Abstract
Background
Local anesthetics (LAs) are typically used for regional anesthesia but can be given systemically to mitigate postoperative pain, supplement general anesthesia or prevent cardiac arrhythmias. However, systemic application or inadvertent intravenous injection can be associated with substantial toxicity, including seizure induction. The molecular basis for this toxic action remains unclear.
Methods
We characterized effects of different LAs on homomeric and heteromeric K+ channels containing TASK-1 (K2P3.1, KCNK3) and TASK-3 (K2P9.1, KCNK9) subunits in a mammalian expression system. In addition, we used TASK-1/TASK-3 knockout mice to test the possibility that TASK channels contribute to LA-evoked seizures.
Results
LAs inhibited homomeric and heteromeric TASK channels in a range relevant for seizure induction; channels containing TASK-1 subunits were most sensitive and IC50 values indicated a rank order potency of bupivacaine > ropivacaine ⟫ lidocaine. LAs induced tonic-clonic seizures in mice with the same rank order potency, but higher LA doses were required to evoke seizures in TASK knockout mice. For bupivacaine, which produced the longest seizure times, seizure duration was significantly shorter in TASK knockout mice; bupivacaine-induced seizures were associated with an increase in electroencephalogram power at frequencies <5 Hz in both wild type and TASK knockout mice.
Conclusions
These data suggest that increased neuronal excitability associated with TASK channel inhibition by LAs contributes to seizure induction. Since all LAs were capable of evoking seizures in TASK channel deleted mice, albeit at higher doses, the results imply that other molecular targets must also be involved in this toxic action.
INTRODUCTION
Local anesthetics (LAs) can be used to complement surgical anesthesia and for acute or chronic pain management.1,2 Although typically employed for regional anesthesia, it is also well known that LAs have beneficial effects when provided systemically in low and moderate doses. For example, perioperative intravenous lidocaine can decrease postoperative and neuropathic pain,3,4 lower postoperative morphine consumption,3-5 reduce requirements for general anesthetics6-9 and counter their arrhythmogenic actions.10,11 Although modern LAs are generally safe, risks persist and toxic reactions remain a problem with accidental intravascular injection, inadvertent intrathecal injection, or administration of an excessive systemic dose of these drugs.12 An early expression of LA toxicity is seizure activity due to central nervous system (CNS) excitation;13 subsequent manifestations are associated with CNS or cardiovascular depression.13
The potency for toxic reactions is different with different LAs.14 For example, lidocaine has been in clinical use for close to 60 years and remains one of the safest LA agents ever manufactured.15 Bupivacaine, on the other hand, is a very long acting LA with a greater potential for toxic reactions;15,16 ropivacaine shares many characteristics with bupivacaine but is less toxic.15,16 It is clear that the major mechanism accounting for LA-induced regional anesthesia involves inhibition of voltage-gated sodium (NaV) channels,17 but it is not certain that inhibition of NaV channels can account for systemic toxic effects of the drugs, including the initial CNS excitation and pro-convulsive actions.18
TASK channels (TASK-1, K2P3.1; TASK-3, K2P9.1) are members of the K2P family of potassium channels that are prominently and differentially expressed throughout the brain.19-21 TASK channels generate neuronal pH-sensitive, background (or ‘leak’) K+ currents22-24 and, of relevance here, they are inhibited by LAs18,25 at toxic systemic concentrations (~10-100 μM).18,26 Inhibition of TASK channels causes membrane depolarization and increased neuronal excitability,23-24 which could contribute to central LA toxicity. To this point, however, behavioral consequences associated with inhibition of TASK channels by LAs have not been examined.18,25
In this study, we explored the hypothesis that neuronal excitation due to TASK channel inhibition contributes to pro-convulsive actions of LAs.18 We show that cloned TASK channels are inhibited by LAs with a potency profile consistent with their known toxic actions (i.e., bupivacaine > ropivacaine >> lidocaine). Importantly, we find that knockout mice with global deletion of TASK channels are less susceptible to LA-induced seizures. These data suggest that TASK channels are an important molecular target for central toxic effects of LAs.
MATERIALS AND METHODS
Animals
Adult TASK-1−/−:TASK-3−/− double knockout and wild-type C57BL/6 mice (2-3 months) used in this study were age, sex and weight-matched. The double knockout mice (hereafter called TASK−/−) were generated, validated and moved onto a C57BL/6 background, as described previously.27-29 All procedures involving animals were approved by the University of Virginia Animal Care and Use Committee.
Cell culture and transfections
Human embryonic kidney (HEK) 293 cells stably expressing the thyrotropin-releasing hormone receptor (E2 cells) were maintained in DMEM/F-12 containing 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) and supplemented with G418 (400 μg/ml; Invitrogen Inc., Carlsbad, CA). Cells were transfected with TASK channel constructs using LipofectAMINE 2000 (Invitrogen); constructs were co-transfected (TASK-1) or tagged with enhanced green fluorescent protein (TASK-3 and TASK-1/TASK-3). Cells were plated onto poly-L-lysine (100 μg/ml)-coated glass coverslips ~12-16 h after transfection and allowed to adhere for 30-40 min at 37°C before recording; they were visualized under infrared differential interference contrast and epifluorescent optics, and individual transfected cells with green fluorescence were selected for recording.
