Abstract
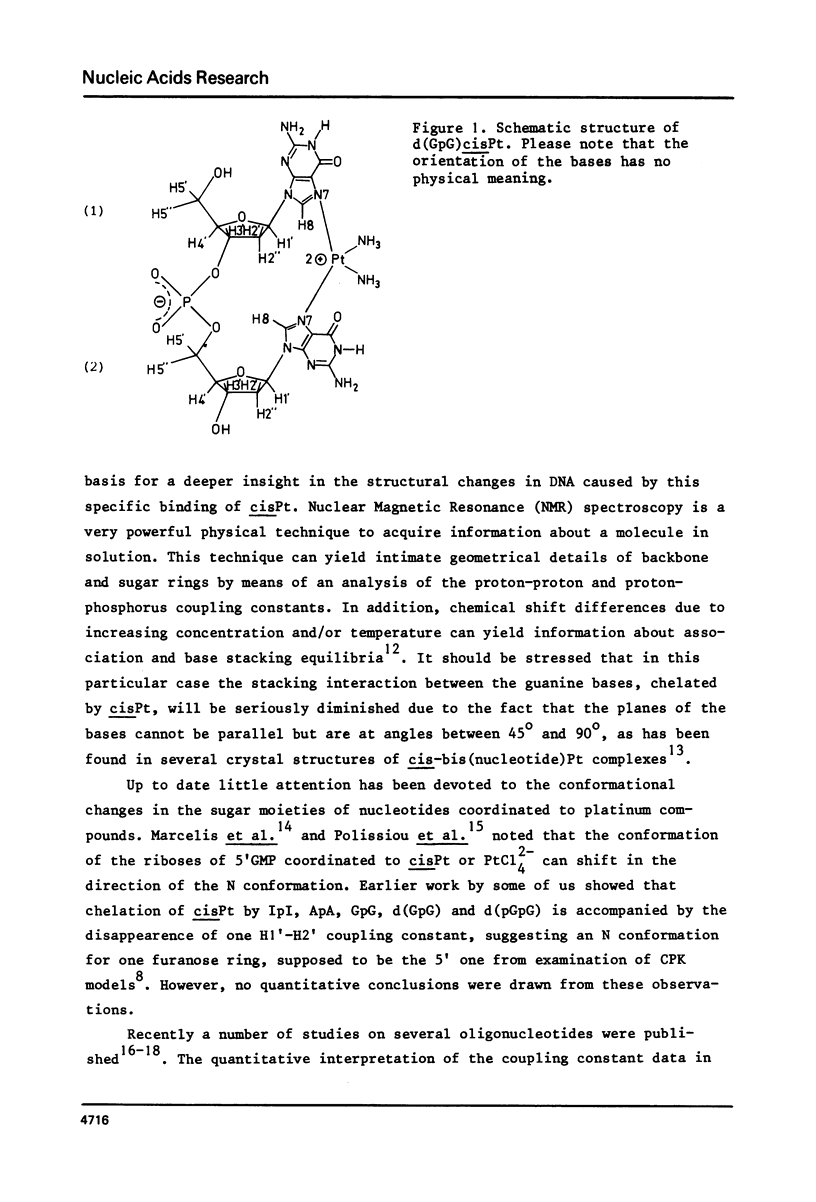
A 500, 400 and 300 MHz proton NMR study of the reaction product of cis-Pt(NH3)2Cl2 or cis-[Pt(NH3)2 (H2O)2] (NO3)2 with the deoxydinucleotide d(GpG): cis-[Pt(NH3)2 d(GpG)] was carried out. Complete assignment of the proton resonances by decoupling experiments and computer simulation of the high field part of the spectrum yield proton-proton and proton-phosphorus coupling constants of high precision. Analysis of these coupling constants reveal a 100% N (C3'-endo) conformation for the deoxyribose ring at the 5'-terminal part of the chelated d(GpG) moiety. In contrast, the 3'-terminal -pG part of the molecule displays the normal behaviour for deoxyriboses: the sugar ring prefers to adopt an S (C2'-endo) conformation (about 70%). Extrapolating from this model compound, it is suggested that Pt chelation by a -dGpdG- sequence of DNA would require a S to N conformational change of one deoxyribose moiety as the main conformational alteration and lead to a kink in one strand of the double-helical structure of DNA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. Improved method for the interpretation of proton magnetic resonance coupling constants. J Am Chem Soc. 1973 Apr 4;95(7):2333–2344. doi: 10.1021/ja00788a038. [DOI] [PubMed] [Google Scholar]
- Altona C., van Boom J. H., de Jager J., Koeners H. J., Van Binst G. Conformational analysis of N6-methyladenylyl-uridine. Nature. 1974 Feb 22;247(5442):558–561. doi: 10.1038/247558a0. [DOI] [PubMed] [Google Scholar]
- Doornbos J., den Hartog J. A., van Boom J. H., Altona C. Conformational analysis of the nucleotides A2'-5'A, A2'-5'A2'-5'A and A2'-5'U from nuclear magnetic resonance and circular dichroism studies. Eur J Biochem. 1981 May 15;116(2):403–412. doi: 10.1111/j.1432-1033.1981.tb05349.x. [DOI] [PubMed] [Google Scholar]
- Galliard T., Phillips D. R., Matthew J. A. Enzymic reactions of fatty acid hydroperoxides in extracts of potato tuber. II. Conversion of 9- and 13-hydroperoxy-octadecadienoic acids to monohydroxydienoic acid, epoxyhydroxy- and trihydroxymonoenoic acid derivatives. Biochim Biophys Acta. 1975 Nov 21;409(2):157–171. doi: 10.1016/0005-2760(75)90151-4. [DOI] [PubMed] [Google Scholar]
- Girault J. P., Chottard G., Lallemand J. Y., Chottard J. C. Interaction of cis-[Pt(NH3)2(H2O)2](NO3)2 with ribose deoxyribose diguanosine phosphates. Biochemistry. 1982 Mar 16;21(6):1352–1356. doi: 10.1021/bi00535a038. [DOI] [PubMed] [Google Scholar]
