Abstract
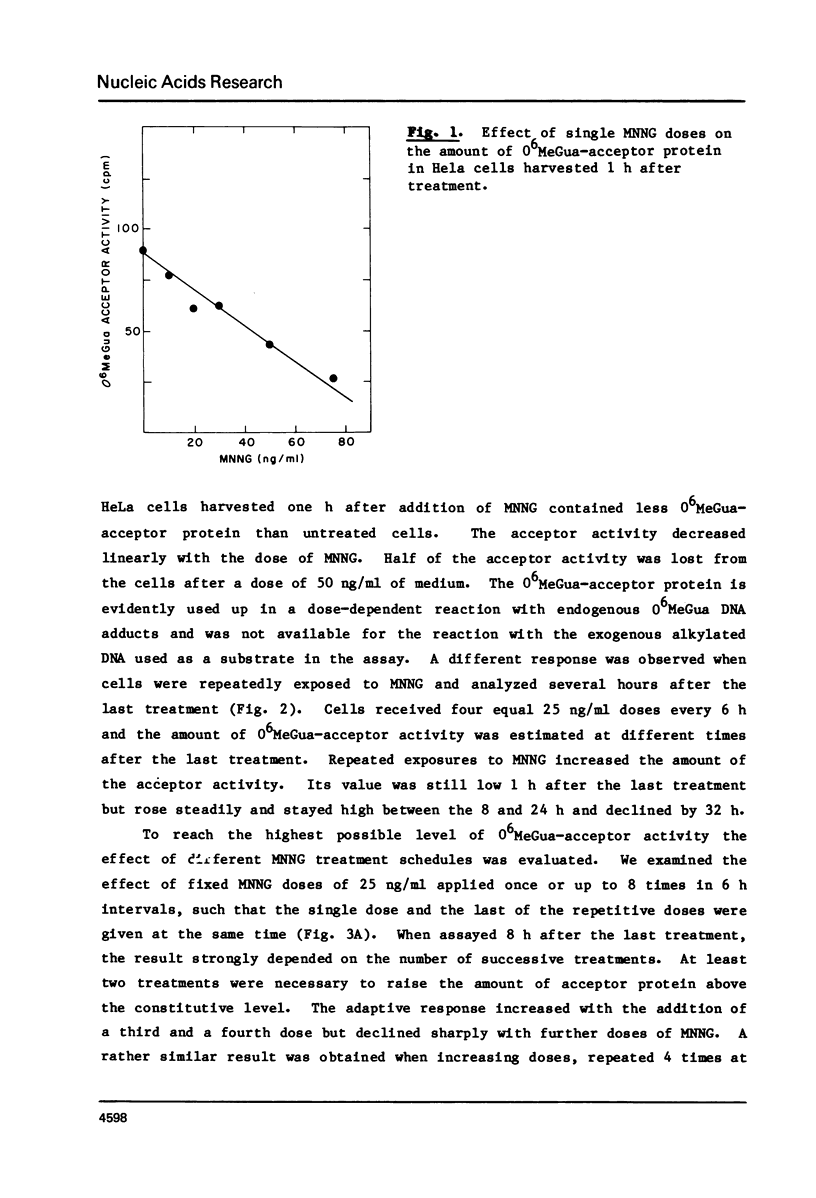
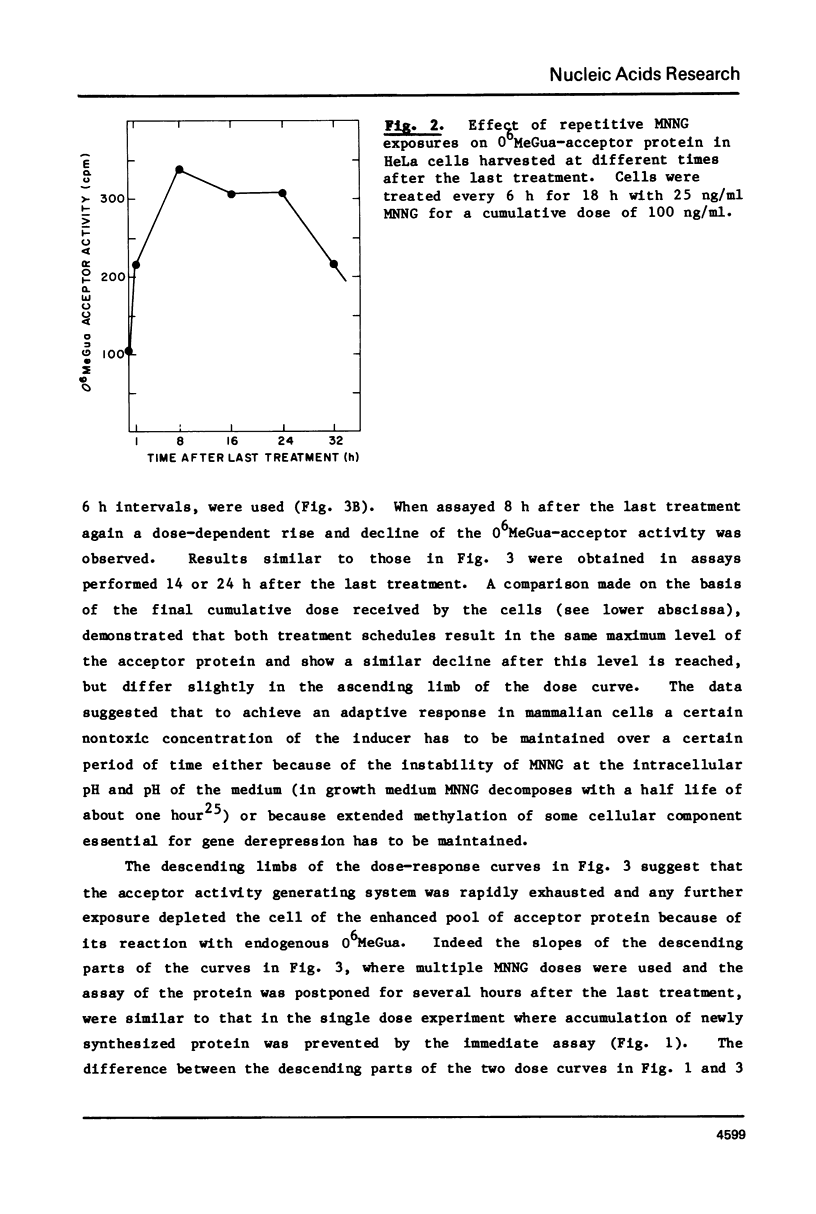
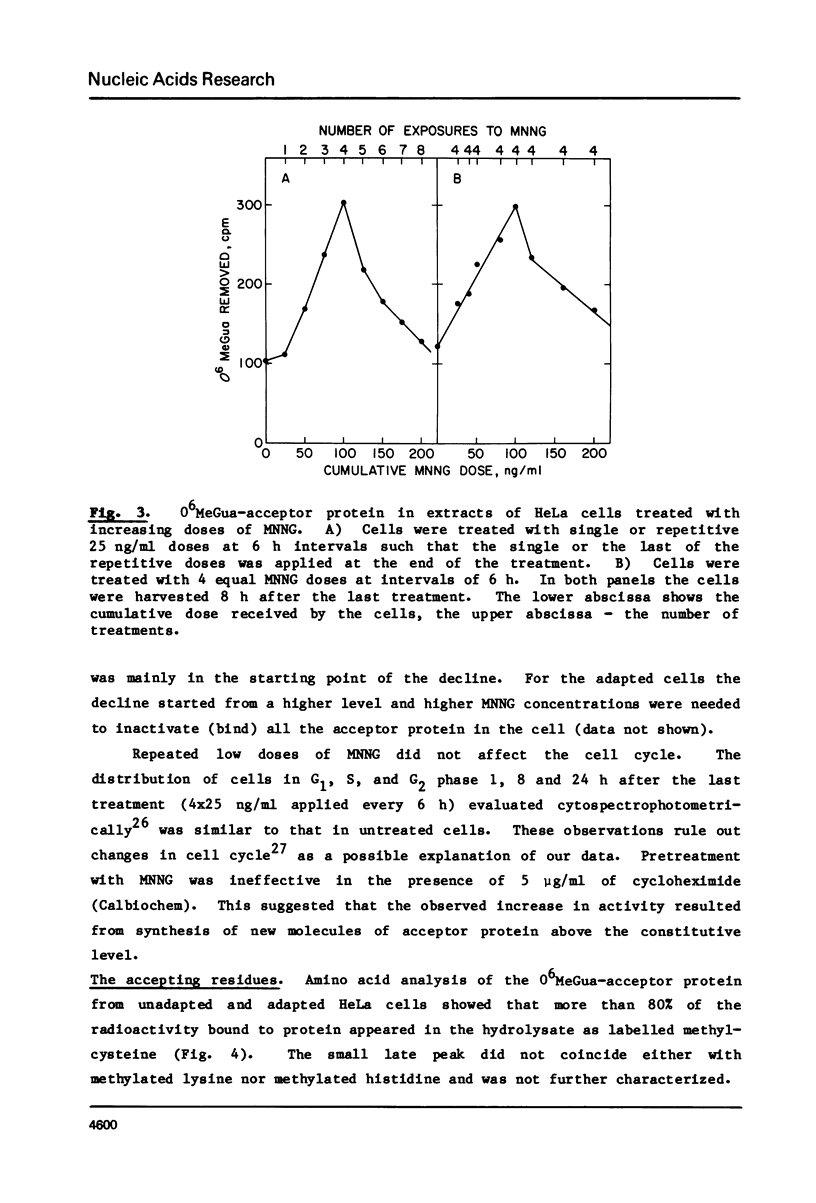
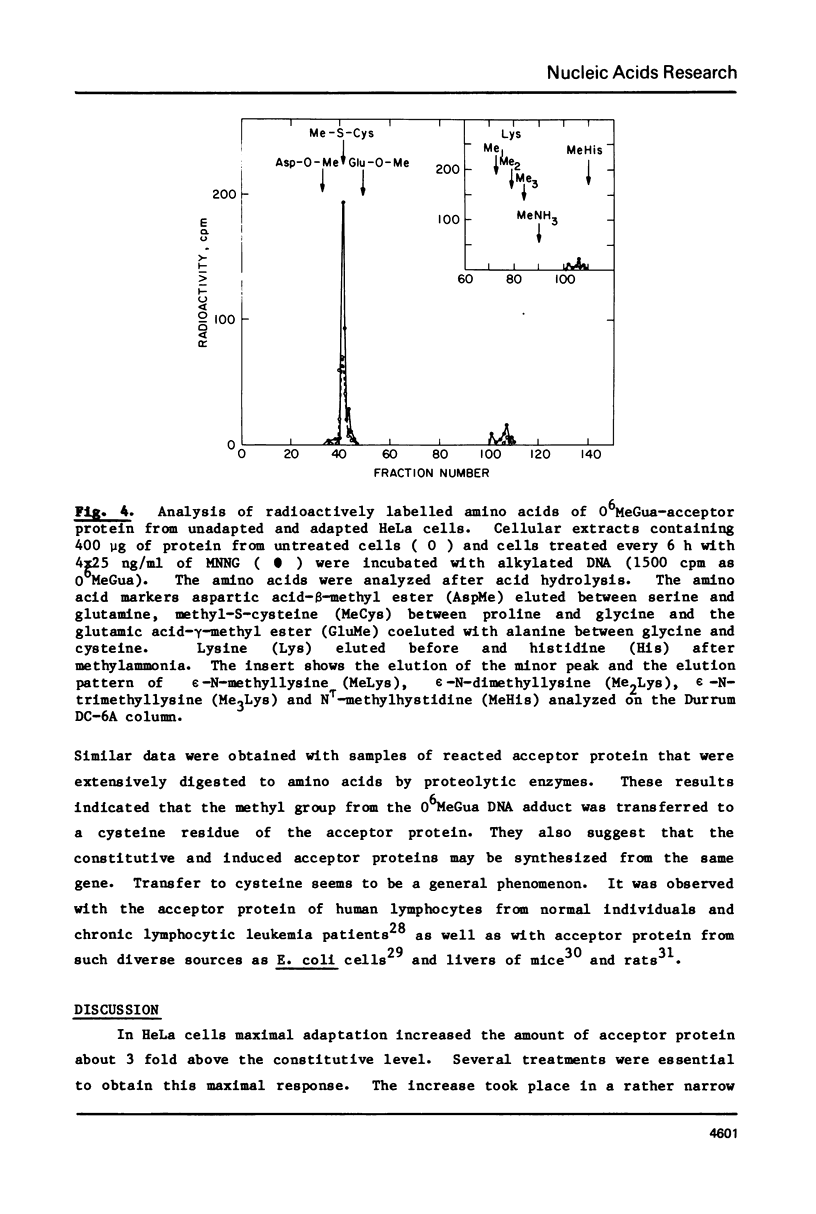
We have assayed in extracts of HeLa cells the amount of acceptor protein that removes O6-methylguanine adducts from alkylated DNA. Cells were treated with single or multiple nontoxic doses of N-methyl-N'-nitrosoguanidine (MNNG) and the extracts were analyzed up to 32 h after the last exposure. The acceptor activity assayed immediately (1 h) after single exposures decreases linearly with dose indicating that the acceptor protein is used up by endogenous O6-methylguanine adducts in a stoichiometric reaction. Multiple exposures, assayed 8-24 h after the last exposure, increase the amount of acceptor protein in a dose dependent fashion followed by a decrease above a cumulative dose of 100 ng/ml. Under conditions of maximum induction, there are about 300,000 acceptor protein sites per cell, approximately 3 fold above the constitutive level. Both in adapted and unadapted cells the methyl group from O6-methylguanine adducts in the alkylated DNA is transferred to cysteine residues of the acceptor protein(s).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrows L. R., Shank R. C. Aberrant methylation of liver DNA in rats during hepatotoxicity. Toxicol Appl Pharmacol. 1981 Sep 15;60(2):334–345. doi: 10.1016/0041-008x(91)90236-8. [DOI] [PubMed] [Google Scholar]
