specificity. The hydrolysis of the natural substrates raffinose, stachyose, and melibiose were of particular interest to us. Because the three enzymes were purified to different purities, the calculated Vmax values are meant for comparison of the activity toward each substrate for each particular enzyme. All three enzymes showed Michaelis-Menten kinetics at concentrations up to 40 mm melibiose, raffinose, or stachyose. Alkaline form I exhibited a nearly 2-fold higher activity, as well as higher affinity for raffinose compared with stachyose. This enzyme also showed substantial activity to hydrolyze melibiose, although with a high Km (Table II).
Table II.
Characterization of partially purified acidic and alkaline forms I and II α-galactosidases from C. melo fruit
| α-Galactosidase | Substrate | Km | Vmax | Vmax/Km |
|---|---|---|---|---|
| mm | μmol mg−1 protein min−1 | |||
| Acid form | Stachyose | 10.5 | 0.23 | 0.02 |
| Raffinose | 4.2 | 0.71 | 0.17 | |
| Melibiose | 0.7 | 0.19 | 0.27 | |
| pNPG | 0.3 | 1.5 | 5.00 | |
| Alkaline form I | Stachyose | 4.0 | 0.24 | 0.06 |
| Raffinose | 1.5 | 0.56 | 0.37 | |
| Melibiose | 20 | 0.3 | 0.02 | |
| pNPG | 1.2 | 1.4 | 1.17 | |
| Alkaline form II | Stachyose | 3.6 | 2.2 | 0.61 |
| Raffinose | 26.3 | 0.26 | 0.01 | |
| Melibiose | 18.7 | 0.21 | 0.01 | |
| pNPG | 3.0 | 7.9 | 2.63 |
The acid α-galactosidase also exhibited a higher activity with raffinose compared with stachyose or melibiose. The affinity of the acid enzyme toward the three substrates decreased with increasing substrate size, being highest (low Km) for the disaccharide melibiose and lowest for the tetrasaccharide stachyose. In contrast, alkaline form II was relatively specific to stachyose, with little activity toward raffinose and melibiose (Table II). Hydrolysis of the synthetic substrate pNPG did not give any indication of natural substrate specificity. When we used pNPG as a substrate, the acid α-galactosidase showed slight inhibition above 5 mm pNPG, whereas the two alkaline forms followed Michaelis-Menten kinetics up to substrate concentrations of 20 mm pNPG.
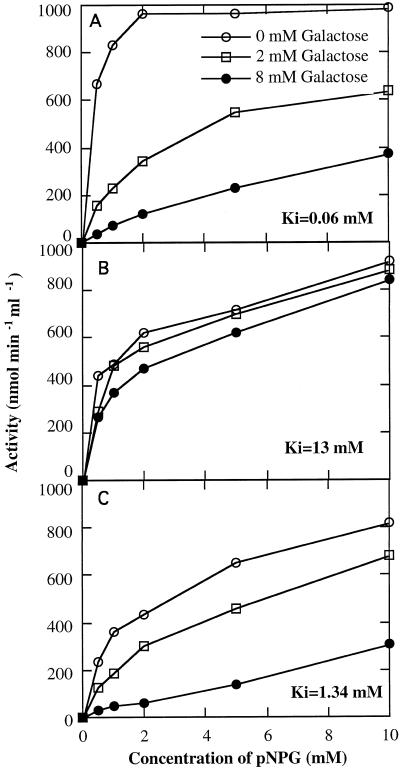
Gal was a strong competitive inhibitor of the acid and alkaline form II enzymes when assayed with pNPG (Fig. 3). The alkaline form I, however, was relatively insensitive to inhibition by Gal, with a Ki value of 13 mm Gal, compared with 1.3 mm for form II and 0.06 mm for the acid form.
Figure 3.
Kinetics of the acid form (A), alkaline form I (B), and alkaline form II (C) of α-galactosidase with pNPG as the substrate in the presence of varying concentrations of the inhibitor Gal.
