Abstract
Background and Objective
Pancreatic cancer is notoriously difficult to treat and resistant to virtually all therapeutics including gemcitabine, the standard front line agent for palliative chemotherapy. Early clinical studies point to the promise of photodynamic therapy (PDT) for management of this deadly disease. Here we examine PDT with verteporfin for treatment of cells that are non-responsive to gemcitabine and identify intracellular and extracellular factors that govern sensitivity to each modality.
Study Design
Using MTS we assess cytotoxicity of verteporfin-PDT in gemcitabine-treated non-responsive populations from a panel of five pancreatic cancer cell lines representing a range of tumor histopathology and origin. We conduct western blots for pro-/anti-apoptotic proteins bax and Bcl-XL to identify factors relevant to PDT and gemcitabine sensitivity. To examine the role of extracellular matrix influences we compare response to each modality in traditional cell culture conditions and cells grown on a laminin-rich basement membrane.
Results
All cell lines have gemcitabine non-responsive populations (17 to 33%) at doses up to 1mM while moderate total verteporfin PDT doses (1–6µMJ/cm2) produce nearly complete killing. Our data shows that cells that are non-responsive to sustained gemcitabine incubation are sensitive to verteporfin PDT indicating that the latter is agnostic to gemcitabine sensitivity. Verteporfin-based PDT decreases Bcl-XL and increases the bax/Bcl-XL ratio toward a pro-apoptotic balance. Insensitivity to gemcitabine is increased in cells that are adherent to basement membrane relative to traditional tissue culture conditions.
Conclusions
Collectively these results indicate the promise of verteporfin-based PDT to bypass intracellular and extracellular cues leading to gemcitabine resistance and point to the emerging role of this therapy for treatment of pancreatic cancer.
INTRODUCTION
Pancreatic adenocarcinoma is an aggressive, invasive disease that is resistant to virtually all available therapeutics including chemo- and radiotherapy. With a median survival of less than 6 months, it is one of the most dismal prognoses of any human cancer (1,2). The disease typically presents at a late stage such that potentially curative surgery is feasible in only 15–20% of patients, and even in this subset of patients five year survival is only 20% (3). Since the landmark clinical study of Burris et al (4), the standard of care for patients with advanced disease consists of palliative chemotherapy with the nucleoside analog gemcitabine. Gemcitabine was shown to provide a modest survival enhancement over 5-Fluorouracil and some mitigation of disease-related symptoms, however, this clinical benefit is obtained by only ~24% of patients who are responsive to this agent. More recently, a chemotherapy combination consisting of oxaliplatin, irinonectan, fluorouracil and leucovorin (FOLFIRINOX) has been shown to prolong survival relative to gemcitabine, but with higher toxicity that is suitable only for patients with good performance status (5). The disappointing response to gemcitabine (and other chemotherapeutics) in pancreatic tumors likely arises from a complex set of factors including intracellular anti-apoptotic signaling (6,7), the complexity of gemcitabine metabolism, which provides numerous mechanisms that can be exploited to develop resistance (8,9) and interactions between the extracellular matrix and other stromal components (10–16). This bleak scenario, where surgery is usually not an option, combined with the failure of chemotherapy to meaningfully improve outcomes, points to the urgent need for exploration of new approaches that can bypass resistance and/or synergize with traditional therapeutics.
Photodynamic therapy (PDT) is a light-based therapeutic modality for several human cancer and non-cancer pathologies which has demonstrated promise for treatment of pancreatic cancer in clinical and pre-clinical studies (17–21). In PDT, a light-activatable molecule known as a photosensitizer (PS) is used to generate cytotoxic reactive chemical species with spatial specificity governed by both the localization of the PS itself and the delivery of light of a specific wavelength required for therapeutic excitation of the PS (22). Early pre-clinical and clinical studies demonstrated the capability of PDT to induce controlled regions of necrosis in localized pancreatic tumors and enhance survival (19,20,23,24). More recently the second generation PS, benzoporphyrin derivative monoacid ring-A (BPD) has emerged as a potent PS with superior pharmacokinetics and high singlet oxygen yield. PDT with, verteporfin, a liposomal formulation of BPD has already been used safely and effectively in millions of patients worldwide for treatment of age-related macular degeneration (25,26), and has recently shown promise in a Phase I clinical study in human pancreatic cancer patients (18).
PDT using PS for which the mitochondria is a major cellular target may hold promise as an adjuvant to chemotherapies targeting other sites and pathways that can be more readily bypassed by tumor drug resistance strategies. A crucial point, as articulated by Kessel, is that PDT can act as a mitochondrial inducer of apoptosis, bypassing numerous potential mutations in tumors driven to suppress apoptotic signaling by triggering release of cytochrome c by direct insult to the mitochondria (27). Indeed, this mechanism of cytotoxicity has been exploited by our lab and others to develop mechanism-based combinations of PDT with other therapeutics simultaneously targeting more than one non-overlapping pathway to achieve enhanced efficacy (28–32). This scenario is especially potentially promising for PDT with verteporfin, which preferentially accumulates in mitochondria (33–36), for treatment of cancer cells that are not responsive to gemcitabine, for which resistance has been shown to be dependent on mitochondria mediated apoptosis (6,7).
In this study we demonstrate that verteporfin PDT eradicates the challenging sub-populations that are non-responsive to even high doses of gemcitabine alone. We specifically contrast the efficacy of PDT with gemcitabine as individual treatments and conduct a systematic evaluation of verteporfin uptake/localization and PDT efficacy in a panel of pancreatic cancer cell lines derived primary tumors, ascites, and metastases, with varying histologic differentiation and immunocytochemical features (37–39). Finally, in light of the noted importance of tumor microenvironment, and specifically interactions with the extracellular matrix in pancreatic cancer, we compare gemcitabine and verteporfin PDT response in traditional tissue culture conditions and in cells grown on basement membrane.
MATERIALS AND METHODS
Cell lines and reagents
AsPC-1, BxPC-3, Capan-1, Capan-2, and PANC-1 cell lines were obtained from American Type Culture Collection (ATCC, Rockville, MD) and grown according to ATCC descriptions. Media and fetal bovine serum were obtained from Mediatech (Herndon, VA) and Invitrogen (Carlsbad, CA), respectively. All media was supplemented with 50 IU/mL penicillin and 50 µg/mL streptomycin (Mediatech). Photodynamic therapy (PDT) treatments using the photosensitizer benzoporphyrin derivative monoacid ring-A (BPD) were conducted using a liposomal formulation of this agent (verteporfin, trade name Visudyne®) provided for these studies by Quadra Logic Technologies (QLT Inc, Burnaby, British Columbia, CA). Quoted concentrations of verteporfin were confirmed by absorption measurements based on the well-characterized photophysical properties of the free BPD content of verteporfin (40).
