Abstract
Background and Objective
Recent studies have demonstrated an effect of photodamage on the endocytic pathway involved in recycling of membrane components. Using a series of agents with known sub-cellular targets, we explored the determinants of photodynamic inhibition of endocytic processes in three cell lines: a murine leukemia, a murine hepatoma and a non-malignant epithelial cell line of human origin.
Study Design/Materials and Methods
The PI-3 kinase antagonist wortmannin blocks endosomal processing pathway dependent on this enzyme, providing an indication of the ‘flux’ of endocytosis. Microscopic observations were used to assess the effect of photodamage on this pathway. Photosensitizing agents specific for mitochondrial, endoplasmic reticulum (ER), lysosomal and endosomal photodamage were employed.
Conclusions
Sub-lethal photodamage directed against endosomes or lysosomes interrupted early steps in this endocytic process in the hepatoma cell line. A mechanism for these effects is proposed. Mitochondrial photodamage could interrupt endocytosis, but at levels that also induced apoptosis. ER photodamage did not affect endocytosis even at lethal levels. Somewhat similar results were obtained with other cell lines, but there were sufficient differences to indicate that the cell phenotype is, in part, a determinant of the endocytic response to PDT. Further work will be needed to delineate the role of these endocytic effects in the array of responses to photodynamic therapy.
Keywords: endosomes, membrane trafficking, photodynamic therapy
INTRODUCTION
Endocytosis is responsible for nutrient uptake, receptor signaling, growth, differentiation, adhesion and migration [1]. This pathway involves migration of portions of the plasma membrane to endosomes, where this membrane material can be re-utilized to form new plasma membrane or trafficked to lysosomes for degradation. At nanomolar concentrations, wortmannin is an irreversible antagonist of a phosphoinositol-3-kinase (PI-3K) that is necessary for some endocytic processes, [2–4], although not receptor-independent endocytosis [5]. When PI-3K is inhibited, a series of cytoplasmic vacuoles form, derived from endocytosed plasma membrane whose normal processing is thereby blocked [6,7]. The mechanism was proposed to involve continued endocytic plasma membrane influx into endosomes while membrane traffic between late endosomes and lysosomes is blocked [8]. The vacuoles therefore represent swollen late endosomes reflecting an inhibition of membrane traffic from endosomes to lysosomes [6].
We have reported that photodamage directed against lysosomes > mitochondria ≫ the ER could inhibit endocytic processes that require PI-3K activity in the 1c1c7 murine hepatoma [8], while mitochondrial photodamage was more effective at inhibiting endocytosis in a murine leukemia cell line [9]. We have now extended the latter studies to include other PDT targets. Additional research involving the hepatoma was carried out using an asymetrically-sulfonated phthalocyanine known to target endosomes [10]. This and similar agents were examined by Berg’s group and form the basis for a therapeutic program termed ‘photochemical internalization’ (PCI). In PCI, irradiation of cells with photosensitized endosomes leads to endosomal disruption. This leads to an enhanced therapeutic effect when these endosomes also contain sequestered chemotherapeutic agents which are thereby released into the cytoplasm [11–13].
Prior studies from this laboratory indicated that while lysosomal photodamage suppressed elements of the membrane trafficking process in the hepatoma cell line, lysosomal proteases played no role in the process. Studies involving other cell lines indicate that effects of PDT on endocytosis can be a function of both the photosensitizer target and the cell line.
MATERIALS AND METHODS
Drugs and chemicals
Disulfonated aluminum phthalocyanine (AlPcS2a), with -SO3H groups on adjacent rings, was provided by Frontier Scientific Co., Logan UT. Benzoporphyrin derivative (Verteporfin, BPD) was purchased from VWR (West Chester, PA). Frontier Scientific (Logan, UT) provided m-tetrahydroxyphenyl chlorin (mTHPC). N-aspartyl chlorine e6 (NPe6) was provided by Prof. Kevin M. Smith, Louisiana State University, Baton Rouge, LA. Tissue culture media were purchased from Sigma-Aldrich, St. Louis, MO. Fluorescent probes and TDPH (trimethylamino-diphenylhexatriene) were obtained from Invitrogen/Molecular Probes, Eugene OR. Other chemicals were provided by Fisher Scientific Co. (Chicago IL) and Sigma-Aldrich (St. Louis, MO).