There was an inhibitory interaction between the substrates raffinose and stachyose when either the acid or the alkaline form II were assayed (Fig. 4). Raffinose inhibited the stachyose-specific alkaline form II, and the addition of 80 mm raffinose to the assay medium containing 10 mm stachyose caused 35% inhibition of the activity, as measured by the release of Gal. For the acid form, 80 mm stachyose added to the assay medium of the acid α-galactosidase containing 10 mm raffinose caused a 45% inhibition in the free Gal release. However, this inhibitory interaction was negligible for alkaline form I, and the addition of excess amount of stachyose did not lead to a decrease in released Gal (Fig. 4).
Figure 4.
The inhibition of the α-galactosidases by either raffinose or stachyose. For the assay of inhibition of the acid form and alkaline form I, the assay medium contained 10 mm raffinose and increasing amounts of stachyose were added. For alkaline form II, the assay medium contained 10 mm stachyose and increasing amounts of raffinose were added. The activity was measured by the production of free Gal.
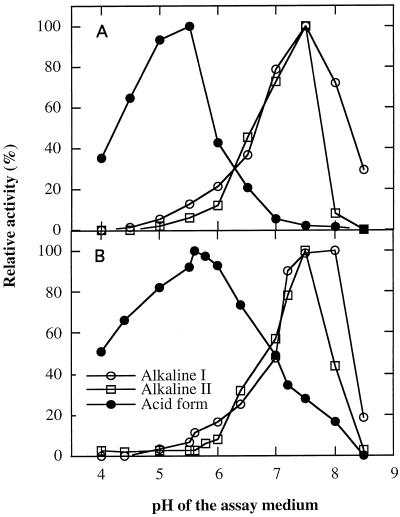
The acid form exhibited a narrow pH range of maximal activity, between 5 and 5.5, with only approximately 5% of maximal activity at pH 7.0, when measured with its preferred substrate, raffinose (Fig. 5A). When we used pNPG as a substrate, the acid form exhibited activity over a broader pH range, from 4.0 to 8.0, with maximal activity at pH 5.8 and approximately 35% maximal activity remaining at pH 7.0 (Fig. 5B). Both alkaline forms had maximal activity at pH 7.5 with raffinose and stachyose, which was similar to that when pNPG was the substrate. Little activity was measured at pH 5.0 with the two alkaline forms.
Figure 5.
Effect of pH on activity of acid and alkaline forms I and II α-galactosidase with pNPG, raffinose, or stachyose as the substrate. The buffers used were citrate-phosphate (pH 4.0–pH 7.0), Hepes-KOH (pH 7.0–pH 8.0), and Tris (pH 8.0–pH 8.5). All data were adjusted relative to maximum activity for each enzyme. A, Raffinose was used as substrate for the acid form and alkaline form I, and stachyose was used for alkaline form II. B, pNPG as the substrate.
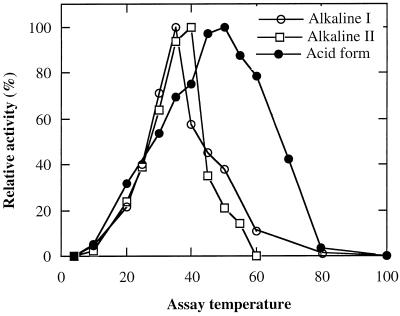
Both alkaline forms I and II exhibited the highest activity in the temperature range of 35°C to 40°C and activity was significantly decreased above 40°C (Fig. 6). The acid α-galactosidase was relatively thermophilic, with maximal activity at 50°C, and retained 40% of its activity at 70°C (Fig. 6).
Figure 6.
Effect of reaction temperature on the activity of the acid form and alkaline forms I and II α-galactosidases with pNPG as the substrate. All data were adjusted relative to maximum activity for each enzyme.