Electrophysiology
Whole-cell recordings were obtained at room temperature using 3-5 MΩ patch pipettes and an Axopatch 200B amplifier in a bath solution consisted of (in mM): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, with pH adjusted using NaOH or HCl. Different concentrations of bupivacaine (5, 25, 50, 100, and 200 μM; Sigma-Aldrich, St. Louis, MO), ropivacaine (5, 25, 50, 100, 200 and 400 μM; Naropin®; APP Pharmaceuticals, LLC, Schaumburg, IL), lidocaine (4, 20, 100, 200, 400 and 800 μM; Sigma-Aldrich), and picrotoxin (50, 100, 300 μM; Sigma-Aldrich) were added to the perfusate. Internal solution contained (in mM): 120 KCH3SO3, 4 NaCl, 1 MgCl2, 0.5 CaCl2, 10 HEPES, 10 EGTA, 3 MgATP, and 0.3 GTP-Tris, pH 7.2. Voltage commands were applied and currents recorded and analyzed with pClamp software (Molecular Devices, Sunnyvale CA). Cells were held at −60 mV and depolarizing ramps (0.2 V/s, from −130 to +20 mV) were applied at 5 s intervals. Slope conductance was determined by linear fits to currents from −130 to −60 mV. Concentration-response data for channel activity were fitted and analyzed statistically in Prism 5.0 using a Boltzmann equation of the form: Y=max/(1+exp((IC50-X)/slope)) with three free parameters (slope, IC50 and maximum) and a fixed origin.
Behavioral toxicity
As a measure of CNS toxicity, we quantified effects of increasing doses of intravenous bupivacaine (2.5, 3.75, 5, 6.25 and 7.5 mg/kg), ropivacaine (2.5, 3.75, 5, 6.25, 7.5, 8.75 and 10 mg/kg) and lidocaine (5, 10, 15 and 17.5 mg/kg) on seizure induction and duration in wild type (n=14 for bupivacaine and ropivacaine, and 15 for lidocaine) and TASK−/− mice (n=12 each). In addition, we determined effects of picrotoxin (0.5, 0.75, 1 & 2 mg/kg, iv), a γ-aminobutyric acid (GABA) receptor antagonist, on seizure duration and incidence in C57BL/6 and TASK−/− mice. For each drug, the dosage was chosen based on preliminary data to span a range that encompassed doses that caused no observable seizure to those that consistently evoked seizures. Individual mice received increasing doses of a drug intravenously, with a single dose administered on any given day and at least a week interval between applications. If death ensued following high dose LA administration, data from those animals was not available for the lethal dose and any higher doses. In some cases, mice received only a single dose of the LA; data obtained with single and multiple administration protocols were not different. The incidence and duration of clonic/tonic convulsions were recorded. Convulsive signs were characterized by whole-body jumps or bursts of running motions (clonic seizure) and rigidity with forelimbs and hindlimbs extended caudally (tonic seizure).30 A series of quantal dose-response curves were fitted and analyzed statistically in Prism 5.0 using a logistic equation of the form: Y= max/(1+10((logEC50-X)*slope)) with three free parameters (slope, EC50 and maximum) and a fixed origin.
Electroencephalogram recordings
Bipolar insulated stainless steel electrodes were implanted over motor cortex under ketamine/xylazine anesthesia and secured to the skull with dental acrylic. After a 5- to 7-day recovery period, each mouse was administered a single intravenous dose of bupivacaine (3.75, 5 or 6.25 mg/kg). Electroencephalogram and video monitoring began 10 min prior to the LA administration and terminated when the electroencephalogram returned to normal baseline or showed irregular spikes without recurrence of seizures in a subsequent observation period of 15 min. After the experiments, animals were euthanized by deep anesthesia. Electrographically recorded seizures were obtained from three wild-type and TASK-deleted mice at each bupivacaine dose; seizure episodes of 15 s duration immediately following bupivacaine injection were subjected to power spectral analysis (Spike 2 software, v. 5.14; Cambridge Electronic Design, Cambridge, United Kingdom).
Statistical analysis
Results are presented as mean ± SEM. Data were analyzed statistically using χ2 analysis, Student’s t test or one-way and two-way repeated measures ANOVA, with Bonferroni’s post hoc test as appropriate. Differences were considered significant if P<0.05 in a two-tailed analysis.
RESULTS
Concentration-dependent inhibition of pH-sensitive TASK currents by local anesthetics
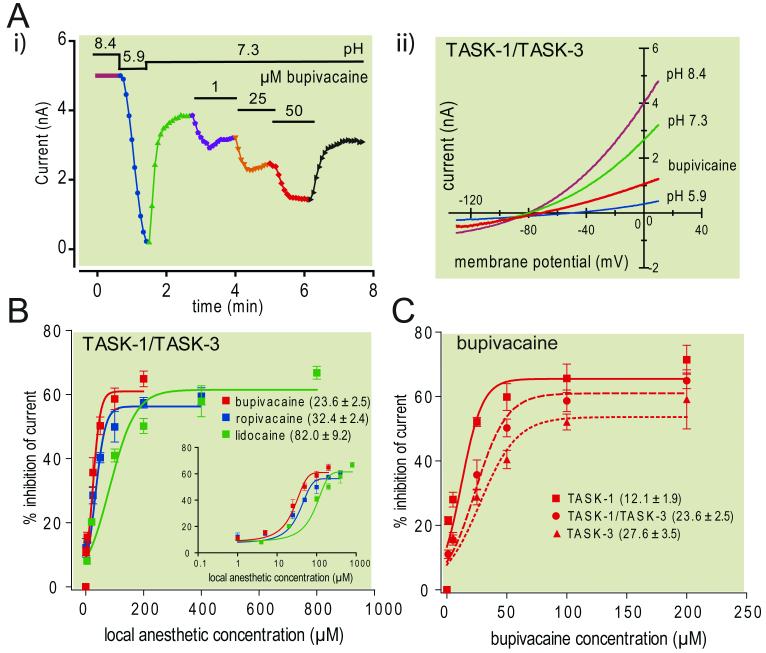
