Abstract
A new hybrid material consisting of a polyhedral oligomeric silsesquioxane (POSS) and carbon nanotube (CNT) was synthesized by a simple and versatile approach entailing click coupling between azide moiety-functionalized POSS and alkyne-functionalized multi-walled CNTs. This approach provides a simple and convenient route to efficiently functionalize a wide variety of nanoscale nanostructure materials on the surface of CNTs.
Introduction
A hybrid nanomaterial can be broadly depicted as a multi-component system where two or more nanomaterials are unified to form a new nanomaterial fabricated with the aim of realizing attractive multi-functional properties. Hybrid nanomaterials of carbon nanotubes (CNTs) with metals, metal oxides, and biological compounds have been developed for various applications such as sensors, actuators, solar cells, biosensors, and light emitting devices [1,2]. CNTs offer diverse optical, electrical, and mechanical properties [3,4], making them attractive building blocks for realizing novel functionality via hybridization [5,6].
Polyhedral oligomeric silsesquioxane (POSS), a type of inorganic nanostructured molecule [7-9], contains Si-O cores that have a special cage structure and good solubility. Surrounded by various organic groups, POSS is a strong candidate for further functionalization to develop nanohybrid materials [10-12]. The functionalization of CNTs has been one of the most intensively explored methods to produce CNT-based nanostructure materials. Various functionalization strategies for CNTs can be performed with non-covalent bonding, such as van der Waals and π-π interaction, as well as by covalent bonding, such as acid treatment, oxidation, esterification, amidation, radial coupling, anionic coupling, and click coupling [13,14]. These functionalization methods are dependent on the type, distribution, and concentration of compounds, i.e., polymers, metals, or inorganic nanoparticles, on the surface of the CNTs [15]. Since a landmark report by Sharpless and co-authors [16], Cu(I)-catalyzed [3+2] Huisgen cycloaddition reaction of azides and alkynes moieties, referred to as "click chemistry," has received a great deal of attention from researchers in fields ranging from organic synthesis to materials chemistry.
This article describes the synthesis of a CNT-POSS nanohybrid material using a click chemistry reaction. It is anticipated that this approach can be utilized to prepare nanohybrids with high interfacial bonding.
Experimental
Materials
Multi-walled carbon nanotubes (MWNTs) used in this study were purchased from Iljin Nano Tech, Seoul, Korea. Their diameter and length ranges were approximately 10-20 nm and 20 μm, respectively. EP0402-epoxycyclohexyllsobutyl POSS (Hybrid Plastic Co. Hattiesburg, MS, USA), propargyl bromide, p-nitrophenol, terabutylammonium bromide, 3-methyl butyl nitrite, copper iodide, and 1,8-diazabicyclo[5,4]undecene-7-ene were used without further purification.
Characterization
Fourier transform-infrared (FT-IR) spectroscopic measurements were performed using a Jasco FT-IR 300E device. Elemental analysis was determined by Perkin-Elmer analyzer model 2400 CHN analyzer. 1H NMR and 13C NMR spectra were measured on a 400-MHz instrument by Bruker on CDCl3 solutions at room temperature. Raman spectroscopy (LabRam HR Ar-ion laser 514 nm, Jobin-Yvon, Longjumeau, France) was used to confirm the functionalization of MWNTs. X-ray photoelectron spectroscopy (XPS, ESCSA 2000) was used to analyze the surface composition of the nanotubes. Observation of the surface morphology and energy dispersive X-ray spectrum (EDX) measurement of the MWNT-POSS nanohybrid was carried out by transmission electron microscopy (TEM, JEM 2100F, JEOL). Thermogravimetric analysis (TGA) was carried out in a TA Q 50 system TGA.
Preparation of alkyne-functionalized MWNTs
For the click reaction, p-aminophenyl propargyl ether was first synthesized according to a procedure reported in the literature [17] to introduce alkyne-functionality on the CNTs. Initially, 60 mg of MWNTs and 3.0 g of p-aminophenyl propargyl ether were placed in a two-necked flask fitted with a reflux condenser and a magnetic stirrer bar under a N2 atmosphere. Then, 3.0 g 3-methyl butyl nitrite was slowly injected via a syringe, and the reaction mixture was stirred at 60°C for 5 h. The resulting product was washed three times with 100 ml of dimethylformamide (DMF), and dried under vacuum at 60°C for 80 h, and the product yield was 80%.
