Abstract
Heart failure with preserved ejection fraction (HFPEF) is increasing in prevalence with the aging of the population, and morbidity and mortality rates are comparable to that of heart failure with reduced ejection fraction (HFREF). The diagnosis can be difficult to make, especially in older adults, stemming from the presence of multiple comorbid illnesses with confounding symptoms. New diagnostic tools have resulted in guidelines proposed to define and diagnose HFPEF. Recent literature focusing on the pathophysiology underlying this disease suggests multiple mechanisms are involved in the generation of the phenotype, such as abnormal relaxation and ventricular-vascular coupling, chronotropic incompetence, volume overload, and redistribution and /or endothelial dysfunction. Currently, no clinically proven treatments are shown to decrease morbidity and mortality in this population; however, there may be a novel multidisciplinary and multistage treatment strategy that can be studied to address this complex disease which incorporates pharmacologic and non-pharmacologic therapeutics.
Keywords: Heart failure, Diastolic heart failure, Heart failure preserved ejection fraction, Heart failure normal ejection fraction, Heart failure diagnosis, Heart failure treatment
Introduction
Heart failure with preserved ejection fraction (HFPEF) has overtaken heart failure in the setting of reduced ejection fraction (HFREF; also known as systolic heart failure) as the most common form of heart failure and now comprises more than 50% of all patients with heart failure [1••, 2–4]. HFPEF is predominantly a disorder of older adults, with women more often afflicted than men, especially those with longstanding hypertension. Morbidity and mortality rates approach that of patients with reduced ejection fraction (EF) [1••]. As the population ages, HFPEF will continue to be a growing public health problem. Recognition of HFPEF can be confounded by multiple co-morbid conditions that can impair exercise capacity and mimic the signs and symptoms of heart failure, thereby limiting a clinician’s ability to make early diagnoses and initiate therapeutic interventions. Additionally, recent studies highlight the heterogeneity of the syndrome with regard to underlying pathophysiologic mechanisms. Completed clinical trials have not resulted in any evidence-based treatments available for improving survival. Given the disappointing results of these investigations, there has been renewed interest in developing interventions with novel study designs and treatment methodologies for this cohort of patients, such as targeting underlying co-morbidities and using multimodality treatment interventions as has been applied successfully in other geriatric syndromes [5]. Additionally, non-pharmacologic interventions such as diet and exercise have shown promise in early, small clinical investigations. Finally, methods to more rationally group patients in order to identify cohorts who could respond to targeted intervention are essential. This review highlights the tools currently available for diagnosing HFPEF, recent landmark studies comparing treatment regimens, and novel approaches that may enhance survival and quality of life.
Epidemiology
The growing prevalence and incidence of HFPEF is predominately due to the aging of the population because it is a disease seen predominantly in older adults. As treatment for hypertension and coronary artery disease have advanced and lifespan has increased, there are growing numbers of patients with this syndrome. For the past few decades, there have been several debates surrounding HFPEF, in part because very little was known about the prevalence and underlying mechanisms of this syndrome. Initially, even its recognition as a distinct entity was questioned [6]. As more data accrued, the high prevalence was appreciated and heart failure in the setting of normal EF (>55%) or preserved EF (40%–55%) is now accepted as a common disease by national geriatric and cardiovascular societies [7, 8].
It is now recognized that more than 50% of patients with heart failure have preserved EF based on prospective and population studies, which is approximately 3 million Americans [1••]. This disease is more common not only in the elderly (average age 73–79 years), but also in women (women constitute 61%–76% of affected patients and 8%–10% of women >80 years have HFPEF, whereas only 4%–6% of men in the same age cohort are affected) and in those with hypertension (Table 1) [9]. Heart failure is the most common cause for hospitalizations in Medicare recipients and is a major public health concern [10••]. Trends show that the hospitalization rate for HFPEF is rising at a rate faster than the rate for systolic heart failure, as the average prevalence of HFPEF hospitalizations increased from 38% to 54% in a 15-year time span without concomitant significant increase in hospitalizations for HFREF [4]. There is a 50% chance of re-hospitalization for heart failure within 6 months in patients with HFPEF, which has become a major focus of quality care initiatives.
Table 1.
