Abstract
Many biologically active macrocycles contain a C–C double bond through which various other derivatives are prepared; the stereochemical identity of the alkene or the resulting moieties can be critical to the beneficial properties of such molecules. Catalytic ring-closing metathesis (RCM) is a widely employed method for the synthesis of large unsaturated rings;1,2 however, cyclizations often proceed without control of alkene stereochemistry.2 Such shortcoming is particularly costly with complex molecules when cyclization is performed after a long sequence of transformations.2 Here, we outline a reliable, practical and general approach for efficient and highly stereoselective synthesis of macrocyclic alkenes by catalytic RCM; transformations deliver up to 97% Z selectivity due to control induced by a tungsten-based alkylidene. Utility is demonstrated by stereoselective preparation of anti-cancer epothilone C [Ref. 3–5] and anti-microbial nakadomarin A [Ref. 6], previously reported syntheses of which have been marred by late-stage non-selective RCM.7–15 The tungsten alkylidene can be manipulated in air, promoting reactions carried out in a fume hood to deliver products in useful yields and high Z selectivity. As a result of efficient RCM and re-incorporation of side products into the catalytic cycle with minimal alkene isomerization, desired cyclizations proceed in preference to alternative pathways even under relatively high concentration (0.1 molar).
Catalytic ring-closing metathesis (RCM) of alkenes is indispensable to the preparation of cyclic structuresi; it is used extensively in the synthesis of biologically active moleculesii. RCM is broadly employed in accessing large rings, despite the lack of a reliably stereoselective variant, the availability of which would substantially enhance the value of this critical class of reactions. The absence of stereochemical control originates from the dependency of the catalytic ring closure on the energetic attributes of the product stereoisomers (vs. dictated by the catalyst). With small- or medium-rings, Z alkenes are generated exclusively; this is not so with sizeable rings, since, frequently, either the energy difference between the two isomeric alkenes is insufficient for achieving high stereoselectivity by thermodynamic control, or, if one isomer is adequately lower in energy, the catalyst is unable to promote facile equilibration.
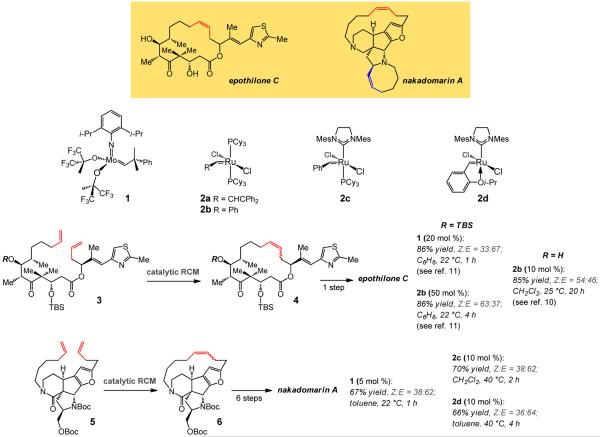
The severe shortcoming in the state-of-the-art is illustrated by the two sets of non-selective catalytic RCM, shown in Fig. 1, performed en route to macrocyclic natural products epothilone Ciii,iv,v and nakadomarin Avi. Efforts from several laboratories have focused on catalytic RCM for synthesis of the macrocyclic moiety of different members of the epothilone family; popular catalysts, like those derived from alkylidene 1vii and carbenes 2a–dviii,ix (Fig. 1), deliver little or no stereoselectivityx,xi. Initiatives regarding nakadomarin A (cf. 5→6, Fig. 1), consisting of four different routes that incorporate a late-stage catalytic macrocyclic ring closure, have met with equally unsatisfactory outcomesxii,xiii,xiv,xv.
Figure 1. Two cases in natural product total synthesis where catalytic RCM with some of the most commonly used complexes (1, 2b–d) affords the macrocyclic alkene with minimal stereoselectivity and often with a preference for generation of the undesired E isomer.
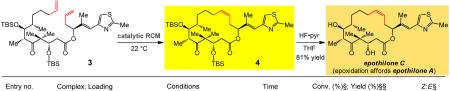
Difficulties in stereoselective ring closure are particularly detrimental since the catalytic RCM takes place late in the synthesis route, inflicting substantial loss in efficiency. For example, diene 3, used in the total synthesis of anti-cancer agent epothilone C, is prepared by a 16-step sequence. TBS = t-butyldimethylsilyl; Boc = t-butoxycarbonyl.
