Abstract
Tracheary element differentiation requires strict coordination of secondary cell wall synthesis and programmed cell death (PCD) to produce a functional cell corpse. The execution of cell death involves an influx of Ca2+ into the cell and is manifested by rapid collapse of the large hydrolytic vacuole and cessation of cytoplasmic streaming. This precise means of effecting cell death is a prerequisite for postmortem developmental events, including autolysis and chromatin degradation. A 40-kD serine protease is secreted during secondary cell wall synthesis, which may be the coordinating factor between secondary cell wall synthesis and PCD. Specific proteolysis of the extracellular matrix is necessary and sufficient to trigger Ca2+ influx, vacuole collapse, cell death, and chromatin degradation, suggesting that extracellular proteolysis plays a key regulatory role during PCD. We propose a model in which secondary cell wall synthesis and cell death are coordinated by the concomitant secretion of the 40-kD protease and secondary cell wall precursors. Subsequent cell death is triggered by a critical activity of protease or the arrival of substrate signal precursor corresponding with the completion of a functional secondary cell wall.
Most terminally differentiated cells fulfill specialized functions until they die, but for some cell types, function does not begin until after death. The developmental programs producing such functional cell corpses involve the coordination of cell differentiation with PCD. For example, the outermost layer of human skin is composed of specialized cell corpses (squams) derived from subtending keratinocytes. During terminal differentiation the surface-migrating keratinocytes undergo a process of cornification that involves the synthesis of specialized keratin proteins and extensive protein cross-linking (Rice and Green, 1977). This process is coordinated with PCD (Polakowska et al., 1994) and ultimately produces a tough, flattened cell corpse that serves a protective function.
The classic example of terminal differentiation in plants is the TE, a functional cell corpse that forms a single unit of the water-conducting vessels of the xylem. We previously used a cell-culture system in which mechanically isolated mesophyll cells differentiate as TEs in vitro to characterize morphological changes during PCD of TEs (Groover et al., 1997). During differentiation the living TE constructs a rigid, interlacing secondary cell wall between the primary cell wall and the plasma membrane. Secondary cell wall synthesis is accompanied by the synthesis of nucleases and proteases (Thelen and Northcote, 1989; Minami and Fukuda, 1995; Ye and Droste, 1996; Ye and Varner, 1996; Beers and Freeman, 1997), vacuolization of the cytoplasm (Groover et al., 1997), and influx of Ca2+ (Roberts and Haigler, 1989, 1990). An average of 6 h after secondary cell wall thickenings become visible, the large central vacuole collapses rapidly, cytoplasmic streaming ceases abruptly, and the contents of the hydrolytic vacuole mix with the cytoplasm (Groover et al., 1997). Enzymatic degradation of the cell contents ensues and nDNA degradation can be detected in single cells with TUNEL both in vitro (Groover et al., 1997) and in vivo (Mittler and Lam, 1995a, 1995b). Individual cells in culture can complete differentiation in the absence of direct contact with other cells, demonstrating that the hydrolysis of the cell contents is a cell-autonomous process (Groover et al., 1997).
The molecular mechanisms controlling TE differentiation are largely unknown, but the nature of the differentiated cell argues that secondary cell wall synthesis must be coordinated with PCD. A continuous column of water is drawn through the center of interconnected TE corpses by a tensile force generated from transpiration. As a result, the secondary cell wall must resist large (<1 MPa) negative pressures (Holbrook et al., 1995; Pockman et al., 1995). Furthermore, incomplete autolysis would leave cellular debris that could directly occlude a vessel or nucleate vessel cavitation. Although further wall modifications may occur after cell death, secondary cell wall synthesis and preparation for autolysis is accomplished by the living protoplast. Failure or mistiming of PCD relative to secondary cell wall synthesis would produce a nonfunctional corpse, which could occlude a length of vessel and cause detrimental ramifications beyond the single cell.
The importance of PCD during the life cycle of plants is well established (for review, see Greenberg and Sussex, 1996; Jones and Dangl, 1996; Pennell and Lamb, 1997), although the underlying molecular mechanisms are poorly defined. Investigations have been hindered by the inability to identify and distinguish central morphological or molecular PCD events from confounding concurrent developmental events, and no basal PCD machinery has yet been identified in plants analogous to the well-defined caspase pathway for apoptosis in animals (Cohen, 1997). Although the evolutionary relationship of plant and animal PCD is uncertain, the examination of plant PCD can be guided by a few fundamental questions established by animal cell death research: What are the signals initiating cell death? Is cell death the result of suicide or murder? How is cell death executed? And how is the cell corpse processed?
As in animal systems, there are indications that the signals initiating PCD in plants vary among cell types. Developmental programs culminating in cell death are initiated by ethylene in aerenchyma formation (He et al., 1996), by GA3 in aleurone cells (Wang et al., 1996), and by auxin in TEs (Dalessandro and Roberts, 1971), although it is not clear if these hormones modulate PCD directly or if they initiate developmental programs in which PCD is a subroutine. The extracellular matrix is an important component of at least some types of plant PCD. The hypersensitive response involves the PCD of plant cells surrounding sites of pathogen ingress (Dangl et al., 1996), and can be induced by protein or carbohydrate “elicitor” molecules derived from the cell walls of the plant or pathogen (Ebel and Cosio, 1994; Dobinson et al., 1997). Cell wall degradation is also involved in the PCD of aerenchyma, aleurone, suspensor, TE, and tapetal cells, but except for the regulatory role of hypersensitive-response elicitors, cell wall degradation has been assumed to be a downstream consequence of these types of plant PCD. In contrast, the extracellular matrix has been identified as a regulatory component of various types of PCD in animals (see Discussion).
For a cell to “commit suicide,” catabolic processes must overwhelm the metabolic processes that normally sustain it. Although it is not known how this is regulated by plant cells, most if not all animal cells irreversibly commit to (execute) PCD through the action of the caspase family of Cys proteases (Nicholson and Thornberry, 1997). Although protease activity in plants has been correlated with developmental events culminating in PCD, including the hypersensitive response (Levine et al., 1996) and TE cell autolysis (Minami and Fukuda, 1995; Ye and Varner, 1996; Beers and Freeman, 1997), it is not known if proteolysis plays a role in regulating or executing cell death.