Prevalence of co-morbidities in heart failure with preserved ejection fraction
| Parametera | Controlled trials
|
Epidemiologic studies
|
Inpatient registries
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHARM-Preserved | SENIORS | PEP-CHF | I-PRESERVE | Average for all controlled trials | CHS | Framingham | Mayo | Average for all epidemiological studies | OPTIMIZE | ADHERE | Average for all inpatient registries | |
| Age, y | 67 | 76 | 76 | 72 | 72 | 75 | 80 | 74 | 76 | 75 | 74 | 75 |
| Hypertension | 64% | 62% | 79% | 89% | 74% | 59% | 59% | 63% | 60% | 76% | 77% | 77% |
| Diabetes mellitus | 28% | 26% | 21% | 28% | 26% | 27% | 22% | 33% | 27% | 38% | 45% | 42% |
| CAD | 44% | 68% | 27% | 25% | 41% | 58% | 37% | 53% | 49% | 38% | 50% | 44% |
| Atrial fibrillation | 29% | 36% | 20% | 29% | 29% | 15% | 29% | 41% | 28% | 33% | 21% | 27% |
| TIA/CVA | 28% | 0.1% | – | 10% | 13% | 5% | – | – | 5% | 15% | 17% | 16% |
| GFR, mL/min | 72 | 56 | 62 | 72 | 66 | 56 | 42 | 40 | 46 | 51 | 37 | 44 |
| Hemoglobin, g/dL | 13 | – | – | 14 | 14 | 14 | 12 | 12 | 13 | 12 | 12 | 12 |
| BMI, kg/m2 | 29 | – | 28 | 30 | 29 | 27 | 27 | 30 | 28 | – | 30 | 30 |
Average value from trials
BMI body mass index; CAD coronary artery disease; GFR glomerular filtration rate; TIA/CVA transient ischemic attack/cerebrovascular accident
Given the population affected, most subjects also have multiple co-morbid illnesses that are either causal or contribute to the observed phenotype, including hypertension, diabetes mellitus, coronary artery disease, chronic kidney disease, anemia, and obesity (Table 1). In population-based studies and registries, non-cardiac disorders are more common in HFPEF subjects than HFREF given their advanced age, including chronic pulmonary disease, anemia, liver and kidney disease, thyroid dysfunction, and cancer [9]. Such disorders impact on long-term outcomes and explain the observation that, although mortality rates in HFPEF and HFREF are similar, the mode of death is more often attributable to non-cardiovascular causes in HFPEF subjects [11]. In clinical trials, the prevalence of co-morbid conditions in the subjects with HFPEF is significantly lower than what is observed in the general population based on cohort studies and national and international registries (Table 1) [10••].
Pathophysiology
The cumulative changes in cardiovascular structure and function with normal human aging predispose to the development of HFPEF [12–14]. Foremost among these changes are alterations in central conduit artery stiffness with resulting increases in left ventricular hypertrophy, shifts toward a reliance on atrial filling of the left ventricle, and progressive enlargement of the left atrium. These age-related changes are superimposed on the development of several important changes in the pulmonary, renal, endocrine, and autonomic nervous system [15–17] that result in alterations in salt and water handling and increased susceptibility to acute pulmonary edema because of altered pulmonary capacitance and change in the distribution of blood volume [15].
A substantial amount of research over the past few decades has revealed that HFPEF is heterogeneous in regard to underlying pathophysiologic mechanisms with both cardiac and non-cardiac mechanisms. Among the cardiovascular processes are those that contribute to diastolic dysfunction, including left ventricular hypertrophy, concentric remodeling, improper calcium handling, and abnormal relaxation. Underlying causes of these “diastolic mechanisms” have included ischemia [18], alteration in cardiomyocyte myocardial stiffness [19–21] including intrinsic cardiomyocyte stiffness [20, 22] related to abnormal calcium homeostasis [23], the cytoskeleton (eg, micro-tubules and intermediate filaments [24, 25] or titin [26, 27]) as well as abnormalities in the extracellular matrix related to collagen and elastin [28–31].
More recently, additional mechanisms have been discovered that are unrelated to diastolic function, including chronic volume overload, veno-constriction with resultant volume redistribution, abnormal ventricular-vascular coupling, and chronotropic incompetence/autonomic dysfunction. These mechanisms can lead to impaired heart rate response to stress, pulmonary arterial hypertension, and endothelial dysfunction that have been identified as causal or contributing factors to the development of HFPEF [32••]. With the diversity of underlying mechanisms, several concepts derived from geriatric medicine are emerging that could advance our understanding of this condition. First, there is a recognition that multiple physiologic domains of cardiovascular function are abnormal in afflicted subjects, resulting in a depressed reserve capacity that contributes in an integrated fashion to produce the observed phenotype. Second is the realization that differing aspects of this syndrome (eg, exercise limitations, pulmonary edema, and labile blood pressure) likely each have distinct physiologic causes, further contributing to the complexity [33, 34]. As a result of the heterogeneous nature of the pathophysiologic processes and co-morbid illnesses in this population, there is a wide range of clinical outcomes. Accordingly, appreciation of the global nature of HFPEF will ideally better inform optimal design for future diagnostic and therapeutic strategies.
Lack of a Standard Definition of HFPEF
The controversy surrounding HFPEF has, in part, been attributable to varying definitions that have been employed to define the presence of the syndrome. After the initial description [35], the debate in the early 1980s and 1990s centered on whether or not the clinical syndrome even existed and had a cardiac basis. After multiple studies delineated that the syndrome indeed was common and increasing in prevalence [7, 36, 37], there was a focus on the pathophysiology. Seminal work showed that many of the cardiac pathophysiologic characteristics of heart failure in the setting of a reduced EF, including altered left ventricular structure and function, reduced exercise capacity, neurohormonal activation, and lower quality of life, were present in patients afflicted with HFPEF, shifting the focus to methods to identify subjects with the syndrome in order to facilitate treatment [38].
