Abstract
The vascular response to injury is a well-orchestrated inflammatory response triggered by the accumulation of macrophages within the vessel wall leading to an accumulation of lipid-laden intra-luminal plaque, smooth muscle cell proliferation and progressive narrowing of the vessel lumen. The formation of such vulnerable plaques prone to rupture underlies the majority of cases of acute myocardial infarction. The complex molecular and cellular inflammatory cascade is orchestrated by the recruitment of T lymphocytes and macrophages and their paracrine effects on endothelial and smooth muscle cells.1
Molecular imaging in atherosclerosis has evolved into an important clinical and research tool that allows in vivo visualization of inflammation and other biological processes. Several recent examples demonstrate the ability to detect high-risk plaques in patients, and assess the effects of pharmacotherapeutics in atherosclerosis.4 While a number of molecular imaging approaches (in particular MRI and PET) can image biological aspects of large vessels such as the carotid arteries, scant options exist for imaging of coronary arteries.2 The advent of high-resolution optical imaging strategies, in particular near-infrared fluorescence (NIRF), coupled with activatable fluorescent probes, have enhanced sensitivity and led to the development of new intravascular strategies to improve biological imaging of human coronary atherosclerosis.
Near infrared fluorescence (NIRF) molecular imaging utilizes excitation light with a defined band width (650-900 nm) as a source of photons that, when delivered to an optical contrast agent or fluorescent probe, emits fluorescence in the NIR window that can be detected using an appropriate emission filter and a high sensitivity charge-coupled camera. As opposed to visible light, NIR light penetrates deeply into tissue, is markedly less attenuated by endogenous photon absorbers such as hemoglobin, lipid and water, and enables high target-to-background ratios due to reduced autofluorescence in the NIR window. Imaging within the NIR 'window' can substantially improve the potential for in vivo imaging.2,5
Inflammatory cysteine proteases have been well studied using activatable NIRF probes10, and play important roles in atherogenesis. Via degradation of the extracellular matrix, cysteine proteases contribute importantly to the progression and complications of atherosclerosis8. In particular, the cysteine protease, cathepsin B, is highly expressed and colocalizes with macrophages in experimental murine, rabbit, and human atheromata.3,6,7 In addition, cathepsin B activity in plaques can be sensed in vivo utilizing a previously described 1-D intravascular near-infrared fluorescence technology6, in conjunction with an injectable nanosensor agent that consists of a poly-lysine polymer backbone derivatized with multiple NIR fluorochromes (VM110/Prosense750, ex/em 750/780nm, VisEn Medical, Woburn, MA) that results in strong intramolecular quenching at baseline.10 Following targeted enzymatic cleavage by cysteine proteases such as cathepsin B (known to colocalize with plaque macrophages), the fluorochromes separate, resulting in substantial amplification of the NIRF signal. Intravascular detection of NIR fluorescence signal by the utilized novel 2D intravascular NIRF catheter now enables high-resolution, geometrically accurate in vivo detection of cathepsin B activity in inflamed plaque.
In vivo molecular imaging of atherosclerosis using catheter-based 2D NIRF imaging, as opposed to a prior 1-D spectroscopic approach,6 is a novel and promising tool that utilizes augmented protease activity in macrophage-rich plaque to detect vascular inflammation.11,12 The following research protocol describes the use of an intravascular 2-dimensional NIRF catheter to image and characterize plaque structure utilizing key aspects of plaque biology. It is a translatable platform that when integrated with existing clinical imaging technologies including angiography and intravascular ultrasound (IVUS), offers a unique and novel integrated multimodal molecular imaging technique that distinguishes inflammatory atheromata, and allows detection of intravascular NIRF signals in human-sized coronary arteries.
