Phospholipids provide an amphipathic barrier between lipid droplets and the cytoplasm of cells. In this issue of Cell Metabolism, Krahmer and colleagues define a role for phosphatidylcholine in preventing lipid droplet coalescence, and show that the rate-limiting enzyme in phosphatidylcholine synthesis is activated through binding to lipid droplets.
Lipid droplets function as a storage depot for neutral lipids, providing a source of substrates for ATP generation, building materials for membrane synthesis and repair, and precursors of hormones and cellular second messengers for intracellular signaling pathways. The neutral lipids, primarily triglycerides and cholesterol esters, are packaged into the core of the lipid droplet; a monolayer of phospholipids covers the surface of the droplet and provides an amphipathic interface with the cytosol. Various proteins decorate lipid droplets, including specific structural proteins (perilipins), enzymes with functions in the metabolism and trafficking of lipids, and a number of proteins of unknown function. How and where lipid droplets are assembled and how they expand to accommodate more lipids when excess calories are consumed and dietary lipids are plentiful are open questions. The report from Krahmer, et al., in this issue of Cell Metabolism sheds considerable light on the mechanisms by which the phospholipid content of lipid droplets is maintained (Krahmer, et al., 2011).
The most abundant phospholipid in the limiting monolayer of the lipid droplet, as in other cellular membranes, is phosphatidylcholine (Bartz, et al., 2007). In animals, phosphatidylcholine is synthesized via two biosynthetic pathways. The major pathway is depicted in Figure 1. The rate-limiting step in phosphatidylcholine biosynthesis is catalyzed by CCT, which is encoded by two genes in animals and Drosophila, yielding two isoforms, CCT α and CCTβ. CCT β is the predominant isoform in most tissues and cycles between the nucleus and cytoplasm. It forms a homodimer and is activated upon binding to nuclear membranes or the cytoplasmic surface of the endoplasmic reticulum (ER) (Taneva et al., 2008). CCT binds to membranes depleted of phosphatidylcholine and enriched in anionic phospholipids via an amphipathic β-helical domain. Conformational changes induced upon membrane binding activate CCT. Krahmer and colleagues now show that CCT is recruited to the phospholipid monolayer of lipid droplets when fatty acids are added to cells to promote the synthesis and storage of triglycerides in lipid droplets (Krahmer, et al., 2011). Recruitment requires the β-helical domain of CCT and is likely facilitated by depletion of phosphatidylcholine at the surfaces of lipid droplets as they expand to accommodate newly synthesized triglyceride. When CCT binds to enlarging lipid droplets, CCT activity on lipid droplets increases by more than 30-fold and total cellular CCT activity increases by 4-fold (Krahmer,et al., 2011). Thus, the present work identifies a major new mechanism of CCT regulation and novel compartment for CCT binding and activation.