Cell lines
Maintenance of the murine hepatoma Hepa 1c1c7 cell line [14] the human epithelial MCF10A [15] and the P388 murine leukemia line in suspension culture [16] have been reported.
PDT Protocols
1c1c7 and MCF10A cells cultured on 22 mm square glass coverslips and then treated with 0.5μM mTHPC or 5 μM AlPcS2a for 16h; 0.5 μM BPD or 40 μM NPe6 for 60 min, all at 37°C. This was followed by incubation of cells in fresh medium for 1 h, to permit photosensitizers to diffuse from plasma membranes. Similar studies were carried out with the P388 cell line in suspension culture. Irradiation was carried out using a 600-watt quartz-halogen lamp, with IR radiation attenuated by a 10-cm layer of water. The bandwidth of irradiation was confined to 690 ± 10 nm (BPD), 660 ± 10 nm (mTHPC, NPe6) or 670 ± 10 nm (AlPcS2a) by interference filters. LD20 light doses for 1c1c7 and MCF10A cells in terms of mJ/cm2 were 20 (NPe6), 60 (BPD), 25 (mTHPC) 20 (AlPcS2a). The corresponding values for P388 cells were 15 (NPe6), 38 (BPD), 15 (mTHPC) and 12 (AlPcS2a).
Viability
1c1c7 cells were sparsely plated on 60 mm dishes. After 24 h, cultures were incubated with photosensitizers for the times specified above in the PDT protocol. At the end of the loading period, the medium was replaced and the cultures irradiated as specified in the text, then returned to a humidified 37° incubator (5% CO2) for several days to allow colonies to form. After staining with crystal violet, colonies were counted with an Oxford Optronix ‘Gelcount’ colony counter. All such experiments were carried out in triplicate. Similar studies were carried out with the P388 cell line.
Wortmannin-induced vacuole formation
Inhibition of PI-3K-dependent processes was achieved by treatment of cultures with 20 nM wortmannin for 30 min. In studies involving PDT, cultures were treated with wortmannin for varying intervals at 37 °C directly after irradiation (unless stated differently in the text), then examined by phase-contrast and fluorescence microscopy.
TDPH labeling
This reagent was originally prepared for examining fluorescence polarization of membranes [17]. A 30 sec incubation with 1 μM TDPH resulted in labeling of only the plasma membrane if cultures were immediately washed with cold medium and then examined by fluorescence microscopy. Alternatively, a subsequent ‘chase’ in fresh medium at 37°C was carried out so as to examine the migration of the probe into sub-surface regions as membrane endocytosis proceeded. In specified studies, cells were incubated with photosensitizers and the medium replaced. This was followed by irradiation, exposure to TDPH (30 sec) and a final incubation in fresh medium for varying intervals at 37°C. This permitted monitoring of PDT effects on movement of TDPH into sub-cellular loci.
Microscopy
Phase-contrast microscopy was used to demonstrate the effect of different experimental conditions on cell morphology. Images were acquired with a Nikon E-600 microscope using a Photometrics CoolSnap HQ CCD camera. AlPcS2a fluorescence was determined using 360–400 nm excitation and assessing 600–700 nm emission. Representative microscopic fields are shown from typical experiments. All studies reported here were repeated at least three times with essentially identical outcomes.