The fate of the cell corpse varies among different types of animal PCD (Clarke, 1990), but is accomplished during one type, apoptosis, by fragmentation of the cell into membrane-bound “apoptotic bodies” that are engulfed and degraded by other cells (Kerr et al., 1972). The fate of the cell corpse also varies among different types of plant PCD. In aerenchyma cell death the entire cell, including the cell wall, is degraded, whereas during the hypersensitive response the cell corpse collapses or is crushed by surrounding tissue. In TE cell differentiation, the secondary cell wall persists while the cytoplasm of the cell is degraded by an autolytic process (Groover et al., 1997).
We present evidence that cell death during TE differentiation is controlled by a signaling mechanism coordinated with secondary cell wall synthesis. We correlate cell death with the secretion of a 40-kD Ser protease and provide data implicating this protease as a primary trigger of cell death. Execution of cell death requires an influx of Ca2+, and is morphologically marked by collapse of the hydrolytic vacuole and the mixing of the vacuole with the cytoplasm. We propose a model in which execution of cell death is coordinated with completion of a functional secondary cell wall by the requirement of either a critical extracellular concentration of protease or the arrival of a substrate whose proteolytic cleavage produces a signaling product.
MATERIALS AND METHODS
Seedlings of zinnia (Zinnia elegans L. cv Green Envy; Stokes Seed, Buffalo, NY) were grown in a growth chamber at 25°C and 60% RH with 14 h of light (110 μmol photons m−2 s−2) per day. Approximately 60 leaves were harvested 12 to 15 d after sowing and surfaced sterilized with 0.525% sodium hypochlorite and 0.01% Triton X-100 for 1 min, transferred to 0.118% sodium hypochlorite and 0.01% Triton X-100 for 10 min, and then repeatedly rinsed with sterile distilled water. The leaves were macerated for 30 s in the medium described by Fukuda and Komamine (1980) using a rheostat-controlled blender (model 7011S, Waring) and the minimum speed necessary to shred the leaves and release living mesophyll cells. The mesophyll cells were isolated from the shredded leaf pieces and organelles by filtration through a 50-μm-mesh nylon filter, followed by two rounds of low-speed centrifugation.
The isolated cells were cultured in 250-mL Erlenmeyer flasks containing 50 mL of the medium described by Fukuda and Komamine (1980) containing the plant-growth regulators α-naphthalene-acetic acid (0.1 mg L−1) and 6-benzylaminopurine (0.2 mg L−1). Vital staining with fluorescein diacetate, detection of DNA fragmentation with TUNEL, and video microscopy were performed as described by Groover et al. (1997). For estimation of the percentage of divided cells, cells were stained with Calcofluor white (0.01%) and divided cells were identified by the presence of one or more cell walls separating daughter cells. Staurosporine and A23187 were purchased from Calbiochem; trypsin (crystallized three times) was from Worthington Biochemicals (Freehold, NJ); Mas 7 and Mas 17 were synthesized and purified by the Peptide Synthesis Facility of the University of North Carolina at Chapel Hill. Peptide composition was checked by MS. All other drugs and reagents of the highest available purity were purchased from Sigma. All experiments were repeated at least once with similar results.
Intracellular proteins were isolated by homogenizing cells in extraction buffer (50 mm Tris-HCl, pH 7.5, 2 mm DTT, 250 mm Suc) at 4°C, followed by centrifugation at 12,000g at 4°C for 15 min to pellet cell debris. For concentration of proteins from the medium, cultures were centrifuged twice to remove cells, and the supernatant was passed through a 2-μm filter. The proteins in the filtered supernatant were concentrated at 4°C using a pressure cell concentrator (Amicon, Beverly, MA) with a 10-kD cutoff filter (YM10, Amicon).
Protein concentrations were estimated using the method of Bradford (1976). Protein samples were mixed with an equal volume of the sample buffer described by Ye and Droste (1996) without heating and loaded onto 0.75-mm-thick 12% SDS acrylamide gels containing heat-denatured gelatin (0.1 mg mL−1), and electrophoresed as described by Laemmli (1970). For expression of protease activity, gels were incubated overnight at room temperature in 50 mL of 50 mm sodium citrate, pH 5.0, 5 mm DTT, 5 mm CaCl2, and 1 mm ZnCl2. For determination of pH optima, the activity buffer pH was adjusted as described by Ye and Droste (1996). The next day, gels were rinsed for 5 min three times with distilled water, and then silver stained as described by Blum et al. (1987). Protease activities were identified by hydrolysis of the gelatin substrate, which produced clear bands against the uniform background staining of the copolymerized gelatin.
RESULTS
Cell Death Is Marked by the Rapid Collapse of the Vacuole and Leads to Autolysis and nDNA Fragmentation
Cultures were initiated from mesophyll cells mechanically dissociated from leaves of zinnia seedlings, as shown in Figure 1A. Typically, 10% to 35% of the cells are killed by the isolation procedure and 15% to 60% of the isolated cells differentiate as TEs (Fig. 1B). The first morphological manifestation of differentiation occurs approximately 72 h after isolation, when nascent TEs synthesize an elaborate secondary cell wall between their primary cell wall and the plasma membrane (Fig. 1C). Approximately 6 h after the appearance of visible cell wall thickenings, the large central vacuole collapses rapidly and cytoplasmic streaming ceases simultaneously (Groover et al., 1997), marking the irreversible termination of normal metabolism and providing a distinct morphological marker of a critical event during PCD, the execution of cell death (video microscopy of vacuole collapse can be viewed at http://www.unc.edu/depts/biology/joneslhp/pcd/). The contents of the hydrolytic vacuole mix with the cytoplasm (Fig. 1, D and E), leading to active degradation of organelles by hydrolytic enzymes synthesized during differentiation. nDNA is degraded and can be assayed in individual cells using TUNEL (Fig. 1F) (Groover et al., 1997), an in situ labeling method.
Figure 1.
Morphological markers of TE PCD. A, Isolated mesophyll cells introduced into culture and observed with phase-contrast light microscopy. Bar = 10 μm. B, Cultured cells 96 h after isolation viewed with fluorescence microscopy. Excitation = 470 nm; emission = 510 nm. Differentiated TE cells are distinguishable by the yellow autofluorescence from their lignified secondary cell walls. Undifferentiated cells are noted by red autofluorescence from their chloroplasts. Bar = 20 μm. C, Differentiated TE stained with Calcofluor white to reveal the cellulose-containing secondary cell wall. Excitation = 370 nm; emission = 400 nm. Bar = 10 μm. D, Optical section of TE before vacuole collapse. Staining with the vital dye fluorescein diacetate shows clear separation of cytoplasm and vacuole. Excitation = 470 nm; emission = 510 nm. Bar = 10 μm. E, Optical section of TE at the developmental stage after vacuole collapse. Staining with fluorescein diacetate shows cytoplasm and vacuole contents mixed. The vacuole was not present in serial sections of this cell (not shown). Excitation = 470 nm; emission = 510 nm. Bar = 10 μm. F, TE containing fragmented nDNA revealed by TUNEL (arrow). Also seen is yellow autofluorescence from the secondary cell wall and red autofluorescence from the chloroplasts. Bar = 10 μm.