Catalytic stereoselective RCM of dienes 3 and 5 (Fig.1) constitutes particularly compelling objectives for several reasons. Epothilone C (precursor to epothilone A), as well as nakadomarin A, belong to important classes of natural products that exhibit exceptional biological activityiv,v,vi,xvi. Epothilones are potent naturally occurring tubulin polymerization and microtubule stabilizing agents that have been investigated extensively. The geometry of the macrocyclic alkene has been shown to influence their activityxvi; the Z macrocyclic alkene of epothilone C is needed for the desired stereochemical outcome in the preparation of epothilone A through epoxidationx,xi. Nakadomarin A is a potent anti-microbial and anti-cancer agent isolated only in minute quantitiesvi,xv. An effective method for laboratory synthesis of such important targets leads to larger quantities of these molecules or their analogs, which might not be easily accessible by fermentationxvii. As synthesis of the large rings in epothilone C or nakadomarin A entails the use of extensively functionalized substrates and occurs late in a multi-step sequence, a non-selective transformation inflicts a costly diminution in efficiency; this difficulty is exacerbated by the fact that the two alkene isomers of epothilone C and nakadomarin A are difficult to separatexviii. Moreover, with structurally complicated dienes such as 3 or 5, the tactic of carrying out preliminary studies involving simpler structural variants to help establish the feasibility of an RCM strategy is unreliable; substituents and their stereochemical identities play a pivotal role in the efficiency and stereoselectivity of catalytic ring closures and their absence often has a major influence on the cyclization processx. In a catalyst-controlled RCM, stereoselectivity would become far less dependent on the attributes of the diene starting material and therefore more predictable.
At the time the present investigations were initiated, efforts to address the above complications had centered on the more common but less efficient detour of altering substrate structure (vs. identification of a catalyst that generates the desired alkene stereoselectively). One relatively established two-step approach consists of W- or Mo-catalyzed alkyne RCM followed by Pd-catalyzed partial hydrogenationxix,xx: the first process affords the ring system and the other adjusts the oxidation state. Syntheses of the methyl-substituted alkyne precursors, required to enhance catalyst longevity and avoid oligomerizationxxi, necessitate additional manipulations; elevated temperatures (80–140 °C) are often required for ring closure, and the presence of Lewis basic alkylamines can lead to a need for high loadings of the metal complex (e.g., 50 mol %xxii) or complete catalyst inhibitionxxiii. More recently, macrocyclic RCM of a limited range of substrates involving reactions between an internal vinylsilane and a terminal alkene, followed by protodesilylation, has been disclosedxxiv. Two additional steps are again needed: the requisite vinylsilanes are prepared by Ru-catalyzed alkyne hydrosilylation, and the resulting trisubstituted silyl-substituted alkenes are converted to the cyclic Z alkene by treatment with a mixture of an ammonium fluoride, a silver fluoride salt and acetic acid. High catalyst loadings (20 mol %) are used in the latter RCM strategy, partly because of the intermediacy of a trisubstituted alkene.
We have introduced several types of intermolecular Z-selective olefin metathesis reactions promoted by molybdenum and, less commonly, tungsten alkylidenes that bear a pyrrolide and an alkoxide or an aryloxide ligand. Such stereogenic-at-metalxxv catalysts initiate Z-selective alkene formation by ring-opening/cross-metathesisxxvi, homocouplingxxvii or the more complicated cross-metathesis (CM)xxviii. Stereochemical models that provide a mechanistic foundation for high Z selectivity have been proposedxxviii and are based on the size differential between the large aryloxide and the smaller imido group (metallacyclobutane substituents oriented towards the latter; see the Supplementary Information for details). Successful design of stereoselective macrocyclic RCM reactions, however, requires addressing challenges that are distinct from those pertaining to stereoselective CM reactions. When RCM or CM involves two unhindered alkenes, stereoisomeric purities can be fragile, since the kinetically-generated Z alkene can more readily undergo isomerization to the E isomerxxviii. With many cyclizations, such as those in Fig. 1, there is no allylic substituent to discourage association of the macrocyclic Z alkene with the catalyst to retard the rate of unwanted equilibration; adventitious ring-opening can pose a serious problem. Achieving high stereoselectivity and yield often calls for a catalyst that delivers the subtle and difficult balance that culminates in an efficient and Z- or E-selective cyclization with little or no ring-opening/ring-closing that can cause isomerization. Thus, a complicating factor that is critical to RCM but does not apply to CM relates to the interplay between ring closure and isomerization by ring-opening. Furthermore, a commonly utilized strategy in CM relates to the use of excess amounts of one cross partner to favor formation of the desired product (vs. homocoupling or isomerization)xxviii; in an RCM, on the other hand, the two reacting alkenes can only be present at the same concentration. Whereas the steric and electronic attributes of one alkene may be rendered distinct in CM as the means to minimize homocoupling and enhance the yield of the desired productxxviii, such strategic differentiations are often not possible in RCM (cf. Fig. 1). Unlike CM, conformational preferences can be critical to the facility of RCM, aiding or resisting the influence of the catalyst. Finally, in CM only homocoupling can lead to adventitious substrate consumption, whereas in RCM the same side product continues to deplete the substrate amount through oligomerization.