RESULTS AND DISCUSSION
Wortmannin-induced vacuole formation
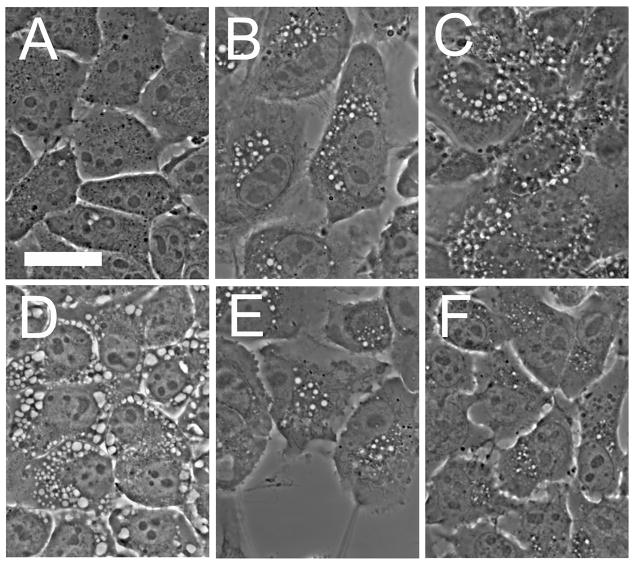
Cytoplasmic vacuoles can readily be detected in 1c1c7 cells 1 hr after treatment with 20 nM wortmannin. After reaching a maximum, the vacuoles began to disappear after 6 hr, with only a few remaining after an additional 2 hr (Fig. 1). We attribute this loss to the continued synthesis of new PI-3K, permitting the endocytic process to resume. While wortmannin is an irreversible enzyme inhibitor, it has only a limited lifetime under aqueous conditions [18]. Vacuole formation was a temperature-sensitive process. The vacuoles persisted for at least 8 hr if the temperature was lowered to 15°C one hour after addition of wortmannin. We have reported [8] that examination of the vacuoles by electron microscopy revealed that wortmannin-induced vacuoles were contained within a single membrane, not the characteristic double membrane of autophagosomes. This was not unexpected since wortmannin is a known antagonist of autophagy [19,20] and a 1c1c7 sub-line of 1c1c7 lacking a critical autophagy protein (Atg7) also showed vacuole formation upon exposure to wortmannin (Kessel and Andrzejak, unpublished observation).
Fig. 1.
Wortmannin-induced vacuole formation in 1c1c7 hepatoma cells. A, controls; B–F, images acquired at various times after addition of 20 nM wortmannin. B, 1 hr; C, 2 hr; D, 3 hr, E, 6 hr; F, 8hr.
Inhibition of vacuoles after photodamage
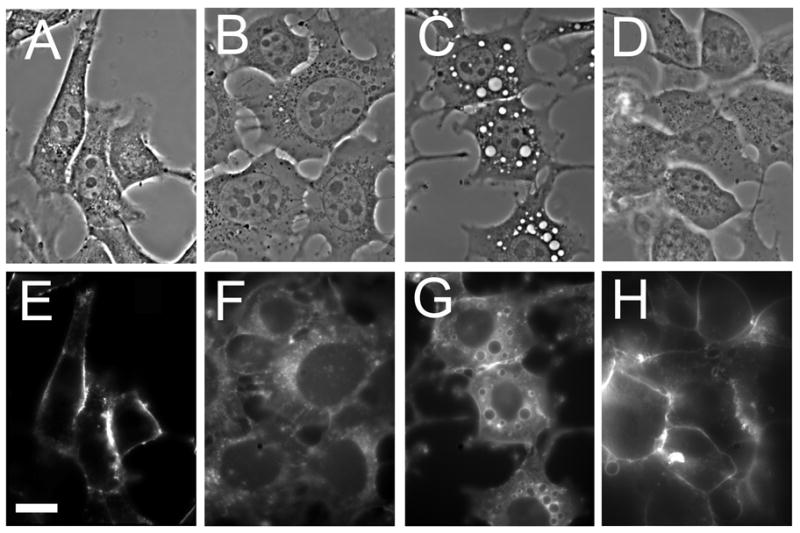
Wortmannin also induced vacuole formation in the P388 (a non-adhering murine leukemia) cell line and in MCF10A, a non-tumorigenic epithelial cell line (Fig. 2, top row). When 1c1c7, MCF10A or P388 cells were treated with LD20 PDT doses using NPe6, wortmannin-induced vacuole formation was prevented (Fig. 2). This agent is known to localize in lysosomes and endosomes [14]. For the mitochondrial-targeting agent BPD, an LD50 PDT dose was required to inhibit vacuole formation in P388 and MCF10A cells, but this PDT dose did not affect endocytosis in 1c1c7 cells. In additional experiments, we found that the ER-targeting photosensitizer mTHPC failed to have any detectable effect on vacuole formation in these cell lines, even at an LD90 PDT dose.