- Haasnoot C. A., Altona C. A conformational study of nucleic acid phosphate ester bonds using phosphorus-31 nuclear magnetic resonance. Nucleic Acids Res. 1979 Mar;6(3):1135–1149. doi: 10.1093/nar/6.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartel A. J., Wille-Hazeleger G., van Boom J. H., Altona C. Conformational analysis of a modified ribotetranucleoside triphosphate: m6(2)A-U-m6(2)A-U studied in aqueous solution by nuclear magnetic resonance at 500 MHz. Nucleic Acids Res. 1981 Mar 25;9(6):1405–1423. doi: 10.1093/nar/9.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellema J. R., Haasnoot C. A., Van Boom J. H., Altona C. Complete assignment and conformational analysis of a deoxyribotetranucleotide. d(TAAT). A 360 and 500 Mhz NMR study. Biochim Biophys Acta. 1981 Sep 28;655(2):256–264. doi: 10.1016/0005-2787(81)90016-2. [DOI] [PubMed] [Google Scholar]
- Olsthoorn C. S., Bostelaar L. J., Van Boom J. H., Altona C. Conformational characteristics of the trinucleoside diphosphate dApdApdA and its constituents from nuclear magnetic resonance and circular dichroism studies. Extrapolation to the stacked conformers. Eur J Biochem. 1980 Nov;112(1):95–110. doi: 10.1111/j.1432-1033.1980.tb04991.x. [DOI] [PubMed] [Google Scholar]
- Patel D. J. d-CpCpGpG and d-GpGpCpC self-complementary duplexes: Nmr studies of the helix-coil transition. Biopolymers. 1977 Aug;16(8):1635–1656. doi: 10.1002/bip.1977.360160804. [DOI] [PubMed] [Google Scholar]
- Remin M., Shugar D. Conformation of the exocyclic 5'-CH 2 OH in nucleosides and nucleotides in aqueous solution from specific assignments of the H 5' and H 5'' signals in the NMR spectra. Biochem Biophys Res Commun. 1972 Aug 7;48(3):636–642. doi: 10.1016/0006-291x(72)90395-6. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Friedlos F. Quantitative aspects of the formation and loss of DNA interstrand crosslinks in Chinese hamster cells following treatment with cis-diamminedichloroplatinum(II) (cisplatin). I. Proportion of DNA-platinum reactions involved in DNA crosslinking. Biochim Biophys Acta. 1981 Sep 28;655(2):146–151. doi: 10.1016/0005-2787(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Thomson A. J. The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol. 1979;22:71–133. doi: 10.1016/s0079-6603(08)60799-0. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Gordon L. K., Haseltine W. A. Use of exonuclease III to determine the site of stable lesions in defined sequences of DNA: the cyclobutane pyrimidine dimer and cis and trans dichlorodiammine platinum II examples. Nucleic Acids Res. 1981 Sep 25;9(18):4595–4609. doi: 10.1093/nar/9.18.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushay H. M., Tullius T. D., Lippard S. J. Inhibition of the BamHI cleavage and unwinding of pBR322 deoxyribonucleic acid by the antitumor drug cis-dichlorodiammineplatinum(II). Biochemistry. 1981 Jun 23;20(13):3744–3748. doi: 10.1021/bi00516a012. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Anderson T., Kohn K. W. DNA-protein and DNA interstrand cross-linking by cis- and trans-platinum(II) diamminedichloride in L1210 mouse leukemia cells and relation to cytotoxicity. Cancer Res. 1979 Feb;39(2 Pt 1):365–369. [PubMed] [Google Scholar]