Although there has been much progress regarding the definition for this syndrome, there remain no clear consensus on its definition. Vasan and Levy [39, 40] developed criteria to distinguish definite and probable HFPEF, which relied on the presence of a clinical syndrome compatible with heart failure, a normal EF (within 72 h of presentation), and evidence of diastolic dysfunction by either catheterization or, more commonly, echocardiography. Subsequently, data on patients suggested that the timing of the echocardiogram was not essential in defining a normal EF because very few patients who initially presented with a normal EF and heart failure either in the emergency room [41] or in the community [42] subsequently had an abnormal EF. Additionally, measuring objective evidence of diastolic dysfunction was not routine in most echocardiography laboratories and, when assessed, was often difficult to interpret due to the load lability of many of these parameters. The near universal presence of Doppler abnormalities of diastolic function [43] and the lack of specificity of these measures for shifts in the EDPVR [44], suggested that the presence of Doppler echocardiography evidence of diastolic dysfunction was not required to make the diagnosis. Although cardiac catheterization remained the gold standard for identifying abnormalities associated with this condition, the invasive nature of the procedure made it unfeasible to obtain in every patient suspected of having HFPEF, despite recent data suggesting it can significantly enhance identification, especially with exercise [45].
Subsequently, reports began to emerge about the non-specific nature of the diagnosis of HFPEF, highlighting several issues related to the criteria used to establish the diagnosis [44, 46–49]. First, many older adults have concomitant co-morbid conditions that could mimic the symptoms of heart failure, such as dyspnea, fatigue, and leg swelling, and reduce the specificity of the diagnosis when made only on clinical grounds. Second, physical examination findings are often difficult to assess and lack specificity. For example, jugular venous distension is often difficult to measure in this population who often are overweight or obese, and other physical examination findings may be absent in well-compensated or well-treated patients. In addition, edema in isolation is not specific for the syndrome of heart failure. Finally, the criteria to define a “normal” or “preserved” EF have been variably employed. Accordingly, with these uncertainties, calls for standardized criteria to establish the diagnosis increased.
The European Society of Cardiology published formal criteria to establish the diagnosis of HFPEF. It focuses initially on the identification of the clinical syndrome based on symptoms of heart failure, such as dyspnea, leg swelling, paroxysmal nocturnal dyspnea, and orthopnea. Subsequently, to further identify patients with HFPEF requires not only an EF >50%, but objective evidence of abnormal left ventricular filling or relaxation, or diastolic distensibility or stiffness, that can be supported by cardiac catheterization, echocardiographic mitral Doppler flow profiles, tissue Doppler measures, ventricular hypertrophy, elevated natriuretic peptides, or concomitant atrial fibrillation. In cases where the diagnosis may be ambiguous, response to heart failure treatment may be used to support the diagnosis of heart failure. Although these criteria have increased the specificity with which the diagnosis of HFPEF is made, these criteria have been criticized. For example, very little data exist on the negative and positive predictive value of brain natriuretic peptide (BNP), and in fact some patients who are well compensated may not have elevation of BNP despite having obvious symptoms of heart failure. In addition, in the Mayo Clinic and Framing-ham Heart Study, it was noted that BNP levels vary with age and sex and therefore normal age-based and sex-based reference values must be obtained before BNP levels can be interpreted correctly [50, 51]. Accordingly, most clinicians currently employ a combination of the previously promulgated approaches in defining patients with HFPEF, including a clinical syndrome compatible with heart failure, a normal or preserved EF, and structural evidence of cardiovascular abnormalities including left ventricular hypertrophy and an increased left atrial size [52].
In many patients with HFPEF, symptoms of heart failure may develop only with exertion with no evidence of the phenotype at rest. In such cases, invasive cardiac catheterization or exercise hemodynamic evaluation can reveal the abnormal elevated filling pressures that are characteristic of this clinical syndrome [45, 53]. Although cardiac catheterization may reveal normal filling pressures at rest, it should be appreciated that there is dissociation between pulmonary venous pressure and left ventricular end-diastolic pressure that is more marked with greater prolongations in tau and at higher heart rates [54]. Accordingly, normal resting left ventricular end-diastolic pressures do not exclude the presence of HFPEF. Supine exercise at low workload may be required to reveal the significant increases in pulmonary venous pressures leading to manifestation of dyspnea that are diagnostic of HFPEF. Invasive measurements are currently reserved for ambiguous cases and not utilized routinely to identify patients with HFPEF.
Treatment
Treatment options for HFPEF remain mainly empiric. Lifestyle modifications including diet and exercise remain a mainstay of therapeutic recommendations. Recent studies suggest a strong role for these interventions in subjects with HFPEF [55]. Because intermittent episodes of decompensation have been linked to volume overload and because sodium is a major driver of volume expansion, low-salt diets have been strongly endorsed [1••, 56]. Despite these recommendations, HFPEF subjects were significantly less likely than systolic heart failure patients to receive discharge recommendations for weight monitoring (33% vs 43%) and sodium-restricted diet (42% vs 53%). However, HFPEF subjects who received a documented sodium-restricted diet recommendation had decreased odds of 30-day combined death and readmission (odds ratio 0.43; 95% CI, 0.24–0.79; P=0.007) in a recently conducted cohort study [57••]. Accordingly, further research revisiting the biological, clinical, and epidemiologic consequences of dietary sodium in HFPEF appear warranted.
A randomized trial of older patients with HFPEF has shown that exercise training is beneficial [58]. Subjects (n= 53) who were 60 to 82 years of age with isolated HFPEF (eg, no significant coronary artery, valvular, or pulmonary disease) were randomized to receive directly observed exercise 3 days per week for 16 weeks or standard care. The primary outcome of peak exercise oxygen uptake increased significantly in the exercise group compared to the attention-controlled group (who received only phone calls 3 times a week from investigators), as did secondary outcomes such as peak power output, exercise time, 6-minute walk distance, ventilator anaerobic threshold, and physical quality of life. Accordingly, the application of cardiac rehabilitation programs remains potentially one of the most under-utilized therapies for this population.