Azidation of POSS molecules
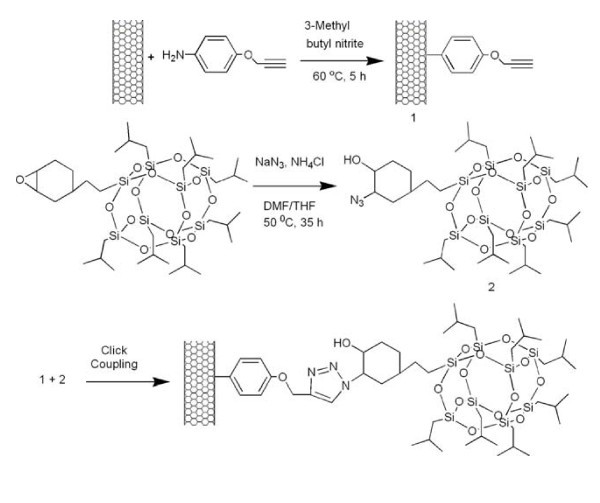
The azidation of the POSS molecule was carried out with sodium azide in the presence of ammonium chloride, as shown in Figure 1. Typically, a solution of POSS (1.0 g 3.19 mmol) in tetrahydrofuran (THF) (5 ml) was added to a solution of sodium azide (208 mg 3.19 mmol) and ammonium chloride (170 mg 3.18 mmol) in DMF (5 ml), and the mixture was stirred for 35 h at 50°C. The mixture was precipitated into 200 ml of water and the product was vacuum dried at 40°C for 60 h. The yield of azide-functionalized POSS obtained was 85%.
Figure 1.
Strategy for "clicking" POSS molecule onto MWNTs.
Synthesis of MWNT-POSS nanohybrid by click coupling
Coupling of an azide moiety-containing POSS and alkyne-functionalized MWNTs was carried out via Cu(I)-catalyzed click chemistry. Typically, 20 mg of alkyne-functionalized MWNTs was dispersed in 15 ml of DMF. The MWNTs solution was added to a two-necked flask containing a 400 mg (0.43 mmol) solution of POSS-N3 in 15 ml of DMF. The flask was equipped with a magnetic stirrer bar with a reflux condenser. 162 mg (0.85 mmol) of copper iodide and 6.4 g (42.5 mmol) of 1,8-diazabicyclo[5,4]undecene-7-ene were charged to the above homogenous solution, which was then heated at 60°C with continuous stirring for 24 h under a nitrogen atmosphere. The product was precipitated into 200 ml of water followed by 100 ml of THF for three times to remove unreacted POSS molecules. The product was dried overnight under vacuum at room temperature, and the product yield was 75-80%.