Examining the ability of different catalysts to promote the RCM of diene 11 to afford sixteen-membered ring lactone Z-12 (Table 1) was first on our agenda. Preliminary DFT calculations (see the Supplementary Information) revealed that the E isomer is 1.2 kcal/mol lower in energy, suggesting that, at equilibrium, there would exist an approximately 12:88 Z:E mixture. A previously disclosed attempt involving ruthenium carbene 2a delivered 12 with 77% E selectivity (i.e., 23% Z; 4.0 mol %, 22 °C, 30 h)xxix. As demonstrated in entries 1–3, E-12 is formed preferentially with complexes 1 or 2c; reduced pressure, a strategy used to minimize isomerizationxxviii, does not improve selectivity (cf. entries 1 vs. 2). In contrast, Z-12 is generated with moderate preference when RCM is carried out with monopyrrolides 7a–b (entries 4–6). Adamantylimido 8 furnishes 85% of the Z isomer under vacuum (7.0 torr; 62% yield; entry 7); stereoselectivity increases to 92% Z with 1.2 mol % catalyst loading (entry 8; vs. 3.0 mol % in entry 7) while generating similar efficiency, presumably because isomerization of the cyclic Z alkene is reduced when the catalyst is less available. Reaction with tungsten alkylidene 9 leads to equally high yield and stereochemical control (62% and 91% Z; entry 9). There is exceptional stereoselectivity with dichloroimido tungsten alkylidene 10xxvii (95% Z; entry 10, Table 1), but the reaction proceeds only to 14% conversion with this less active and sterically more demanding complex; longer reaction times do not result in significantly higher conversion. The preference for generation of the Z macrocycle is likely due to similar principles that result in stereoselective homocoupling and cross-metathesis reactions (see the Supplementary Information for a proposed model).
Table 1.
Examination of various metal complexes for RCM of diene 11 to generate sixteen-membered ring macrocycle 12 stereoselectivity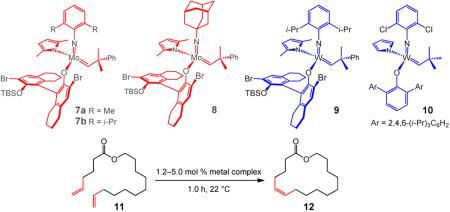
| Entry no. | Metal Complex | Catalyst Loading (mol %)† | Pressure | Conv. (%)§; Yield (%)§§ | Z:E§ |
|---|---|---|---|---|---|
| 1 | 1 | 5.0 | ambient | 85; 60 | 22:78 |
| 2 | 1 | 5.0 | 7.0 torr | 96; 58 | 21:79 |
| 3 | 2c | 5.0 | ambient | 75; 61 | 21:79 |
| 4 | 7a | 5.0 | ambient | 56; 45 | 70:30 |
| 5 | 7a | 5.0 | 7.0 torr | 97; 56 | 77:23 |
| 6 | 7b | 5.0 | 7.0 torr | 91; 55 | 72:28 |
| 7 | 8 | 3.0 | 7.0 torr | 80; 62 | 85:15 |
| 8 | 8 | 1.2 | 7.0 torr | 75; 56 | 92:8 |
| 9 | 9 | 5.0 | 7.0 torr | 80; 62 | 85:15 |
| 10 | 10 | 5.0 | 7.0 torr | 14; 10 | 95:5 |
The reactions were carried out in toluene (5.0 mM) at 22 °C for one hour under an atmosphere of nitrogen gas or under vacuum, as noted; reaction in entry 3 performed in CH2Cl2 at 40 °C. See the Supplementary Information for details.