We used whole cell recording to test inhibition by three different local anesthetics (bupivacaine, ropivacaine and lidocaine) of pH-sensitive homodimeric and heterodimeric TASK channels expressed in mammalian cells. The records presented in Fig. 1A are from an exemplar experiment in which increasing concentrations of bupivacaine were administered to a cell expressing TASK-1/TASK-3 tandem heterodimeric channels. TASK channel currents were evoked by using ramp voltage commands (0.2 V/sec, from −130 to +20 mV) and peak current obtained from the corresponding I-V curves (Fig. 1A, i) was plotted as a function of time (Fig. 1A, ii). From an initial alkalized bath solution (pH=8.4), currents were decreased by bath acidification (to pH 5.9) and partially restored at physiological pH (pH=7.3); subsequent exposure to bupivacaine caused a concentration-dependent decrease in TASK currents. Similar data were obtained for all LAs when tested with any of the TASK channel constructs, but significant differences were observed in the potency of inhibition by the different drugs on each channel type and among channel conformations for each compound. Again, this is exemplified for TASK-1/TASK-3 (Fig. 1B) and for bupivacaine (Fig. 1C). We derived IC50 values from concentration-dependent inhibition of TASK-1/TASK-3 currents by different anesthetics that indicated a rank order of potency of bupivacaine > ropivacaine ⟫ lidocaine (Fig. 1B). A similar rank order potency of these anesthetics was also obtained for TASK-1 and TASK-3 homodimeric constructs (Table 1); indeed, across TASK channels, bupivacaine was ~1.4- to 1.7-fold more potent than ropivacaine and ~3.1- to 4.4-fold more potent than lidocaine. As shown for bupivacaine (Fig. 1C), each compound was also more potent at inhibiting homomeric TASK-1 than the other two TASK channel conformations; in this case, the rank order potency across drugs was TASK-1 ⟫ TASK-1/TASK-3 > TASK-3 (Table 1). Taken together, these data indicate that bupivacaine is the most potent of the LAs tested, regardless of TASK channel conformation, and channels that include TASK-1 subunits are most sensitive to LA inhibition.
Figure 1. Inhibition of different TASK channel constructs by multiple local anesthetics.
A. Representative recording from a human embryonic kidney 293 cell transfected with a concatenated TASK-1/TASK-3 heterodimeric construct. Ramp voltage commands (-130 mV to 20 mV, 0.1 V/s at 0.2 Hz) were used to elicit currents under alkalized (pH 8.4) and acidified (pH 5.9) bath conditions, and then at neutral pH 7.3 during exposure to increasing concentrations of bupivacaine (1, 25 & 50 μM). i) Time series of peak TASK-1/TASK-3 currents obtained under indicated conditions. ii) I-V curves of TASK-1/TASK-3 current under the indicated conditions. B. Concentration-response curves for inhibitory effects of three different LA compounds (bupivacaine, n=5-7; ropivacaine, n=4-15; lidocaine, n=6-15) on TASK-1/TASK-3 heterodimeric channel currents. Inset: Same data on log scale. C. Concentration-response curves for effects of bupivacaine on homomeric (TASK-1, n=8-11; TASK-3, n=4-10) and heteromeric (TASK-1/TASK-3, n=5-7) TASK channel constructs. In B & C, overlaid lines represent logistic curves that were fitted to concentration-response data in order to derive values for IC50 (provided on figures) and maximal inhibition (see also Table 1 for concentration-response data derived for all constructs and LA compounds).
Table 1.
Effects of different local anesthetics on distinct TASK channel conformations
| bupivacaine (IC50, max) | ropivacaine (IC50, max) | lidocaine (IC50, max) | |
|---|---|---|---|
| TASK-1 | 12.1 ± 1.9 μM, 65.5 ± 2.3% n = 8-11 |
17.6 ± 2.3 μM, 60.2 ± 1.6% n = 5-10 |
53.8± 7.7 μM†, 58.7 ± 2.3 % n = 4-13 |
| TASK-1/TASK-3 | 23.6 ± 2.5 μM*, 61.0 ± 2.0% n = 5-7 |
32.4 ± 2.4 μM*†, 56.3 ± 1.5% n = 4-15 |
82.0 ± 9.2 μM*†, 61.5 ± 1.8% n = 6-15 |
| TASK-3 | 27.6 ± 3.5 μM*, 53.6 ± 2.6% n = 4-10 |
46.3 ± 5.9 μM*†, 70.5 ± 2.9% n = 8-11 |
86.5 ± 9.9 μM*†, 51.2 ± 2.3% n = 5-10 |
IC50 value is concentration at half maximal inhibition, in μM; max value is maximum inhibition, as % of total pH-sensitive current; n value represents minimum and maximum numbers of cells tested at any concentration.
P<0.05 vs. TASK-1
P<0.05 vs. bupivacaine.
TASK knockout mice are less sensitive to seizures induced by systemic local anesthetics
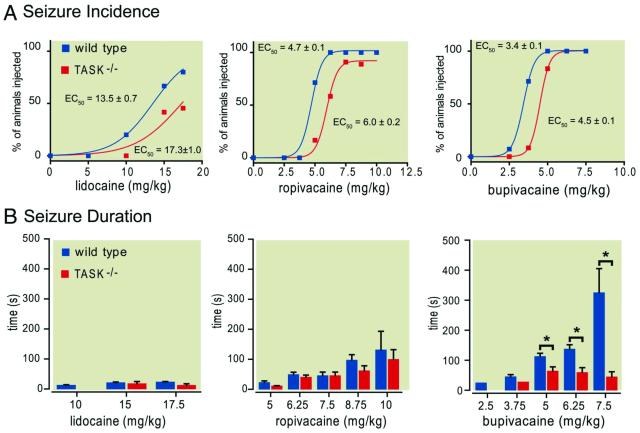
Our recordings reveal a rank order of potency for inhibition of TASK channels by LAs of bupivacaine > ropivacaine ⟫ lidocaine, matching the rank order of potency reported for seizures induced by systemic administration of LAs.5,14,31-34 In order to test whether TASK channels contribute to this toxic effect of LAs, we determined seizure incidence and duration induced by increasing intravenous doses of bupivacaine (2.5 to 7.5 mg/kg), ropivacaine (2.5 to 10 mg/kg) and lidocaine (5 to 17.5 mg/kg) in wild-type and TASK-deleted mice.
