Abstract
Background
Standard therapy (ST) for postoperative pain after knee and hip replacement at the Hamilton Health Sciences Henderson Hospital consists of epidural analgesia or patient-controlled analgesia for the first 48 hours, followed by oral or parenteral analgesics, or both, on an as-needed basis. We compared the efficacy and safety of scheduled controlled-release (CR) oxycodone hydrochloride (OxyContin; Purdue Pharma, Pickering, Ont.) and ST for postoperative pain 48 hours after primary knee and hip replacement.
Methods
In 2 separate 3-week studies of similar design, pain intensity, pain relief, length of hospital stay, analgesic use and side effects of CR oxycodone (n = 70) and ST (n = 101) were evaluated. In the CR oxycodone trial, a dose de-escalation protocol was used.
Results
At the time of discharge from hospital, patients in the CR oxycodone group recorded lower mean (and standard deviation) pain intensity scores than the ST group (20.2 [17.9] v. 27.7 [21.5] mm on a 100-mm visual analogue scale; p = 0.021). Length of hospital stay was 5.5 and 6.4 days for the CR oxycodone and ST groups respectively (p < 0.001). CR oxycodone patients used less opioid (morphine equivalent) while in hospital than ST patients (p < 0.001), and the average number of daily administrations of analgesics in hospital was 2.1 and 3.5 for CR oxycodone and ST patients respectively (p < 0.001). ST patients reported more nausea and vomiting, pruritus and fever than the CR oxycodone patients, but less somnolence, constipation, dizziness, confusion and tachycardia.
Conclusions
CR oxycodone every 12 hours is as effective as ST in treating postoperative pain but length of hospital stay was shorter and analgesic administration in the hospital was used less frequently, providing potential hospital cost savings and reduced use of health care resources.
Abstract
Contexte
La thérapie standard (TS) contre la douleur postopératoire après une arthroplastie du genou et de la hanche à l'Hôpital Henderson des sciences de la santé de Hamilton consiste en une analgésie péridurale ou contrôlée par le patient pendant les 48 premières heures, suivie d'une analgésie orale ou parentérale, ou les deux, au besoin. Nous avons comparé l'efficacité et l'innocuité du chlorhydrate d'oxycodone à libération contrôlée (LC) périodique (OxyContin; Purdue Pharma, Pickering [Ontario]) et celle de la TS contre la douleur postopératoire 48 heures après une arthroplastie primitive du genou et de la hanche.
Méthodes
Au cours de deux études distinctes de conception semblable, d'une durée de trois semaines, on a évalué l'intensité de la douleur, le soulagement de la douleur, la durée de l'hospitalisation, l'utilisation d'analgésiques et les effets secondaires de l'oxycodone LC (n = 70) et de la TS (n = 101). Au cours de l'essai sur l'oxycodone LC, on a suivi un protocole de réduction graduelle de la dose.
Résultats
Au moment de leur départ de l'hôpital, les patients du groupe recevant l'oxycodone LC ont déclaré des scores d'intensité de la douleur moyenne (et écart type) plus faibles que ceux du groupe TS (20,2 [17,9] c. 27,7 [21,5] mm sur une échelle analogique visuelle de 100 mm; p = 0,021). L'hospitalisation a duré 5,5 et 6,4 jours pour les patients qui ont reçu l'oxycodone LC et la TS respectivement (p < 0,001). Les patients qui ont reçu l'oxycodone LC ont utilisé moins d'opioïdes (équivalent morphine) pendant leur séjour à l'hôpital que ceux du groupe TS (p < 0,001), et le nombre d'administrations quotidiennes d'analgésiques à l'hôpital s'est établi en moyenne à 2,1 et 3,5 pour les patients qui ont reçu l'oxycodone LC et la TS respectivement (p < 0,001). Les patients qui ont reçu la TS ont signalé plus de nausées et de vomissements, de prurit et de fièvre que ceux qui ont reçu l'oxycodone LC, mais moins de somnolence, de constipation, d'étourdissements, de confusion et de tachycardie.
Conclusions
L'oxycodone LC administrée aux 12 heures est aussi efficace que la TS pour traiter la douleur postopératoire, mais l'hospitalisation a duré moins longtemps et l'on a administré des analgésiques à l'hôpital moins souvent, ce qui pourrait réduire les coûts des hôpitaux et l'utilisation des ressources du système de santé.
Joint replacement is a well established procedure that provides pain relief and restores mobility and stability to arthritic joints.1 More than 40 000 total hip and knee joint replacements are performed in Canada each year,2 and the volume continues to rise, increasing the demand on hospital resources.
For 1999–2000, the national average length of hospital stay for total hip and total knee replacement was 10.1 days and 8.5 days respectively.3 Improvements in anesthetic, surgical and postoperative pain-management techniques as well as patient education initiatives contributed to these reductions.