Protocol
In vivo Animal Model: Generation of Experimental AortoIliac Atherosclerosis
1) Baseline Angiography and Balloon Denudation
Prior to obtaining baseline angiography and balloon denudation, a New Zealand white rabbit is fed a high cholesterol (1%) diet for 1 week. This animal is utilized for translational relevance as 1) the aorto-iliacs vessels in rabbits are the same caliber as human coronary arteries (2.5-3.5mm) and 2) the hyperlipidemic, balloon-injury model generates inflamed atherosclerosis bearing similar inflammatory cells (macrophages) and molecules (cathepsins) as in human atherosclerosis.
Following cholesterol feeding, the animal is anesthetized with propofol and ketamine. A one-inch ventral midline neck incision is made using using a size 15-scalpel blade. Using blunt dissection techniques, the muscles underneath the fascia on the right side of the trachea is exposed. The left sternocephalicus muscle is separated along its connective tissue junction, and the right common carotid artery is exposed. The artery is separated from the vagus nerve. Proximal and distal suture loops are placed on the artery to allow for retraction and occlusion. A 1 to 2mm beveled arteriotomy is made through which a 5 French (outer diameter 1.67mm) vascular sheath is inserted and heparin (1000μ/mL, ~150units/kg) is administered intra-arterially via the sheath.
Contrast dye (Ultravist) is then injected (1 to 2mL) over a 2 second period to obtain a control angiogram of the distal aorta and both iliac arteries.
The iliofemoral arteries and aorta are then injured by endothelial denudation. Using standard fluoroscopy methods, a 3Fr Fogarty embolectomy catheter is placed in the distal iliofemoral artery and inflated with 0.3 to 0.5 cc of contrast (50% contrast/50% saline) or air. The catheter is then withdrawn proximally in its inflated state a distance along the right iliac and distal aorta up to the take-off of the left renal artery. Following the balloon denudation, angiography is repeated to document vessel patency. Following angiography, all catheters and sheaths are removed and the proximal right common carotid artery is ligated, the muscle and fascia are sutured with a 4/0 absorbable suture, and the skin incision closed with a 4/0 non-absorbable suture.
The animal is then allowed to recover with administration of one dose antibiotics (Cephazolin, 0.5 grams IM). Pain medications including 0.01 mg/kg buprenorphine IM (twice a day as needed). Animals are then continued on 1% cholesterol for 4 weeks post-balloon denudation. At week 5, animals are transitioned to 0.3% cholesterol diet.
Integrated Multi-modal Imaging of Rabbit Atheromata
2) Labeling of proteolytically active inflamed plaque using injectable nanosensor; Angiography, intravascular ultrasound (IVUS), and in vivo intravascular NIRF imaging of Rabbit Atheroma
Eight weeks following balloon injury and 24 hours prior to imaging, the rabbit is injected with intravenous 500 nmol/kg Prosense/VM110 (PerkinElmer) via ear vein.
Twenty-four hours after injection, animals are anesthetized and arterial access is obtained via left common carotid artery (see step 1.2). Intra-arterial heparin is administered (150 units/kg). Baseline angiography is obtained as above.
An IVUS catheter is loaded onto a clinical coronary artery capable 0.014 inch wire and inserted into the sheath. Using fluoroscopic guidance, the radiopaque tip of the wire is positioned distally into the right iliac artery. The IVUS catheter is then advanced into the proximal iliac artery using a standard clinical monorail technique.
A 100 mm pullback is initiated and images are recorded. Longitudinal reconstruction of the vessel is obtained and luminal plaque is identified.
The NIRF catheter11,12 is loaded onto the 0.014 inch wire (monorail system), and the catheter is carefully inserted into the sheath and imaging head is positioned distally into right iliac artery.
Multiple automated pullbacks (1 mm/sec longitudinal pullback, 30 rounds per minute) are performed and fluorescence signals within zones of atherosclerosis are noted. Images are recorded and further processing with appropriate scaling and windowing based on range of signal is achieved.
3) Euthanasia and isolation of ex vivo aorto-iliac tissue
Euthanasia is accomplished with 1cc of euthanasia agent (solution of 390mg sodium pentobarbital and 50mg phenytoin sodium), intravenous, single injection.