Complexes 1, 2c–d and 9–10 were prepared prior to use; alkylidenes 7a–b and 8 were synthesized in situ from the bis-pyrrolide and aryl alcohol, which proceeds in >98% yield for 7a–b but in 60% (±5%) yield in the case of 8 (thus, catalyst loading for the latter complex is 3.0 mol %). See the Supplementary Information for details.
Conversion and Z:E ratios measured by analysis of 400 MHz 1H NMR spectra of unpurified mixtures; the variance of values are estimated to be ≤±2%.
Yield of isolated product after purification; the variance of values are estimated to be ≤±5%.
Boc = tert-Butoxycarbonyl; ND = not determined.
Next, we turned our attention to the challenge of achieving high Z selectivity in RCM reactions that lead to epothilones C and A (cf. Fig. 1). We prepared diene 3 along the lines of a formerly devised sixteen-step sequencex. Treatment of 3 with Ru-based 2d leads to preferential formation of the E isomer (66%; entry 1, Table 2). Use of 10 mol % arylimido Mo alkylidene 7a gives rise to 57% conversion to macrocyclic alkene 4 within three hours, but the Z isomer is only generated with 64% selectivity (entry 2). When adamantylimido 8 is employed under the same conditions (entry 3), efficiency and stereoselectivity improve (87% conv. in 1.5 h and 85% Z), presumably as a result of a more accessible metal center and larger size differential between the aryloxide and the alkylimido unit (cf. stereochemical model in the Supplementary Information). There is only a limited enhancement of conversion and stereoselectivity under reduced pressure (entry 4 vs. 3, Table 2). When ring closure is carried out under reduced pressure with tungsten alkylidene 10, which bears a 2,6-dichlorophenylimido and a bulky 2,5-di-[2,4,6-(i-Pr)3]-phenoxy ligand (vs. aryloxides in 7–9), (entry 5) the reaction proceeds to near completion in the same amount of time (2.5 h, 97% conv.), allowing the desired macrocycle to be isolated in 85% yield (96% Z). As the data in entry 6 of Table 2 illustrate, with the reaction mixture fifty times more concentrated (0.05 M), 3.0 mol % of the same alkylidene can be used to synthesize the desired product (4) in 63% yield and 97% Z selectivity. The wider gap between percent conversion and yield values (97% and 63%, respectively) is largely the result of adventitious oligomerization, likely facilitated by the increased substrate concentration. Lactone 4 is converted to epothilone C upon silyl ether removal (81% yield; Table 2); epoxidation of epothilone C generates epothilone Ax,xi.
Table 2.
| Entry no. | Complex; Loading | Conditions | Time | Conv. (%)§; Yield (%)§§ | Z:E§ |
|---|---|---|---|---|---|
| 1 | 2d; 5.0 mol % | ambient; 1.0 mM | 16 h | 96; ND | 34:66 |
| 2 | 7a; 10 mol % | 1.0 torr; 1.0 mM | 3.0 h | 57; ND | 64:36 |
| 3 | 8; 10 mol % | ambient; 1.0 mM | 1.5 h | 87; ND | 85:15 |
| 4 | 8; 10 mol % | 1.0 torr; 1.0 mM | 1.5 h | 91; ND | 90:10 |
| 5 | 10; 10 mol % | 1.0 torr; 1.0 mM | 2.5 h | 97; 85 | 96:4 |
| 6 | 10; 3.0 mol % | 1.0 torr; 0.05 M | 3.0 h | 97; 63 | 97:3 |
| 7 | 10; 7.5 mol %** | 0.02 torr; 6.0 mM | 4.0 h | 96; 82 | 94:6 |
The reactions were carried out at 22 °C in purified benzene (under an atmosphere of nitrogen gas) or toluene (vacuum), except for entry 7 (in mesitylene); see the Supplementary Information for details.
Conversion and Z:E ratios measured by analysis of 500 MHz 1H NMR spectra of unpurified mixtures; the variance of values are estimated to be ≤±2%.
Yield of isolated product after purification; the variance of values are estimated to be ≤±5%.
Catalyst was weighed in air and reaction performed in a typical fume hood under argon; see the Methods Summary for details.
THF = tetrahydrofuran; ND = not determined.