The Process Executing Cell Death Influences Postmortem Development and Is Distinct from Necrosis
The immediate question centers on the significance of cell death during PCD. Specifically, does the endogenous mechanism used to end normal metabolism (i.e. to execute cell death) significantly influence postmortem developmental events, including autolysis? A related question is whether vacuole collapse and DNA fragmentation (assayed by TUNEL) discern PCD from necrotic death under our experimental conditions. We reasoned that these questions could be addressed directly by treating cultures containing nascent TEs (before the onset of cell death during PCD) with drugs that modulate specific components of cell signaling or metabolic pathways and assaying for premature collapse of the vacuole and TUNEL.
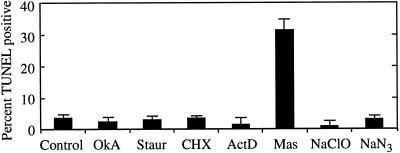
Among the various drugs tested, only mastoparan induced significant numbers of cells to prematurely fragment nDNA, as shown in Figure 2. Concentrations of other drugs tested included lethal doses, but did not induce DNA fragmentation detectable with TUNEL, immediately suggesting that cell death must be executed in a specific fashion for postmortem DNA fragmentation to occur, and showing that TUNEL is a robust marker of PCD in this system. Mastoparan is an activator of heterotrimeric G-proteins that stimulate enzymes or ion channels in response to ligand-mediated receptor activation in both animals and plants (e.g. Legendre et al., 1992; Munnik et al., 1995).
Figure 2.
Premature nDNA fragmentation resulting from drug treatments. Aliquots of cultures containing nascent TEs (after synthesis of hydrolytic enzymes commenced, but before cell death during PCD) were treated with three different concentrations of the indicated agents. Concentrations used for each drug were established in preliminary experiments, with the lowest concentration causing little or no cell death and the highest causing significant cell death. Cells were incubated for 6 h to allow the drugs to exert their effects and to allow time for nuclease activity (if any) to produce detectable amounts of DNA fragmentation. Cells treated with different concentrations of the same agent were then combined and the percentage of cells exhibiting DNA fragmentation was determined using TUNEL. Combining samples allowed a larger number of drugs and concentrations to be surveyed. Drug treatments included the protein phosphatase inhibitor okadaic acid (OkA; 0.01, 0.04, and 0.08 μm), the protein kinase inhibitor staurosporine (Staur; 1, 10, and 100 μm), the protein synthesis inhibitor cycloheximide (CHX; 1, 10, and 100 μm), the RNA synthesis inhibitor actinomycin D (ActD; 50 and 500 nm), the heterotrimeric G-protein activator mastoparan (Mas; 1, 2, and 5 μm), the respiration inhibitor sodium azide (NaN3; 4, 20, and 40 μm), and sodium hypochlorite (bleach) (NaClO; 0.3, 3, and 6 mm). Control cells were not treated with drugs before labeling. Cells treated with DNAse 1 h after fixation and permeabilization as a positive control displayed 88% TUNEL positive cells. Cells processed without addition of terminal transferase did not label. Error bars (se) represent the variation between two samples taken from the same culture and processed in parallel.
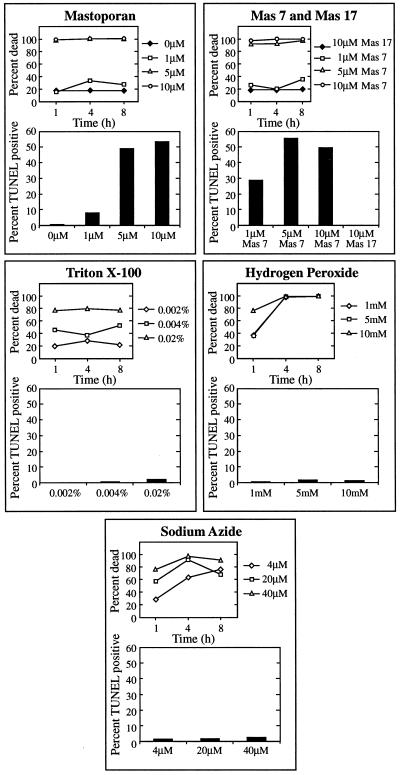
Mastoparan activates an endogenous process required for the rapid collapse of the vacuole, leading to autolysis and fragmentation of DNA. Figure 3 shows that low levels of Mas 7, an active synthetic analog of mastoparan, and mastoparan-induced cell death and DNA fragmentation occur in a dose-dependent manner, whereas Mas 17, an inactive synthetic analog, had no effect above control levels, showing that the effects of mastoparan were specific and not attributable to contaminating substances. Other agents that killed cells with similar kinetics and efficacy as mastoparan did not induce DNA fragmentation. Sodium azide, an inhibitor of Cyt oxidase and thus a general inhibitor of metabolic respiration, also caused rapid, high levels of cell death but did not result in DNA fragmentation. Similarly, Triton X-100 and hydrogen peroxide, both of which would be expected to disrupt the plasma membrane, caused high levels of cell death without DNA fragmentation.
Figure 3.
DNA fragmentation and kinetics of cell death induced by pharmacological agents and chemical insults. Aliquots of cultures containing nascent TEs were treated with the indicated concentrations of drug or chemical and assayed for cell death using fluorescein diacetate staining at the indicated times after treatment. For determination of DNA fragmentation in response to treatments, cells were cultured for 68 h with the indicated concentrations of drug or chemical and assayed with TUNEL 6 h later.
Furthermore, as observed with time-lapse videomicroscopy, 83% of cells (n = 12) dying in response to mastoparan treatment displayed the rapid vacuole collapse characteristic of TE cell death within minutes of treatment, with cytoplasmic streaming ending instantaneously with collapse of the vacuole. Cells dying from hydrogen peroxide treatment (10 mm; n = 6) gradually slowed cytoplasmic streaming without collapse of the vacuole; cells dying from sodium azide treatment (40 μm; n = 14) rapidly stopped cytoplasmic streaming but did not display vacuole collapse; cells dying from Triton X-100 treatment (0.02%; n = 13) stopped streaming gradually, plasmolyzed, then showed dissolution of chloroplast membranes.