Several seminal randomized clinical trials using pharmacologic therapy have revealed inconclusive evidence for the benefit of agents classically used to treat HFREF in patients with HFPEF (Table 2). Digoxin is one of the oldest medications used for the treatment of heart failure. An ancillary study within the larger Digitalis Investigation Group (DIG) trial compared composite rates of death or hospitalizations for heart failure in those subjects with preserved EF (EF>45%) taking a median dose of digoxin of 0.25 mg (n=492) with those assigned to placebo (n= 496) [59]. There was no significant change in the primary outcome (risk ratio was 0.82; 95% CI, 0.63–1.07)). Angiotensin receptor blockers (ARBs) were studied in the Candesartan in Heart Failure-Assessment of Reduction of Mortality and Morbidity (CHARM) Preserved study, where 3,023 patients were randomized to receive candesartan to target dose of 32 mg daily or placebo [60]. There were modest decreases in hospitalizations for heart failure in those receiving candesartan compared to placebo, but no difference in the composite primary outcome of cardiovascular death or heart failure hospitalization in the candesartan group compared to placebo. The effect of beta-blockers was studied in the elderly population with heart failure in the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure (SENIORS) trial [61]. The primary outcome was composite of all-cause mortality or cardiovascular hospitalizations. A subgroup analysis of this trial with subjects with EF >40% revealed a hazard ratio of 0.83 (95% CI, 0.62–1.11; P=0.203), indicating that there was no benefit of nebivolol in preserved EF or the elderly. In the randomized controlled trial Perindopril for Elderly People With Chronic Heart Failure (PEP-CHF), which enrolled 850 subjects, perindopril, an angiotensin-converting enzyme inhibitor (ACEI), was administered to elderly patients (age >70 years) with LVEF >40%, left atrial enlargement, and Doppler evidence of impaired LV filling [62]. In this trial, perindopril did not have overall benefit in the primary endpoint of composite all-cause mortality or hospitalizations due to heart failure. Similarly, the Irbesartan in Heart Failure with Preserved Systolic Function (I-PRESERVE) trial enrolled 4,128 subjects older than 60 years who were randomized to receive 300 mg of irbesartan or placebo in order to determine the effect on primary outcome of the composite of all-cause mortality or hospitalizations due to cardiovascular causes [63]. The hazard ratio for the primary outcome comparing both groups was 0.95 (95% CI, 0.86–1.05; P=0.35), indicating there was no significant benefit of irbesartan compared to placebo. The only current standard therapy for systolic heart failure that has not been formally evaluated in the HFPEF population is aldosterone antagonists, which are currently the focus of an ongoing NIH-funded trial called Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT).
Table 2.
Clinical trials for treatment in subjects with heart failure with preserved ejection fraction
| DIG | CHARM-Preserved | SENIORS | PEP-CHF | I-PRESERVE | |
|---|---|---|---|---|---|
| Subjects, n | 988 | 3023 | 2128 (all groups) | 850 | 4128 |
| Average age, y | 63 (all groups) | 67 | 76 (all groups) | 75 | 72 |
| Inclusion criteria | No age cutoff LVEF <45% in main study, LVEF >45% in ancillary study CHF diagnosis |
Age >18 y LVEF >40% Cardiac hospitalization at any time in past NYHA class II–IV |
Age ≥70 LVEF <35% or CHF symptoms Cardiac hospitalization within past 12 mo |
Age ≥70 LVEF ≥40% Cardiac hospitalization within last 6 months Heart failure symptoms LA enlargement Impaired LV filling |
Age ≥60 y LVEF ≥45% Cardiac hospitalization within [past 6 mo NYHA class II–IV |
| Primary outcome | Composite of deaths or heart failure hospitalizations | Composite of cardiovascular death or heart failure hospitalization | Composite of all-cause mortality or cardiovascular hospitalizations | Composite of all-cause mortality or heart failure hospitalizations | Composite of all-cause mortality or cardiovascular hospitalization |
| Results (HR or P value) | For EF >45%: HR 0.82 (95% CI, 0.63–1.07) | HR 0.89 (95% CI 0.77–1.03; P=0.118) | For EF >40%: HR 0.83 (95% CI, 0.62–1.11), P=0.203 | HR 0.92 (95% CI, 0.70–1.21; P=0.545 | HR 0.95 (95% CI, 0.86–1.05; p=0.35) |
CHF congestive heart failure; LA left atrium; LV left ventricle; LVEF left ventricular ejection fraction.; NYHA New York Heart Association
Future Considerations
The failure of various treatment modalities in randomized clinical trials for HFPEF subjects have resulted in calls for a different approach to addressing this complex clinical syndrome. Among the many issues being debated is the question of the lower limit of EF used as the basis for including patients in this group; indeed, definitions have varied considerably among clinical studies (see Table 2). Most single center studies define HFNEF when EF is >50% or 55%, however, several completed and ongoing clinical trials have used a lower limit of 45%, 40%, or even 35%. The use of broad and differing EF criteria to define this syndrome have the potential to confound results by including patients with different pathologies and, at the very least, make it difficult to compare results from different studies. This could also confound the translation of results obtained from different investigations to clinical practice.