The higher stereoselectivities furnished by W-based complex 10 are likely because, as stated above, it possesses the desired activity level. The tungsten alkylidene promotes efficient RCM at reasonable catalyst loading within being too active for it to react readily with the macrocyclic alkene to cause Z-to-E isomerization – even at late stages of the transformation when diene concentration is low. In contrast, the more active Mo-based variants likely initiate a similarly Z-selective RCM but are sufficiently active to engender subsequent ring-opening/isomerization. It is possibly due to such attenuated activity that – contrary to the commonly held perception – tungsten alkylidene 10 proves to be sufficiently stable so that it can be easily handled in air. An example is shown in entry 7 of Table 3: with 7.5 mol % 10, weighed in air under up to 80% humidity, and all manipulations performed in a fume hood with standard glassware, macrocyclic alkene 4 is delivered in 82% yield and 94% Z selectivity (219 mg scale). It should be mentioned that the faster acting Mo complexes, superior to W-based alkylidenes in effecting intermolecular CM reactionsxxviii, are more sensitive to air and moisture.
Table 3.
| Entry no. | Catalyst; Loading | Conditions; Concentration | Time | Conv. (%)§; Yield (%)§§ | Z:E§ |
|---|---|---|---|---|---|
| 1 | 7b; 5.0 mol % | 7.0 torr; 5.0 mM | 2.0 h | 10; ND | ND |
| 2 | 8; 6.0 mol % | 7.0 torr; 5.0 mM | 2.0 h | 95; 71 | 69:31 |
| 3 | 9; 5.0 mol % | 7.0 torr; 5.0 mM | 2.0 h | 26; ND | ND |
| 4 | 10; 5.0 mol % | 7.0 torr; 5.0 mM | 2.0 h | 98; 90 | 97:3 |
| 5 | 10; 5.0 mol % | 7.0 torr; 0.1 M | 0.5 h | >98; 39 | 90:10 |
| 6 | 10; 5.0 mol % | ambient; 0.1 M | 2.0 h | 95; 52 | 94:6 |
The reactions were carried out in purified toluene under an atmosphere of nitrogen gas or under vacuum, as noted. The stereochemical identity of 6 was determined by X-ray crystallography. See the Supplementary Information for details.
Conversion and Z:E ratios measured by analysis of 400 MHz 1H NMR spectra of unpurified mixtures; the variance of values are estimated to be ≤±2%.
Yield of isolated product after purification; the variance of values are estimated to be ≤±5%.
Boc = tert-Butoxycarbonyl; ND = not determined.
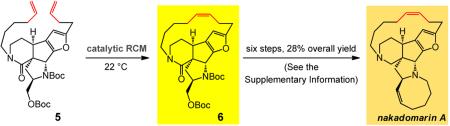
The above findings broach the question as to whether the low stereoselectivity in the synthesis of the fifteen-membered ring moiety of nakadomarin A (cf. Fig. 1) can be addressed through the use of monopyrrolide complexes. As indicated by the data in entries 1–2 of Table 3, arylimido molybdenum alkylidene 7b affords only 10% conversion to 6, a precursor to the natural product. In contrast, the sterically more accessible adamantylimido 8 readily converts 5 to pentacycle 6, but with only 69:31 Z:E selectivity. Similarly, reaction with tungsten alkylidene 9 is inefficient likely due to slow rate of initiation (entry 3, Table 3). The robust tungsten alkylidene 10, on the other hand, again emerges as the source of a facile and uniquely stereoselective catalyst (entry 4, Table 3): the desired pentacycle 6 is obtained in 90% yield after purification and with 97% Z selectivity (performed with 107 mg of 5).
It is striking that under conditions (0.1 M; entry 5, Table 3) routinely used to perform a typical chemical transformation (vs. high dilution typically required for marcocyclic RCM), reaction proceeds to furnish 6 in 52% yield and 94% Z selectivity. Equally noteworthy is that when cyclization of 5 is carried out at 0.1 M concentration, reduced pressure is not necessary (i.e., ethylene is not removed). Otherwise, 6 is obtained in lower yield and selectivity (39% and 90% Z under 7.0 torr, entry 6, Table 3). Since at higher concentration of the diene, homocoupling is rampant, it is likely that the ethylene formed as the byproduct raises the availability of the highly reactive methylidene complexxxviii, which converts the homocoupled product to the monomeric RCM substrate, thus increasing the yield of the desired product. The above scenario, and the fact that Z selectivity remains high at 0.1 M concentration (94% Z), implies that the tungsten methylidene reacts with the acyclic alkene of the homocoupled triene preferably (vs. with the cyclic alkene 6 to promote isomerization). The somewhat lower stereoselectivity observed under vacuum (90:10 vs. 94:6 Z:E, entries 5–6, Table 3) might be because some macrocyclic product is formed through RCM involving the alkylidene derived from the terminal alkene of the homocoupled byproduct. The latter pathway to pentacyclic 6 can be less selective than RCM via diene 5, arising from reaction of two terminal alkenes. It is consequently as a result of several delicate reactivity preferences that the RCM with complex 10 in a 0.1 M solution can be performed efficiently and selectively.