The arterial tree is perfused with 0.9% normal saline until the inferior vena cava is clear of blood. The atherosclerotic aorta and iliac arteries are identified and dissected free from the surrounding tissues. In addition, small 2 x 2 cm pieces of liver, kidney, spleen and heart are also obtained.
Ex vivo NIRF imaging with the intravascular NIRF imaging catheter can be done at this stage. The vessel is elongated and the NIRF catheter is re-inserted into proximal aorta until the imaging head is positioned at the right iliac artery or bifurcation. Multiple automated pullbacks are performed as above (see 2.6).
4) Ex vivo Fluorescence Reflectance Imaging (FRI) of dissected aorta and iliac arteries
Dissected tissue is placed in 10-20 cc of normal saline and transported for FRI analyses (Kodak Image Station 4000MM Pro, Carestream Health, Inc.).
Aorta, iliac vessels are elongated to approximate real-time lengths and images are obtained at multiple wavelengths [white light, green fluorescent channel (ex 495 nm, em 515 nm), Cy5 (ex 565 nm, em 670 nm) and Cy7 (ex 650 nm, em 760 nm)] channels. A series of exposure times are utilized for each wavelength (0.1-30sec) and acquired images are exported as DICOM or 16-bit unscaled TIFF files for further analyses. As positive and negative controls, organs (liver, spleen, kidney and heart) are imaged at similar channels and exposure times.
Areas of increased signal in the near-infrared channel (780nm+) are noted in atherosclerotic arteries.
5) Tissue Embedding for Sectioning and immunohistochemical analysis
Areas of normal (non-injured tissue; i.e. left iliac artery) and areas of plaque are identified and small 5-10 mm rings of tissue are embedded in OCT (Optimal Cutting Temperature) media. Blocks are stored at -80 C until sectioning.
Standard techniques for sectioning and immunohistochemical analyses are performed. Hematoxylin and eosin stain, Ram-11 and Cathepsin B staining are performed.
Analyses and Integration of multi-modal Images (Angiography, IVUS, NIRF and FRI)
6) Processing of NIRF and FRI images
DICOM files containing imaging data from NIRF and FRI (taken at near infrared 780 nm channel) pullbacks are processed using MATLAB and Osirix software, respectively. Proper windowing to display full range of signal intensity is achieved. Final images are exported as TIFF files.
Files are imported into standard image processing software (Keynote can be used). Images are aligned based on reference points (i.e. vertebrae on angiogram, iliac bifurcation, and renal artery). Areas of normal vessel and plaque are identified.
Regions of interest (ROI) are manually traced (for normal tissue and areas of plaque) and mean signal intensities are acquired using Osirix and MATLAB, respectively for both FRI and NIRF images. To guide appropriate tracing, the longitudinal IVUS image of the vessel is used and identification of normal vessel and plaque are easily identified.
Target-to background (TBR) ratios are calculated for plaque zones.
Representative Results:
Upon completion of above protocol, we can identify and characterize areas of augmented cathepsin protease activity in inflammatory plaque within the aorta and iliac vessels. Injection of an activatable nanosensor (Prosense/VM110) allows us to identify proteolytically active plaque. These appear as bright or signal intense zones when imaged using FRI in the near infrared channel (750 nm). The NIRF pullbacks correlate with increased signal intensity by FRI and alignments with IVUS which allow anatomical registration of NIRF signals. Calculated plaque TBR's obtained from FRI and NIRF were similar (see Figure 3: mean NIRF TBR 4.2, mean FRI TBR 2.9). Immunohistochemical analysis of bright plaque confirms intense presence of RAM-11 and Cathepsin B activity in areas of plaque (data not shown).