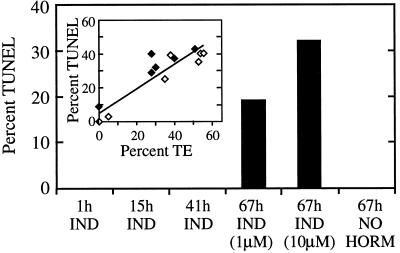
Mastoparan did not cause DNA fragmentation directly, and only cells differentiating as TEs fragmented DNA in response to mastoparan treatment. Cells cultured in medium without exogenous hormones did not differentiate as TE, undergo PCD, or fragment DNA in response to mastoparan treatment, as shown in Figure 4. Cells induced to differentiate with hormones fragmented DNA in response to mastoparan treatment only after reaching a developmental stage within approximately 6 h before the appearance of secondary cell wall thickenings visible with light microscopy. Mastoparan induced a high rate of cell death in all of the cultures (data not shown), but the percentage of dying cells fragmenting DNA in response to mastoparan treatment was correlated with the percentage of cells differentiating as TEs (Fig. 4, inset), suggesting that mastoparan treatment leads to DNA fragmentation only in cells differentiating as TEs. The ability of mastoparan to trigger premature vacuole collapse and DNA fragmentation suggests that it activates part of the endogenous mechanism that executes cell death. Because cell death was also induced in cells not differentiating as TEs, mastoparan must activate cellular components used during PCD that are not unique to differentiating TEs (probably Ca2+ channels; see below).
Figure 4.
Correlation of mastoparan-induced DNA fragmentation and TE differentiation. Cells cultured either in medium containing inductive levels of hormones (IND) supporting TE differentiation or in medium lacking hormones (NO HORM) (which does not support TE differentiation) were treated with 2.5 μm mastoparan at the indicated times. The percentage of cells exhibiting DNA fragmentation was determined using TUNEL 6 h later. To determine the correlation between the percentage of cells differentiating as TE and the percentage of cells fragmenting DNA in response to mastoparan (inset), cells from two separate cell isolations (♦ and ⋄) were cultured in different media with hormone levels ranging from zero to fully inductive to cause variations in the percentage of cells differentiating as TEs. Cells were treated with 2.5 μm mastoparan at the onset of differentiation and assayed for DNA fragmentation with TUNEL 6 h later. The percentage of TEs formed was determined for both sets of cultures after approximately 96 h of culture.
Execution of Cell Death Requires Ca2+ Influx
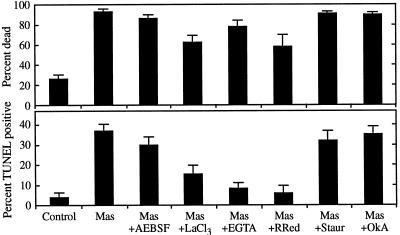
The rapid collapse of the vacuole and the cessation of cytoplasmic streaming that occur during PCD of TEs and in response to mastoparan treatment likely represent changes in cell turgor and membrane potential that might be explained by ion flux across the plasma membrane. Consistent with this notion, pretreatment of cultures containing nascent TEs with either EGTA (to chelate extracellular Ca2+) or La3+ or ruthenium red (to inhibit Ca2+ influx) reduced both cell death and DNA fragmentation resulting from mastoparan treatment, as shown in Figure 5. The antagonistic effect on cell death by inhibiting Ca2+ influx was limited, although the level of DNA fragmentation was reduced to near control levels. This may indicate that DNA fragmentation has a more stringent requirement for Ca2+ influx than cell death during PCD. Regardless, these results indicate that mastoparan prematurely induces cell death during PCD by a mechanism requiring an influx of Ca2+ into the cell, probably through plasma membrane channels.
Figure 5.
Inhibition of mastoparan-induced cell death and DNA fragmentation by antagonists of Ca2+ influx. Five-hundred-microliter aliquots of cultures containing nascent TEs were pretreated for 30 min with 0.1 mm 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF; a Ser protease inhibitor), 150 μm LaCl3 (a Ca2+ channel antagonist), 500 μm ethylene glycol-bis(β-aminoethyl ether)-EGTA (a Ca2+ chelator), 50 μm ruthenium red (RRed; a Ca2+ channel antagonist), 10 μm staurosporine (Staur; a protein kinase inhibitor), or 40 nm okadaic acid (OkA; a protein phosphatase inhibitor), and were then treated with 2.5 μm mastoparan (Mas). The percentage of dead cells was determined 1 h later using fluorescein diacetate; the percentage of cells exhibiting DNA fragmentation was determined using TUNEL 3.5 h after treatment. Control cells were not treated with drugs before processing. Error bars represent the se of two samples treated in parallel.
Figure 6 shows that imposing Ca2+ influx directly is sufficient to prematurely initiate vacuole collapse leading to DNA fragmentation. Cultures containing nascent TEs were treated with the Ca2+ ionophore A23187. Cells in medium containing 1 mm CaCl2 treated with A23187 died (approximately 55%) and fragmented DNA (approximately 20%), whereas about one-half as many cells treated with A23187 in medium lacking supplemental CaCl2 died and fragmented DNA. A23187 (0.1 mm) caused vacuole collapse in 57% of dying cells (n = 28) cultured in 1 mm CaCl2 (videomicroscopy not shown).
Figure 6.
Cell death and DNA fragmentation induced by the Ca2+ ionophore A23187. Five-hundred-microliter aliquots of cultures containing nascent TEs were pelleted by centrifugation three times and resuspended in either standard culture medium containing 1 mm CaCl2 (control and A23187) or medium lacking added CaCl2 (A23187, low [Ca++]) before treatment with 0.1 mm A23187. The percentage of dead cells was determined 4 h later using fluorescein diacetate; the percentage of cells exhibiting DNA fragmentation was determined 7 h after treatment using TUNEL. Error bars represent the se of two samples treated in parallel.
TE Cell Death Can Be Manipulated by Extracellular Proteolysis
We envisioned that extracellular changes could coordinate cell wall synthesis and PCD. For example, the synthesis of a secondary cell wall between the primary wall and the plasma membrane could sever connections between the cytoskeleton and the extracellular matrix, which triggers cell death. Alternatively, the hydrolysis of the primary cell wall during TE differentiation could release a signal molecule triggering cell death, as during cell death in response to wall-derived elicitor molecules during the hypersensitive response. To test these possibilities, cultures containing nascent TEs were treated with exogenous hydrolytic enzymes targeting specific components of the extracellular matrix and assayed for cell death and DNA fragmentation.