Cell culture on basement membrane
In some cases where noted specifically in the text, cells were grown in overlay on a bed of Growth Factor Reduced (GFR) Matrigel™ (BD Biosciences, Bedford, MA, USA) as a basement membrane to model stromal interactions in pancreatic cancer. For these studies, which included internal comparison with growth on traditional tissue culture treated plastic, Matrigel beds were prepared by adding 250µL GFR Matrigel to selected wells of black-walled, Equiglass coverslip botttom 24-well dishes (Genetix, San Jose, CA, USA) and incubating at 37°C for 15–20 minutes for Matrigel to undergo sol-gel transition. A 1 mL volume of single cell suspension (7500cells/mL) was added to each well and plates were returned to the incubator for 24 hours prior to initiation of treatment.
Verteporfin (BPD) uptake measurements
Cells were incubated with 250 nM verteporfin (liposomal BPD) for various time intervals. Following incubation cells were washed with PBS twice and lysed using 0.1 M NaOH/1% SDS for two hours with shaking. Lysates were sonicated to eliminate interference from DNA. 100 µL of each sonicated lysate was aliquoted to a 96-well white-walled plate, and BPD concentration was quantified in a SpectraMax Gemini EM fluorescent plate reader (excitation 436 nm, emission 690 nm) with appropriate standards. The resulting BPD concentrations were normalized to protein concentrations in the lysates, which were determined using the Lowry protein assay (BioRad, Hercules, CA), according to the manufacturer’s instructions.
Quantitative photosensitizer co-localization analysis
To quantify verteporfin localization with mitochondria, an imaging based method was developed for three-dimensional quantitative co-localization of the presence of BPD and Mitotracker Green (Invitrogen, Carlsbad, CA) in confocal fluorescence z-stacks. Images were obtained using an Olympus FV-1000 confocal microscope. Cells in 35mm glass bottom dishes (MatTek corporation, Ashland, MA) BPD and Mitotracker Green were simultaneously excited with a 488nm line from an argon ion laser. Optimal dichroic mirror, filter and detector settings for each fluorescence channel were established imaging each dye separately and the spectrum of each dye was verified in hyperspectral scans. Three-dimensional stacks were saved for offline processing using routines that we developed in MATLAB. Briefly, our analysis routine would generate a mask at each z-position by segmentation of the mitochondria fluorescence channel and multiply this mask and its inverse respectively by the BPD channel to determine the relative BPD fluorescence intensity from mitochondria co-localized pixels and non-mitochondria pixels. The ratio of the summations of the mitochondrial co-localized BPD fluorescence and total BPD fluorescence were then reported for each z-stack to obtain fractional co-localization.
Gemcitabine and PDT Treatments
Cells seeded onto 35 mm culture dishes were allowed to grow for 24 hours prior to treatment for 72 hours with the indicated concentrations of gemcitabine (Chemiceuticals LLC, Apex, NC, USA). For PDT treatments, cells seeded onto 35 mm culture dishes were incubated with medium containing 250 nM BPD for one hour. Prior to irradiation, medium was replaced with BPD-free medium. Cells were irradiated at specified doses using a 690 nm laser diode source (Model 7401; High Power Devices, Inc.) at a fluence rate of 100 mW/cm2 measured via VEGA laser power energy meter (Ophir Laser Measurement Group, LLC) and allowed to incubate for 24 hours prior to evaluation. Laser irradiation was delivered through the bottom of each culture dish on a clear plastic tray via a vertically mounted Thorlabs FT600EMT multimode fiber (Thorlabs, Newton, New Jersey, USA), which was collimated to overfill the dish area for nearly uniform light delivery. In the gemcitabine + PDT combination treatment experiments, all cells were treated for 72 hours with 500µM gemcitabine (as described above) prior to a media change with gemcitabine-media with or without BPD depending on treatment group. Media was again changed prior to irradiation (as for the PDT protocol described above) with media also containing 500µM gemcitabine.
Cell viability measurements
With the exception of experiments in basement membrane culture conditions described below, viability was measured using the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI) for all treatment response assays. The protocol was modified for 35 mm and 6-well plates as follows. Phenazine methosulfate (PMS) and 3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) solutions from the kit were mixed at a ratio of 1:20. The resulting solution was diluted 1:6 in culture medium to form MTS/PMS medium. Cells were incubated with 2 mL of MTS and PMS medium for 30 minutes. After incubation three 200 µL aliquots of medium were transferred to clear 96-well flat-bottom plates, and the absorbance at 490 nm was read by a plate reader.
In experiments comparing treatment response in cells grown on tissue culture treated plastic with cells grown on basement membrane, the MTS assay was not suitable for use due to binding of the reagents to the Matrigel as previously described (31,41). For this set of experiments an imaging-based approach to enumerate cells positively stained with Calcein AM (Invitrogen, Carlsbad, CA) as a fluorescent label for viable cells was used. This method was applied for both the Matrigel overlaid cells as well as the relevant comparative groups of cells grown directly on tissue culture (TC)-treated plastic for internally consistent quantification of viability in this subset of studies. Briefly, following PDT or Gemcitabine treatment, cells were incubated with Calcein AM for 40 minutes prior to imaging on the FV-1000 microscope using a 4X objective to obtain large fields containing many cells and the 488nm line from an Argon ion laser for excitation of the cleaved Calcein. Images were processed in Matlab using a routine to segment the fluorescent images and count viable cells, based on a methodology previously described (41). Fractional viability was reported as a ratio of the number of viable cells in a given treatment group to the no treatment group for the same plating condition (Matrigel, or directly on TC-treated plastic).
Western blots for Bcl-XL and Bax
In an effort to identify some of the key factors that may provide the basis for the effectiveness of verteporfin PDT against cells that are non-responsive to gemcitabine we investigated the treatment-dependent status of Bax, a pro-apoptotc factor, and Bcl-XL, an anti-apoptotic factor in the bcl-2 family of proteins that is important to both gemcitabine (7) and PDT response (42–44). AsPC-1 cells were plated in 35 mm culture dishes and incubated for 24 hours. Cells were treated with PDT, BPD only, or gemcitabine as described above, at sub-curative doses; 7 plates were used for each treatment regimen. Cell killing was verified using one plate for MTS assay. After treatment, the remaining 6 plates of cells were lysed using RIPA buffer for 1 hour with shaking, and the lysates were combined.