Among the population of patients with heart failure, a significant percentage has an EF that is not normal, but rather is mildly reduced (eg, 40%–55%). An analysis of a large registry of hospitalized patients with heart failure documented significant differences in demographic and clinical characteristics of those with mildly reduced EF (40%–55%) compared to moderately to severely reduced EF (<40%) [64]. Additionally, using non-invasive pressure-volume analysis, distinct physiologic differences between those with a normal (>55%) and a mildly reduced (40%–55%) EF were found [65]. Subjects with mildly depressed ejection fraction had eccentric left ventricular hypertrophy with remodeling such that there was a rightward shift of the end diastolic pressure-volume relation with concomitant decreased contractility. Such changes were similar to what was seen in subjects with HFREF. Accordingly, subgroup analysis of completed clinical trials in subjects with HFPEF stratified by the presence of a normal (eg, >55%) and a preserved (40%–55%) EF could yield important insights into the efficacy of certain therapies in these two distinct cohorts.
The lack of progress in improving heart failure outcomes has recently been linked to an age bias in heart failure research [10••]. Despite the growing epidemic, there is only one large ongoing NIH-funded clinical trial for HFPEF compared with multiple trials for HFREF, and the average age of patients recruited to these trials is 10 to 15 years younger than the average patient with systolic heart failure. Although calls for proactive measures to enhance recruitment of older adults in clinical trials have included requiring investigators to include older adults based on pre-specified criteria (similar to what NIH requires for inclusion of ethnic minorities), including subjects with complex clinical phenotypes and developing protocols that address the needs of older adults (eg, performing examinations outside traditional settings in order to address mobility issues), one major issue has been left unaddressed, which is the need to embrace different trial designs to deal with the complex phenotype and multiple pathophysiologic mechanisms that constitute HFPEF. Randomized clinical trials for heart failure typically evaluate the efficacy of a single intervention compared with placebo or usual care on endpoints that focus on mortality, and in particular cardiovascular mortality, and heart failure hospitalizations. As illustrated in this article, treatments that have been shown to be beneficial in patients with HFREF do not appear to have mortality or morbidity benefit in HFPEF. However, mortality remains high in HFPEF based on community studies, and the discrepancy in treatment outcomes may be because the underlying causes in approximately 50% of patients with HFPEF are non-cardiac [11, 66]. An epidemiologic study in Minnesota revealed that 49% of deaths in subjects with EF >50% with a median age of 76 years were from pulmonary disease, malignancy, central nervous system disease, gastrointestinal or genito-urinary disease, diabetes, or other endocrine diseases. To more effectively manage patients and design clinical trials, these co-morbid illnesses must be embraced in the actual design of trials rather than excluding such patients. Such designs will require cardiovascular experts to collaborate with colleagues across multiple disciplines in order to evaluate potentially effective treatments. Similar to the approaches that geriatrics has employed for managing syndromes such as falls, delirium, and incontinence, a multimodal approach will likely be required to delineate management strategies in HFPEF that have meaningful impacts on outcomes. Additionally, alternative outcome measures are needed that focus on function, quality of life, and cost effectiveness.
Knowing that patients with HFPEF have a multitude of underlying co-morbid illnesses and causes of death are often related to these chronic illnesses, it may be prudent to establish alternative treatment strategies that target these comorbidities [66]. Anemia, chronic renal disease, chronic pain secondary to arthritis, and diabetes are very common, and if they are well-controlled may prolong life and improve quality of life. Large studies such as the Ancillary DIG Study as well as community-based multicenter studies revealed that most hospitalizations in HFPEF patients are due to non-heart failure causes. Accordingly, better managing these chronic conditions holds promise for reducing the rate of re-hospitalization. Additionally, treatment that is organized in a staged manner using a multidisciplinary approach instead of a dichotomous approach may provide opportunities for delineating which combination of intervention is most successful for an individual patient (Fig. 1). Treatments in such trials can be offered after assessments suggest a threshold has been reached in which treatment would be expected to be effectual (eg, a hemoglobin level at which treatment of anemia would be appropriate or an evaluation using polysomnography suggests a role for intervention). Response to this multimodal treatment plan can be assessed at each stage in order to evaluate the efficacy of the components of the approach. Such a multidisciplinary approach to treatment could include targets such as non-cardiovascular co-morbidities (e.g. sleep-disordered breathing, depression, anemia, low physical activity resulting in sarcopenia, among others). Shifting the focus from the heart to the periphery may delineate important targets for treatment because many patients with HFPEF have impaired endothelial and vascular function as well as co-morbid conditions that, by through loading conditions, have dramatic effects on cardiovascular structure and function [12]. Finally, focusing on dietary and lifestyle interventions as key components in the prevention and treatment of heart failure would be essential. A low-sodium diet such as the Dietary Approaches to Stop Hypertension (DASH) diet has been shown in a large study to significantly decrease the incidence of heart failure [67]. Women in the Swedish Mammography Cohort who were compliant with the DASH diet after 7 years of follow-up had a 37% less chance of developing heart failure. The DASH diet is also beneficial in decreasing the incidence of many of the co-morbid illnesses seen in HFPEF, such as hypertension, hyperlipidemia, and stroke [55, 68]. Non-pharmacologic treatment options such as yoga and devices that mimic this approach [69] hold promise in the treatment of hypertension that often leads to HFPEF without the side effects and expense of medications.