Result and discussion
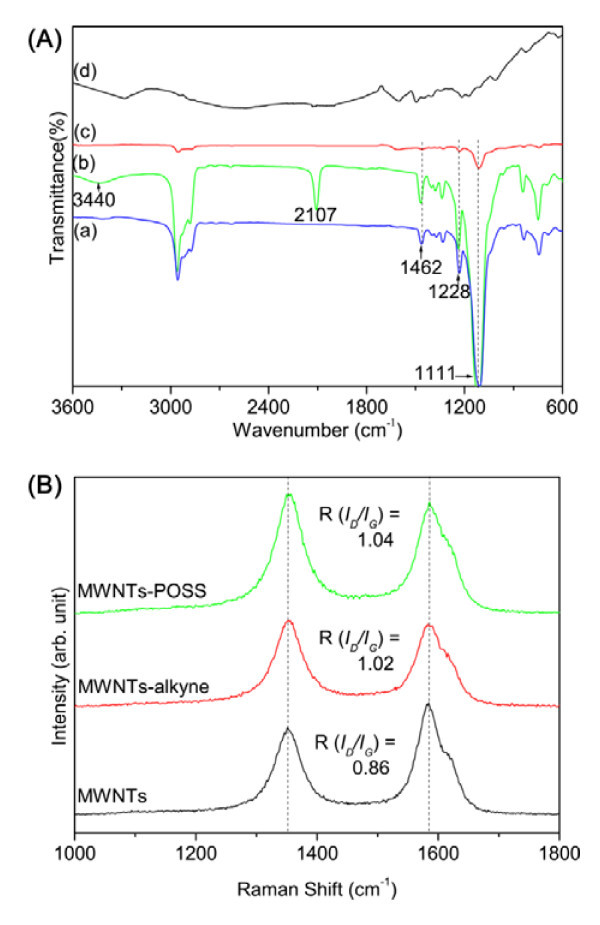
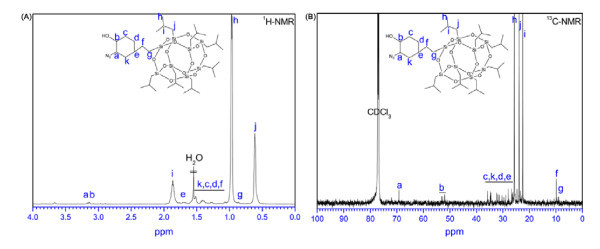
The aim of this study is to prepare covalently functionalized MWNT-POSS nanohybrids by click chemistry between azide-functionalized POSS (POSS-N3) and alkyne-functionalized MWNTs (Figure 1). Alkyne-functionalized MWNTs are prepared via a solvent-free diazotization reaction and a coupling reaction between MWNTs and p-aminophenyl propargyl ether. POSS-N3 is prepared by a simple reaction of POSS with sodium azide in the presence of ammonium chloride. The success of click cycloaddition is supported by evidence from FT-IR, Raman, XPS, TEM, EDX, and TGA. As a confirmation of the reactions, Figure 2a shows the IR spectra of pure POSS, which has characteristic peaks at 1111 cm-1 for Si-O-Si stretching [18], 1462 cm-1 for CH2 stretching of cyclohexyl [19], and 1228 cm-1 for Si-CH2 stretching [20]. The azidation of the POSS molecule was also confirmed by comparison of the IR spectrum of pure POSS with that of POSS (POSS-N3) with azide-functionality. A new peak at 2107 cm-1 corresponding to the azide group [21], and simultaneously another peak at 3440 cm-1 for OH stretching were observed. The results of 1H NMR and 13C NMR measurements reveal clearly the POSS-N3 structure (Figure 3a,b). The charecteristic signals at δ = 3.18 and 3.12 ppm in 1H NMR, and δ = 69.2 and 52-53 in 13C NMR are assigned to the -CH proton and carbon of cyclohexane combined with -OH and N3 groups, respectively. Elemental anlysis results are also in good agreement with experimental values (Table 1), confirming the successful azidation of POSS. The click coupling between the alkyne-functionalized MWNTs and azide-functionalized POSS in the presence of Cu(I) catalyst provided a 1,2,3-triazole ring. This indicates that the POSS molecule is successfully attached to the surface of the MWNTs. Thus, the IR spectra of MWNT-POSS nanohybrid, featuring a azide peak of POSS molecules at 2107 cm,-1 completely disappeared, indicating the formation of 1,2,3-triazole after the click reaction.
Figure 2.
FT-IR and Raman spectra of nanomaterials. (A) IR spectra of pure POSS (a), POSS-N3 (b), MWNT-POSS nanohybrid (c), and MWNTs-alkyne (d). (B) Raman spectra of pristine MWNTs, MWNTs-alkyne, and MWNT-POSS nanohybrid.
Figure 3.
1H NMR and 13C NMR spectra of azide functionalized POSS. (a) 1H NMR spectrum of POSS-N3 and (b) 13C NMR spectrum of POSS-N3.
Table 1.