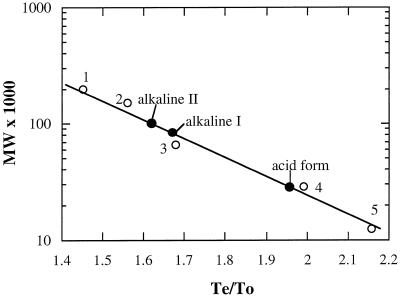
We estimated the pI values of the two alkaline forms at 5.0 and 4.7 for forms I and II, respectively, by activity staining of IEF gels (data not shown). The molecular masses of the native proteins were 27, 84, and 102 kD for the acid form and alkaline forms I and II, respectively (Fig. 7).
Figure 7.
The calibration curve of Superdex 200H 10/30 from which the native molecular mass of the acid form and alkaline forms I and II α-galactosidases were determined. The molecular markers were: 1, β-amylase (200 kD); 2, alcohol dehydrogenase (150 kD); 3, BSA (66 kD); 4, carbonic anhydrase (29 kD); and 5, Cyt c (12.4 kD).
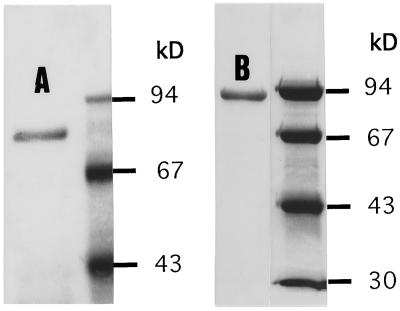
The proteins were further purified using native electrophoresis, as described in Methods. Following the respective native electrophoresis, the two alkaline α-galactosidase forms appeared to be nearly homogeneous, as shown in the SDS-PAGE gel (Fig. 8). The denatured molecular masses were calculated at 79 and 92 kD for forms I and II, respectively, and comparison with the native molecular masses implied that the alkaline forms existed in the native state as monomers.
Figure 8.
The purified alkaline α-galactosidase form I (lane A) and II (lane B) in SDS-PAGE gel showing the denatured molecular masses of 79 kD and 92 kD, respectively. The partially purified proteins, described in Table II, were further purified with steps of native electrophoresis, as described in Methods. Next to each of the purified proteins is a lane showing the separation of markers of known molecular mass.
Changes of Acid and Alkaline α-Galactosidases in Developing C. melo Fruits
The substrate preference (Table II) and pH profile (Fig. 5) from the partially purified acid and alkaline α-galactosidases I and II allowed us to measure and estimate their activities even in crude extracts of C. melo fruit by using their natural substrates. Very little overlap in activity occurred between pH 5.5 and 7.5 (Fig. 5A), and at pH 7.5 the activities of alkaline α-galactosidase I and II in the crude extracts could be distinguished by the activity with raffinose or stachyose as the substrate. The activity with raffinose at pH 7.5 was a good indicator of form I activity, because form II is relatively specific for stachyose. Although there should be an overestimation of form II activity when using stachyose, due to the hydrolysis of this substrate by form I, distinct patterns of α-galactosidase activities were apparent when using these two substrates.
Stachyose hydrolysis at alkaline pH was highest in the pre-anthesis fruit ovary and progressively declined throughout development (Table III). In contrast, raffinose hydrolysis at alkaline pH (form I) increased from pre-anthesis to anthesis. In the mature fruit mesocarp tissue the major α-galactosidase activity was toward raffinose at an alkaline pH (Table III), and the activity of this enzyme also increased during the Suc-accumulating stage (data not shown). Raffinose and stachyose hydrolysis at acid pH declined during fruit development, but the relative hydrolysis of the two substrates remained the same at each stage measured (Table III), as would be expected from a single enzyme. These changes in activities were observed also after Mono-Q separation (data not shown). From anthesis to 10 DAA the activity of alkaline form I increased from approximately 300 to 400 nmol g−1 fresh weight min−1, whereas that of form II sharply decreased from approximately 550 to 200 nmol g−1 fresh weight min−1, in correlation with the results from the differential assay of the crude extract with raffinose or stachyose.