Although several of the hydrolases tested caused an increase in the percentage of dead cells, only trypsin caused cell death leading to DNA fragmentation (Table I). Moreover, trypsin (0.5%) caused vacuole collapse in 87% of killed cells (n = 15) observed with time-lapse videomicroscopy (not shown). The observation that other proteases did not trigger DNA fragmentation suggested that specific proteolysis of the extracellular matrix is required to trigger cell death mimicking PCD of TEs.
Table I.
Cell death and DNA fragmentation induced by hydrolytic enzymes
| Hydrolase | Concentration (w/v) | Dead | TUNEL |
|---|---|---|---|
| % | |||
| Control | 25 | 2.7 | |
| Macerozyme | 1 | 40 | 4.8 |
| 0.1 | 27 | 2.5 | |
| Pectinase | 1 | 40 | 0.5 |
| 0.1 | 39 | 3.4 | |
| Cellulase | 1 | 58 | 0 |
| 0.1 | 37 | 1.5 | |
| Proteinase K | 1 | 48 | 0 |
| 0.1 | 17 | 0 | |
| Protease XIV | 1 | 31 | 0 |
| 0.1 | 22 | 1.1 | |
| Protease XXIV | 1 | 54 | 0 |
| 0.1 | 30 | 0 | |
| Protease XVII-B | 1 | 21 | 0 |
| 0.1 | 35 | 0 | |
| Papain | 1 | 61 | 3.2 |
| 0.1 | 19 | 1.1 | |
| Chymotrypsin | 1 | 28 | 0 |
| 0.1 | 22 | 0 | |
| Trypsin | 1 | 96 | 21.9 |
| 0.1 | 55 | 6.0 | |
Cells were treated with the indicated concentration of each hydrolase 67 h after isolation and scored for the percentage of dead cells 6 h later. At least 200 cells were scored for each treatment.
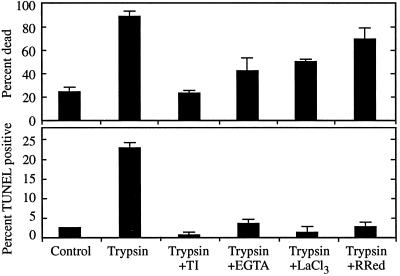
Trypsin initiated cell death via an influx of Ca2+, which is consistent with the activation of the endogenous mechanism executing cell death. As shown in Figure 7, trypsin-induced death and DNA fragmentation were inhibited by chelating extracellular Ca2+ with EGTA or by blocking Ca2+ channels with La3+ or ruthenium red. Trypsin-induced death was also inhibited by soybean trypsin inhibitor (Fig. 7), indicating that cell death resulted from the proteolytic activity of trypsin, not from contaminating substances.
Figure 7.
Cell death and DNA fragmentation induced by trypsin in the presence or absence of Ca2+ influx antagonist. Two-hundred-fifty-microliter aliquots of cultures containing nascent TE were pretreated for 15 min with either 4 mg/mL soybean trypsin inhibitor (TI), 500 μm EGTA, 150 μm LaCl3, or 50 μm ruthenium red (RRed) before treatment with 0.5% trypsin. The percentage of dead cells was determined 1 h later using fluorescein diacetate staining; the percentage of cells exhibiting DNA fragmentation was determined 4 h after treatment with TUNEL. Error bars represent the se of two samples treated in parallel.
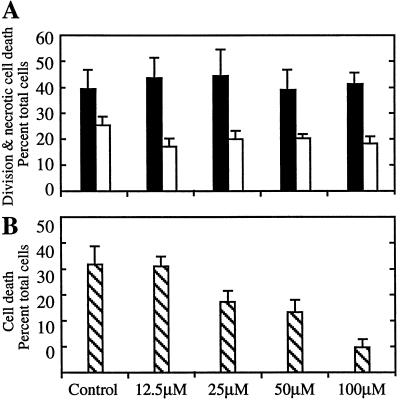
Selective inhibition of extracellular proteolysis specifically inhibited PCD. Cells at different points in development were treated with soybean trypsin inhibitor. As shown in Figure 8A, when present between 24 and 70 h of culture, soybean trypsin inhibitor did not cause necrosis or inhibit cell division, indicating that the inhibitor had negligible toxicity in this system. In contrast, soybean trypsin inhibitor present between 48 and 96 h effectively inhibited TE differentiation and PCD in a dose-dependent fashion (Fig. 8B). The 21-kD soybean trypsin inhibitor would not be expected to cross the plasma membrane, suggesting that its inhibitory effects on TE cell death were exerted in the extracellular matrix.
Figure 8.
Effect of soybean trypsin inhibitor on cell division and cell death. A, Five-hundred-microliter aliquots of cells from three independent isolations were treated with the indicated concentrations of soybean trypsin inhibitor at 24 h of culture. Cells were scored for percentage of divided cells (black bars) and dead cells (white bars) at 70 h. B, Five-hundred-microliter aliquots of cells from three independent isolations were treated at 48 h and scored for percentage of dead cells at 96 h. The increase in cell death in the untreated control at this later time point reflects the PCD of differentiated TEs.
A Ser Protease Is Secreted Coincident with PCD
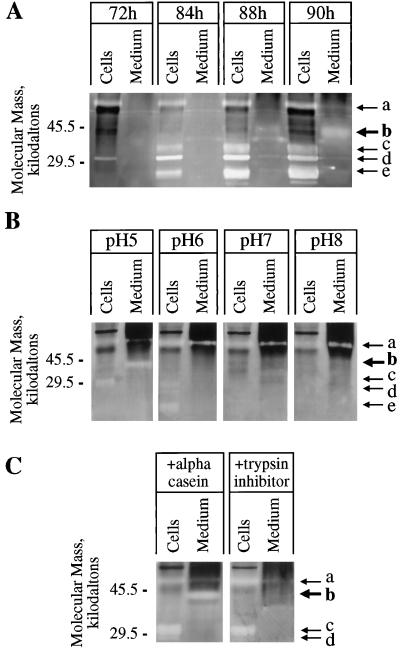
A secreted protease whose properties implicated it as an activator of cell death was identified with substrate-activity gels (see Methods). As shown in Figure 9, several intracellular proteases were recognized in protein preparations from cells, as in previous reports (Ye and Varner, 1996; Beers and Freeman, 1997), whereas the activity of a unique protease of approximately 40 kD (Fig. 9A) increased in the medium of cultures as PCD progressed. Although several strong protease activities were detected in intracellular protein samples, the 40-kD activity did not accumulate intracellularly, which is consistent with secretion. Leakage of intracellular proteases could be detected in the culture supernatants at later time points. However, leakage of protease from dying cells was not responsible for the 40-kD activity, because the abundant intracellular proteases showed little activity in the medium (Fig. 9A).