Seizure incidence increased with increasing doses of LAs for all genotypes and, as expected, higher concentrations were required for induction of seizures by lidocaine than for ropivacaine than for bupivacaine (Fig. 2A). Of particular importance, however, TASK knockout mice were markedly less likely to have seizures when injected with any of these LAs. For example, whereas 10 of 14 wild-type mice exhibited seizure activity at 3.75 mg/kg bupivacaine (~71%), only 1 of 12 TASK−/− mice had seizures at this same dose (~8%; P<0.001). Likewise, at 5 mg/kg ropivacaine, seizures were observed in ~72% of the wild-type mice (n=10/14) but in only ~17% of the TASK−/− mice (n=2/12; P<0.005), while at 6.25 mg/kg, seizures occurred in all wild-type mice (n=14/14) but in only ~58% of TASK knockout animals (n=7/12; P<0.007). Although seizure sensitivity with lidocaine was much less than for the other drugs, similar differences in incidence were noted between wild type and TASK knockout mice. Quantal dose-response curves describing seizure incidence for increasing doses of bupivacaine, ropivacaine and lidocaine were well fitted by using a logistic equation; for all drugs, rightward shifts in dose-response curves were obtained with TASK channel deletion, with significantly higher EC50 values for induction of seizures by comparison to wild type mice (lidocaine: 17.3 ± 1.0 mg/kg vs. 13.5 ± 0.7 mg/kg; ropivacaine: 6.0 ± 0.2 mg/kg vs. 4.7 ± 0.1 mg/kg; bupivacaine: 4.5 ± 0.1 mg/kg vs. 3.4 ± 0.1 mg/kg; P<0.0006, P<0.0001 & P<0.0003 respectively). In both wild type and TASK-deleted mice, bupivacaine was ~1.4-fold more potent for induction of seizures than ropivacaine and ~4-fold more potent than lidocaine, indicating that TASK channel deletion did not change the relative toxicity among these compounds. Also, note that LAs remained capable of inducing seizures in most TASK channel knockout mice, even if at higher drug concentrations.
Figure 2. Incidence and duration of seizures induced following systemic administration of local anesthetics in wild type and TASK knockout mice.
Incrementing intravenous doses of lidocaine (5 to 17.5 mg/kg), ropivacaine (2.5 to 10 mg/kg) and bupivacaine (2.5 to 7.5 mg/kg) were administered to wild type and TASK−/− mice and the incidence and duration of seizures were observed. A. The percentage of wild type and TASK−/− mice that experienced seizures was determined and a series of quantal dose-response curves were constructed and compared statistically in order to ascertain differences in EC50 and maximal incidence values for each compound. For each compound, the derived EC50 values (provided on figures) obtained for TASK−/− mice were significantly greater than those for wild type mice. B. Plots illustrate averaged duration of seizures (± SEM) for those mice in which seizures were observed (i.e., excluding zero values). For bupivacaine, F3,57 = 31.8 and F1,57 = 10.9 for genotype and interaction effect by two-way ANOVA, both P<0.0001; *, P<0.01 for TASK−/− vs. wild type mice. N=seizure/total for wild type vs. knockout for each anesthetic and dose (lidocaine, 5 mg/kg: 0/15 vs. 0/12; 10 mg/kg: 4/15 vs. 0/12; 15 mg/kg: 10/15 vs. 5/12; 17.5 mg/kg: 13/15 vs. 5/11; ropivacaine, 2.5 mg/kg: 0/14 vs. 0/12; 3.75 mg/kg: 0/14 vs. 0/12; 5 mg/kg: 10/14 vs. 2/12; 6.25 mg/kg: 14/14 vs. 7/12; 7.5 mg/kg: 11/11 vs. 10/11; 8.75 mg/kg: 9/9 vs. 8/9; 10 mg/kg: 3/3 vs. 4/4; bupivacaine, 2.5 mg/kg: 1/14 vs. 0/12; 3.75 mg/kg: 10/14 vs. 1/12; 5 mg/kg: 14/14 vs. 10/12; 6.25 mg/kg: 12/12 vs. 9/9; 7.5 mg/kg: 4/4 vs. 5/5). Reduced sample sizes at higher doses reflect LA-induced death; data from mice at lethal doses were not included in any analysis.
In animals that experienced seizures, we determined the duration of the seizure at each dose of LA (Fig. 2B). For lidocaine and ropivacaine, the LA-induced seizures were relatively brief in duration (typically <25 s and <150 s, respectively), and little difference could be discerned between wild type and TASK knockout mice. By contrast, seizures evoked by bupivacaine were more prolonged in general than with the other LAs, especially at higher doses in wild type mice where they were also significantly longer in duration than those observed in TASK−/− mice (P<0.0001). Thus, TASK knockout mice were less sensitive to seizures induced by any of the tested anesthetics and, for bupivacaine in particular, both seizure incidence and the duration were reduced by TASK channel deletion.