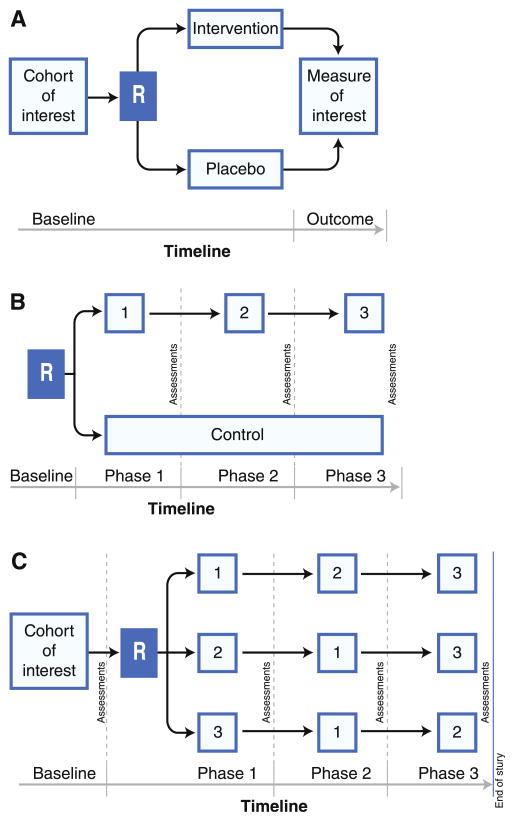
Fig. 1.
Alternative clinical trial designs for older adult subjects with heart failure with preserved ejection fraction (HFPEF). a Flow chart of traditional randomized placebo-controlled clinical trial (R). b Trial in which several interventions (numbered in the example as 1, 2, and 3) are instituted sequentially with assessment of outcome of interest in comparison to control. Interventions could include diet and lifestyle modifications (eg, salt restriction or cardiac rehabilitation), pharmacologic therapy (eg, treatment aimed at cardiovascular target or non-cardiac target, such as chronic pain) and/or treatment of extra-cardiac factors (eg, mood disorders, sleep-disordered breathing, diabetes). c Trial design in which cohort of interest is randomized to various interventions (indicated by 1, 2 and 3) and the order of interventions is varied in order to evaluate the efficacy of multiple interventions, which are likely to be required in complex clinical conditions such as heart failure with a preserved ejection fraction and to determine if the order of treatment intervention has an effect on outcomes
Finally, optimization of outpatient care is integral to successful treatment in patients with HFPEF as it is with other chronic illnesses in the geriatric population. It is important that patients or their caregivers are provided with the appropriate training in skills required for chronic disease management. These skills include crafting accurate medication lists, assessing volume status with either daily weights or other devices, and interpreting food labels to ensure adherence to low-sodium diets, all of which are essential for the ongoing management of HFPEF. However, programs to formally assess a patient’s ability to comply and, if unable, a caregiver’s ability to provide needed support are not part of current clinical practice. Given the fact that a large number of hospital admissions are attributable to medication errors, with up to 14.1% of hospital discharges noted to have post-discharge medication discrepancies and 14% of those patients requiring re-hospitalization within 30 days of discharge [70], programs to ensure adequate adherence are likely to have a huge impact on relevant outcomes. Early post-discharge follow-up, ideally by a team that specializes in heart failure and the needs of older adults, has also been problematic and contributes to poor outcomes in older adults with HFPEF [71••]. Outcomes in patients with HFPEF may be dramatically improved not by novel therapies, but by developing systems of care that ensure basic needs of patients are provided, including education about their illness, medication regimen, earlier detection and intervention of heart failure symptoms, and timely and consistent outpatient follow-up, even at home if necessary.
Conclusions
HFPEF is a common syndrome, and given the epidemiology this disease will continue to increase in prevalence. Although initially there was hesitance in acknowledging the disease entity, it is now recognized that about half of all patients with heart failure have HFPEF, and the syndrome is associated with a morbidity and mortality rate that matches that of HFREF. Guidelines have been established to assist clinicians and researchers in better defining and diagnosing HFPEF. There are currently few known effective treatments available for this multifactorial and complex disorder. Novel approaches to understanding the underlying mechanisms for this disease as well as alternative treatment strategies such as treating the co-morbid illness with a multidisciplinary approach may be the key to attaining successful treatment outcomes in this complex disease.
Acknowledgments
Dr. Maurer is supported by grants from the NIH/ NIA K24AG036778-01A1 and R01AG027518.
Footnotes
Disclosure No conflicts of interest relevant to this article were reported.
Contributor Information
Taslima Bhuiyan, Division of Cardiology, Columbia University Medical Center, New York, NY, USA.