Table III.
Activities of α-galactosidases in developing C. melo ovaries and fruits
| Fruit Stage | Stachyose pH 7.5 | Raffinose pH 7.5 | Stachyose pH 5.5 | Raffinose pH 5.5 |
|---|---|---|---|---|
| nmol Gal g−1 fresh wt min−1 | ||||
| DAA | ||||
| −3 | 490.3 ± 29.8 | 314.7 ± 40.0 | 184.2 ± 20.0 | 239.7 ± 36.1 |
| 2 | 363.9 ± 41.9 | 399.6 ± 57.8 | 118.4 ± 8.7 | 167.6 ± 14.7 |
| 10 | 175.5 ± 12.7 | 379.9 ± 61.1 | 63.6 ± 2.4 | 109.4 ± 8.9 |
| 45 | 39.5 ± 4.8 | 146.6 ± 17.1 | 33.5 ± 3.0 | 65.4 ± 5.1 |
Citrate-phosphate buffer, pH 5.5, and Hepes buffer, pH 7.5, were used for the assays with stachyose or raffinose as the substrate. Data are means ± se (n = 4).
α-Galactosidase Activity in Other Species
We compared the hydrolytic activity toward raffinose and stachyose in a number of galactosyl-Suc-translocating species, including additional cucurbits and C. blumei (Table IV). We assayed the crude extract without the step of (NH4)2SO4 precipitation, and measured the free Gal released. In contrast to C. annuum leaves, which showed no hydrolytic activity at alkaline conditions, all the other tissues assayed showed significant raffinose hydrolysis. In young leaves of C. melo cv C-8 and of C. blumei, alkaline hydrolysis was higher with raffinose as substrate than with stachyose.
Table IV.
Raffinose and stachyose hydrolysis of crude extracts of various plant tissues
| Plant | Source | Raffinose pH 5 | Raffinose pH 7.5 | Stachyose pH 7.5 |
|---|---|---|---|---|
| nmol Gal mg−1 protein min−1 | ||||
| C. melo cv C-8 | Young leaves | 10.7 | 27.8 | 23.5 |
| Mature leaves | 8.6 | 11.6 | 8.2 | |
| Roots | 1.3 | 10.8 | 34.5 | |
| C. melo cv ESH | Mature leaves | 12.6 | 6.2 | 7.2 |
| C. maxima cv Big max | Young leaves | 25.0 | 17.9 | 63.7 |
| Ovary, preanthesis | 16.1 | 47.6 | 126.6 | |
| L. ciceravia | Young leaves | 28.7 | 17.4 | 44.1 |
| C. blumei | Young leaves | 7.8 | 12.4 | 8.6 |
| C. annum | Young leaves | 4.2 | 0 | 0 |
Raffinose hydrolysis was assayed at both pH 5.5 and 7.5; stachyose hydrolysis was assayed only at pH 7.5. Data are the means of assays from two to four extractions.
DISCUSSION
Although acid α-galactosidase often exists in multiple forms in leaves and seeds (Thomas and Webb, 1978; Smart and Pharr, 1980; Dey et al., 1983; Gaudreault and Webb, 1983), to our knowledge, only one form of alkaline α-galactosidase has been reported in plants (Gaudreault and Webb, 1983, 1986). Results from the present study clearly demonstrated that there were three α-galactosidases in the fruit tissue of C. melo as resolved by ion-exchange chromatography (Figs. 1 and 2). Two of the partially purified α-galactosidases exhibited maximum activity at neutral-alkaline conditions (Fig. 5). In addition to the pH optima, the two alkaline α-galactosidases showed similar temperature sensitivity (Fig. 6) and were nonglycosylated, in contrast to the acid α-galactosidase in C. melo fruit. The glycoprotein nature of the C. melo fruit acid α-galactosidase was common to the enzyme from a number of legume seeds (Dey et al., 1983; Porter et al., 1990).