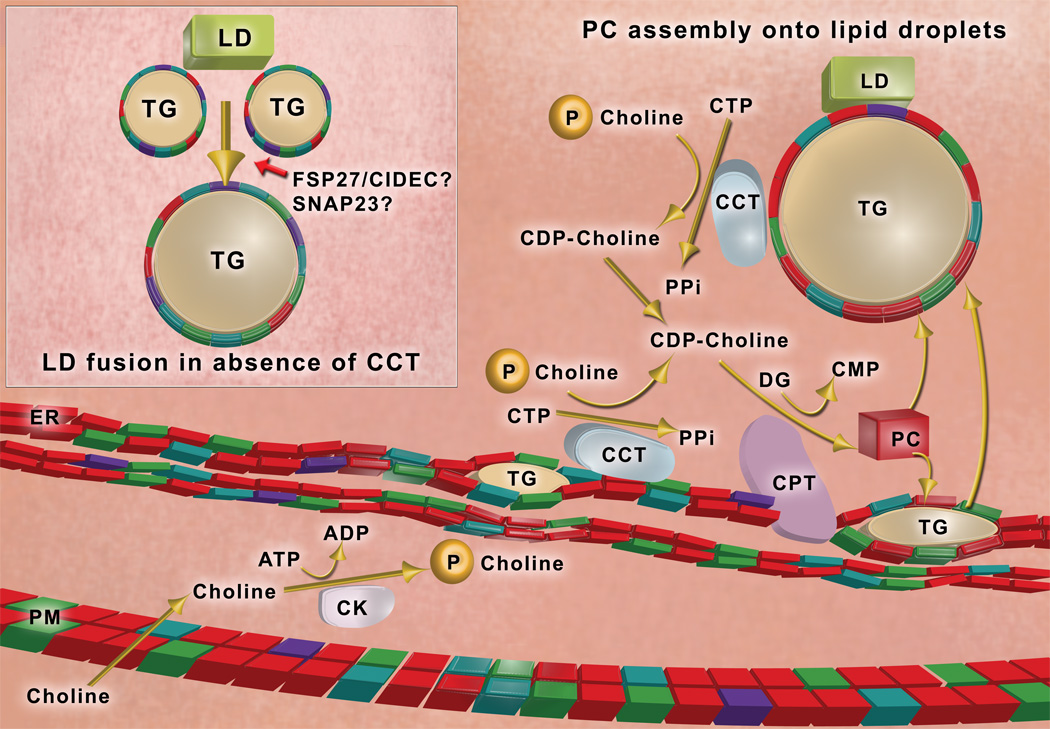
Figure 1. Phosphatidylcholine synthesis and lipid droplet dynamics.
Dietary choline is taken up across the plasma membrane (PM) and is phosphorylated by choline kinase (CK). Phosphocholine is converted to CDP-choline by CTP:phosphocholine cytidylyltransferase (CCT), which binds to endoplasmic reticulum (ER) membranes and the phospholipid monolayer (colored blocks) of lipid droplets (LD) via an amphipathic helical domain (Krahmer, et al, 2011); membrane binding activates the enzyme. CDP-choline is condensed with diglyceride (DG) to make phosphatidylcholine (PC) by an integral protein of the ER membrane, CDP-choline:1,2 diacylglycerol cholinephosphotransferase (CPT). Phosphatidylcholine (represented by red blocks) is the most abundant phospholipid in cellular membranes and the phospholipid monolayer of lipid droplets. Lipid droplets are thought to originate as a lens of triglyceride (TG) within the membrane bilayer of the ER. Following expansion of the triglyceride core, lipid droplets bud out of the ER to become independent organelles in the cytoplasm of the cell. When phosphatidylcholine is limiting in the phospholipid monolayer coating the lipid droplet (Inset), adjacent lipid droplets coalesce into larger lipid droplets (Krahmer, et al., 2011). Lipid droplet-associated proteins may also promote lipid droplet fusion; candidates include SNAP23, and Fsp27/CIDEC. (Illustration by R. Hasney)
How do lipid droplets form? The enzymes catalyzing the final steps of neutral lipid synthesis reside in the ER (Yen et al., 2008). Thus, the most popular hypothetical model for lipid droplet formation posits that lipid droplets originate as a lens of neutral lipid within the membrane bilayer of the ER membrane (Figure 1). This model proposes that the phospholipid monolayer that coats the emerging droplet is derived from the ER. As the core of the lipid droplet expands, the nascent droplet recruits specific proteins and eventually buds from the ER to form a distinct structure, which nonetheless remains closely associated with ER membranes.
When cells are exposed to fatty acids, rapid import and esterification of fatty acids increases triglycerides and the formation of new lipid droplets, but may also lead to the expansion of preexisting lipid droplets. How triglycerides and phospholipids are added to pre-formed lipid droplets is unclear. Although CCT forms CDP-choline at the surfaces of lipid droplets (Krahmer, et al., 2011), the final step of phosphatidylcholine synthesis occurs on the ER. The mechanisms for the transport of phospholipids to lipid droplets remain to be determined. Possible mechanisms include transfer through ER contact sites with lipid droplets or via cytosolic phospholipid transfer proteins.