Protein was quantified using Lowry or Bradford assay and separated on a 12% SDS-PAGE gel. Protein in the gels was transferred to nitrocellulose membranes. Membranes were blocked using 5% nonfat dry milk in TBS-T overnight. Membranes were then incubated overnight at 4° Celsius with primary antibody (rabbit anti-Bax or rabbit anti-Bcl-XL, Cell Signaling, Danvers, MA, USA), then incubated overnight at 4° Celsius with anti-rabbit antibody horseradish peroxidase (HRP) conjugate. For visualization, blots were exposed to HRP substrates (Amersham, Little Chalfont, Buckinghamshire, UK) and subjected to autoradiography using XAR-5 film (Kodak, Rochester, NY). Densitometric analysis of the blots was performed using ImageJ software (National Institutes of Health) to analyze scanned images of the film and obtain the ratio of Bax to Bcl-XL for each treatment group.
RESULTS
Verteporfin uptake and intracellular localization in pancreatic cancer cell lines
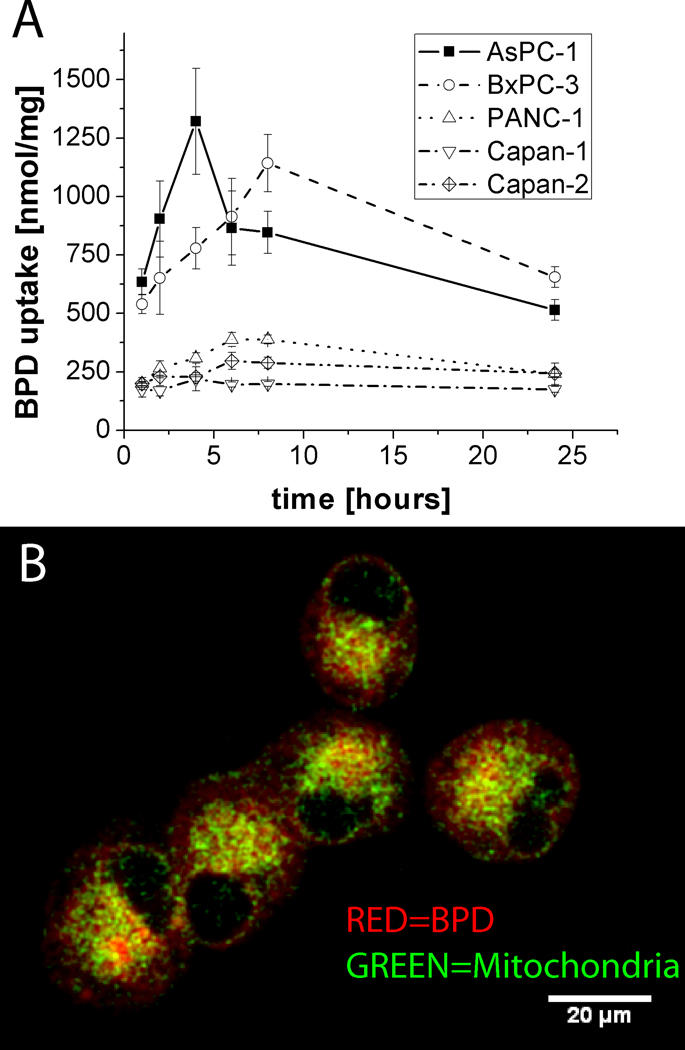
We examined uptake and intracellular localization of liposomal verteporfin in pancreatic cancer cell lines using extraction to quantify total verteporfin uptake complemented by a quantitative imaging approach to examine co-localization with mitochondria. The extraction analysis (Figure 1A) reveals significant variation in total verteporfin uptake, reported in nmol/mg of total protein, across cell lines. AsPC-1 and BxPC-3 cells have peak total verteporfin levels in the µmol/mg regime in contrast to PANC-1, Capan-1 and Capan-2 cells which all exhibit lower uptake with peak verteporfin levels from 200–400 nmol/mg.
Figure 1.
Analysis of total verteporfin uptake and mitochondrial co-localization in pancreatic cancer cell lines. (A) Time series of total verteporfin uptake measured by extraction of BPD from lysed cells normalized to total protein content at each time point. In (B) mitochondrial co-localization in live cells is visualized by overlaid verteporfin (BPD) fluorescence (red) and Mitotracker green (green) channels from representative slices through confocal z-stacks of each cell line. A single representative (multichannel) slice is shown from one of the PANC-1 image datasets. Quantitative analysis of 3D datasets shows that there is no significant difference in the proportion of mitochondrial co-localization as a fraction of total photosensitizer uptake for PANC-1 and AsPC-1 (55 +/− 4 %, and 62 +/− 18%, respectively), as described in the text.
The mitochondria is considered a preferential (though not exclusive) intracellular site of localization for BPD and an important target for verteporfin PDT induced cytotoxicity (33–36). We therefore further probed whether the total amount of verteporfin uptake (and cytotoxicity, below) in pancreatic cancer cell lines is correlated with the extent of mitochondrial co-localization (Figure 1, B) using a custom confocal imaging-based 3D co-localization routine. Analysis of mitochondrial co-localization after 60 minutes in AsPC-1 (relatively high verteporfin uptake) and PANC-1 (relatively low verteporfin uptake) shows that there is no significant difference in the proportion of PS localized to the mitochondria (as a percentage of total PS fluorescence) with 55 +/− 4 % and 62 +/− 18% respectively, exhibiting partial localization to the mitochondria, but also diffuse cytosolic distribution consistent with previous reports (45).