Elemental analysis data of POSS-N3
| POSS-N3 | C% | H% | N% |
|---|---|---|---|
| Calculated | 43.90 | 7.88 | 4.26 |
| Found | 44.26 | 7.65 | 4.01 |
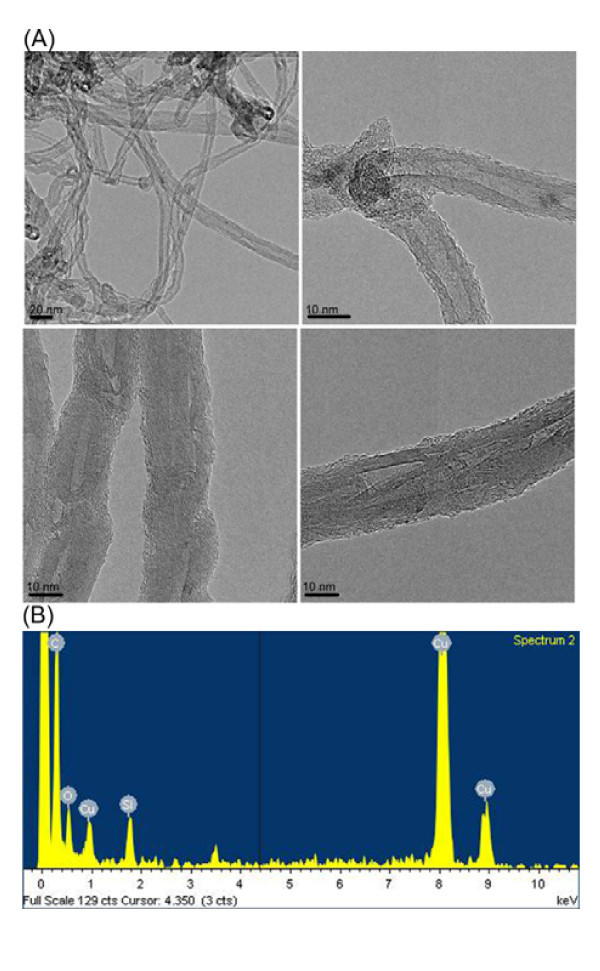
Raman spectroscopy can be used as a powerful tool for characterizing functionalized CNTs. Figure 2b shows that the pristine MWNTs, MWNTs-alkyne, and the MWNT-POSS nanohybrid have two characteristic bands at 1352 cm-1 (D band) and 1585 cm-1 (G band) [22]. The D band is attributed to a disordered graphite structure or sp3-hybridized carbons of the nanotubes, whereas the G band corresponds to a splitting of the E2g stretching mode of graphite, which reflects the structural intensity of the sp2-hybridized carbon atoms. The increase in the band intensity ratio (ID/IG) of the functionalized MWNTs reflects the relative degree of functionalization or defects in the nanotubes, indicating covalent functionalization MWNT-POSS nanohybrids. TEM images of the MWNT-POSS nanohybrid (Figure 4a) show that MWNTs are grafted by the POSS molecules. This shows strong evidence that the POSS molecules are well coated on the surface of the MWNTs. These results are also strongly supported by the EDX with copper as a substrate (Figure 4b).
Figure 4.
TEM images and EDX spectra of nanohybrid. (a) TEM images of MWNT-POSS nanohybrid and (b) EDX spectra of MWNT-POSS nanohybrid.