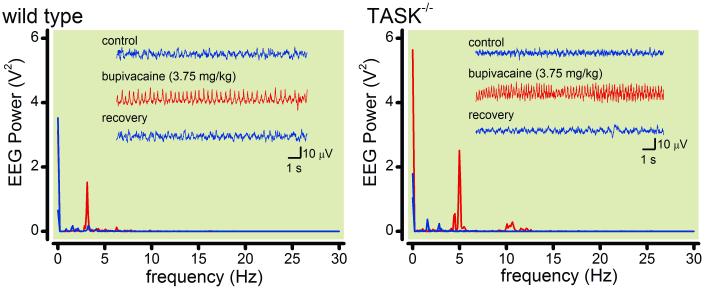
In order to verify seizures electrographically, we recorded electroencephalogram activity under control conditions and immediately after an i.v. injection of bupivacaine (at 3.75, 5 & 6.25 mg/kg) in both wild-type and TASK knockout mice. As exemplified in Fig. 3 for representative mice of each genotype that seized following a 3.75 mg/kg bupivacaine dose, electroencephalogram activity was characterized by a pronounced increase in peak power at frequencies ≤5 Hz during the seizure; electroencephalogram power in this delta frequency range was enhanced during LA-induced seizures in animals from both genotypes and at all tested doses of bupivacaine (by at least 7-fold at peak seizure frequency, as compared to respective baseline conditions). There was a tendency for slightly higher peak frequency at the two lower bupivacaine doses during seizures in TASK-deleted mice (e.g., see Fig. 3; 3.4 ± 0.4 Hz vs. ~4.4 ± 0.2 Hz in control and TASK−/− mice, n=6), but this trend was not observed at the higher bupivacaine dose and its significance remains unclear.
Figure 3. Electroencephalographic characteristics of seizures induced by bupivacaine in wild type and TASK knockout mice.
Mice were fitted with electrodes for measuring cortical electroencephalogram (EEG) and injected with bupivacaine (3.75, 5 & 6.25 mg/kg, iv). Inset: Representative EEG recordings from a wild type and a TASK−/− mice are depicted, under control conditions, during a seizure induced by 3.75 mg/kg i.v. bupivacaine, and then following recovery. A Fast Fourier transform of the EEG data reveals a large increase in power at ≤ 5 Hz specifically during the bupivacaine-induced seizure.
Picrotoxin does not inhibit TASK channels and TASK channel deletion has no effect on picrotoxin-induced seizures
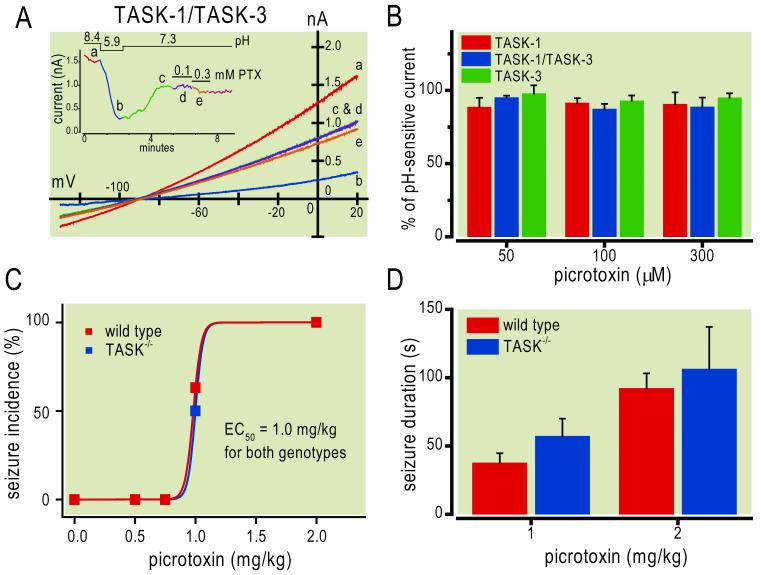
In order to rule out the possibility of non-specific effects of TASK gene deletion on seizure susceptibility, we tested the effect of another seizure-inducing pharmacological agent, specifically one that is silent at TASK channels. For this, we found that picrotoxin, a GABAA receptor antagonist,35 has essentially no effect on homomeric or heteromeric TASK channels, even at concentrations up to 300 μM (Figs. 4A, 4B). As shown in Fig. 4A for an exemplar cell expressing TASK-1/TASK-3 heterodimeric channels, picrotoxin had no discernible effect at 100 μM and caused only a slight decrease in peak current at 300 μM (<10%). Averaged data for all tested TASK channel constructs, at three different picrotoxin concentrations, revealed non-significant decreases in current amplitude of no greater than ~13% (Fig. 4B). In light of this, we examined the incidence and duration of seizures induced by picrotoxin (0.5 to 2.0 mg/kg, iv) in wild type and TASK−/− mice (Fig. 4C); we found that both genotypes were equally susceptible to picrotoxin-induced seizures (EC50~1.0 mg/kg), with similar durations for the two doses at which seizures were induced consistently. These data indicate that deletion of TASK channels does not non-specifically alter sensitivity to seizures evoked by picrotoxin, a drug that does not appreciably modulate TASK channels.
Figure 4. Picrotoxin does not modulate TASK channels and sensitivity to picrotoxin-induced seizures is unaltered in TASK knockout mice.
A. Representative recording of a human embryonic kidney 293 cell transfected with a concatenated TASK-1/TASK-3 heterodimeric construct. Ramp voltage commands (-130 mV to 20mV, 0.1 V/s at 0.2 Hz) were used to elicit TASK currents under alkalized (pH 8.4) and acidified (pH 5.9) bath conditions, and then at neutral pH 7.3 during exposure to picrotoxin (100 μM & 300 μM). Inset: Time series of peak currents obtained under indicated conditions; letters correspond to time points represented in the I-V curves of the main panel. B. Averaged residual current (± SEM) for TASK-1 (n=4-6), TASK-3 (n=4) and TASK-1/TASK-3 (n=5-6) constructs during exposure to picrotoxin (50, 100 & 300 μM), normalized to total pH-sensitive current. There was essentially no effect of picrotoxin on homomeric or heteromeric TASK channels, even at the highest concentration. C & D. Wild type and TASK−/− mice were injected with picrotoxin (0.5-2 mg/kg, i.v.) and the incidence (C) and duration (D) of picrotoxin-induced seizures were determined; there was no difference among genotypes in either of these measures of picrotoxin sensitivity (N for wild type & knockout at each dose; 0.5 mg/kg: 11 & 11; 0.75 mg/kg: 11 & 12; 1 mg/kg: 19 & 18; 2 mg/kg: 8 & 6).