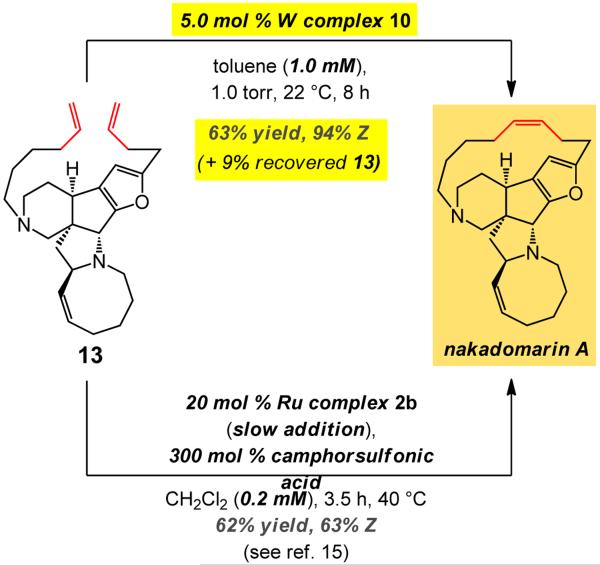
Total synthesis of nakadomarin A might alternatively be accomplished by a late-stage stereoselective RCM (vs. at an earlier point as in the pathway in Table 3); such a plan, however, can present additional complications and a non-selective RCM translates to loss of a more valuable advanced intermediate. One route proceeds through the especially demanding RCM (vs. 5→6) of azacene-containing 13xv (Fig. 2): the higher ring strain within the pentacyclic diene substrate is not only expected to discourage ring closure, it likely lowers the barrier to undesired rupture of the macrocyclic alkene. Accordingly, past attempts at achieving conversion of 13 to nakadomarin A, as shown in Fig. 2, have involved the significantly less reactive ruthenium carbene 2bviii (vs. 2c–d) in order to minimize post-RCM isomerization of the macrocyclic alkene. Use of such a reluctant catalyst, which must be introduced slowly, translates to high loadings and elevated temperatures (20 mol %, 40 °C). Extremely dilute conditions (0.2 mM) are needed as well, since it is unlikely that under such conditions any homocoupled byproducts that would otherwise be formed can be reverted back to the monomeric dienes or converted directly to the desired macrocycle. Additionally, the presence of substantial quantities (300 mol %) of camphorsulfonic acid, a strong BrØnsted acid, is required for achieving 63% Z selectivity (otherwise, slight excess of the E alkene is obtained)xv. In sharp contrast, treatment of 13 with 5.0 mol % 10 at 22 °C affords nakadomarin A in 94% Z selectivity and 63% yield (plus 9% recovered diene). Finally, it should be noted that attempts to effect alkyne RCM of the diyne derivative of 13 (Me-substituted), bearing two Lewis basic tertiary amines, with either Mo- or W-based alkylidynes leads to <5% conversion even with 30–50 mol % of a metal complex and at 80 °C (up to 24 h); this latter approach must therefore involve the use of the derived bisamide (20–25 mol % catalyst, 80 °C, 16–18 h).
Figure 2. Total synthesis of nakadomarin A realized through late-stage tungsten-catalyzed RCM of pentacyclic 13 and comparison with results delivered by Ru catalysts.
RCM of the strained 13 with tungsten complex 10 affords the natural product in 63% yield (69% based on recovered substrate) and 94% Z selectivity. This is in contrast to previous attempts, the best of which involves 20 mol % of a Ru carbene added slowly to a highly dilute solution (0.2 mM) to generate only 63:37 Z:E mixture.
The investigations described above point to stereogenic-at-tungsten alkylidenes as practical and uniquely effective catalysts for Z-selective macrocyclic RCM. We demonstrate that, in planning a multi-step pathway for the preparation of a complex molecule, such complexes can be relied upon to deliver the desired outcome at the late stages of an extended route. The impact of stereoselective W-catalyzed marcocyclizations reaches beyond the target structures probed in this study, as there are numerous other total synthesesxxii, xxx of biologically active molecules that would similarly benefit from the protocols disclosed here.