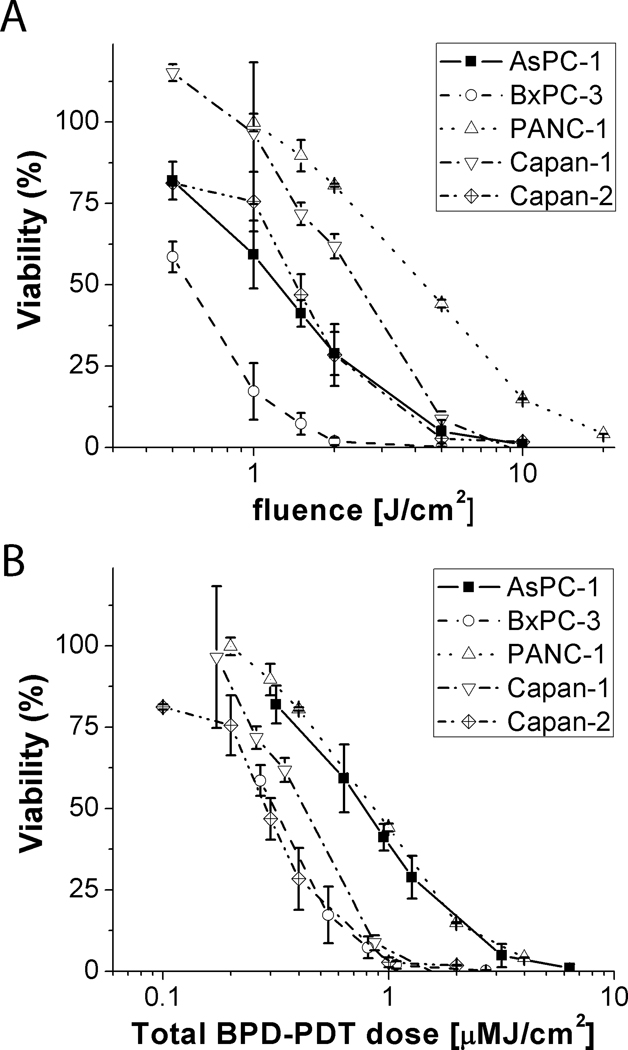
Verteporfin PDT cytotoxicity in pancreatic cancer cells
To examine sensitivity to verteporfin-based PDT treatment all cell lines were incubated with 250nM verteporfin for 60 minutes prior to irradiation at 690nm at varying total fluence as shown in Figure 2A. Although sensitivity to verteporfin PDT treatment varies between cell lines, in most cases there is nearly complete killing with a 10J/cm2 treatment. This representation however, does not reflect cell killing with respect to the amount of verteporfin taken up in each cell type. In Figure 2B, viability is plotted against total verteporfin PDT dose, with dimensions of PS concentration [µM] × total fluence [J/cm2] or [µM·J/cm2]. In this more complete representation, which essentially shows sensitivity to verteporfin-based PDT per molecule of BPD taken up by each cell type, dose response collapses into a much more compact range, and the dose response for all 5 cell lines lie on two distinct curves with PANC-1 and AsPC-1 exhibiting significantly less sensitivity than the other three. In all cells there is nearly complete cell killing at a total verteporfin PDT dose of ~ 6µM·J/cm2 in all cases. As shown in Supplemental Figure 2, there was little or no toxicity in control groups exposed to light alone (at the highest fluence used) or from incubation with verteporfin only.
Figure 2.
Verteporfin PDT dose response curves. In (A), percentage viability is plotted against total fluence delivered (at 690nm) showing wide variation in response across the five cell lines reported (AsPC-1, BxPC-3, PANC-1, Capan-1 and Capan-2 cells). In (B), total verteporfin PDT dose deposited is reported as the product of photosensitizer concentration and fluence accounting for the actual amount of BPD taken up by each cell line at the time of light activation (1 hour) using the extraction data shown in Figure 1.
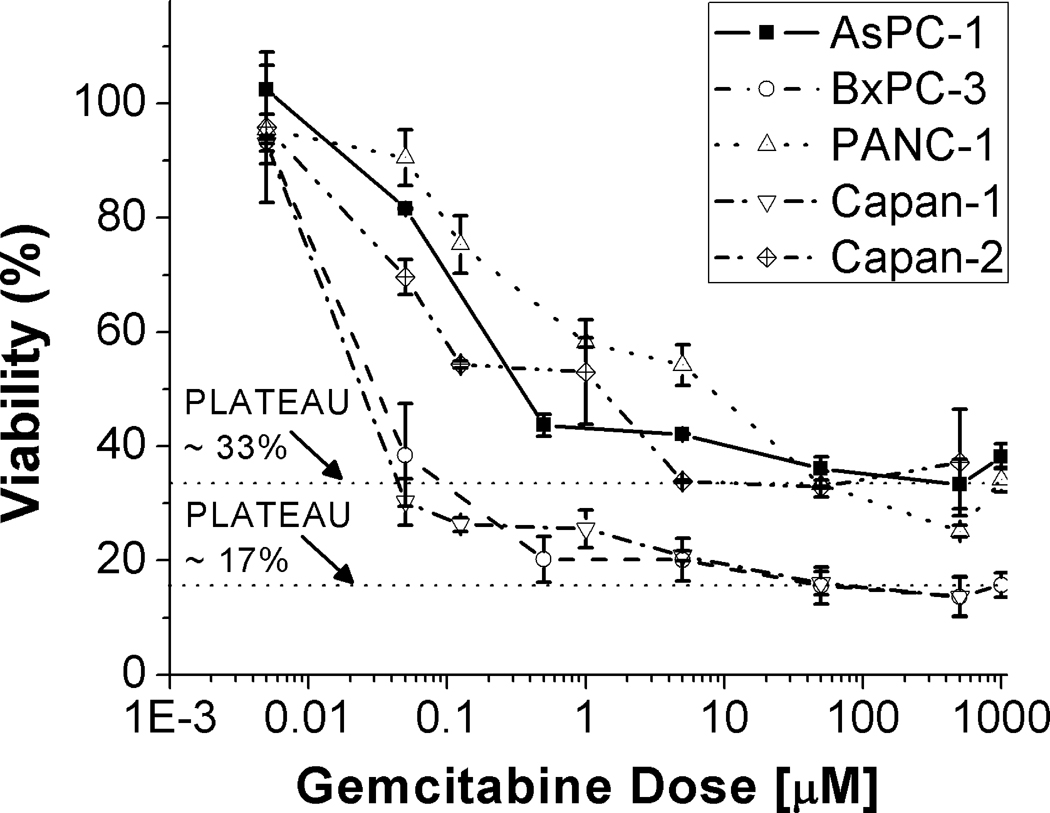
Gemcitabine treatment leaves residual surviving fractions
Dose response to gemcitabine was examined by MTS assay in all five cell lines tested in this study over more than 5 orders of magnitude from 5nM to 1 mM concentrations for 72 hour incubation periods (Figure 3). In all cases there is a modest cytotoxic response evidenced by a roughly 50% reduction in viability in the nM regime, consistent with previous reports of the in vitro IC50 value for this drug. However, all cell lines harbor highly gemcitabine-resistant fractions which persist even at the highest doses examined and with no increased cytotoxicity evident from increased doses from 1µM to 1 mM. The most pronounced surviving fractions of approximately 40%, are observed in the AsPC-1, Capan-2 and PANC-1 cells, while BxPC-3 and Capan-1 cells are relatively more sensitive to gemcitabine, with more precipitous response at low doses and a smaller fraction of residual non-responsive cells (~20%).
Figure 3.
Gemcitabine dose response curves. Percentage viability is plotted against a nearly 6 order of magnitude range gemcitabine dose where in each case the indicated concentration was incubated for 72 hours. The results indicate that in each case there is an initial response at sub micromolar doses, while non-responsive sub-populations of each cell type persist even to millimolar concentrations of the drug as evidence by the extended plateaus in each dose response curve. Horizontal dashed lines are included to guide the eye indicating an approximately 33% gemcitabine non-responsive fraction of AsPC-1, Capan-2 and PANC-1 cells and 17% non-responsive fraction of Capan-1 and BxPC-3 cells.