DISCUSSION
In this work, we characterized concentration-dependent inhibition by various LAs of TASK channels in different conformations, including a heteromeric TASK-1/TASK-3 construct. Among TASK channel subunits, TASK-1 conferred the greatest sensitivity to LAs (TASK-1 ⟫ TASK-1/TASK-3 > TASK-3) with a rank order potency of channel inhibition by the LAs tested that matched the expected toxicity profile of those drugs (bupivacaine > ropivacaine ⟫ lidocaine). We also showed that intravenous LA administration caused behavioral (tonic/clonic) and electrographic seizures in mice, with seizures induced by this panel of LAs following the same potency profile. In TASK-1−/−:TASK-3−/− double knockout mice, a rightward shift in the dose-response curves for seizure incidence was obtained for these three LAs, indicating that mice lacking TASK channels are less sensitive to LA-induced seizures. For bupivacaine, the longest acting agent tested, seizure duration was significantly reduced in TASK−/− mice. We found no differences in incidence or duration of picrotoxin-evoked seizures between wild type and TASK knockout mice, as expected given our observation that picrotoxin does not modulate TASK channels and arguing against simple, non-specific effects of TASK gene deletion on excitability. In sum, these results suggest that increased neuronal excitability due to LA-mediated inhibition of TASK channels likely facilitates seizure induction by those drugs; since LAs retained the ability to evoke seizures in TASK−/− mice, with the same rank order potency, it appears that other targets must also be involved in this CNS toxic action of LAs.
A common concern when studying complex behavioral phenotypes in knockout mice is that a measured difference in drug action reflects some non-specific effect on neuronal function secondary to gene deletion, rather than loss of a specific molecular target. Our results suggest that this is unlikely to be the case here. First, we performed control experiments with picrotoxin, a drug that we showed is silent at TASK channels and induced seizures equally well in wild type and TASK−/− mice. Although this argues against some general change in seizure susceptibility in the knockout mice, the neural mechanisms underlying picrotoxin-induced and LA-induced seizures are not known to overlap, and it is therefore possible that non-specific changes in excitability could have taken place in the circuits involved in LA-induced seizures but not in those mediating picrotoxin-induced seizures. It is noteworthy in this respect, however, that ablation of TASK channels led to a decrease in LA-induced seizure susceptibility despite the fact that loss of these neuronal background K+ channels would be predicted to have an opposite non-specific effect (i.e., enhance excitability and be pro-convulsive). Thus, together with the relatively benign unchallenged phenotypes of these and most other K2P channel knockout mice,24,29,36-41 it seems most likely that TASK channel deletion led to a general homeostatic compensation that preserved neuronal excitability. In this context, the simplest explanation of our results is that TASK channels represent a behaviorally relevant target for LA toxicity; according to this idea, LA-induced TASK channel inhibition enhances neuronal excitability to promote seizure induction in wild type mice, but loss of this drug target in TASK−/− mice renders animals less sensitive to LA-induced seizures.
It should be noted that systemic administration of LAs was effective at evoking seizures in TASK−/− mice, even if higher doses were required than with wild type mice. In addition, there were no obvious differences in the electroencephalogram characteristics of seizures produced by higher doses of LAs in wild type and TASK−/− mice. Thus, TASK channel inhibition by LAs may play a permissive, rather than mediating, role in this toxic central action. Interestingly along these lines, we found that the rank order potency of LA-induced seizures was also retained in TASK knockout mice. This suggests that the rank order potency shared between LA modulation of TASK channel currents in vitro and LA induction of seizures in vivo may be coincidental, rather than causative. Indeed, if the relative sensitivity of TASK channel inhibition to different LAs was responsible for differential seizure susceptibility to those same LAs, then one would have predicted that those drugs would be equipotent in TASK−/− mice. This also suggests that additional targets of LA action relevant for seizure induction may share this same rank order drug sensitivity. In this respect, NaV channel inhibition by these LAs follows the same rank order potency14,31-34 even though it is unlikely that LA-evoked seizures result from NaV channel inhibition.18,25,42 Although expressed in the brain at much lower levels than TASK-1 or TASK-3,19,43 it is possible that modulation of the distantly-related (but similarly named) alkaline-activated TASK-2 channel18,25 could also contribute to LA-induced seizures.
It is clear from previously published reports that both TASK-1 and TASK-3 are inhibited by various LAs18,25 at concentrations relevant for toxic effects of these drugs.18,26 However, in general, these data have been obtained in different laboratories with distinct expression systems, and they usually have focused on effects of a single compound in the context of one of the homomeric TASK channels; there has been no information regarding LA actions on heterodimeric TASK channels, which appear to represent a predominant native conformation.28,36,37,44-47 Our whole cell recordings in mammalian cells confirm inhibition by three different LAs of homomeric TASK-1 and TASK-3 channels,18 and indicate that TASK-1 is the more sensitive subunit. Further, we find that all three drugs inhibit heterodimeric TASK-1/TASK-3 channels (by ~55-65%, with IC50 values of ~24 μM, ~32 μM and ~82 μM for bupivacaine, ropivacaine and lidocaine). Thus, these data indicate that all of these TASK channel conformations are sensitive to LA-mediated inhibition in a clinically relevant range.33,48-50
In conclusion, these results indicate a prominent contribution of TASK channel inhibition in facilitating seizures induced by LA compounds, a major toxic effect associated with systemic actions of those drugs. Thus, our data further suggest that counter-screens against TASK channel subunits could be employed in development of new LA compounds with reduced CNS toxicity.