METHODS SUMMARY
General procedure for catalytic Z-selective macrocyclic RCM
A 250-ml Schlenk flask, fitted with a connecting adapter attached to an argon-filled manifold, was flame-dried and charged with diene 3 (0.219 g, 0.298 mmol). After azeotropic distillation with dry benzene (three times; freeze-pump), the apparatus was charged with tungsten complex 10 (21.9 mg, 22.4 μmol, weighed in air), evacuated, back-filled with argon and charged with mesitylene (50.0 ml). The mixture was exposed to vacuum (0.02 torr) and allowed to stir for four hours at 22 °C, after which the reaction was quenched by the addition of wet diethyl ether (~1 ml). Purification by silica gel chromatography (hexanes:diethyl ether 20:1) afforded 4 (0.172 g, 0.243 mmol, 82% yield, 94:6 mixture of Z:E isomers, determined by 500 MHz 1H NMR) as a white foam and 9.3 mg of the recovered starting material (13 μmol, 3.0%).
Supplementary Material
Acknowledgements
This research was supported by the United States National Institutes of Health, Institute of General Medical Sciences (Grant GM-59426 to A. H. H. and R. R. S.). M. Y. is a John LaMattina Graduate Fellow, A. F. K. the recipient of an EPSRC-GlaxoSmithKline Synthesis Studentship, and P. J. an EPSRC Postdoctoral Fellow. D. J. D. is grateful for an EPSRC Leadership Fellowship. We thank S. J. Meek, S. J. Malcolmson, R. V. O'Brien, T. J. Mann and E. T. Kiesewetter for valuable discussions. We are grateful to A. R. Zhugralin, S. Torker and D. L. Silverio for DFT calculations, to K. Wu for experimental assistance and to Boston College for providing access to computational facilities. The X-ray facilities at Boston College are supported by the United States National Science Foundation (CHE-0923264).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions M. Y. and C. W. were involved in the discovery, design and development of the Z-selective macrocyclic ring-closing metathesis strategies and applications to the natural product syntheses. A. F. K., P. J. and D. J. D. devised routes for and performed enantioselective syntheses of precursors 5 and 13. R. R. S. and A. H. H. were involved in the discovery and development of the catalysts used in the study; A. H. H. conceived and directed the investigations and composed the manuscript with revisions provided by M. Y. and C. W.
R. R. S. and A. H. H. declare competing financial interests.
References
- i.Hoveyda AH, Zhugralin AR. The remarkable metal-catalyzed olefin metathesis reaction. Nature. 2007;450:243–251. doi: 10.1038/nature06351. [DOI] [PubMed] [Google Scholar]
- ii.Gradillas A, Perez-Castells J. Macrocyclization by ring-closing metathesis in the total synthesis of natural products: reaction conditions and limitations. Angew. Chem. Int. Edn. 2006;45:6086–6101. doi: 10.1002/anie.200600641. [DOI] [PubMed] [Google Scholar]
- iii.Höfle G, et al. Epothilone A and B – novel 16-membered macrolides with cytotoxic activity: Isolation, crystal structure, and conformation in solution. Angew. Chem. Int. Edn. 1996;35:1567–1569. [Google Scholar]
- iv.Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (taxol) J. Biol. Chem. 1997;272:2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- v.Bollag DM, et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- vi.Kobayashi J, Watanabe D, Kawasaki N, Tsuda M. Nakadomarin A, a novel hexacyclic manzamine-related alkaloid from Amphimedon Sponge. J. Org. Chem. 1997;62:9236–9239. [Google Scholar]
- vii.Schrock RR, Hoveyda AH. Molybdenum and tungsten imido alkylidene complexes as efficient olefin metathesis catalysts. Angew. Chem. Int. Edn. 2003;42:4592–4633. doi: 10.1002/anie.200300576. [DOI] [PubMed] [Google Scholar]
- viii.Scholl M, Ding S, Lee CW, Grubbs RH. Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidine ligands. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- ix.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. Efficient and recyclable monomeric and dendritic Ru-based metathesis catalysts. J. Am. Chem. Soc. 2000;122:8168–8179. [Google Scholar]
