Abstract
Culturing retinoblastoma tumor cells in defined stem cell media gives rise to primary tumorspheres that can be grown and maintained for only a limited time. These cultured tumorspheres may exhibit markedly different cellular phenotypes when compared to the original tumors. Demonstration that cultured cells have the capability of forming new tumors is important to ensure that cultured cells model the biology of the original tumor.
Here we present a protocol for propagating human retinoblastoma tumors in vivo using Rag2-/- immune deficient mice. Cultured human retinoblastoma tumorspheres of low passage or cells obtained from freshly harvested human retinoblastoma tumors injected directly into the vitreous cavity of murine eyes form tumors within 2-4 weeks. These tumors can be harvested and either further passaged into murine eyes in vivo or grown as tumorspheres in vitro. Propagation has been successfully carried out for at least three passages thus establishing a continuing source of human retinoblastoma tissue for further experimentation.
Wesley S. Bond and Lalita Wadhwa are co-first authors.
Keywords: Medicine, Issue 54, retinoblastoma, tumor, xenograft, tumorsphere, mouse, human, eye, cancer stem cell
Protocol
1. Preparation of retinoblastoma tumorspheres
Obtain retinoblastoma tumor sample under an IRB-approved protocol.
Prepare fresh defined stem cell media (Neurobasal-A media, 1X B-27 supplement minus vitamin A, 1X non-essential amino acid solution, 1X L-glutamine, 20 ng/mL EGF, 10 ng/mL bFGF).
Mechanically disaggregate the tumor tissue with a scalpel using a cross-cutting technique in a sterile, tissue culture-treated 6-well plate. Immediately add 100 μL of defined stem cell media to the tissue and gently spread the minced tissue using the scalpel. Add 5 mL of defined stem cell media to the well.
Incubate at 37°C, 5% CO2 in a humidified incubator and inspect daily. Some tumorspheres should arise from the disrupted tumor tissue almost immediately, with increasing numbers over the course of 2-4 days.
Weekly, centrifuge tumorspheres at 300xg for 5 minutes, resuspend in freshly prepared defined stem cell media, and pipet up and down 10 times to disrupt larger, centrally necrotic spheres and allow new, healthy spheres to form.
2. Preparation of tumor cells for injection
If injecting cultured tumorspheres, gently pipet spheres into a 15-mL conical tube. Gently pipet up and down with a serological pipet 10-15 times to disrupt the spheres.
Count the cells to be injected using a hemacytometer, and aliquot at least 50,000 cells per injection into a new tube. Prepare for as many injections as possible based on the number of cells available.
Centrifuge at 300xg for 5 minutes. Carefully aspirate the supernatant and resuspend in 5 mL PBS. Repeat centrifugation and aspiration, and resuspend in 10 μL PBS per injection.
3. Preparation of animals
Using an insulin syringe, administer an intraperitoneal injection of rodent anesthesia cocktail (ketamine 37.6 mg/mL, xylazine 1.92 mg/mL, acepromazine 0.38 mg/mL) at 1 μL per gram body weight (gbw). Wait 5 minutes for the animal to become sedated. Check for heartbeat and place the animal on a heated pad. Maintain animals on a heated pad at all times until recovery.
After sedation, administer 1 drop of proparacaine HCl to the right eye. Replace animal on heated pad and wait 1 minute.
Administer 1 drop of phenylephrine HCl to the right eye. Replace the animal on the heated pad and wait 5 minutes. If dilation of the pupil has not occurred, re-administer 1 drop and wait another 5 minutes.
Monitor animals for signs of movement or twitching, which may occur at >30 minutes post-sedation. At first signs of movement, administer 1 μL/gbw of ketamine HCl (diluted to 10 mg/mL in PBS) and wait 5 minutes.
4. Injection of tumor cells
Place the anesthetized animal under the microscope on its side, with the right eye facing up and with the head resting on gauze and centered to obtain a red reflex of the right eye fundus (pupil should be dilated at this time) when observed through the microscope.