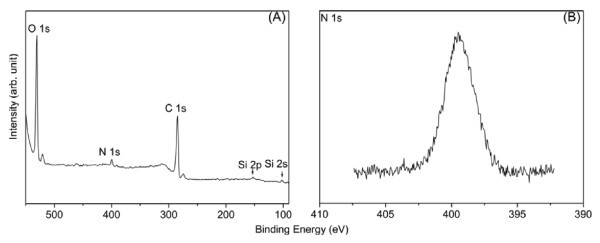
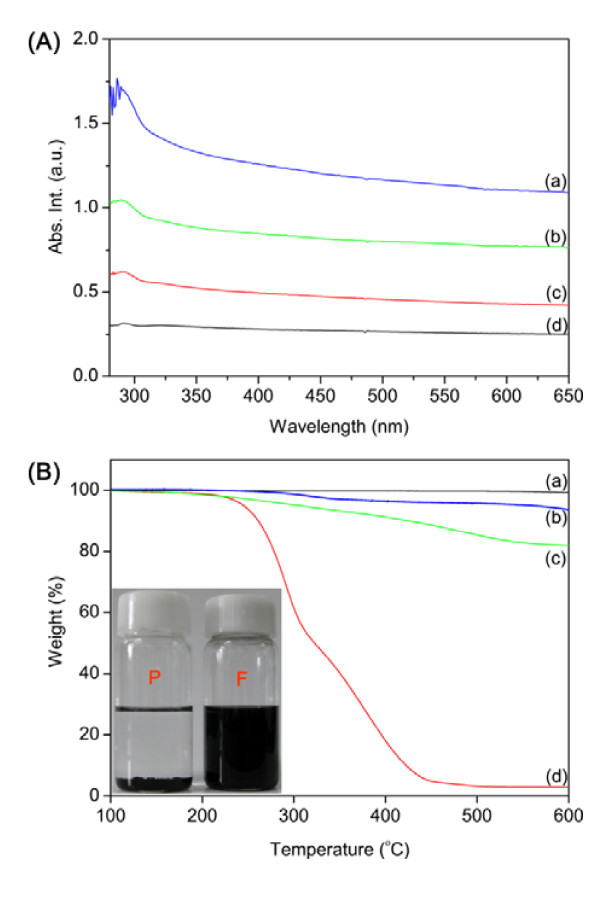
Furthermore, XPS was additionally used to investigate the clicked surface. The XPS spectra of MWNT-POSS nanohybrid material are shown in Figure 5A. Three characteristic peaks at 285, 532, and 400 eV were observed for C 1s, O 1s, and N 1s, respectively. Two relatively weak signals were also observed at 102 and 152 eV, which are characteristic peaks of Si 2s and Si 2p, respectively, from the POSS cage. The N (1s) high-resolution peak for the MWNT-POSS nanohybrid (Figure 5B) suggests the presence of only one oxidation state of the nitrogen atom due to the formation of a 1,2,3-triazole ring [23], which confirms that the POSS-N3 molecule reacted with alkyne-functionalized MWNTs. The atomic percent and weight percent of Si for the MWNT-POSS nanohybrid were calculated by EDX measurment as 3.98 and 8.57%, respectively (Table 2). These results indicate the presence of POSS molecules on the surface of the MWNTs. The MWNT-POSS nanohybrid showed a typical electronic absorption spectrum of solubilized CNTs, and the absorbance decreased gradually in the UV to visible region (Figure 6a). As the POSS molecules have better reactivity and solubility in organic solvent, functionalization of POSS molecule with CNTs can substantially enhance the solubility and processability of the nanohybrid. Figure 6b (inset) shows the solubility test results of pristine MWNTs and the MWNT-POSS nanohybrid in THF at a concentration of 2.5 mg/mL. It is observed that the MWNT-POSS nanohybrid shows better dispersion stability than pristine MWNTs in THF after 4 weeks. The TGA analysis provides further evidence for functionalization of MWNTs with POSS (Figure 6b). TGA results show weight losses of 2, 6, and 19% at 700°C for pristine MWNTs, alkyne-functionalized MWNTs, and the MWNT-POSS nanohybrid, respectively. The difference in weight loss of alkyne-functionalized MWNTs and the MWNT-POSS nanohybrid is attributed to the presence of POSS molecules on the surface of the MWNTs [24,25]. TGA data of POSS show almost complete mass loss at temperatures over 450°C due to its sublimation [10].
Figure 5.
XPS spectra of MWNT-POSS nanohybrid. (a) Wide scan spectra of MWNT-POSS nanohybrid and (b) N (1s) high-resolution peak for MWNT-POSSS nanohybrid.
Table 2.
Atomic % and weight % of MWNT-POSS nanohybrid determined from EDX experimental data
| Element | Weight % | Atomic % |
|---|---|---|
| C | 79.31 | 86.14 |
| O | 12.11 | 9.88 |
| Si | 8.57 | 3.98 |
Figure 6.