- Bogden J. M., Eastman A., Bresnick E. A system in mouse liver for the repair of O6-methylguanine lesions in methylated DNA. Nucleic Acids Res. 1981 Jul 10;9(13):3089–3103. doi: 10.1093/nar/9.13.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley J. D., O'Connor P. J., Craig A. W. Pretreatment with acetylaminofluorene enhances the repair of O6-methylguanine in DNA. Nature. 1979 Oct 4;281(5730):403–404. doi: 10.1038/281403a0. [DOI] [PubMed] [Google Scholar]
- Charlesworth J. D., Chu Y. H., O'Connor P. J., Craig A. W. Effect of pretreatment by feeding acetylaminofluorene on methylated purines formed in rat liver DNA after administration of dimethylnitrosamine. Carcinogenesis. 1981;2(4):329–342. doi: 10.1093/carcin/2.4.329. [DOI] [PubMed] [Google Scholar]
- Chu Y. H., Craig A. W., O'Connor P. J. Repair of O6-methylguanine in rat liver DNA is enhanced by pretreatment with single or multiple doses of aflatoxin B1. Br J Cancer. 1981 Jun;43(6):850–855. doi: 10.1038/bjc.1981.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. K., Hauenstein E., Kolar G. F., Kleihues P. DNA alkylation and neuro-oncogenesis by 3,3-dimethyl-1-phenyltriazene. Acta Neuropathol. 1978 Aug 7;43(1-2):105–109. doi: 10.1007/BF00685004. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H. MNNG-pretreatment of a human kidney carcinoma cell strain decreases its ability to repair MNNG-treated adenovirus 5. Carcinogenesis. 1981;2(3):213–218. doi: 10.1093/carcin/2.3.213. [DOI] [PubMed] [Google Scholar]
- Durrant L. G., Margison G. P., Boyle J. M. Pretreatment of Chinese hamster v79 cells with MNU increases survival without affecting DNA repair or mutagenicity. Carcinogenesis. 1981;2(1):55–60. doi: 10.1093/carcin/2.1.55. [DOI] [PubMed] [Google Scholar]
- Goth-Goldstein R. Inability of Chinese hamster ovary cells to excise O6-alkylguanine. Cancer Res. 1980 Jul;40(7):2623–2624. [PubMed] [Google Scholar]