Prop up the right globe by gently pushing down simultaneously with two fingers on the eyelids and hold this position steadily for the remainder of the procedure.
Using a sterile 30-gauge needle attached to a Luer-lock syringe, pierce the globe laterally through the conjunctiva and sclera adjacent to the corneal limbus in the area of the pars plana (Figure 1) and just through the choroid into the vitreous cavity. Remove the needle from the opening.
Sterilize the Hamilton needle with an alcohol swab. Draw the cell suspension into the Hamilton syringe and insert the needle into the opening until the needle is visualized through the microscope behind the lens in the vitreous near the retina. With the help of a second person while holding the needle steadily in this position, depress the plunger slowly. Remove the needle. If necessary, use a cotton swab to absorb any fluid from the opening.
Gently release the pressure from the eyelids to close the mouse eyelids.
Administer 1 drop of hypromellose to each eye and return animal to heated pad for recovery.
5. Monitoring of injected mice and harvesting of tumors
Daily, examine the injected eye for leukocoria (white papillary reflex) and/or periocular distention that usually becomes apparent 2-4 weeks post-injection.
Once tumor growth is detected, euthanize animal according to institutional guidelines.
If the eye and tumor are covered by the eyelids, use the scalpel to make two incisions orthogonal to the palpebral fissure and lift the skin flaps.
Under visualization using the stereoscopic microscope, make a circumferential incision at the limbus, removing the cornea and the lens, and carefully dissect the bulk of the tumor mass from the other tissues using tweezers. Place the tumor in sterile RPMI-1640 media.
Use tweezers to slowly pull the open globe from the socket. To ensure an attached optic nerve so tumor invasion can be visualized, pull the globe until the nerve is exposed and use scissors to cut the nerve as long as possible. Place in 10% formalin for regular processing for histological examination.
6. Representative Results:
Retinoblastoma tumorspheres will begin to appear from the disaggregated tissue almost immediately as they are liberated from the tumor mass. Within 2-4 days, more tumorspheres will begin to form and will increase in size. The spheres tend to be regular and exhibit a well-defined, secondary membrane surrounding the aggregate (Figure 2).
The animal typically presents with leukocoria within 4 weeks post-injection (Figure 3b), followed by enlargement of the globe and distention of the surrounding tissues 5-8 weeks after injection as the tumor grows (Figure 3c).
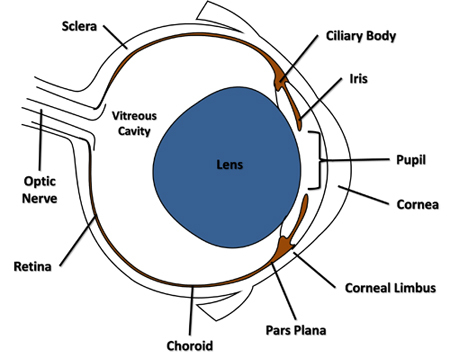 Figure 1. Cross-sectional diagram of the mouse eye highlighting features referenced in the protocol.
Figure 1. Cross-sectional diagram of the mouse eye highlighting features referenced in the protocol.
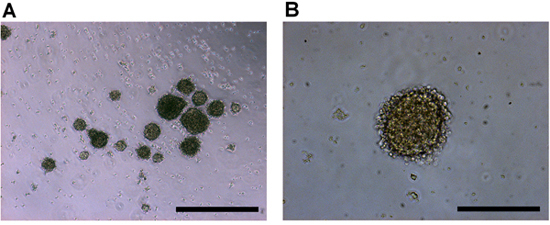 Figure 2. Culture of human retinoblastoma cells in vitro imaged at a) 4x, and b) 10x objective magnification. Retinoblastoma primary tumor cells produce tumorspheres with a regular spheroid shape and a crisp outer membrane. Scale bars represent a) 500 μm, and b) 200 μm.