Verteporfin PDT is highly cytotoxic in gemcitabine-resistant cells
Building on the observation that verteporfin PDT is a highly efficient cytotoxic modality in otherwise treatment resistant pancreatic cancer cells, we evaluated sensitivity of residual cells to gemcitabine non-responsive populations to subsequent verteporfin PDT. Modeling the clinical scenario, in which tumors would be exposed to gemcitabine as a first line therapy, all cultures were incubated with high dose gemcitabine (500µM) to generate residual non-responsive populations which could then be subject to PDT and/or continued gemcitabine. For combinations, an effective fluence for PDT treatment of each cell line was selected based on the data in Figure 2A (10J/cm2 for AsPC-1, Capan-1 and Capan-2, 4J/cm2 for BxPC-3 and 20J/cm2 for PANC-1). In Figure 4, response to sustained gemcitabine treatment only is compared with the result of gemcitabine followed by either verteporfin only (250nM), light only, or complete verteporfin PDT treatment in all five cell lines probed here. Gemcitabine after the initial 72 hour regimen is shown to provide no additional benefit with or without light or verteporfin (BPD) controls. In contrast PDT treatment is highly cytotoxic in the gemcitabine resistant populations, producing nearly complete cell killing within the sensitivity of the MTS assay.
Figure 4.
Bar graph showing response of gemcitabine non-responsive cells to subsequent verteporfin PDT treatment normalized to cells which received only gemcitabine. The effective PDT dose for each cell line was selected based on the treatment response data in Figure 2A ((10J/cm2 for AsPC-1, Capan-1 and Capan-2, 4J/cm2 for BxPC-3 and 20J/cm2 for PANC-1). Sustained gemcitabine treatment at 500µM produces no benefit in treatment of residual non-responsive cells, which however exhibit approximately the same response to PDT treatment as cells that are naïve to gemcitabine treatment (Figure 2).
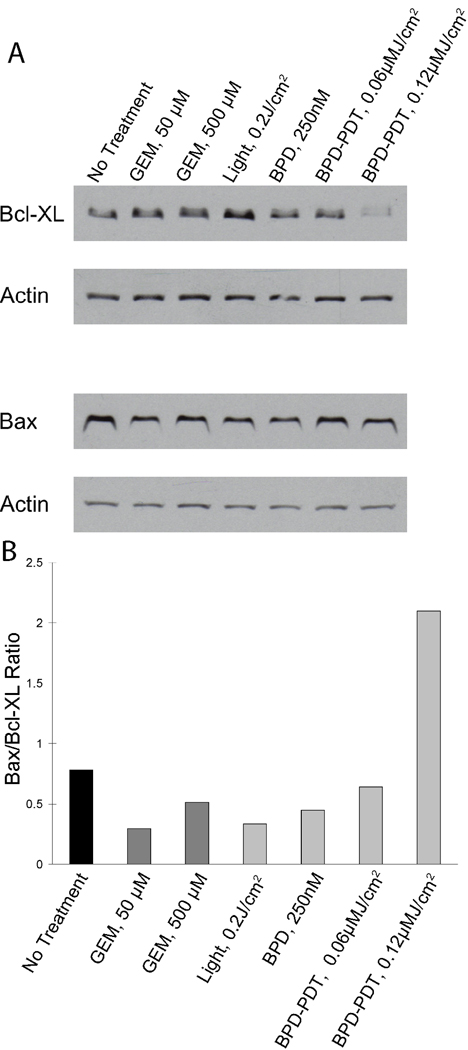
Gemcitabine and PDT sensitivity correlated with relative expression of pro and anti-apoptotic proteins
To examine the mechanisms underlying the contrasting sensitivities to gemcitabine and verteporfin PDT treatments observed in this study, we conducted western blots and densitometry analysis for the relative expression of anti-apoptotic factor Bcl-XL, and pro-apoptotic factor bax following sub-lethal doses of each modality. Bcl-XL expression levels were observed to be high in all of the cell lines tested (Supplemental Figure 1), a trait which could be associated with a protective effect against gemcitabine treatment (6). As seen in Figure 5 (AsPC-1 cells), the intensity of the Bax band shows little variation relative to the loading control across all the treatment groups. In contrast, there is a clear decrease in Bcl-XL in response to PDT treatment indicating direct loss of this mitochondrial regulator of apoptosis which has been linked with gemcitabine resistance (7). Densitometry analysis further illustrates that this leads to a roughly 3-fold increase the ratio of Bax to Bcl-XL in the 0.2 J/cm2 PDT treated cells, indicating a PDT-induced shift in the balance toward pro-apoptotic factors, while for both gemcitabine treatment arms, the value of the ratio remains in the same range as that of the control groups.
Figure 5.
Western blots for Bcl-XL and Bax protein content. Cells were treated with sub-curative doses of PDT and gemcitabine, and extracts were probed for Bcl-XL and Bax protein content by Western blot. In (A), from left to right, the lanes in the blot correspond to untreated cells, 50 µM gemcitabine, 500 µM gemcitabine, 0.2 J/cm2 light dose only (no verteporfin), 250 nM verteporfin only, no light, verteporfin PDT with 0.1 J/cm2 light dose, and with 0.2 J/cm2 light dose. In (B), the ratio of Bax to Bcl-XL is shown from densitometry analysis of the western blot data in (A).