- Harris G., Lawley P. D., Olsen I. Mode of action of methylating carcinogens: comparative studies of murine and human cells. Carcinogenesis. 1981;2(5):403–411. doi: 10.1093/carcin/2.5.403. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mak S. Mammalian cell cycle analysis using microspectrophotometry combined with autoradiography. Exp Cell Res. 1965 Aug;39(1):286–289. doi: 10.1016/0014-4827(65)90030-3. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Curtin N. J., Snell K., Craig A. W. Effect of chronic N,N-diethylnitrosamine on the excision of O6-ethylguanine from rat liver DNA. Br J Cancer. 1979 Nov;40(5):809–813. doi: 10.1038/bjc.1979.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margison G. P. Effect of pretreatment of rats with N-methyl-N-nitrosourea on the repair of O(6)-methylguanine in liver DNA. Carcinogenesis. 1981;2(5):431–434. doi: 10.1093/carcin/2.5.431. [DOI] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B., Renard A., Verly W. G. Repair of O6-ethylguanine in DNA by a chromatin fraction from rat liver: transfer of the ethyl group to an acceptor protein. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6766–6770. doi: 10.1073/pnas.78.11.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Brésil H., Margison G. P. Increased excision of O6-methylguanine from rat liver DNA after chronic administration of dimethylnitrosamine. Cancer Res. 1979 May;39(5):1798–1802. [PubMed] [Google Scholar]