Summary Statement.
We show that local anesthetics (bupivacaine > ropivacaine >> lidocaine) inhibit cloned TASK background K+ channels (TASK-1 >> TASK-1/TASK-3 > TASK-3); we also find that TASK knockout mice are less susceptible ⟫ to local anesthetic-induced seizures.
Acknowledgements
The authors thank Samuel L. Kowalski, Undergraduate Student Lab Aide, University of Virginia, Charlottesville, Virginia, for assistance in electroencephalogram data collection and analysis.
Support Statement: Supported by GM66181 (to Dr. Bayliss) from the National Institutes of Health (Bethesda, Maryland); a Research Scholarship (to Dr. Du) from China Scholarship Council (Beijing, China); and the Young Investigator Award Program (to Dr. Chen) from National Alliance for Research on Schizophrenia and Depression (Great Neck, New York).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Catterall WA, Mackie K. In: Goodman and Gilman’s The Pharmacological Basis of Therapeutics, Local Anesthetics. 12 edition Brunton LL, Chabner BA, Knollmann BC, editors. McGraw-Hill; 2011. pp. 565–82. [Google Scholar]
- 2.Moraca RJ, Sheldon DG, Thirlby RC. The role of epidural anesthesia and analgesia in surgical practice. Ann Surg. 2003;238:663–73. doi: 10.1097/01.sla.0000094300.36689.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koppert W, Weigand M, Neumann F, Sittl R, Schuettler J, Schmelz M, Hering W. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg. 2004;98:1050–5. doi: 10.1213/01.ANE.0000104582.71710.EE. [DOI] [PubMed] [Google Scholar]
- 4.Rowbotham MC, Reisner-Keller LA, Fields HL. Both intravenous lidocaine and morphine reduce the pain of postherpetic neuralgia. Neurology. 1991;41:1024–8. doi: 10.1212/wnl.41.7.1024. [DOI] [PubMed] [Google Scholar]
- 5.Kingery WS. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain. 1997;73:123–39. doi: 10.1016/S0304-3959(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 6.DiFazio CA, Neiderlehner JR, Burney RG. The anesthetic potency of lidocaine in the rat. Anesth Analg. 1976;55:818–21. doi: 10.1213/00000539-197611000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Himes RS, Jr, DiFazio CA, Burney RG. Effects of lidocaine on the anesthetic requirements for nitrous oxide and halothane. Anesthesiology. 1977;47:437–40. doi: 10.1097/00000542-197711000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Pypendop BH, Ilkiw JE. The effects of intravenous lidocaine administration on the minimum alveolar concentration of isoflurane in cats. Anesth Analg. 2005;100:97–101. doi: 10.1213/01.ANE.0000139350.88158.38. [DOI] [PubMed] [Google Scholar]
- 9.Vandermeulen E. Systemic analgesia and co-analgesia. Acta Anaesthesiol Belg. 2006;57:113–20. [PubMed] [Google Scholar]
- 10.Pinter A, Dorian P. Intravenous antiarrhythmic agents. Curr Opin Cardiol. 2001;16:17–22. doi: 10.1097/00001573-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Trujillo TC, Nolan PE. Antiarrhythmic agents: Drug interactions of clinical significance. Drug Saf. 2000;23:509–32. doi: 10.2165/00002018-200023060-00003. [DOI] [PubMed] [Google Scholar]
- 12.Brown DL, Ransom DM, Hall JA, Leicht CH, Schroeder DR, Offord KP. Regional anesthesia and local anesthetic-induced systemic toxicity: Seizure frequency and accompanying cardiovascular changes. Anesth Analg. 1995;81:321–8. doi: 10.1097/00000539-199508000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Graf BM. The cardiotoxicity of local anesthetics: The place of ropivacaine. Curr Top Med Chem. 2001;1:207–14. doi: 10.2174/1568026013395164. [DOI] [PubMed] [Google Scholar]
- 14.Feldman HS, Arthur GR, Covino BG. Comparative systemic toxicity of convulsant and supraconvulsant doses of intravenous ropivacaine, bupivacaine, and lidocaine in the conscious dog. Anesth Analg. 1989;69:794–801. [PubMed] [Google Scholar]
- 15.Finucane BT. Ropivacaine cardiac toxicity--not as troublesome as bupivacaine. Can J Anaesth. 2005;52:449–53. doi: 10.1007/BF03016520. [DOI] [PubMed] [Google Scholar]
- 16.Akerman B, Hellberg IB, Trossvik C. Primary evaluation of the local anaesthetic properties of the amino amide agent ropivacaine (LEA 103) Acta Anaesthesiol Scand. 1988;32:571–8. doi: 10.1111/j.1399-6576.1988.tb02788.x. [DOI] [PubMed] [Google Scholar]
- 17.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–8. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- 18.Kindler CH, Yost CS. Two-pore domain potassium channels: New sites of local anesthetic action and toxicity. Reg Anesth Pain Med. 2005;30:260–74. doi: 10.1016/j.rapm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karschin C, Wischmeyer E, Preisig-Muller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A. Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K+ channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci. 2001;18:632–48. doi: 10.1006/mcne.2001.1045. [DOI] [PubMed] [Google Scholar]
- 21.Vega-Saenz de Miera E, Lau DH, Zhadina M, Pountney D, Coetzee WA, Rudy B. KT3.2 and KT3.3, two novel human two-pore K+ channels closely related to TASK-1. J Neurophysiol. 2001;86:130–42. doi: 10.1152/jn.2001.86.1.130. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–84. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- 23.Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- 24.Enyedi P, Czirjak G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 25.Kindler CH, Yost CS, Gray AT. Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. Anesthesiology. 1999;90:1092–102. doi: 10.1097/00000542-199904000-00024. [DOI] [PubMed] [Google Scholar]