Interaction with basement membrane enhances gemcitabine resistance
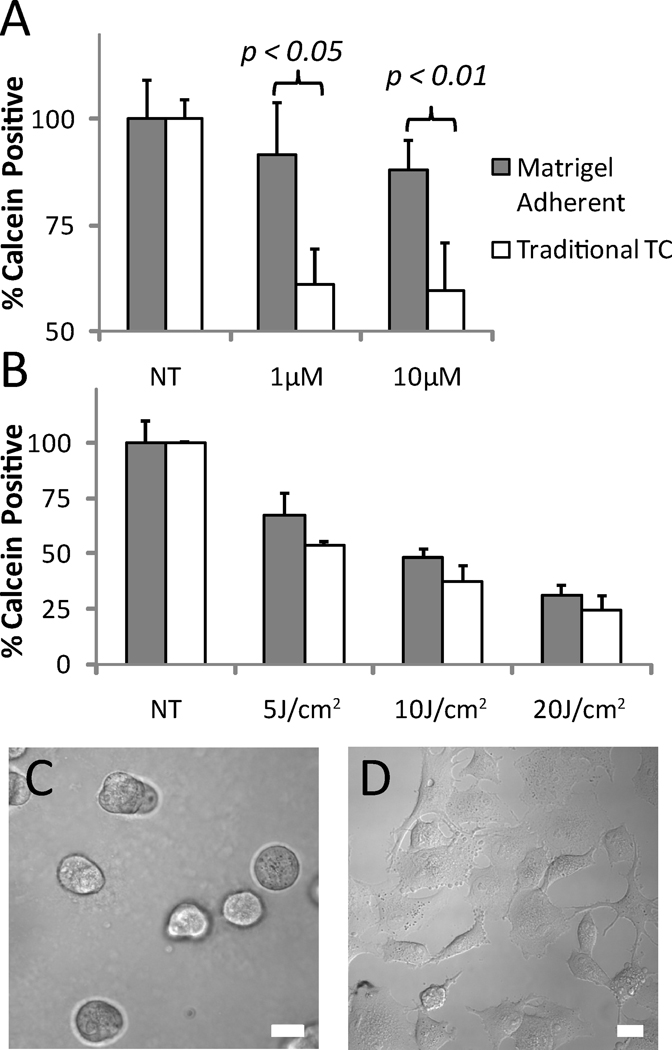
To examine the role of ECM interactions in gemcitabine and PDT sensitivity, we contrasted treatment response in PANC-1 cells plated side by side on either tissue culture treated plastic (standard monolayer growth conditions) or on beds of GFR Matrigel. In both cases, single cell suspensions of equal density were allowed 24 hours to adhere and equilibrate prior to initiation of treatment. In the basement membrane overlay cultures, the presence of the underlying matrix bed, which is included to recapitulate early events in physiological tumor-stroma signaling, poses no barrier to drug penetration, and the 24 hour time interval does not allow for formation of multicellular 3D nodules on the Matrigel surface as previously described (31,46,47). As seen in Figure 6A, the presence of the GFR Matrigel induces a significant decrease in sensitivity to gemcitabine in contrast to cells grown in monolayer cultures which exhibit several-fold higher fractional loss in the number of viable (calcein positive) cells upon incubation with 1µM (p<0.05) and 10µM doses (p<0.01) of gemcitabine. There is also some evidence of a decrease in sensitivity to verteporfin PDT in the basement membrane adherent cells, though the magnitude of the apparent influence of matrix adhesion is interaction is less than in the case of gemcitabine treatment. We note that PANC-1 monolayer dose response to verteporfin PDT and gemcitabine as reported in Figure 6 are not numerically identical to those values in Figures 2 and 3 respectively due to the different methodology as necessitated by the side-by-side comparison with Matrigel adherent cells that could not be assayed by MTS The methodology of enumerating calcein positive cells in these experiments will report fractional viability values that are higher than MTS, as any cell with sufficient esterase activity to convert calcein AM above an arbitrary fluorescence threshold will be enumerated in a binary manner, in contrast to MTS, for which the contribution to the total signal from damaged or dying cells may be considerably lower.
Figure 6.
A comparison of sensitivity to gemcitabine treatment (A) and verteporfin PDT treatment (B) in PANC-1 cells grown in traditional cell culture conditions versus those grown in overlay on beds of GFR Matrigel, a commercial basement membrane. The morphology of PANC-1 cells (40X magnification, DIC) at the time of treatment on GFR Matrigel (C) and traditional tissue culture (TC) plastic (D) reveal that the matrix adherent cells are more compact and less extended in the plane. In (A), cells on basement membrane exhibit decreased sensitivity to gemcitabine treatment, while in (B) the presence of the basement membrane has less impact on sensitivity to verteporfin PDT treatment. Scale bars equal 20µm.
DISCUSSION
Collectively, the results of this study point to the promising role of verteporfin-based PDT to eradicate stubborn populations that are non-responsive to gemcitabine and perhaps other standard chemotherapy agents for treatment of pancreatic cancer. Our results indicate that PDT with verteporfin overcomes multiple mechanisms of gemcitabine resistance and is effective against the troublesome pancreatic cancer cell populations that are non-responsive to gemcitabine. It has been previously shown that gemcitabine resistant pancreatic cancer cells are also more invasive and migratory, thus further underscoring the importance of our finding that verteporfin PDT can target this potentially lethal population (48). Gemcitabine resistance in pancreatic cancer cells has previously been linked with Bcl-XL, a mitochondrial anti-apoptotic factor (6). Our results (Figure 5) show that verteporfin PDT targets this very factor, thus optimally leveraging the inherent preferential localization of this photosensitizer to the mitochondria to attack a key site for undermining this gemcitabine resistance mechanism exploited by pancreatic cancer cells. Interestingly, comparison of PDT treatment in naïve cells (Figure 2) and as part of a regimen in cells that have been subject to high dose gemcitabine (Figure 4) indicates that PDT efficacy is agnostic to prior exposure to gemcitabine treatment. Furthermore the contrasting sites and mechanisms of each modality may have interesting implications that warrant further investigation in more complex preclinical models that would allow for a broader application.
The results of this study in five pancreatic cancer cell lines point to the broad conclusion that these cells are generally responsive to PDT with significant populations that are non-responsive to gemcitabine. However, evaluation of response in this diverse and well-characterized panel of cell lines exhibiting a range of disease histopathology allows for focused correlation between treatment response and tumor grade, stage, and specific immunocytochemical features (37–39). In general, our results are consistent with an inverse correlation between the grade and histologic differentiation and sensitivity to therapeutics. Of the cell lines tested, the two adenocarcinoma lines derived from tumors with poor histologic differentiation (AsPC-1 and PANC-1) (39), both very clearly exhibit the least sensitivity to PDT and gemcitabine treatment. AsPC-1 and PANC-1 are also derived from the highest grade tumors (G2 and G3 respectively, WHO grading system) (38). Capan-2, which are of moderate histologic differentiation, also exhibit less sensitivity to gemcitabine than either Capan-1 (well differentiated) or BxPC-3 (adenosquamous) but share approximately the same verteporfin PDT dose response relationship with these cell lines. Of the five cell lines used in this study there is also a correlation between the cell lines which have been reported by Sipos et al to have the strongest expression of vimentin (38), AsPC-1 and PANC-1, and the least sensitivity to therapeutics. Additionally, Capan-2, AsPC-1 and PANC-1, the cell lines with poor gemcitabine response, and with the exception of Capan-2, poor response to verteporfin PDT, have all been shown to overexpress cytokeratin (CK) 8, CK18, and CK19, in contrast to Capan-1 and BxPC-3 which stain positively for these markers in less than 10% of cells. While it is too early to make any firm conclusions from these in vitro studies these findings point to the utility of further investigation of these targets in more elaborate in vivo studies to identify populations that will benefit most from verteporfin PDT and gemcitabine treatments.