The two alkaline α-galactosidases are distinct from each other with respect to a number of characteristics, including pI (Table II), molecular mass (Fig. 8), and inhibition by d-Gal (Fig. 3, B and C). The most significant difference between the two alkaline isoforms was in their distinct preferences to hydrolyze the natural substrates raffinose and stachyose, although both forms hydrolyzed the synthetic substrate pNPG (Table II). Form II was relatively specific for stachyose, whereas form I showed preferred activity to raffinose. By comparison, the partially purified alkaline α-galactosidase from leaves of C. pepo showed substrate preference for stachyose (Gaudreault and Webb, 1983), similar to alkaline α-galactosidase II. The partially purified acid form was similar to the smaller-molecular form of acid α-galactosidase isolated from C. sativus leaves with respect to pH optima and the Km for raffinose and stachyose (Smart and Pharr, 1980). To the best of our knowledge, this is the first report of an alkaline α-galactosidase with higher affinity and activity toward raffinose than to stachyose, yet with a broad spectrum of substrates, allowing it to hydrolyze stachyose as well. The alkaline form I may have escaped detection in previous studies of alkaline α-galactosidase (Gaudreault and Webb, 1983) in plant tissues due to its sensitivity to (NH4)2SO4, which had been used in the purification scheme of the enzyme.
Our survey of alkaline hydrolytic activity of raffinose and stachyose in other species (Table IV) indicated that in the absence of (NH4)2SO4, significant activity of raffinose hydrolysis can be observed. The only tissue that showed no hydrolysis of either raffinose or stachyose was from leaves of the Suc-translocating C. annuum. Because form I can hydrolyze stachyose as well as raffinose, the higher amount of Gal released with stachyose as a substrate could be at least partially due to double hydrolysis by form I.
The importance of alkaline α-galactosidase form I in cucurbit carbohydrate metabolism may be significant. Madore (1995) has pointed out the dilemma of stachyose and raffinose catabolism in cucurbits. Previous studies showed that the alkaline activity assayed with pNPG had physiologically significant differences in activity during, for example, the leaf sink-to-source transition (Thomas and Webb, 1978; Pharr and Sox, 1984) and along the C. sativus pedicel (Pharr and Hubbard, 1994), whereas changes in the acid activity did not indicate similar involvement in sink function. This suggested that alkaline hydrolysis of imported photoassimilate, rather than hydrolysis at acid pH, was the metabolic pathway controlling photoassimilate partitioning. However, the presence of an alkaline α-galactosidase specific toward stachyose presented a dilemma, because the complete hydrolysis of stachyose would indicate the unlikely situation of the hydrolysis of stachyose in an alkaline compartment, followed by the continued hydrolysis of raffinose in an acid compartment. Based on a study of stachyose metabolism in Peperomia camptotricha leaves, Madore (1995) hypothesized a “raffinose hydrolase” that could continue the metabolism of raffinose to Suc and galactinol. However, the form I, raffinose-preferring, broad-spectrum alkaline α-galactosidase that we report here could account either for stachyose and raffinose metabolism in cucurbits alone or in concert with the stachyose-specific form II enzyme.
The different levels of inhibition by Gal may also have physiological significance. The strong inhibition of the acid α-galactosidase with low Ki of 0.064 mm (Fig. 3A) suggests that the activity of acid α-galactosidase in vivo may be regulated by the level of Gal concentration, as proposed in Stachys sieboldii tubers (Keller and Matile, 1985). On the other hand, the alkaline form I enzyme is unlikely to be regulated by Gal levels, due to a weak inhibition and high Ki for the inhibitor. Similarly, the inhibition by raffinose of stachyose hydrolysis by alkaline form II and the inhibition of raffinose hydrolysis by stachyose of the acid α-galactosidase (Fig. 5) may be physiologically significant. Mitchell et al. (1992) reported concentrations of approximately 50 mm stachyose and 10 mm raffinose in the phloem exudate of C. melo. Depending on the compartmentation of these sugars with respect to the α-galactosidase enzymes, inhibition by these substrates may play a regulatory role in photoassimilate partitioning. Further study of the localization of these enzymes should shed light on this question.