Figure 2. Culture of human retinoblastoma cells in vitro imaged at a) 4x, and b) 10x objective magnification. Retinoblastoma primary tumor cells produce tumorspheres with a regular spheroid shape and a crisp outer membrane. Scale bars represent a) 500 μm, and b) 200 μm.
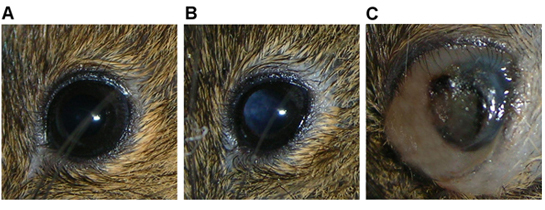 Figure 3. Eye of Rag2-/- mouse showing a) normal features, b) leukocoria indicative of a tumor mass in vitreous cavity, and c) a large tumor mass filling the globe with associated periocular distension, intraocular hemorrhage.
Figure 3. Eye of Rag2-/- mouse showing a) normal features, b) leukocoria indicative of a tumor mass in vitreous cavity, and c) a large tumor mass filling the globe with associated periocular distension, intraocular hemorrhage.
Discussion
The technique described herein facilitates the propagation retinoblastoma tumors in their intraocular, intravitreal milieu. The intraocular injection technique has been used in the past to create tumors from retinoblastoma-derived cell lines1 as well as to deliver viral vectors for intraocular gene therapy2,3. This technique has now been successfully utilized to produce human retinoblastoma tumors by direct injection of cells from the primary tumor and injection of tumorspheres as well as serial propagation of xenograft tumors. Visible evidence of tumor formation (usually leukocoria) is usually first noted within 4 weeks, after which intraocular hemorrhage and distention of the globe and/or tissues surrounding the orbit develop within 5-8 weeks. A minority of injected mice injure the injected eye, leading to permanent closure of the eyelids. In these cases, distention is the only sign of tumor formation.
Establishment of murine xenograft tumors has not been successful with all human retinoblastoma tumors, though propagation of established xenograft tumors is highly successful. This observation suggests that certain characteristics of the primary tumor, such as invasiveness and level of differentiation or some other unknown factor, may be influencing the ability of these tumors to persist in the murine ocular environment.
The amount of tissue that can be acquired from a human retinoblastoma tumor is quite small, and there are significant limitations on the ability to culture human retinoblastoma cells in vitro such as limited life span, loss of solid tumor histology and changes in cellular phenotype. This protocol provides a relatively simple way to propagate the human tumor and establish a murine model of the human disease. This allows further in vitro and in vivo experimentation of the biology of retinoblastoma and longer maintenance of the tumor outside of the patient.
Disclosures
Experiments on animals were performed in accordance with the guidelines and regulations set forth by the Baylor College of Medicine IACUC Committee.
Acknowledgments
Funding for this project is provided by the Clayton Foundation for Research and the Retina Research Foundation.
References
- Chèvez-Barrios P. Metastatic and Nonmetastatic Models of Retinoblastoma. Am. J. Pathol. 2000;157:1405–1412. doi: 10.1016/S0002-9440(10)64653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suber ML, Hurwitz MY, Chèvez-Barrios P, Hurwitz RL. Immune consequences of intraocular administration of modified adenoviral vectors. Hum. Gene Ther. 2001;12:833–838. doi: 10.1089/104303401750148801. [DOI] [PubMed] [Google Scholar]
- Mallam JN, Hurwitz MY, Mahoney T, Chèvez-Barrios P, Hurwitz RL. Efficient Gene Transfer into Retinal Cells Using Adenoviral Vectors: Dependence on Receptor Expression. Invest. Ophthalmol. Vis. Sci. 2004;45:1680–1687. doi: 10.1167/iovs.03-0730. [DOI] [PubMed] [Google Scholar]