This study also brings to light provocative observations on the critical role of the tumor microenvironment in drug response that are relevant to the present discussion as to the role of the rigid fibrotic stroma that surrounds pancreatic tumors serving potentially, as both a barrier to drug penetration and a signaling partner. While recent studies have made the case that enhancing gemcitabine delivery through the hypovascular stroma improves treatment response (14,16), it has also been asserted that the role of drug penetration is overstated as pancreatic cancer cells are innately non-responsive to therapeutics, even when barriers to delivery are not present (11). In this study we show that PANC-1 cells, a primary tumor derived cell line (which would exist in this characteristic stroma-rich environment) actually become more resistant to gemcitabine treatment when grown in contact with a bed of laminin-rich basement membrane. In these experiments examining the role of stromal signaling in a culture geometry where drug penetration is explicitly not a factor, we demonstrate that indeed the mere presence of extracellular matrix changes treatment sensitivity. It is important to note that in contrast to previous studies in which we and others have utilized overlay growth on GFR Matrigel for three-dimensional cell culture, the brief period of overlay growth gel surface in the present study allows for adhesion to the matrix and early events in the change of phenotype in the matrix rich environment without formation of large multicellular 3D nodules, which require extended periods in culture. Our results (Figure 6), using the laminin-rich basement membrane Matrigel, are consistent with a previous report from Miyamoto et al that the presence of laminin in cultures of PANC-1 and other pancreatic cell lines reduced cytotoxicity of 5-FU (15). This observation could be explained by increased treatment resistance through interaction of cancer cells with the stroma via cell-matrix receptors such as integrins (49). It is further interesting to note that while gemcitabine treatment is clearly less effective in Matrigel adherent cells, verteporfin PDT efficacy is only modestly decreased indicating that the mechanism of cytotoxicity of verteporfin PDT, by direct damage to the mitochondria, may bypass the mechanisms through which matrix interactions lead to an apparent decrease in gemcitabine sensitivity observed here. Runnels et al have shown that BPD-PDT decreases the function of α5β1 integrin, thus potentially disrupting this resistance pathway (45). This observation suggests the utility of further investigation using more fully developed methods for three-dimensional culture of pancreatic cancer cell lines in extracellular matrix and quantitative methods for analysis of growth and treatment response in 3D systems, building on recent studies from our own lab in other 3D and in vivo disease models (31,41).
These early studies, combined with ongoing clinical work point to the promising role of PDT in the therapeutic armamentarium for pancreatic cancer. The demonstrated capability of verteporfin-based PDT to bypass gemcitabine resistance could be especially important given the widespread lack of response to this front line therapeutic. Building on the central findings of this study, further investigation into PDT and gemcitabine combination regimens is warranted to identify the optimal dose and scheduling of each modality in more sophisticated pre-clinical models and also to investigate different routes of delivery. For example, nanoconstructs that allow delivery of multiple therapeutics (50) could be used for simultaneous delivery of BPD, gemcitabine and other agents.
Supplementary Material
Western blot data showing strong expression of Bcl-XL relative to Actin loading control in all pancreatic cancer cell lines investigated in this study.
ACKNOWLEDGMENTS
We thank Imran Rizvi, Adnan Abu-Yousif, and Adam R. Blanden of the Wellman Center for Photomedicine and Virginie Cerec of University College London for insights resulting from discussion of this work. We gratefully acknowledge funding from the National Institutes of Health, P01CA084203-06 (to T.H.).
Footnotes
SUPPLEMENTARY MATERIALS
Figure S1 can be found at DOI: 10.1562/2006-xxxxxx.s1.
REFERENCES
- 1.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2(12):897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 2.Warshaw A, Fernandez-del Castillo C. Pancreatic carcinoma. New England Journal of Medicine. 1992;326(7):455. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 3.Ahrendt S, Pitt H. Surgical management of pancreatic cancer. Oncology (Williston Park) 2002;16(6):725–734. [PubMed] [Google Scholar]
- 4.Burris H, 3rd, Moore M, Andersen J, Green M, Rothenberg M, Modiano M, Cripps M, Portenoy R, Storniolo A, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of Clinical Oncology. 1997;15(6):2403. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 6.Bold RJ, Chandra J, McConkey DJ. Gemcitabine-induced programmed cell death (apoptosis) of human pancreatic carcinoma is determined by Bcl-2 content. Ann Surg Oncol. 1999;6(3):279–285. doi: 10.1007/s10434-999-0279-x. [DOI] [PubMed] [Google Scholar]
- 7.Schniewind B, Christgen M, Kurdow R, Haye S, Kremer B, Kalthoff H, Ungefroren H. Resistance of pancreatic cancer to gemcitabine treatment is dependent on mitochondria-mediated apoptosis. Int J Cancer. 2004;109(2):182–188. doi: 10.1002/ijc.11679. [DOI] [PubMed] [Google Scholar]
- 8.Mackey JR, Yao SY, Smith KM, Karpinski E, Baldwin SA, Cass CE, Young JD. Gemcitabine transport in xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters. J Natl Cancer Inst. 1999;91(21):1876–1881. doi: 10.1093/jnci/91.21.1876. [DOI] [PubMed] [Google Scholar]
- 9.Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T, Kohgo Y. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96(3):457–463. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101(4):887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 11.Garber K. Stromal depletion goes on trial in pancreatic cancer. J Natl Cancer Inst. 2010;102(7):448–450. doi: 10.1093/jnci/djq113. [DOI] [PubMed] [Google Scholar]
- 12.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6(4):1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 13.Ohuchida K, Mizumoto K, Murakami M, Qian LW, Sato N, Nagai E, Matsumoto K, Nakamura T, Tanaka M. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004;64(9):3215–3222. doi: 10.1158/0008-5472.can-03-2464. [DOI] [PubMed] [Google Scholar]
- 14.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28(1):38–44. doi: 10.1097/00006676-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, Tuveson DA. Stromal biology and therapy in pancreatic cancer. Gut. 2010 doi: 10.1136/gut.2010.226092. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Pereira SP. Photodynamic therapy for pancreatic and biliary tract carcinoma. San Jose, CA, USA: SPIE; 2009. pp. 71640J–71610J. [DOI] [PubMed] [Google Scholar]