Patients who undergo joint replacement generally experience intense pain in the immediate postoperative period, resulting in delayed discharge, unanticipated hospital readmission and frequent contact with the physician.4,5,6 Opioid analgesics are the mainstay of treatment for moderate to severe pain. Although opioid analgesics may be administered by a variety of routes, oral dosing is usually the most convenient and least expensive route and is appropriate as soon as the patient can tolerate oral intake.7
Fixed-dose, immediate-release, combination preparations containing codeine or oxycodone plus acetaminophen, prescribed on an as-needed basis, are often used for the management of postoperative surgical pain once the patient can tolerate oral opioids. These preparations must be given every 4–6 hours. A delay in administration, especially when ordered on an as-needed basis, may result in lower plasma opioid concentrations and the re-emergence of pain.8 Since patients will usually only request medication once pain levels have risen to a level sufficient to make a request, re-emergence of pain almost invariably occurs. There are further delays while the nurse responds to the call, obtains the medications, completes the appropriate documentation and administers the medication. This is followed by a delay until therapeutic blood levels of the active drug are attained.
Controlled-release (CR) opioid preparations provide a reliable means of maintaining stable serum concentrations and avoiding the erratic fluctuations that may characterize immediate-release formulations; they also free patients from the onus of requesting as-needed pain medication. Use of CR preparations may result in improved pain relief and shorter convalescence. Cheville and associates9 reported superior improvement in active and passive joint range-of-motion and accelerated discharge in patients who underwent total knee arthroplasty and were managed postoperatively with CR oxycodone.
OxyContin (Purdue Pharma, Pickering, Ont.) is a single-entity CR preparation that is designed to provide controlled delivery of oxycodone over a 12-hour period and is indicated for the management of moderate to severe pain. The CR delivery system used in this formulation provides a biphasic absorption profile.10,11 This is characterized by initial prompt release, which promotes early absorption with an onset of action within 1 hour for most patients, similar to the onset of action for immediate-release combination preparations.12 Although frequently used in the management of chronic pain, oxycodone has been shown to be effective in the management of shorter- term pain conditions.8,9,12
An open-label evaluation of the efficacy and safety of oral CR oxycodone (OxyContin) for postoperative pain after hip or knee surgery (phase I) was conducted by the Hamilton Arthroplasty Group at the Hamilton Health Sciences (HHS) Henderson Hospital.
To compare these results with standard therapy (ST), evaluation of ST for postoperative pain relief after hip or knee replacement was also conducted (phase II).
The objective of these studies was to assess and compare the efficacy, safety and benefits of CR oxycodone and ST in the treatment of postoperative pain beginning 48 hours after primary knee or hip replacement.
Methods
Two separate, 3-week studies of similar design were conducted. Phase I examined the treatment of postoperative pain with CR oxycodone. Phase II examined the treatment of postoperative pain with ST.
Subjects
In phase I, 93 patients were enrolled between September 1999 and January 2000, and 70 patients completed the study. In phase II, 101 patients were enrolled in the study between January 2001 and September 2001. Patients scheduled to undergo elective primary unilateral total knee or hip replacement secondary to osteoarthritis and able to comply with the study protocol and complete study diaries were permitted to enter the study. Patients were excluded for the following reasons: allergy to any opioid, a history of drug abuse, ingestion of opioid analgesics within 24 hours before the operation, recipient of workers' compensation benefits, inflammatory arthritis or significant pain of other origin.
The protocols and informed consent were approved by the research ethics board at the study centre. The patients gave prior, written and informed consent.
Medication
For the first 48 hours postoperatively, patients received intravenous morphine through patient-controlled analgesia (PCA) or epidural administration of a combination of morphine, fentanyl and bupivacaine. When this was discontinued, patients received the following:
Phase I: oral, CR oxycodone (OxyContin 10-, 20- and 40-mg tablets); rescue medication: morphine 7.5–10 mg intramuscularly every 3–4 hours as needed for severe pain (in hospital) and acetaminophen 325–650 mg orally every 4 hours as needed (after discharge), or
Phase II: standard analgesics, according to the physician's written orders. The most common regimen was acetaminophen plus codeine (A/C; 300 mg/30 mg) 1–2 tablets orally every 3–4 hours as needed. Rescue medication was meperidine intramuscularly every 3–4 hours as needed (in hospital) for severe pain and acetaminophen 325 mg as needed (after discharge). Alternative oral opioid analgesics included acetaminophen plus codeine (A/C; 300 mg/15 mg) and oxycodone and acetaminophen combinations.
No other analgesics were permitted during the study. All patients were instructed not to add other prescription or nonprescription analgesic agents to their therapeutic regimen.
Procedures
Phase I was a randomized, balanced, parallel group comparison between CR oxycodone administered every 12 hours and CR oxycodone administered 12-hourly, as required, with respect to degree of analgesia, clinical effectiveness and severity of side effects. As there were no statistical differences in any outcomes between these 2 groups, including pain levels, dose of oxycodone or frequency of oxycodone administration, the data were pooled.