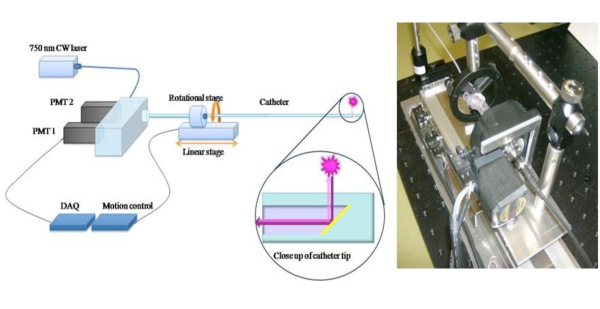 Figure 1. Schematic of 2D NIRF Catheter To extend the clinical potential of a 1D NIRF sensing approach6, we constructed a novel 2-D NIRF-catheter for intravascular imaging.11,12 The custom-built catheter consists of an optical fiber (125 micron diameter housed in polyethylene tubing: 2.9F) that illuminates using a 750 nm laser excitation source. Laser light is emitted at a 90 degree angle relative to fiber axis. The system utilizes two automated motors (rotational and translational) to enable concomitant 360 degree imaging and longitudinal pullback to obtain true 2D imaging. Images used with permission from reference 11.
Figure 1. Schematic of 2D NIRF Catheter To extend the clinical potential of a 1D NIRF sensing approach6, we constructed a novel 2-D NIRF-catheter for intravascular imaging.11,12 The custom-built catheter consists of an optical fiber (125 micron diameter housed in polyethylene tubing: 2.9F) that illuminates using a 750 nm laser excitation source. Laser light is emitted at a 90 degree angle relative to fiber axis. The system utilizes two automated motors (rotational and translational) to enable concomitant 360 degree imaging and longitudinal pullback to obtain true 2D imaging. Images used with permission from reference 11.
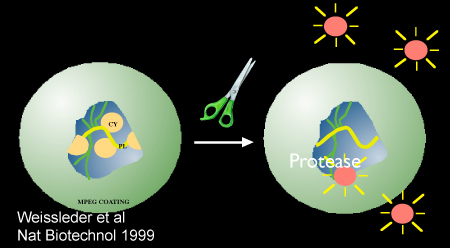 Figure 2. Schematic demonstrating protease-mediated activation of the nanosensor, Prosense/VM110. Image used with permission from reference 10.
Figure 2. Schematic demonstrating protease-mediated activation of the nanosensor, Prosense/VM110. Image used with permission from reference 10.
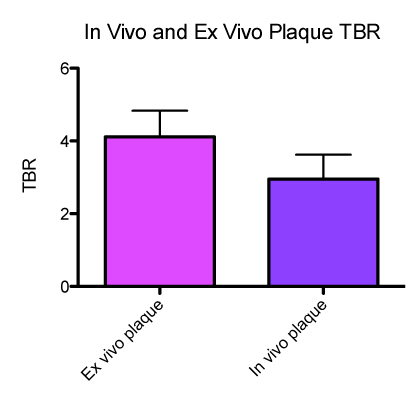 Figure 3
. In vivo and ex vivo Plaque TBRs (target-to background ratios)
Figure 3
. In vivo and ex vivo Plaque TBRs (target-to background ratios)
Discussion
Inflamed high-risk or vulnerable plaques are likely responsible for the majority of myocardial infarctions. The identification of such plaques prior to onset of symptoms has important clinical implications both in predicting outcomes and guiding medical therapy. Conventional coronary arterial imaging modalities such as x-ray angiography typically focus on characterization of luminal narrowings rather than illuminating the underlying biological profiles of high-risk, usually non-stenotic lesions. Intravascular NIRF molecular imaging offers a cardiac catheterization laboratory translatable approach that incorporates the biology of plaque inflammation by utilizing nanosensors that identify active macrophages within plaque, a cellular hallmark of an inflamed plaque prone to rupture.9
The following protocol outlined above utilizes a multimodal integrative approach combining angiography, IVUS and NIRF imaging to identify inflamed plaque. This novel 2D NIRF catheter capitalizes on the favorable optical properties of the near-infrared fluorescence bandwidth to detect molecular signatures through blood, and is a promising in vivo approach to molecular imaging. The nanosensor Prosense/VM110 is coupled to fluorochromes that emit fluroescence at 780 nm and utilize auto-quenching in the absence of proteolytic cleavage or activation by the enzyme cathepsin B (highly expressed in resident macrophages). Detection of in vivo fluorescence signal by the NIRF catheter allows identification of macrophage-laden plaque (TBR 2.9). The use of ex vivo fluorescence reflectance imaging (FRI) confirms the presence of NIR fluorescence signal within areas of plaque (TBR 4.2). Immunohistochemical staining of RAM-11 and cathepsin within areas of plaque confirm the intense infiltration of cathepsin-B positive macrophages (data not shown).