Fig. 2.
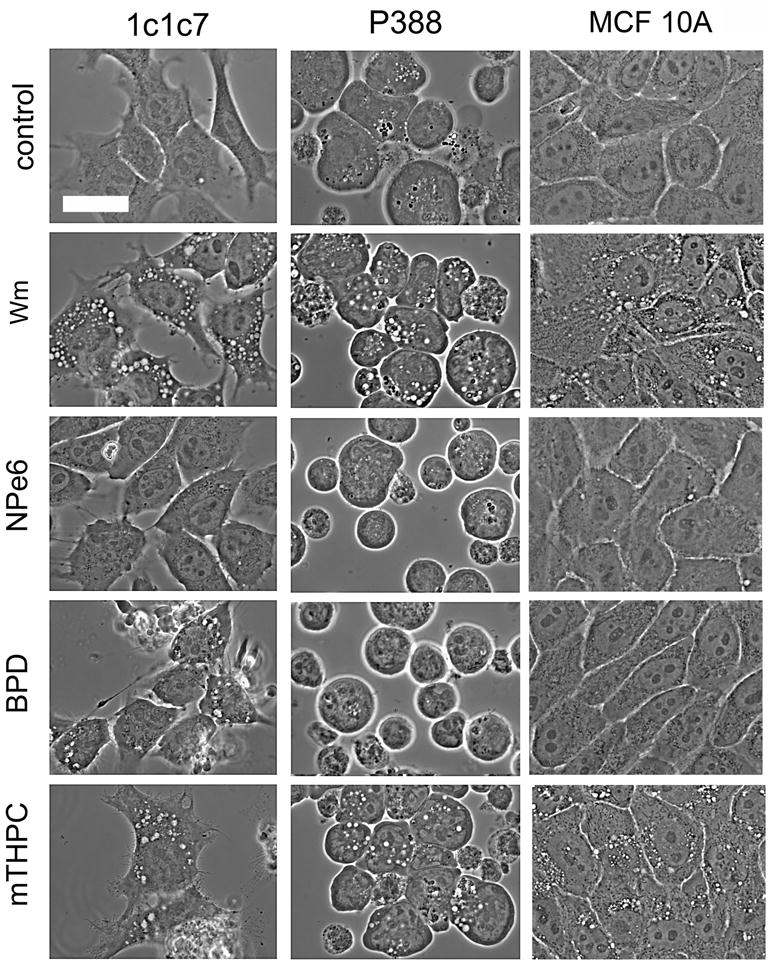
Effect of prior photodamage on wortmannin-induced vacuole formation in 1c1c7 hepatoma, P388 leukemia and MCF10A epithelial cells. From top: control = untreated controls; Wm = 90 min after addition of 20 nM wortmannin; NPe6, BPD & mTHPC = cells given an LD20 PDT dose with the specified photosensitizer, then treated with wortmannin for 90 min.