UV-Vis absorption spectra and TGA analysis of nanomaterials. (A) UV-Vis absorption spectra of MWNT-POSS nanohybrid in different concentrations: (a) 0.01 mg/ml, (b) 0.005 mg/ml, (c) 0.002 mg/ml, and 0.001 mg/ml in THF. (B) TGA analysis of pristine MWNTs (a), MWNT-POSS nanohybrid (b), pure POSS (c), solubility test results (inset) of pristine MWNTs (P), and MWNT-POSS nanohybrid (F).
Conclusion
In summary, the synthesis of a MWNT-POSS nanohybrid was accomplished via Cu(I)-catalyzed azide-alkyne cycloaddition between azide moiety-containing POSS and alkyne-functionalized MWNTs. Click coupling can provide a new strategy for the synthesis of CNT-based nanohybrids.
Abbreviations
CNT: carbon nanotube; DMF: dimethylformamide; EDX: energy dispersive X-ray spectrum; FT-IR: Fourier transform infrared; MWNTs: multi-walled carbon nanotubes; POSS: polyhedral oligomeric silsesquioxane; TEM: transmission electron microscopy; TGA: thermogravimetric analysis; THF: tetrahydrofuran; XPS: X-ray photoelectron spectroscopy.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SKY conducted all the experiments and drafted the manuscript. SSM helped in technical support for experiments and characterization. HJY participated in measurements and data analysis. JWC designed the experiments and supervised the all of the study. All the authors discussed the results and approved the final manuscript.
Contributor Information
Santosh Kumar Yadav, Email: santosh@konkuk.ac.kr.
Sibdas Singha Mahapatra, Email: sibdas202@gmail.com.
Hye Jin Yoo, Email: y821124@konkuk.ac.kr.
Jae Whan Cho, Email: jwcho@konkuk.ac.kr.
Acknowledgements
This study was supported by the Defense Acquisition Program Administration (DAPA) and the Agency for Defense Development (ADD), and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (R11-2005-065).
References
- Yang Y, Qu L, Dai L, Kang T, Durstock M. Electrophoresis coating of titanium dioxide on aligned carbon nanotubes for controlled syntheses of photoelectronic nanomaterials. Adv Mater. 2007;19:1239. [Google Scholar]
- Park HS, Choi BG, Yang SH, Shin WH, Kang JK, Jung D, Hong WH. Ionic-liquid-assisted sonochemical synthesis of carbon nanotube-based nanohybrid control in the structures and interfacial characteristics. Small. 2009;5:1754. doi: 10.1002/smll.200900128. [DOI] [PubMed] [Google Scholar]
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56. [Google Scholar]
- Srivastava SK, Vankar VD, Kumar V. Excellent field emission properties of short conical carbon nanotubes prepared by microwave plasma enhanced CVD process. Nanoscale Res Lett. 2008;3:25. [Google Scholar]
- Ajayan PM, Stephan O, Colliex C, Trauth D. Aligned carbon nanotube arrays formed by cutting a polymer resin nanotube composite. Science. 1994;265:1212. doi: 10.1126/science.265.5176.1212. [DOI] [PubMed] [Google Scholar]
- Moniruzzaman M, Winey KI. Polymer nanocomposites containing carbon nanotubes. Macromolecules. 2006;39:5194. [Google Scholar]
- Letant SE, Maiti A, Jones TV, Herberg JL, Maxwell RS, Saab AP. Polyhedral oligomeric silsesquioxane (POSS) stabilized Pd nanoparticles factors governing crystallite morphology and secondary aggregate structure. J Phys Chem C. 2009;113:19424. [Google Scholar]
- Madbouly SA, Otaigbe JU. Recent advances in synthesis, characterization and rheological properties of polyurethanes and POSS/polyurethane nanocomposites dispersions and films. Prog Polym Sci. 2009;34:1283. [Google Scholar]