- 26.Mather LE, Copeland SE, Ladd LA. Acute toxicity of local anesthetics: Underlying pharmacokinetic and pharmacodynamic concepts. Reg Anesth Pain Med. 2005;30:553–66. doi: 10.1016/j.rapm.2005.07.186. [DOI] [PubMed] [Google Scholar]
- 27.Davies LA, Hu C, Guagliardo NA, Sen N, Chen X, Talley EM, Carey RM, Bayliss DA, Barrett PQ. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci U S A. 2008;105:2203–8. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarenko RM, Willcox SC, Shu S, Berg AP, Jevtovic-Todorovic V, Talley EM, Chen X, Bayliss DA. Motoneuronal TASK channels contribute to immobilizing effects of inhalational general anesthetics. J Neurosci. 2010;30:7691–704. doi: 10.1523/JNEUROSCI.1655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–58. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pericic D, Lazic J, Jazvinscak JM, Svob SD. Stimulation of 5-HT1A receptors increases the seizure threshold for picrotoxin in mice. Eur J Pharmacol. 2005;527:105–10. doi: 10.1016/j.ejphar.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Liu PL, Feldman HS, Giasi R, Patterson MK, Covino BG. Comparative CNS toxicity of lidocaine, etidocaine, bupivacaine, and tetracaine in awake dogs following rapid intravenous administration. Anesth Analg. 1983;62:375–9. [PubMed] [Google Scholar]
- 32.Rutten AJ, Nancarrow C, Mather LE, Ilsley AH, Runciman WB, Upton RN. Hemodynamic and central nervous system effects of intravenous bolus doses of lidocaine, bupivacaine, and ropivacaine in sheep. Anesth Analg. 1989;69:291–9. [PubMed] [Google Scholar]
- 33.Scott DB, Lee A, Fagan D, Bowler GM, Bloomfield P, Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989;69:563–9. [PubMed] [Google Scholar]
- 34.Dony P, Dewinde V, Vanderick B, Cuignet O, Gautier P, Legrand E, Lavand’homme P, De KM. The comparative toxicity of ropivacaine and bupivacaine at equipotent doses in rats. Anesth Analg. 2000;91:1489–92. doi: 10.1097/00000539-200012000-00036. [DOI] [PubMed] [Google Scholar]
- 35.Barker JL, McBurney RN, Mathers DA. Convulsant-induced depression of amino acid responses in cultured mouse spinal neurones studied under voltage clamp. Br J Pharmacol. 1983;80:619–29. doi: 10.1111/j.1476-5381.1983.tb10051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005;25:11455–67. doi: 10.1523/JNEUROSCI.3153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brickley SG, Aller MI, Sandu C, Veale EL, Alder FG, Sambi H, Mathie A, Wisden W. TASK-3 two-pore domain potassium channels enable sustained high-frequency firing in cerebellar granule neurons. J Neurosci. 2007;27:9329–40. doi: 10.1523/JNEUROSCI.1427-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yost CS, Oh I, Eger EI, Sonner JM. Knockout of the gene encoding the K2P channel KCNK7 does not alter volatile anesthetic sensitivity. Behav Brain Res. 2008;193:192–6. doi: 10.1016/j.bbr.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yost CS. Update on tandem pore (2P) domain K+ channels. Curr Drug Targets. 2003;4:347–51. doi: 10.2174/1389450033491091. [DOI] [PubMed] [Google Scholar]
- 40.Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–95. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci. 2008;29:566–75. doi: 10.1016/j.tips.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kindler CH, Paul M, Zou H, Liu C, Winegar BD, Gray AT, Yost CS. Amide local anesthetics potently inhibit the human tandem pore domain background K+ channel TASK-2 (KCNK5) J Pharmacol Exp Ther. 2003;306:84–92. doi: 10.1124/jpet.103.049809. [DOI] [PubMed] [Google Scholar]
- 43.Gabriel A, Abdallah M, Yost CS, Winegar BD, Kindler CH. Localization of the tandem pore domain K+ channel KCNK5 (TASK-2) in the rat chentral nervous system. Brain Res Mol Brain Res. 2002;98:153–63. doi: 10.1016/s0169-328x(01)00330-8. [DOI] [PubMed] [Google Scholar]
- 44.Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;554:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D, Cavanaugh EJ, Kim I, Carroll JL. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol. 2009;587:2963–75. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meuth SG, Aller MI, Munsch T, Schuhmacher T, Seidenbecher T, Meuth P, Kleinschnitz C, Pape HC, Wiendl H, Wisden W, Budde T. The contribution of TWIK-related acid-sensitive K +-containing channels to the function of dorsal lateral geniculate thalamocortical relay neurons. Mol Pharmacol. 2006;69:1468–76. doi: 10.1124/mol.105.020594. [DOI] [PubMed] [Google Scholar]
- 48.Tucker GT. Pharmacokinetics of local anaesthetics. Br J Anaesth. 1986;58:717–31. doi: 10.1093/bja/58.7.717. [DOI] [PubMed] [Google Scholar]
- 49.Moore PA, Hersh EV. Local anesthetics: Pharmacology and toxicity. Dent Clin North Am. 2010;54:587–99. doi: 10.1016/j.cden.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 50.Rousseau GF, Oram M, Barrington J, Priston M, Swart M. Plasma lidocaine concentrations following insertion of 2% lidocaine gel into the uterine cavity after uterine balloon thermal ablation. Br J Anaesth. 2002;89:846–8. doi: 10.1093/bja/aef267. [DOI] [PubMed] [Google Scholar]