Endosomal photodamage
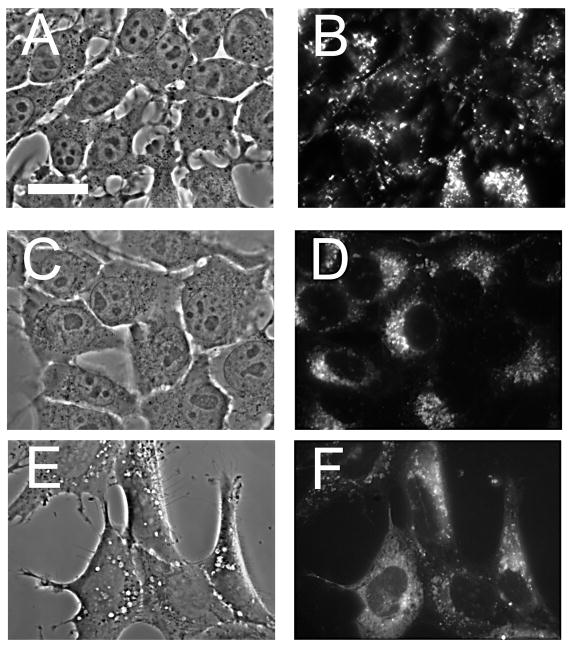
To better delineate the effects of photodamage on 1c1c7 cells, we utilized the asymmetrically-sulfonated phthalocyanine AlPcS2a. This agent labels small cytoplasmic vesicles that were established by Berg’s group to represent endosomes [11,12]. Phase-contrast and fluorescence images of 1c1c7 cells labeled with AlPcS2a are shown in the top panel of Fig. 3. An LD10 light dose left many endosomes intact but prevented the formation of wortmannin-induced vacuoles (center panels). If cells loaded with AlPcS2a were exposed to 20 nM wortmannin in the dark, the photosensitizer was released from the endosomes and entered the cytoplasm. This indicates that the swollen late endosomes induced by wortmannin treatment become leaky and cannot retain AlPcS2a.
Fig. 3.
Phase contrast and fluorescence microscopy showing localization of AlPcS2a in 1c1c7 hepatoma cells. A,B = after a 16 hr incubation with 5 μM AlPcS2a, then washed for 1 hr in fresh medium. C,D = Cells treated as indicated above, then given an LD20 light dose and treated with 20 nM wortmannin for 90 min. E,F = cells labeled with AlPcS2a, then exposed to 20 nM wortmannin for 90 min in the dark.
Endocytic internalization studies with TDPH
An example of the effect of wortmannin on membrane trafficking can be demonstrated if cell membranes are first labeled with a brief pulse of TDPH (Fig. 4). A 30-sec incubation of 1c1c7 cells with TDPH led to a fluorescent labeling pattern that involved only the plasma membrane (panel A). A subsequent 30 minute ‘chase’ in fresh medium at 37°C resulted in migration of fluorescence into the cytoplasm where a variety of structures became labeled (panel B). When the ‘chase’ was carried out in the presence of 20 nM wortmannin, there appeared instead numbers of large vacuoles whose membranes were highly fluorescent (panel C). This result is consistent with the proposal that the wortmannin-induced vacuoles are derived from plasma membrane material that has undergone incomplete recycling. Reduced labeling of the cytoplasm by TDPH after a 30 min chase was observed if cultures were first incubated with AlPcS2a and then irradiated with an LD30 light dose before a 30 sec incubation in TDPH (panel E).
Fig. 4.
Phase-contrast and fluorescence images of 1c1c7 cells showing TDPH labeling patterns. A,E: cell surface labeling by a 30-sec pulse of TDPH. B,F: partition of TDPH into the cell interior when the 30-sec pulse is followed by a 30 min incubation in fresh medium. C,G: effect of the presence of 20 nM wortmannin during the 30 min ‘chase’. D,H: effect of an LD20 PDT dose to cells photosensitized with AlPcS2a showing inhibition of the subsequent cytoplasmic migration of a TDPH pulse.