Figure 9.
Timing of expression and characteristics of proteases expressed by differentiating TEs. A, Intracellular proteins (Cells) and proteins concentrated from media (Medium) of the same culture at the indicated times after culture initiation were assayed on protease activity gels as described in Methods. After development, protease activities are recognized as clear bands resulting from hydrolysis of the gelatin substrate. At the time of harvest, the percentages of dead TEs were 0%, 20%, 49%, and 78% for the 72-, 84-, 88-, and 90-h cultures, respectively. Several intracellular protease activities can be seen (arrows a, c, d, and e), similar to the findings of Beers and Freeman (1997) and Ye and Droste (1996). Protease activity is visible at approximately 40 kD (arrow b) in media after 84 h. The exact time during development that protease secretion commences cannot be determined directly from this technique, and accumulation of detectable protease activity in the medium may significantly lag behind the onset of secretion. Approximately 0.015 μg of medium protein and 0.5 μg of intracellular protein was loaded per sample. B, Aliquots of the same preparations of intracellular proteins (90-h culture) and medium proteins (88-h culture) were run on the same protease activity gel. After fractionation the gel was sliced into four pieces, and each piece was incubated in an activity buffer with the indicated pH overnight (see Methods) before development. The 40-kD activity in medium proteins (arrow b) is detected only at pH 5. C, Aliquots of the same preparations of intracellular proteins (90-h culture) and medium proteins (88.5-h culture) were run on the same protease activity gel. The gel was divided in half, and one-half was immersed in ice-cold activity buffer containing 10 mg/mL soybean trypsin inhibitor (21 kD) and the other half was immersed in ice-cold activity buffer containing 10 mg/mL dephosphorylated α-casein (23 kD) for 45 min to allow the proteins to diffuse into the gels. Gels were then incubated at room temperature overnight before development. α-Casein has no protease inhibitory property, so it was used as a control for increasing background staining attributable to protein infusion into the gel. The soybean-trypsin-infused gel does not show the 40-kD activity in the medium, whereas the α-casein-infused gel does show the 40-kD activity (arrow b), indicating that the activity was not simply obscured by the infused proteins, but was specifically inhibited by soybean trypsin inhibitor. {/ANNT;;;left;top}
The 40-kD protease was active at pH 5.0 but not at a more basic pH (Fig. 9B), which is consistent with the wall pH in planta and in vitro (the culture medium pH was 5.5 at the time of culture initiation). Most importantly, the 40-kD protease was inhibited by soybean trypsin inhibitor (Fig. 9C). The observations that (a) the 40-kD protease was the only detectable secreted protease (Fig. 9A); (b) the appearance of the 40-kD protease activity was coincident with PCD (Fig. 9A); and (c) soybean trypsin inhibitor inhibited both the endogenous TE PCD mechanism (Fig. 8) and the secreted protease (Fig. 9C) provide strong indirect evidence that the 40-kD Ser protease triggers TE cell death.
DISCUSSION
We have addressed two fundamental questions concerning TE differentiation: How is the synthesis of the secondary cell wall coordinated with PCD? And how does the cell execute cell death? We found that a principal part of the mechanism executing cell death is a regulated influx of Ca2+, probably through plasma membrane channels. Death is morphologically manifest by rapid collapse of the hydrolytic vacuole, mixing of the vacuole and the cytoplasm, and immediate cessation of cytoplasmic streaming. This endogenous mechanism does not simply terminate normal metabolism, but also creates an environment necessary for postmortem developmental events, including autolysis, to proceed. Vacuole collapse may result from either a transition from the gradual Ca2+ influx shown to occur during secondary cell wall synthesis (Roberts and Haigler, 1989, 1990) to a rapid influx, or the activation of additional ion channels upon exceeding a threshold level of intracellular Ca2+.
The coordination of secondary cell wall synthesis and PCD begins well in advance of the execution of cell death, with the approximately concurrent commencement of secondary cell wall synthesis and the production of hydrolytic enzymes. All of the inhibitors shown to block PCD also block secondary cell wall synthesis, suggesting that these developmental programs are not only concurrent, but molecularly interdependent. However, we were able to implicate a 40-kD Ser protease as a key coordinating factor by exploiting PCD-specific markers that report cell death independently of cell wall synthesis. The protease was secreted by cells coincident with PCD, and the protease and cell death were both inhibited by soybean trypsin inhibitor. Execution of cell death can be triggered prematurely by exogenous application of another Ser protease, trypsin, which presumably mimics the action of the endogenous protease.
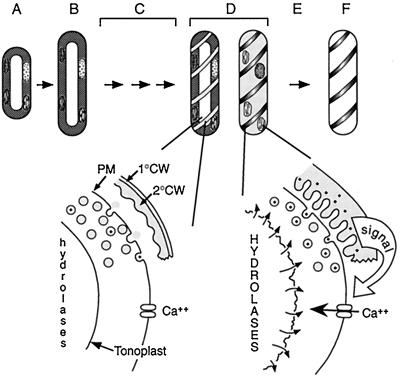
A simple model describes the coordination of cell death with secondary cell wall synthesis (Fig. 10). The secretion of secondary cell wall precursors during differentiation is accompanied by secretion of the 40-kD protease, leading to increasing protease activity in the extracellular matrix as secondary cell wall synthesis proceeds. The secreted protease activates Ca2+ influx, and upon realization of a critical extracellular activity of protease or the arrival of signal substrate, cell death is executed via Ca2+ influx. The accumulation of protease in the extracellular matrix would thus act to measure the progression of secondary cell wall synthesis, and activates cell death only after a critical amount of secondary cell wall synthesis is achieved.
Figure 10.