- Montesano R., Brésil H., Planche-Martel G., Margison G. P., Pegg A. E. Effect of chronic treatment of rats with dimethylnitrosamine on the removal of O6-methylguanine from DNA. Cancer Res. 1980 Feb;40(2):452–458. [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Pegg A. E. Dimethylnitrosamine inhibits enzymatic removal of O6-methylguanine from DNA. Nature. 1978 Jul 13;274(5667):182–184. doi: 10.1038/274182a0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Formation and subsequent repair of alkylation lesions in tissues of rodents treated with nitrosamines. Arch Toxicol Suppl. 1980;3:55–68. doi: 10.1007/978-3-642-67389-4_5. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Perry W. Stimulation of transfer of methyl groups from O6-methylguanine in DNA to protein by rat liver extracts in response to hepatotoxins. Carcinogenesis. 1981;2(11):1195–1200. doi: 10.1093/carcin/2.11.1195. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Shuttleworth K., Hibasami H. Specificity of mammalian spermidine synthase and spermine synthase. Biochem J. 1981 Aug 1;197(2):315–320. doi: 10.1042/bj1970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. R., Landolph J. R., Peterson H., Spears C. P., Heidelberger C. Oncogenic transformation and mutation of C3H/10T 1/2 clone 8 mouse embryo fibroblasts by alkylating agents. Cancer Res. 1981 Aug;41(8):3095–3099. [PubMed] [Google Scholar]
- Robins P., Cairns J. Quantitation of the adaptive response to alkylating agents. Nature. 1979 Jul 5;280(5717):74–76. doi: 10.1038/280074a0. [DOI] [PubMed] [Google Scholar]