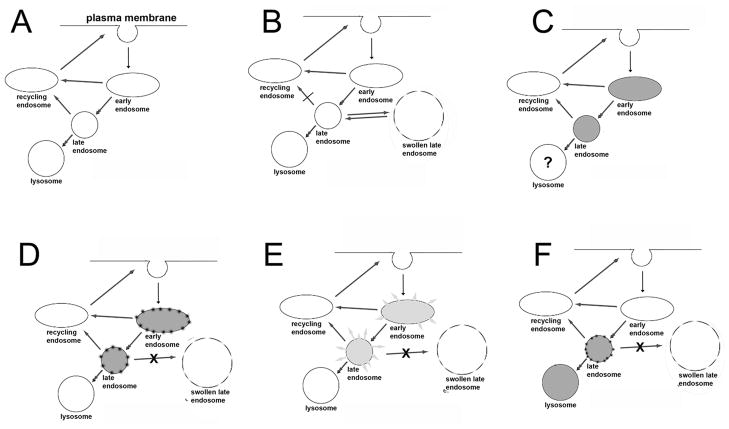
Model building
A model for the effects observed with the hepatoma cell line is seen in Fig. 5. It is not yet known whether this will also apply to other cell types. The endocytic pathway functions normally (panel A) with membrane components being recycled, unless this process is perturbed by wortmannin (panel B). The result of inhibition of PI-3K activity is formation of swollen late endosomes, indicated by the broken peripheral line to be somewhat leaky. This is a reversible effect: these structures gradually disappear with time. An incubation with AlPcS2a loads both early and late endosomes, and possibly lysosomes, with this fluorescent phthalocyanine (panel C). We propose that low-dose irradiation alters endosomal membranes such that addition of wortmannin no longer elicits swollen endosomal formation (Panel D). A higher light dose leads to endosomal breakage and release of their contents into the cytoplasm (panel E), as established by Berg’s group [11–13]. With NPe6, an agent that targets both lysosomes and late endosomes, the wortmannin effect was also inhibited (panel F).
Fig. 5.
Proposed model for effects of endosomal photodamage on endocytosis in 1c1c7 cells. A = A normal membrane recycling pattern. B = Formation of swollen late endosomes when further processing of late endosomes is blocked by wortmannin. C = AlPcS2a labeling pattern. D = Proposed effect of an LD20 light dose on photosensitized endosomes resulting in imperviousness to ‘swollen endosome’ formation. E= Endosomal fragmentation and release of encapsulated AlPcS2a into the cytoplasm after a greater light dose. F = postulated effect of low-dose PDT on cells photosensitized with NPe6. This is based on studies indicating that AlPcS2a targets both lysosomes and late endosomes [14]. Accumulation of photosensitizer is indicated by shading of the organelles;
How is BPD able to inhibit vacuole formation in all three cell lines? Perhaps the specificity is broad enough so that some endosomal photodamage occurs. It is noteworthy that a substantially higher PDT dose is required with BPD than with NPe6 or AlPcS2a. The inability of mTHPC to effectively inhibit vacuole formation suggests that this agent is very specific for ER, sparing endosomes from photodamage.
While this model is designed to explain effects of wortmannin and PDT on endocytosis, it should be pointed out that wortmannin does not inhibit all endocytic processes. Receptor-mediated endocytosis is unaffected [5] by lack of PI-3K activity.
Conclusions
The significance of these events in the photodynamic process leading to tumor eradication is not yet clear. Cells that receive a sufficient PDT dose to initiate apoptosis or a lethal autophagic response will die, but for those receiving a sub-lethal dose, interference with membrane recycling can occur. The overall contribution of such effects to the overall PDT response remains to be determined.
Acknowledgments
Ann Marie Santiago provided excellent technical assistance.
Grant sponsor: National Cancer Institute; grant number: R01-CA 23378.