The incorporation of IVUS and NIRF signal can be fused to map signal intensity within visible plaque along the length of the vessel (data not shown) and offers a unique opportunity to further elucidate luminal plaque morphology and macrophage content. Current limitations of the above technique include the inability to exactly co-register the IVUS and NIRF pullbacks. Simultaneous pullback via an integrated dual-modal catheter would increase the accuracy of signal location and potentially enable resolution of the location of signal within vessel wall (i.e. depth of signal within lumen, media, or adventitia). Dual modal NIRF/IVUS or NIRF/optical coherence tomography (OCT) fusion catheters are anticipated therefore to provide further delineation of signal and incorporate plaque architecture with biology.
Disclosures
FAJ - Former Consultant, VisEn Medical; Honoraria, Boston Scientific
Acknowledgments
Support for this work was provided by National Institutes of Health grant #R01 HL 108229, American Heart Association Scientist Development Grant #0830352N, Howard Hughes Medical Institute Career Development Award, Broadview Ventures, European Community's Seventh Framework Programme (FP7/2007-2013 under grant agreement #235689), and the MGH William Schreyer Fellowship.
References
- Andersson J, Libby P. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134:33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Calfon MA, Vinegoni C. Intravascular near-infrared fluorescence molecular imaging of atherosclerosis: toward coronary arterial visualization of biologically high-risk plaques. Journal of Biomedical Optics. 2010;15:011107–011107. doi: 10.1117/1.3280282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tung C-H. In Vivo Imaging of Proteolytic Activity in Atherosclerosis. Circulation. 2002;105:2766–2771. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- Jaffer FA, Libby P. Molecular Imaging of Cardiovascular Disease. Circulation. 2007;116:1052–1061. doi: 10.1161/CIRCULATIONAHA.106.647164. [DOI] [PubMed] [Google Scholar]
- Jaffer FA, Libby P. Optical and Multimodality Molecular Imaging: Insights Into Atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1017–1024. doi: 10.1161/ATVBAHA.108.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffer FA, Vinegoni C. Real-Time Catheter Molecular Sensing of Inflammation in Proteolytically Active Atherosclerosis. Circulation. 2008;118:1802–1809. doi: 10.1161/CIRCULATIONAHA.108.785881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-E, Kim J-Y. Protease Imaging of Human Atheromata Captures Molecular Information of Atherosclerosis, Complementing Anatomic Imaging. Arterioscler Thromb Vasc Biol. 2010;30:449–456. doi: 10.1161/ATVBAHA.109.194613. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Naghavi M, Libby P. From Vulnerable Plaque to Vulnerable Patient: A Call for New Definitions and Risk Assessment Strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Tung C-H. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotech. 1999;17:375–375. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- Razansky RN, Rosenthal A. Near-infrared fluorescence catheter system for two-dimensional intravascular imaging in vivo. Optics Express. 2010;18:11372–11381. doi: 10.1364/OE.18.011372. [DOI] [PubMed] [Google Scholar]
- Jaffer FA, Calfon MA. Two-Dimensional Intravascular Near-Infrared Fluorescence Molecular Imaging of Inflammation in Atherosclerosis and Stent-Induced Vascular Injury. Journal of the American College of Cardiology. 2011;57:2516–2526. doi: 10.1016/j.jacc.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]