- x.Nicolaou KC, et al. The olefin metathesis approach to epothilone A and its analogues. J. Am. Chem. Soc. 1997;119:7960–7973. [Google Scholar]
- xi.Meng D, et al. Total synthesis of epothilones A and B. J. Am. Chem. Soc. 1997;119:10073–10092. [Google Scholar]
- xii.Nagata T, Nakagawa M, Nishida A. The first total synthesis of nakadomarin A. J. Am. Chem. Soc. 2003;125:7484–7485. doi: 10.1021/ja034464j. [DOI] [PubMed] [Google Scholar]
- xiii.Ono K, Nakagawa M, Nishida A. Asymmetric total synthesis of (−)-nakadomarin A. Angew. Chem. Int. Edn. 2004;43:2020–2023. doi: 10.1002/anie.200453673. [DOI] [PubMed] [Google Scholar]
- xiv.Young IS, Kerr MA. Total synthesis of (+)-nakadomarin A. J. Am. Chem. Soc. 2007;129:1465–1469. doi: 10.1021/ja068047t. [DOI] [PubMed] [Google Scholar]
- xv.Jakubec P, Cockfield DM, Dixon DJ. Total synthesis of (−)-nakadomarin A. J. Am. Chem. Soc. 2009;131:16632–16633. doi: 10.1021/ja908399s. [DOI] [PubMed] [Google Scholar]
- xvi.Altmann KH, et al. The total synthesis and biological assessment of trans-epothilone A. Helv. Chim. Acta. 2002;85:4086–4110. [Google Scholar]
- xvii.Starks CM, Zhou Y, Liu F, Licari PJ. Isolation and characterization of new epothilone analogues from recombinant Myxococcus xanthus fermentation. J. Nat. Prod. 2003;66:1313–1317. doi: 10.1021/np030218+. [DOI] [PubMed] [Google Scholar]
- xviii.Shinzer D, et al. Total synthesis of (−)-epothilone A. Chem. Eur. J. 1999;5:2483–2491. [Google Scholar]
- xix.Fürstner A, Mathes C, Lehmann CW. Alkyne metathesis: development of a novel molybdenum-based catalyst system and its application to the total synthesis of epothilone A and C. Chem. Eur. J. 2001;7:5299–5317. doi: 10.1002/1521-3765(20011217)7:24<5299::aid-chem5299>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- xx.Nilson MG, Funk RL. Total synthesis of (−)-nakadomarin A. Org. Lett. 2010;12:4912–4915. doi: 10.1021/ol102079z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxi.Coutelier O, Mortreux A. Terminal alkyne metathesis: A further step towards selectivity. Adv. Synth. Catal. 2006;348:2038–2042. [Google Scholar]
- xxii.Smith BJ, Sulikowski GA. Total synthesis of (±)-haliclonacyclamine C. Angew. Chem. Int. Edn. 2010;49:1599–1602. doi: 10.1002/anie.200905732. [DOI] [PubMed] [Google Scholar]
- xxiii.Zhang W, Moore JS. Alkyne metathesis: Catalysts and synthetic applications. Adv. Synth. Catal. 2007;349:93–120. [Google Scholar]
- xxiv.Wang Y, et al. Control of olefin geometry in macrocyclic ring-closing metathesis using a removable silyl group. J. Am. Chem. Soc. 2011;133:9196–9199. doi: 10.1021/ja202012s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxv.Malcolmson SJ, Meek SJ, Sattely ES, Schrock RR, Hoveyda AH. Highly efficient molybdenum-based catalysts for alkene metathesis. Nature. 2008;456:933–937. doi: 10.1038/nature07594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxvi.Ibrahem I, Yu M, Schrock RR, Hoveyda AH. Highly Z- and enantioselective ring-opening/cross-metathesis reactions catalyzed by stereogenic-at-Mo adamantylimido complexes. J. Am. Chem. Soc. 2009;131:3844–3845. doi: 10.1021/ja900097n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxvii.Jiang AJ, Zhao Y, Schrock RR, Hoveyda AH. Highly Z-selective metathesis homocoupling of terminal olefins. J. Am. Chem. Soc. 2009;131:16630–16631. doi: 10.1021/ja908098t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxviii.Meek SJ, O'Brien RV, Llaveria J, Schrock RR, Hoveyda AH. Catalytic Z-selective olefin cross-metathesis for natural product synthesis. Nature. 2011;471:461–466. doi: 10.1038/nature09957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxix.Fürstner A, Langemann K. Macrocycles by ring-closing metathesis. Synthesis. 1997:792–803. [Google Scholar]
- xxx.Fürstner A, Stelzer F, Rumbo A, Krause H. Total synthesis of the turrianes and evaluation of their DNA-cleaving properties. Chem. Eur. J. 2002;8:1856–1871. doi: 10.1002/1521-3765(20020415)8:8<1856::AID-CHEM1856>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.