Model of the coordination of PCD and secondary cell wall synthesis during TE differentiation. A, Mechanically isolated mesophyll cells were induced to differentiate with auxin and cytokinin. B, After 24 h, the cells expand and some cells divide (not a prerequisite for differentiation). C, A number of molecular events presumably precede visible manifestations of differentiation, including the synthesis of hydrolytic enzymes that are likely sequestered in the vacuole. D, At approximately 72 h, differentiation is visibly manifested by the appearance of secondary cell wall thickenings. A 40-kD Ser protease (represented by dots in vesicles and cell wall) is secreted concomitantly with secondary cell wall materials (shaded vesicles), leading to an increase of extracellular protease activity as secondary cell wall synthesis proceeds. Approximately 6 h after the first appearance of secondary cell wall thickenings, a critical activity of protease is reached in the extracellular matrix, which triggers cell death, ending secondary cell wall synthesis. Cell death is initiated by an influx of Ca2+, leading to vacuole collapse and cessation of cytoplasmic streaming. E, Mixing of the hydrolytic vacuole with the cytoplasm leads to autolysis. F, The cell is completely cleared within 8 to 12 h, leaving the functional cell corpse composed of secondary cell wall. The secondary cell wall, represented here as having a stylized reticulate pattern, is drawn separated from the plasma membrane for clarity. 1°CW, Primary cell wall; 2°CW, secondary cell wall; PM, plasma membrane.
There are three possible mechanisms by which the secreted protease could activate Ca2+ influx. First, the protease may release a signal molecule from the extracellular matrix that activates ligand-gated or receptor-activated Ca2+ channels. Precedence for this mechanism is found in the hypersensitive response, which can be induced by “elicitor” molecules released from cell walls of pathogen or plant cells (Ebel and Cosio, 1994). Second, the protease might sever connections between the cytoskeleton and the extracellular matrix, which signals cell death. Connections between the primary cell wall and the plasma membrane have been demonstrated for plant cells (Roberts, 1990), and these connections must be severed or modified in TEs in the areas where secondary cell wall is synthesized between the primary cell wall and the plasma membrane. Specific arabinogalactan proteins have been immunolocalized to TEs (Dolan et al., 1995) and to cells predisposed to cell death in embryogenic suspension cultures (Pennell et al., 1992), and represent potential targets for protease action. Third, it is possible that the protease could activate channels by cleavage of an associated receptor. The thrombin and PAR-2 receptors are activated by proteolysis of the amino terminus, leading to exposure of a self-tethered ligand (Vu et al., 1991; Santulli et al., 1995; Verrall et al., 1997).
The extracellular matrix is of fundamental importance for the PCD of at least some animal cell types, and can be a primary regulator of apoptosis (Meredith et al., 1993; Frisch and Francis, 1994; Ruoslahti and Reed, 1994). Disruption of the extracellular matrix is involved in PCD during normal development in mammals (Talhouk et al., 1992; Bourdreau et al., 1995; Coucouvanis and Martin, 1995) and during Xenopus laevis metamorphosis (Patterson et al., 1995). Abnormal development, which leads to the loss of proper integrin-mediated contacts with the extracellular matrix, triggers PCD and may be a primary mechanism inhibiting neoplasia (Frisch and Francis, 1994). The epidermal karatinocyte, an animal cell type that undergoes terminal differentiation and PCD, has been proposed to use a secreted Ser protease to trigger cell death (Marthinuss et al., 1995), which is similar to the scenario we propose for TE cells in the present study.
The effects of the secreted protease must be confined to the dying cell, because neighboring cells are not killed by differentiating TEs. Previous observations indicate that such mechanisms exist. TEs contain a complement of hydrolases that completely degrade the contents of the cell, yet neighboring cells are not killed. The middle lamella is not hydrolyzed when a TE abuts a living parenchyma cell, but is hydrolyzed when two TEs differentiate side by side (O'Brien, 1970). This observation could indicate that hydrolysis is actively inhibited by living neighboring cells. A putative Ser protease inhibitor gene has been cloned from zinnia and is down-regulated in cultures at the mRNA level just before secondary cell wall synthesis and PCD (Ye and Varner, 1996). We found that exogenous soybean trypsin inhibitor (a Ser protease inhibitor) blocks TE differentiation and cell death, raising the possibility that the endogenous Ser protease inhibitor could negatively regulate differentiation or cell death.
Extracellular proteases and their inhibitors have been shown to be vital components of fundamental developmental processes in animals. For example, a secreted Ser protease in Drosophila melanogaster encoded by the Easter gene proteolytically releases a ligand (derived from the product of the Spatzle gene) that activates the receptor encoded by Toll (Morisato and Anderson, 1995; Misra et al., 1998). This pathway is responsible for establishing the dorsal-ventral asymmetry of the embryo. Our results indicate that secreted proteases may play important roles during plant development.
ACKNOWLEDGMENTS
We thank Drs. J. Dangl, R. Dietrich, and D. Boyes for critical reading of the manuscript and S. Whitfield for preparation of the figures. We also thank Drs. E. Beers, H. Fukuda, P. Low, and Z. Ye for helpful comments and criticisms.
Abbreviations:
- PCD
programmed cell death
- TE
tracheary element
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
Footnotes
Supported in part by the Development Mechanisms Program of the National Science Foundation. A.G. was supported by fellowships from the Institute of Marine and Agricultural Research and by the Graduate School and Department of Biology of the University of North Carolina at Chapel Hill.