- Rydberg B., Lindahl T. Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-L-methionine is a potentially mutagenic reaction. EMBO J. 1982;1(2):211–216. doi: 10.1002/j.1460-2075.1982.tb01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Samson L., Schwartz J. L. Evidence for an adaptive DNA repair pathway in CHO and human skin fibroblast cell lines. Nature. 1980 Oct 30;287(5785):861–863. doi: 10.1038/287861a0. [DOI] [PubMed] [Google Scholar]
- Schendel P. F., Robins P. E. Repair of O6-methylguanine in adapted Escherichia coli. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6017–6020. doi: 10.1073/pnas.75.12.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. N-nitroso alkylating agents: formation and persistence of alkyl derivatives in mammalian nucleic acids as contributing factors in carcinogenesis. J Natl Cancer Inst. 1979 Jun;62(6):1329–1339. [PubMed] [Google Scholar]
- Smith G. J., Kaufman D. G., Grisham J. W. Decreased excision of O6-methylguanine and N7-methylguanine during the S phase in 10T1/2 cells. Biochem Biophys Res Commun. 1980 Feb 12;92(3):787–794. doi: 10.1016/0006-291x(80)90772-x. [DOI] [PubMed] [Google Scholar]
- Swann P. F., Mace R. Changes in O6-methylguanine disappearance from rat liver DNA during chronic dimethylnitrosamine administration. A possible similarity between the system removing O6-methylguanine from DNA in rat liver and in Escherichia coli adapted to N-methyl-N'-nitro-N-nitrosoguanidine. Chem Biol Interact. 1980 Aug;31(2):239–245. doi: 10.1016/0009-2797(80)90012-5. [DOI] [PubMed] [Google Scholar]
- Warren W., Crathorn A. R., Shooter K. V. The stability of methylated purines and of methylphosphotriesters in the DNA of V79 cells after treatment with N-methyl-N-nitrosourea. Biochim Biophys Acta. 1979 Jun 20;563(1):82–88. doi: 10.1016/0005-2787(79)90009-1. [DOI] [PubMed] [Google Scholar]