Mathew S. Maurer, Email: msm10@columbia.edu, Division of Cardiology, Columbia University Medical Center, New York, NY, USA. Clinical Cardiovascular Research Laboratory for the Elderly, Allen Hospital of New York Presbyterian Hospital, 5141 Broadway, 3 Field West, Room 037, New York, NY 10034, USA
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1••.Lindenfeld J, Albert NM, Boehmer JPHFSA, et al. Comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. This is a comprehensive document that discusses the epidemiology, pathophysiology, and diagnosis of heart failure in general, including a section on those with preserved EF. It also sets forth guidelines for the management of this complex disease by incorporating the most recent literature and evidence. [DOI] [PubMed] [Google Scholar]
- 2.Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–9. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 3.Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–7. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–7. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 6.Redfield MM, Kitzman DW. Heart failure: a rose by any other name? Congest Heart Fail. 2006;12:166–8. doi: 10.1111/j.1527-5299.2006.05520.x. [DOI] [PubMed] [Google Scholar]
- 7.Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol. 1995;26:1565–74. doi: 10.1016/0735-1097(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 8.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–32. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Kitzman DW, Rich MW. Age disparities in heart failure research. JAMA. 2010;304:1950–1. doi: 10.1001/jama.2010.1592. This commentary highlights the disparities in patient population selection in major clinical trials compared to population characteristics observed in registries who have heart failure with preserved EF. These differences may explain the negative clinical trial results in HFPEF and the poor outcomes observed in this group despite significant advancement of care in systolic heart failure. The authors call for strict criteria to include older subjects and subjects with multiple co-morbidities in large clinical trials because that is a more realistic study population and results are generalizable. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–7. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 13.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 14.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–7. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR. Structural and physiological age-associated changes in aging lungs. Semin Respir Crit Care Med. 2010;31:521–7. doi: 10.1055/s-0030-1265893. [DOI] [PubMed] [Google Scholar]
- 16.Duarte D, Santos-Araujo C, Leite-Moreira AF. Hypertension and angiogenesis in the aging kidney: A review. Arch Gerontol Geriatr. 2010 doi: 10.1016/j.archger.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 17.Hotta H, Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr Gerontol Int. 2010;10 (Suppl 1):S127–36. doi: 10.1111/j.1447-0594.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 18.Vanhecke TE, Kim R, Raheem SZ, McCullough PA. Myocardial ischemia in patients with diastolic dysfunction and heart failure. Curr Cardiol Rep. 2010;12:216–22. doi: 10.1007/s11886-010-0101-1. [DOI] [PubMed] [Google Scholar]
- 19.van Heerebeek L, Borbely A, Niessen HW, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–73. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 20.Borbely A, van der Velden J, Papp Z, et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–81. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 21.Bronzwaer JG, Paulus WJ. Matrix, cytoskeleton, or myofilaments: which one to blame for diastolic left ventricular dysfunction? Prog Cardiovasc Dis. 2005;47:276–84. doi: 10.1016/j.pcad.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 22.van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 23.Lebeche D, Davidoff AJ, Hajjar RJ. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5:715–24. doi: 10.1038/ncpcardio1347. [DOI] [PubMed] [Google Scholar]
- 24.Zile MR, Richardson K, Cowles MK, et al. Constitutive properties of adult mammalian cardiac muscle cells. Circulation. 1998;98:567–79. doi: 10.1161/01.cir.98.6.567. [DOI] [PubMed] [Google Scholar]
- 25.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027–44. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeWinter MM, Granzier H. Cardiac titin: a multifunctional giant. Circulation. 2010;121:2137–45. doi: 10.1161/CIRCULATIONAHA.109.860171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borbely A, Falcao-Pires I, van Heerebeek L, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–6. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez A, Lopez B, Querejeta R, Zubillaga E, Echeverria T, Diez J. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension. 2010;55:1418–24. doi: 10.1161/HYPERTENSIONAHA.109.149112. [DOI] [PubMed] [Google Scholar]
- 29.Bradshaw AD, Baicu CF, Rentz TJ, et al. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation. 2009;119:269–80. doi: 10.1161/CIRCULATIONAHA.108.773424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borbely A, Papp Z, Edes I, Paulus WJ. Molecular determinants of heart failure with normal left ventricular ejection fraction. Pharmacol Rep. 2009;61:139–45. doi: 10.1016/s1734-1140(09)70016-7. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 32••.Bench T, Burkhoff D, O’Connell JB, et al. Heart failure with normal ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6:57–64. doi: 10.1007/s11897-009-0010-z. This review article discusses the major mechanisms that play a role in heart failure with preserved EF. It discusses mechanisms that are unrelated to diastolic function, such as aging, presence o f co-morbidities, volume redistribution, and central and peripheral vascular disconnect, among others. [DOI] [PubMed] [Google Scholar]
- 33.Boyle A, Maurer MS, Sobotka PA. Myocellular and interstitial edema and circulating volume expansion as a cause of morbidity and mortality in heart failure. J Card Fail. 2007;13:133–6. doi: 10.1016/j.cardfail.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Maurer MS, King DL, El-Khoury Rumbarger L, Packer M, Burkhoff D. Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail. 2005;11:177–87. doi: 10.1016/j.cardfail.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Luchi RJ, Snow E, Luchi JM, Nelson CL, Pircher FJ. Left ventricular function in hospitalized geriatric patients. J Am Geriatr Soc. 1982;30:700–5. doi: 10.1111/j.1532-5415.1982.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 36.Gardener J. Partial hospitalisation gives patients with mental health problems. Nurs Times. 1998;94:47. [PubMed] [Google Scholar]