LITERATURE CITED
- Beers E, Freeman T. Proteinase activity during tracheary element differentiation in Zinnia mesophyll cultures. Plant Physiol. 1997;113:873–880. doi: 10.1104/pp.113.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum H, Beier H, Gross H. Improved silver staining of plant proteins, RNA, and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- Bourdreau N, Sympson C, Werb Z, Bissell M. Suppression of ICE in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clarke P. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol. 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Cohen G. Caspases: the executioners of apoptosis. Biochem J. 1997;15:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coucouvanis E, Martin G. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- Dalessandro G, Roberts L. Induction of xylogenesis in pith parenchyma explants of Lactuca. Am J Bot. 1971;58:378–385. [Google Scholar]
- Dangl J, Dietrich R, Richberg M. Death don't have no mercy: cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobinson K, Lecomte N, Lazarovits G. Production of an extracellular trypsin-like protease by the fungal plant pathogen Verticillium dahliae. Can J Microbiol. 1997;43:227–233. doi: 10.1139/m97-031. [DOI] [PubMed] [Google Scholar]
- Dolan L, Linstead P, Roberts K. An AGP epitope distinguishes a central metaxylem initial from other vascular initials in the Arabidopsis root. Protoplasma. 1995;189:149–155. [Google Scholar]
- Ebel J, Cosio E. Elicitors of plant defense responses. Int Rev Cytol. 1994;148:1–35. [Google Scholar]
- Frisch S, Francis H. Disruption of epithelial cell-matrix interactions induce apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Komamine A. Establishment of an experimental system for the study of tracheary element differentiation from single cells isolated from the mesophyll of Zinnia elegans. Plant Physiol. 1980;65:57–60. doi: 10.1104/pp.65.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J, Sussex I. Programmed cell death: a way of life for plants. Proc Natl Acad Sci USA. 1996;93:12094–12097. doi: 10.1073/pnas.93.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groover A, DeWitt N, Heidel A, Jones A. Programmed cell death of plant tracheary elements differentiating in vitro. Protoplasma. 1997;196:197–211. [Google Scholar]
- He C, Morgan P, Drew M. Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol. 1996;112:463–472. doi: 10.1104/pp.112.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N, Burns M, Field C. Negative pressure in plants: a test of the balancing pressure technique. Science. 1995;270:1193–1194. [Google Scholar]
- Jones A, Dangl J. Logjam at the Styx. Trends Plant Sci. 1996;1:114–119. [Google Scholar]
- Kerr J, Wyllie A, Currie A. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legendre L, Heinstein P, Low P. Evidence for participation of GTP-binding proteins in elicitation of the rapid oxidative burst in cultured soybean cells. J Biol Chem. 1992;267:20140–20147. [PubMed] [Google Scholar]
- Levine A, Pennell R, Alvarez M, Palmer R, Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;6:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Marthinuss J, Andrade-Gordon P, Seiberg M. A secreted serine protease can induce apoptosis in Pam212 keratinocytes. Cell Growth Differ. 1995;6:807–816. [PubMed] [Google Scholar]
- Meredith J, Fazeli B, Schwartz M. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami A, Fukuda H. Transient and specific expression of a cysteine endopeptidase associated with autolysis during the differentiation of Zinnia mesophyll cells into tracheary elements. Plant Cell Physiol. 1995;36:1599–1606. [PubMed] [Google Scholar]
- Misra S, Hecht P, Maeda R, Anderson K. Positive and negative regulation of Easter, a member of the serine protease family that controls dorsal-ventral patterning in the Drosophila embryo. Development. 1998;125:1261–1267. doi: 10.1242/dev.125.7.1261. [DOI] [PubMed] [Google Scholar]
- Mittler R, Lam E. Identification, characterization, and purification of a tobacco endonuclease activity induced upon hypersensitive response cell death. Plant Cell. 1995a;7:1951–1962. doi: 10.1105/tpc.7.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Lam E. In situ detection of nDNA fragmentation during the differentiation of tracheary elements in higher plants. Plant Physiol. 1995b;108:489–493. doi: 10.1104/pp.108.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisato D, Anderson K. Signaling pathways that establish the dorsal-ventral pattern of Drosophila melanogaster. Annu Rev Genet. 1995;29:371–399. doi: 10.1146/annurev.ge.29.120195.002103. [DOI] [PubMed] [Google Scholar]
- Munnik T, Arisz S, de Vrije T, Musgrave A. G protein activation stimulates phospholipase D signaling in plants. Plant Cell. 1995;7:2197–2210. doi: 10.1105/tpc.7.12.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson D, Thornberry N. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- O'Brien T . Further observations on hydrolysis of the cell wall in the xylem. Protoplasma. 1970;69:1–14. [Google Scholar]
- Patterson D, Hayes W, Shi Y. Transcriptional activation of the matrix metalloproteinase gene stromelysin-3 coincides with thyroid hormone-induced cell death during frog metamorphosis. Dev Biol. 1995;167:252–262. doi: 10.1006/dbio.1995.1021. [DOI] [PubMed] [Google Scholar]
- Pennell R, Janniche L, Scofield G, Booij H, Vries S, Roberts K. Identification of a transitional state in the developmental pathway to carrot somatic embryogenesis. J Cell Biol. 1992;119:1371–1380. doi: 10.1083/jcb.119.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell R, Lamb C. Programmed cell death in plants. Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockman W, Sperry J, O'Leary J. Sustained and significant negative water pressure in xylem. Nature. 1995;378:715–716. [Google Scholar]
- Polakowska R, Piacentini M, Bartlett R, Goldsmith L, Haake A. Apoptosis in human skin development: morphogenesis, periderm, and stem cells. Dev Dyn. 1994;199:176–188. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- Rice R, Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977;11:417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Roberts A, Haigler C. Rise in chlorotetracycline fluorescence accompanies tracheary element differentiation in suspension cultures of Zinnia. Protoplasma. 1989;152:37–45. [Google Scholar]
- Roberts A, Haigler C. Tracheary-element differentiation in suspension-cultured cells of Zinnia requires uptake of extracellular Ca2+ Planta. 1990;180:502–509. doi: 10.1007/BF02411447. [DOI] [PubMed] [Google Scholar]
- Roberts K. Structures at the plant cell surface. Curr Opin Cell Biol. 1990;2:920–928. doi: 10.1016/0955-0674(90)90093-t. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Reed J. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Santulli R, Derian C, Darrow A, Tomko K, Eckardt A, Seiberg M, Scarborough R, Andrade-Gordon P. Evidence for the presence of a protease-activated receptor distinct from the thrombin receptor in human keratinocytes. Proc Natl Acad Sci USA. 1995;92:9151–9155. doi: 10.1073/pnas.92.20.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk R, Bissel M, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M, Northcote D. Identification and purification of a nuclease from Zinnia elegans: a potential molecular marker for xylogenesis. Planta. 1989;179:181–195. doi: 10.1007/BF00393688. [DOI] [PubMed] [Google Scholar]
- Verrall S, Ishii M, Chen M, Wang L, Tram T, Coughlin S. The thrombin receptor second cytoplasmic loop confers coupling to Gq-like G-proteins in chimeric receptors. J Biol Chem. 1997;272:6898–6902. doi: 10.1074/jbc.272.11.6898. [DOI] [PubMed] [Google Scholar]
- Vu T, Hung D, Wheaton V, Coughlin S. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Wang M, Oppedijk B, Lu X, Van Duijn B, Schilperoort R. Apoptosis in barley aleurone during germination and its inhibition by abscisic acid. Plant Mol Biol. 1996;32:1125–1134. doi: 10.1007/BF00041396. [DOI] [PubMed] [Google Scholar]
- Ye Z, Droste D. Isolation and characterization of cDNAs encoding xylogenesis-associated and wounding-induced ribonucleases in Zinnia elegans. Plant Mol Biol. 1996;30:697–709. doi: 10.1007/BF00019005. [DOI] [PubMed] [Google Scholar]
- Ye Z, Varner J. Induction of cysteine and serine proteases during xylogenesis in Zinnia elegans. Plant Mol Biol. 1996;30:1233–1246. doi: 10.1007/BF00019555. [DOI] [PubMed] [Google Scholar]