With respect to developmental patterns of enzyme activity, the initial high activity of alkaline α-galactosidase, together with the decline in activity during development (Table III), is typical of that found in other species of cucurbits (Chrost and Schmitz, 1997; Irving et al., 1997). However, we resolved the general alkaline α-galactosidase activity into its component parts. The decline in activity toward stachyose and the parallel increase in activity with raffinose during the early period of ovary development and fruit set (Table III) might suggest a specific role for alkaline form I in this critical stage of fruit development. Similarly, the observation that form I activity is the major α-galactosidase activity during the latter stage of fruit development may suggest a role for this enzyme in the mature, Suc-accumulating mesocarp.
In conclusion, the discovery of the novel alkaline α-galactosidase form I can contribute to our understanding of galactosyl-Suc metabolism in cucurbits and perhaps in other galactosyl-Suc-metabolizing plant tissues as well. Furthermore, the unique characteristics of the enzyme, particularly its activity at alkaline conditions, its relatively broad spectrum of substrates, and its insensitivity to inhibition by its product and substrates, may make it useful in enzymatic processes in the food and pharmaceutical industries, in which maintaining alkaline conditions can be critical. α-Galactosidases can also contribute to the hydrolysis of raffinose contamination in beet sugar crystallization (Suzuki et al., 1969), the removal of flatulence-associated stachyose and raffinose from soybean milk (Thananunkul et al., 1976), the modification of Gal-containing plant gums (Bulpin et al., 1990), and the seroconversion of type-B blood to type-O blood (Zhu et al., 1996).
ACKNOWLEDGMENTS
The authors thank S. Shen, M. Petreikov, M. Fogelman, and Y. Yeselson for their expert technical assistance and Dr. Rivka Barg for helpful advice.
Abbreviations:
- Con A
concanavalin A
- DAA
days after anthesis
- pNPG
p-nitrophenyl α-galactopyranoside
Footnotes
This research was partially supported by the United States-Israel Binational Agricultural Research and Development Fund (grant no. 2270-93). This work is contribution no. 146/98, 1998 series, from the Agricultural Research Organization, The Volcani Center, Bet Dagan, Israel.
LITERATURE CITED
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bulpin PV, Gidley MJ, Jeffcoat R, Underwood DR. Development of a biotechnological process for the modification of galactomannan polymers with plant α-galactosidase. Carbohydr Polym. 1990;12:155–168. [Google Scholar]
- Chrost B, Schmitz K. Changes in soluble sugar and activity of α-galactosidases and acid invertase during muskmelon (Cucumis melo L.) fruit development. J Plant Physiol. 1997;151:41–45. [Google Scholar]
- Dey PM, Campillo EMD, Lezica RP. Characterization of a glycoprotein α-galactosidase from lentil seeds (Lens culinaris) J Biol Chem. 1983;258:923–929. [PubMed] [Google Scholar]
- Gaudreault PR, Webb JA. Alkaline α-galactosidase in leaves of Cucurbita pepo. Plant Sci Lett. 1982;24:281–288. [Google Scholar]
- Gaudreault PR, Webb JA. Partial purification and properties of an alkaline α-galactosidase from mature leaves of Cucurbita pepo. Plant Physiol. 1983;71:662–668. doi: 10.1104/pp.71.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault PR, Webb JA. Alkaline α-galactosidase activity and galactose metabolism in the family cucurbitaceae. Plant Sci. 1986;45:71–75. [Google Scholar]