Inhibition of phosphatidylcholine biosynthesis affects lipid droplet morphology. When CCT or CK are knocked down in cells, phosphatidylcholine synthesis is attenuated and giant lipid droplets are formed (Guo, et al., 2008, Krahmer, et al., 2011). Experiments with lipid emulsions of varying composition of phospholipids and triglycerides show that phosphatidylcholine stabilizes small micelles that resemble lipid droplets, preventing coalescence of the emulsified lipids into large amorphous pools (Krahmer, et al., 2011). Other species of phospholipids lack this property. Thus, phosphatidylcholine serves a unique function in stabilizing lipid droplets.
Most types of cells produce numerous small lipid droplets; however, adipocytes within white adipose tissue maintain single (unilocular) lipid droplets that can be larger than 100 microns, defining the size of the fat cell. What is the advantage of large, unilocular lipid droplets? The current study shows that when phosphatidylcholine synthesis is limited by knocking down CCT in Drosophila, the enlarged lipid droplets of fat body cells are hydrolyzed very slowly allowing increased survival during starvation. Extending this concept to animals, packaging of fat into unilocular lipid droplets in adipocytes likely enhances fat storage and thus necessitates elaborate control mechanisms to promote lipolysis for release of energy substrates (Lass, et al., 2011).
The study by Krahmer and colleagues suggests that a major determinant of lipid droplet size is the phosphatidylcholine content of the limiting monolayer; however, since phosphatidylcholine is the most abundant phospholipid coating adipocyte lipid droplets, maintenance of unilocular droplets is not a function of deficiency of this phospholipid. Lipid droplet-associated proteins most likely contribute to the control of lipid droplet size through the promotion of fusion events. One study has suggested that SNAP23, a protein involved in secretory vesicle fusion, is required for lipid droplet fusion (Bostrom et al., 2007); however, the current study shows no effect of SNAP23 depletion on lipid droplet size (Krahmer et al., 2011). A promising candidate for a fusion-mediating protein is CIDEC/Fsp27, a protein expressed primarily in adipocytes; when the Fsp27 gene is deleted in mice, adipocytes have smaller, multilocular lipid droplets (Nishino, et al., 2008, Toh, et al., 2008). Mechanisms controlling lipid droplet size and fusion are poorly understood. Perhaps Fsp27 promotes local depletion of phosphatidylcholine, removing the surfactant barrier between adjacent lipid droplets and enabling fusion.
The control of lipid droplet formation, expansion and metabolism has become a lively area of investigation. Elucidation of the role of phosphatidylcholine in lipid droplet dynamics is an important new addition to this increasingly more complex story.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Selected Reading
- Bartz R, Li W-H, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RGW, Liu P, Chapman KD. J. Lipid Res. 2007;48:837–847. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Andersson L, Rutberg M, Perman J, Lidberg U, Johansson BR, Fernandez-Rodriguez J, Ericson J, Nilsson T, Boren J, Olofsson S-O. Nature Cell Biol. 2007;9:1286–1293. doi: 10.1038/ncb1648. [DOI] [PubMed] [Google Scholar]
- Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV., Jr. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahmer N, Guo Y, Wifling F, Hilger M, Lingrell S, Heger K, Newman HW, Schmid-Supprian M, Vance DE, Mann M, Farese RV, Jr., Walther T. Cell Metab. 2011 doi: 10.1016/j.cmet.2011.07.013. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Zimmerman R, Oberer M, Zechner R. Prog. Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Vance DE. J. Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, et al. J. Clin. Invest. 2008;118:2808–2821. doi: 10.1172/JCI34090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneva S, Dennis MK, Ding Z, Smith JL, Cornell RB. J. Biol. Chem. 2008;283:28137–28148. doi: 10.1074/jbc.M802595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh SY, Gong J, Du G, Li JZ, Yang S, Ye J, Yao H, Zhang Y, Xue B, Li Q, Yang H, Wen Z, Li P. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002890. e2890(1–16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV., Jr. J. Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]