References
- 1.Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 2.Kjeken R, Mousavi SA, Brech A, Griffiths G, Berg T. Wortmannin-sensitive trafficking steps in the endocytic pathway in rat liver endothelial cells. Biochem J. 2001;357:497–503. doi: 10.1042/0264-6021:3570497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reaves BJ, Bright NA, Mullock BM, Luzio JP. The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- 4.Shpetner H, Joly M, Hartley D, Corvera S. Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J Cell Biol. 1996;132:595–605. doi: 10.1083/jcb.132.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Wang Z. Regulation of intracellular trafficking of the EGF receptor by wortmannin through activation of Rab5 rather than inhibition of phosphatidylinositol 3-kinase. EMBO Rep. 2001;2:842–849. doi: 10.1093/embo-reports/kve179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reaves BJ, Bright NA, Mullock M, Luzio JO. The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- 7.Bright NA, Lindsay MR, Stewart A, Luzio JP. The relationship between lumenal and limiting membranes in swollen late endocytic compartments formed after wortmannin treatment or sucrose accumulation. Traffic. 2001;2:631–642. doi: 10.1034/j.1600-0854.2001.20906.x. [DOI] [PubMed] [Google Scholar]
- 8.Kessel D, Price M, Caruso C, Reiners J. Effects of photodynamic therapy on the endocytic pathway. Photochem Photobiol Sci. 2011 doi: 10.1039/c0pp00276c. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessel D. Mechanism of cell death in photodynamic therapy. In: Kadish K, Smith KM, Guilard R, editors. Handbook of Porphyrin Science. Vol. 4. Singapore: World Scientific; 2010. pp. 403–423. [Google Scholar]
- 10.Kessel D, Andrzejak M. Effects of Endosomal Photodamage on Membrane Recycling and Endocytosis. Photochem Photobiol. 2011 doi: 10.1111/j.1751-1097.2011.00890.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norum OJ, Selbo PK, Weyergang A, Giercksky KE, Berg K. Photochemical internalization (PCI) in cancer therapy: From bench towards bedside medicine. J Photochem Photobiol B. 2009;96:83–92. doi: 10.1016/j.jphotobiol.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Prasmickaite L, Hogset A, Selbo PK, Engesaeter BO, Hellum M, Berg K. Photochemical disruption of endocytic vesicles before delivery of drugs: A new strategy for cancer therapy. Br J Cancer. 2002;86:652–657. doi: 10.1038/sj.bjc.6600138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weyergang A, Selbo PK, Berg K. Y1068 phosphorylation is the most sensitive target of disulfonated tetraphenylporphyrin-based photodynamic therapy on epidermal growth factor receptor. Biochem Pharmacol. 2007;74:226–235. doi: 10.1016/j.bcp.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Caruso JA, Mathieu PA, Reiners JJ., Jr Sphingomyelins suppress the targeted disruption of lysosomes/endosomes by the photosensitizer NPe6 during photodynamic therapy. Biochem J. 2005;392:325–334. doi: 10.1042/BJ20050313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller FR, Bukowski J. Mammary tumor stimulatory factors as well as mammastatin are produced by the normal human breast epithelial cell line MCF10A. Int J Cancer. 1994;59:296–297. doi: 10.1002/ijc.2910590224. [DOI] [PubMed] [Google Scholar]
- 16.Price M, Terlecky SR, Kessel D. A role for hydrogen peroxide in the pro-apoptotic effects of photodynamic therapy. Photochem Photobiol. 2009;85:1491–1496. doi: 10.1111/j.1751-1097.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prendergast FG, Haugland RP, Callahan PJ. 1-[4-(Trimethylamino)phenyl]-6-phenylhexa-1,3,5-triene: Synthesis, fluorescence properties and use as a fluorescent probe of lipid bilayers. Biochemistry. 1981;20:7333–7338. doi: 10.1021/bi00529a002. [DOI] [PubMed] [Google Scholar]
- 18.Holleran JL, Egorin MJ, Zuhowski EG, Parise RA, Musser SM, Pan SS. Use of high-performance liquid chromatography to characterize the rapid decomposition of wortmannin in tissue culture media. Anal Biochem. 2003;323:19–25. doi: 10.1016/j.ab.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Tassa A, Roux MP, Attaix D, Bechet DM. Class III phosphoinositide 3-kinase--Beclin1 complex mediates the amino acid-dependent regulation of autophagy in C2C12 myotubes. Biochem J. 2003;376:577–586. doi: 10.1042/BJ20030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelárová H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]