- Kannan RY, Salacinski HJ, Groot JD, Clatworthy I, Bozec L, Horton M, Butler E, Seifalian AM. The antithrombogenic potential of a polyhedral oligomeric silsesquioxane (POSS) nanocomposites. Biomacromolecules. 2006;7:215. doi: 10.1021/bm050590z. [DOI] [PubMed] [Google Scholar]
- Cordes DB, Lickiss PD, Rataboul F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem Rev. 2010;110:2081. doi: 10.1021/cr900201r. [DOI] [PubMed] [Google Scholar]
- Ni C, Wu G, Zhu C, Yao B. The preparation and characterization of amphiphilic star block copolymer nano micelles using silsesquioxane as the core. J Phys Chem C. 2010;114:13471. [Google Scholar]
- Lickiss PD, Rataboula F. Fully condensed polyhedral oligosilsesquioxanes (POSS): from synthesis to application. Adv Organomet Chem. 2008;57:1. [Google Scholar]
- Sahoo NG, Cho JW, Li L, Chan SH. Polymer nanocomposites based on functionalized carbon nanotubes. Prog Polym Sci. 2010;35:837. [Google Scholar]
- Jin J, Dong Z, He J, Li R, Ma J. Synthesis of novel porphyrin and its complexes covalently linked to multi-walled carbon nanotubes and study of their spectroscopy. Nanoscale Res Lett. 2009;4:578. doi: 10.1007/s11671-009-9282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury T, Fitzmaurice D. Templated assembly of semiconductor and insulator nanoparticles at the surface of covalently modified multiwalled carbon nanotubes. Chem Mater. 2004;16:3780. [Google Scholar]
- Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Li H, Cheng F, Duft AM, Adronov A. Functionalization of single-walled carbon nanotubes with well-defined polystyrene by "click" coupling. J Am Chem Soc. 2005;127:14518. doi: 10.1021/ja054958b. [DOI] [PubMed] [Google Scholar]
- Leu CM, Chang YT, Wei KH. Synthesis and dielectric properties of polyimide tethered polyhedral oligomeric silsesquioxane (POSS) nanocomposites via POSS diamine. Macromolecules. 2003;36:9122. [Google Scholar]
- Kim CK, Kim BS, Sheikh FA, Lee US, Khil MS, Kim HY. Amphiphilic poly(vinyl alcohol) hybrids and electrospun nanofibers incorporating polyhedral oligosilsesquioxane. Macromolecules. 2007;40:4823. [Google Scholar]
- Sheikh FA, Barakat NAM, Kim BS, Aryal S, Khil MS, Kim HY. Self-assembled amphiphilic polyhedral oligosilsesquioxane (POSS) grafted poly(vinyl alcohol) (PVA) nanoparticles. Mater Sci Eng C. 2009;29:869. doi: 10.1007/s10856-008-3637-5. [DOI] [PubMed] [Google Scholar]
- Ge Z, Wang D, Zhou Y, Liu H, Liu S. Synthesis of organic/inorganic hybrid quatrefoil shaped star cyclic polymer containing a polyhedral oligomeric silsesquioxane core. Macromolecules. 2009;42:2903. [Google Scholar]
- Sato-Berrú RY, Basiuk EV, Saniger JM. Application of principal component analysis to discriminate the Raman spectra of functionalized multiwalled carbon nanotubes. J Raman Spectrosc. 2006;37:1302. [Google Scholar]
- Yadav SK, Mahapatra SS, Cho JW, Lee JY. Functionalization of multiwalled carbon nanotubes with poly(styrene-b-(ethylene-co-butylene)-b-styrene) by click coupling. J Phys Chem C. 2010;114:11395. [Google Scholar]
- Yadav SK, Mahapatra SS, Cho JW, Park HC, Lee JY. Enhanced mechanical and dielectric properties of poly(vinylidene fluoride)/polyurethane/multi-walled carbon nanotube nanocomposites. Fibers Polym. 2009;10:756. [Google Scholar]
- Izuhara D, Swager TM. Electroactive block copolymer brushes on multiwalled carbon nanotubes. Macromolecules. 2009;42:5416. [Google Scholar]