- Gross KC, Pharr DM. A potential pathway for galactose metabolism in Cucumis sativus L., a stachyose-transporting species. Plant Physiol. 1982;69:117–121. doi: 10.1104/pp.69.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard NL, Huber SC, Pharr DM. Sucrose phosphate synthase and acid invertase as determinants of sucrose concentration in developing muskmelon (Cucumis melo L.) fruits. Plant Physiol. 1989;91:1527–1534. doi: 10.1104/pp.91.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving DE, Hurst PL, Ragg JS. Changes in carbohydrates and carbohydrate metabolism enzymes during the development, maturation, and ripening of buttercup squash (Cucurbita maxima D. Delica) J Am Soc Hortic Sci. 1997;122:310–314. [Google Scholar]
- Keller F, Matile P. The role of the vacuole in storage and mobilization of stachyose in tubers of Stachys sieboldii. J Plant Physiol. 1985;119:369–380. [Google Scholar]
- Keller F, Pharr DM (1996) Metabolism of carbohydrates in sink and sources: galactosyl-sucrose oligosaccharides. In E Zamski, AA Schaffer, eds, Photoassimilate Distribution in Plants and Crops. Marcel Dekker, New York, pp 157–183
- Laemmli UK. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madore MA. Carbohydrate metabolism in photosynthetic and nonphotosynthetic tissues of variegated leaves of Coleus blumei Benth. Plant Physiol. 1990;93:617–622. doi: 10.1104/pp.93.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore MA (1995) Catabolism of raffinose family oligosaccharides by vegetative sink tissues. In MA Madore, WJ Lucas, eds, Carbon Partitioning and Source-Sink Interactions in Plants. American Society of Plant Physiologists, Rockville, MD, pp 204–214
- Mitchell DE, Gadus MV, Madore MA. Patterns of assimilate production and translocation in muskmelon (Cucumis melo L.) Plant Physiol. 1992;99:959–965. doi: 10.1104/pp.99.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharr DM, Sox HN. Changes in carbohydrate and enzyme levels during the sink to source transition of leaves of Cucumis sativus L., a stachyose translocator. Plant Sci Lett. 1984;35:187–193. [Google Scholar]
- Pharr M, Hubbard NL (1994) Melons: biochemical and physiological control of sugar accumulation. In Encyclopedia of Agricultural Science, Vol 3. Academic Press, New York, pp 25–37
- Porter JE, Herrmann KM, Ladisch MR. Integral kinetics of α-galactosidase purified from Glycine max for simultaneous hydrolysis of stachyose and raffinose. Biotechnol Bioeng. 1990;35:15–22. doi: 10.1002/bit.260350104. [DOI] [PubMed] [Google Scholar]
- Richardson PT, Baker DA, Ho LC. The chemical composition of cucurbit vascular exudates. J Exp Bot. 1982;33:1239–1247. [Google Scholar]
- Schaffer AA, Pharr DM, Madore MA (1996) Cucurbits. In E Zamski, AA Schaffer, eds, Photoassimilate Distribution in Plants and Crops. Marcel Dekker, New York, pp 729–757
- Smart EL, Pharr DM. Characterization of α-galactosidase from cucumber leaves. Plant Physiol. 1980;66:731–734. doi: 10.1104/pp.66.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Osawa Y, Oota H, Yoshida H. Studies on the decomposition of raffinose by α-galactosidase of mold. Agric Biol Chem. 1969;33:501–513. [Google Scholar]
- Thananunkul D, Tanaka M, Chichester CO, Lee TC. Degradation of raffinose and stachyose in soybean milk by α-galactosidase from Mortierella vinacea. J Food Sci. 1976;41:173–175. [Google Scholar]
- Thomas B, Webb JA. Distribution of α-galactosidase in Cucurbita pepo. Plant Physiol. 1978;62:713–717. doi: 10.1104/pp.62.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Leng L, Monahan C, Zhang ZF, Hurst R, Lenny L, Goldstein J. Characterization of recombinant α-galactosidase for use in seroconversion from blood group B to O of human erythrocytes. Arch Biochem Biophys. 1996;327:324–329. doi: 10.1006/abbi.1996.0129. [DOI] [PubMed] [Google Scholar]