- 18.Sandanayake NS, Huggett MT, Bown SG, Pogue BW, Hasan T, Pereira SP. PDT for locally advanced pancreatic cancer: early clinical results. San Francisco, California, USA: SPIE; 2010. pp. 75510L–75518L. [Google Scholar]
- 19.Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, Jones L, Wyld P, Gillams A, Hatfield AWR. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50(4):549–557. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayaru L, Bown SG, Pereira SP. Photodynamic therapy for pancreatic and biliary tract carcinoma. Int J Gastrointest Cancer. 2005;35(1):1–13. doi: 10.1385/IJGC:35:1:001. [DOI] [PubMed] [Google Scholar]
- 21.Hasan T, Ortel B, Solban N, Moor C, Pogue B. Photodynamic therapy of cancer. In: Kufe DW, Wiechselbaum RR, editors. Cancer Medicine. BC Decker Inc.; 2005. [Google Scholar]
- 22.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem Rev. 2010;110(5):2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikvy P, Messman H, MacRobert AJ, Pauer M, Sams VR, Davies CL, Stewart JC, Bown SG. Photodynamic therapy of a transplanted pancreatic cancer model using meta-tetrahydroxyphenylchlorin (mTHPC) Br J Cancer. 1997;76(6):713–718. doi: 10.1038/bjc.1997.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mlkvy P, Messmann H, Regula J, Conio M, Pauer M, Millson CE, MacRobert AJ, Bown SG. Photodynamic therapy for gastrointestinal tumors using three photosensitizers--ALA induced PPIX, Photofrin and MTHPC. A pilot study. Neoplasma. 1998;45(398383263):157–161. [PubMed] [Google Scholar]
- 25.Brown S, Mellish K. Verteporfin: a milestone in opthalmology and photodynamic therapy. Expert Opin Pharmacother. 2001;2(2):351–361. doi: 10.1517/14656566.2.2.351. [DOI] [PubMed] [Google Scholar]
- 26.Messmer KJ, Abel SR. Verteporfin for age-related macular degeneration. Ann Pharmacother. 2001;35(12):1593–1598. doi: 10.1345/aph.10365. [DOI] [PubMed] [Google Scholar]
- 27.Kessel D, Luo Y. Photodynamic therapy: a mitochondrial inducer of apoptosis. Cell Death Differ. 1999;6(1):28–35. doi: 10.1038/sj.cdd.4400446. [DOI] [PubMed] [Google Scholar]
- 28.Ferrario A, Chantrain CF, von Tiehl K, Buckley S, Rucker N, Shalinsky DR, Shimada H, DeClerck YA, Gomer CJ. The matrix metalloproteinase inhibitor prinomastat enhances photodynamic therapy responsiveness in a mouse tumor model. Cancer Res. 2004;64(7):2328–2332. doi: 10.1158/0008-5472.can-04-0071. [DOI] [PubMed] [Google Scholar]
- 29.Ferrario A, Rucker N, Wong S, Luna M, Gomer CJ. Survivin, a member of the inhibitor of apoptosis family, is induced by photodynamic therapy and is a target for improving treatment response. Cancer Res. 2007;67(10):4989–4995. doi: 10.1158/0008-5472.CAN-06-4785. [DOI] [PubMed] [Google Scholar]
- 30.Ferrario A, von Tiehl K, Wong S, Luna M, Gomer C. Cyclooxygenase-2 Inhibitor Treatment Enhances Photodynamic Therapy-mediated Tumor Response. Cancer Research. 2002;62(14):3956–3961. [PubMed] [Google Scholar]
- 31.Rizvi I, Celli JP, Evans CL, Abu-Yousif AO, Muzikansky A, Pogue BW, Finkelstein D, Hasan T. Synergistic enhancement of carboplatin efficacy with photodynamic therapy in a three-dimensional model for micrometastatic ovarian cancer. Cancer Res. 2010;70(22):9319–9328. doi: 10.1158/0008-5472.CAN-10-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duska L, Hamblin M, Miller J, Hasan T. Combination photoimmunotherapy and cisplatin: effects on human ovarian cancer ex vivo. J Natl Cancer Inst. 1999;91:1557–1563. doi: 10.1093/jnci/91.18.1557. [DOI] [PubMed] [Google Scholar]
- 33.Carthy CM, Granville DJ, Jiang H, Levy JG, Rudin CM, Thompson CB, McManus BM, Hunt DW. Early release of mitochondrial cytochrome c and expression of mitochondrial epitope 7A6 with a porphyrin-derived photosensitizer: Bcl-2 and Bcl-xL overexpression do not prevent early mitochondrial events but still depress caspase activity. Lab Invest. 1999;79(8):953–965. [PubMed] [Google Scholar]
- 34.Granville DJ, Jiang H, An MT, Levy JG, McManus BM, Hunt DW. Overexpression of Bcl-X(L) prevents caspase-3-mediated activation of DNA fragmentation factor (DFF) produced by treatment with the photochemotherapeutic agent BPD-MA. FEBS Lett. 1998;422(2):151–154. doi: 10.1016/s0014-5793(97)01616-5. [DOI] [PubMed] [Google Scholar]
- 35.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasan T, Ortel B, Solban N, Pogue B. Photodynamic therapy of cancer. In: Kufe DW, Bast RCJ, Hait WN, Hong WK, Pollock RE, Weichselbaum RR, Holland JF, Frei EI, editors. Cancer Medicine. 7th ed. Hamilton, Ontario: B.C. Decker, Inc.; 2006. pp. 537–548. [Google Scholar]
- 37.Ulrich AB, Schmied BM, Standop J, Schneider MB, Pour PM. Pancreatic cell lines: a review. Pancreas. 2002;24(2):111–120. doi: 10.1097/00006676-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Sipos B, Moser S, Kalthoff H, Torok V, Lohr M, Kloppel G. A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform. Virchows Arch. 2003;442(5):444–452. doi: 10.1007/s00428-003-0784-4. [DOI] [PubMed] [Google Scholar]
- 39.Loukopoulos P, Kanetaka K, Takamura M, Shibata T, Sakamoto M, Hirohashi S. Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity. Pancreas. 2004;29(3):193–203. doi: 10.1097/00006676-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Aveline B, Hasan T, Redmond RW. Photophysical and photosensitizing properties of benzoporphyrin derivative monoacid ring A (BPD-MA) Photochem Photobiol. 1994;59(3):328–335. doi: 10.1111/j.1751-1097.1994.tb05042.x. [DOI] [PubMed] [Google Scholar]
- 41.Celli JP, Rizvi I, Evans CL, Abu-Yousif AO, Hasan T. Quantitative imaging reveals heterogeneous growth dynamics and treatment-dependent residual tumor distributions in a three-dimensional ovarian cancer model. J Biomed Opt. 2010;15(5):051603. doi: 10.1117/1.3483903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kessel D. Promotion of PDT efficacy by a Bcl-2 antagonist. Photochem Photobiol. 2008;84(3):809–814. doi: 10.1111/j.1751-1097.2007.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kessel D, Arroyo AS. Apoptotic and autophagic responses to Bcl-2 inhibition and photodamage. Photochem Photobiol Sci. 2007;6(12):1290–1295. doi: 10.1039/b707953b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kessel D, Oleinick NL. Initiation of autophagy by photodynamic therapy. Methods Enzymol. 2009;453:1–16. doi: 10.1016/S0076-6879(08)04001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Runnels JM, rtel B, Solban N, Pogue B. BPD-MA-mediated photosensitization in vitro and in vivo: cellular adhesion and beta1 integrin expression in ovarian cancer cells. Br J Cancer. 1999;80(7):946–953. doi: 10.1038/sj.bjc.6690448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1(1):46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5(9):675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 48.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14(12):3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 49.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomed. 2008;3(2):137–140. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot data showing strong expression of Bcl-XL relative to Actin loading control in all pancreatic cancer cell lines investigated in this study.