- 37.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–9. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 38.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 39.Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation. 2000;101:2118–21. doi: 10.1161/01.cir.101.17.2118. [DOI] [PubMed] [Google Scholar]
- 40.Vasan RS, Levy D, Larson MG, Benjamin EJ. Interpretation of echocardiographic measurements: a call for standardization. Am Heart J. 2000;139:412–22. doi: 10.1016/s0002-8703(00)90084-x. [DOI] [PubMed] [Google Scholar]
- 41.Gandhi SK, Powers JC, Nomeir AM, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17–22. doi: 10.1056/NEJM200101043440103. [DOI] [PubMed] [Google Scholar]
- 42.Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–15. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 43.Zile MR, Gaasch WH, Carroll JD, et al. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation. 2001;104:779–82. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 44.Maurer MS, Spevack D, Burkhoff D, Kronzon I. Diastolic dysfunction: can it be diagnosed by Doppler echocardiography? J Am Coll Cardiol. 2004;44:1543–9. doi: 10.1016/j.jacc.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 45.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–95. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanderson JE. Heart failure with a normal ejection fraction. Heart. 2007;93:155–8. doi: 10.1136/hrt.2005.074187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrie MC, Hogg K, Caruana L, McMurray JJ. Poor concordance of commonly used echocardiographic measures of left ventricular diastolic function in patients with suspected heart failure but preserved systolic function: is there a reliable echocardiographic measure of diastolic dysfunction? Heart. 2004;90:511–7. doi: 10.1136/hrt.2003.011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmieri V, Innocenti F, Pini R, Celentano A. Reproducibility of Doppler echocardiographic assessment of left ventricular diastolic function in multicenter setting. J Am Soc Echocardiogr. 2005;18:99–106. doi: 10.1016/j.echo.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Kindermann M, Reil JC, Pieske B, van Veldhuisen DJ, Bohm M. Heart failure with normal left ventricular ejection fraction: what is the evidence? Trends Cardiovasc Med. 2008;18:280–92. doi: 10.1016/j.tcm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Miller VM, Redfield MM, McConnell JP. Use of BNP and CRP as biomarkers in assessing cardiovascular disease: diagnosis versus risk. Curr Vasc Pharmacol. 2007;5:15–25. doi: 10.2174/157016107779317251. [DOI] [PubMed] [Google Scholar]
- 51.Romano S, di Mauro M, Fratini S, et al. Serial BNP assay in monitoring exercise tolerance in patients with diastolic dysfunction. Int J Cardiol. 2011;147:312–3. doi: 10.1016/j.ijcard.2010.12.064. [DOI] [PubMed] [Google Scholar]
- 52.Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 53.Borlaug BA, Kass DA. Invasive hemodynamic assessment in heart failure. Heart Fail Clin. 2009;5:217–28. doi: 10.1016/j.hfc.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hay I, Rich J, Ferber P, Burkhoff D, Maurer MS. Role of impaired myocardial relaxation in the production of elevated left ventricular filling pressure. Am J Physiol Heart Circ Physiol. 2005;288:H1203–8. doi: 10.1152/ajpheart.00681.2004. [DOI] [PubMed] [Google Scholar]
- 55.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–9. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dickstein K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: application of natriuretic peptides. Reply Eur Heart J. 2008 doi: 10.1093/eurheartj/ehn560. [DOI] [PubMed] [Google Scholar]
- 57••.Hummel SL, DeFranco AC, Skorcz S, Montoye CK, Koelling TM. Am J Med. 2009;122:1029–36. doi: 10.1016/j.amjmed.2009.04.025. This study revealed that a low-salt diet in patients with HFPEF is shown to decrease the risk of death and readmission to the hospital; however only a small percentage of patients with HFPEF are being counseled on a low-salt diet at time of discharge compared to patients with systolic heart failure. The authors concluded that if clinicians educate patients with HFPEF about the importance of a low-salt diet, there may be fewer deaths and readmissions to the hospital in this group of patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–67. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 60.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 61.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 62.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–45. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 63.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 64.Sweitzer NK, Lopatin M, Yancy CW, Mills RM, Stevenson LW. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol. 2008;101:1151–6. doi: 10.1016/j.amjcard.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He KL, Burkhoff D, Leng WX, et al. Comparison of ventricular structure and function in Chinese patients with heart failure and ejection fractions >55% versus 40% to 55% versus <40% Am J Cardiol. 2009;103:845–51. doi: 10.1016/j.amjcard.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA. 2008;300:431–3. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 67.Levitan EB, Wolk A, Mittleman MA. Consistency with the DASH diet and incidence of heart failure. Arch Intern Med. 2009;169:851–7. doi: 10.1001/archinternmed.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–20. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 69.Meles E, Giannattasio C, Failla M, Gentile G, Capra A, Mancia G. Nonpharmacologic treatment of hypertension by respiratory exercise in the home setting. Am J Hypertens. 2004;17:370–4. doi: 10.1016/j.amjhyper.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165:1842–7. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- 71••.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–22. doi: 10.1001/jama.2010.533. This study highlights one of the most important reasons for readmission in patients with HFPEF, which is poor follow-up care. In this study, the authors demonstrate that less than half of patients admitted with heart failure are seen within 7 days post-discharge, and less than 5% are seen by the same cardiologist in the hospital and within 7 days post-discharge in the office. [DOI] [PubMed] [Google Scholar]