Phase II was performed at the same centre and was an open-label examination of standard analgesic therapy with respect to degree of analgesia, clinical effectiveness and severity of side effects.
In phase I, surgery occurred on day 0, and all patients received 30 mg of CR oxycodone as their first dose of study medication on the morning of the second day after surgery (day 2). Baseline pain levels were recorded after PCA or epidural analgesia was discontinued once pain was of moderate intensity. Subsequent doses of CR oxycodone followed a structured dose de-escalation schedule. Patients who required rescue medication within the first 12-hour period on day 2 had a dose increase up to 40 mg every 12 hours; on days 4 and 5, these patients received 30 mg every 12 hours; on days 6 and 7, they received 20 mg every 12 hours; and on days 8–21, they received 10 or 20 mg every 12 hours. Patients who did not require rescue medication within the first 12-hour period on day 2, had a dose of 30 mg every 12 hours on days 2 and 3; they received 20 mg every 12 hours on days 4, 5 and 6; and 10 or 20 mg every 12 hours on days 7–21. Patients received CR oxycodone according to the de-escalation schedule either every 12 hours (scheduled group) or every 12 hours as needed, up to twice daily (as-needed group). While in hospital, patients who reported uncontrolled pain (≥40 mm on a 100-mm visual analogue scale [VAS]) following the appropriate dose of CR oxycodone were administered rescue medication in the form of a morphine bolus (7.5–10 mg) intramuscularly. Rescue medication was not administered until at least 1 hour had elapsed after the dose of CR oxycodone. During the post-discharge period, patients were provided rescue medication in the form of 325-mg acetaminophen. Patients were instructed to take 1–2 tablets of acetaminophen every 4 hours for pain unrelieved by CR oxycodone.
In the event of inadequate pain control, unacceptable side effects or the development of postoperative complications requiring medications contraindicated by the protocol, patients were withdrawn from the study by the investigator, and treatment with alternative analgesics was initiated.
In phase II, surgery occurred on day 0 and ST was begun following discontinuation of PCA or epidural analgesia, approximately 48 hours postoperatively. Baseline pain was recorded concomitant with cessation of PCA or epidural administration. ST was based on the physician's written orders.
Efficacy and safety evaluations were based on the patient diary and on assessments completed by patients directly on the case report form during the first 4 hours after the first dose of study medication and during the follow-up visit. Pain intensity was assessed at regular intervals using a 100-mm VAS. The VAS was an unmarked line, bounded on the left by “no pain” and on the right by “excruciating pain.” During the hospital stay, patients were issued a daily diary (diary 1) to complete the visual analogue and categorical scales for pain intensity and pain relief 3 times per day (morning 0700–0900, afternoon 1300–1500 and evening 1700–2100). In phase I, the times to first rescue analgesic, the dose of rescue analgesics and the number of rescue analgesics used by each patient were also recorded. The same measures were recorded in phase II, with the addition of time and type of all analgesics taken.
Discharge criteria for total knee or hip arthroplasty at the HHS Henderson Hospital specify that patients must transfer independently, be able to climb stairs as appropriate, walk safely with a walker and manage an exercise protocol independently. Total hip arthroplasty patients must also demonstrate knowledge and safety in hip precautions with respect to flexion, adduction and rotation. At the time of hospital discharge patients were asked the following question: How satisfied are you with the pain relief treatment you received while you were in the hospital? Patients were also asked to provide an overall pain relief rating (0 = not effective, 1 = slightly effective, 2 = moderately effective, 3 = highly effective).
For a further 2 weeks after discharge, patients in phase I recorded in the daily diary (diary 2) the number of CR oxycodone tablets they took and the date and time they were taken. Also patients were instructed to record the date, time and the number of 325-mg acetaminophen tablets taken to alleviate pain that was not controlled following the appropriate dose of CR oxycodone. Diary 2 contained the same visual analogue and categorical scales as those in diary 1. In phase II, patients recorded the same measures for all analgesics taken.
At 2 weeks postoperatively in both phases, patients were asked to complete the brief pain inventory-(BPI) short form, a structured self-report questionnaire that has reliability and validity as a measure of pain in studies of the effectiveness of pain treatment.13 Most questions were scored on a 0–10 scale with 0 representing no pain or difficulty and 10 representing maximum pain or difficulty. A composite pain score (Pain Intensity) and composite functional ability score (Functional Impairment) were calculated by summing the appropriate individual items for each. In addition, a pain relief measure (% of relief afforded) and hours measure (the number of hours for which pain medications are not required) were reported. For each suspected adverse event noted in the patient diaries or the progress notes, the severity of the event was rated as none, mild, moderate or severe.
At the end of the 3-week study period, patients returned to the Arthroplasty Clinic for a follow-up visit. Patients returned with the completed diary, as well as all unused medications and empty medication containers.
Statistics
In phase I, all patients enrolled who had data from at least the first 14 days of pain assessments in the patient diaries and did not have major protocol violations were included in evaluations of efficacy; all patients were included for evaluation of safety. In phase II, all patients who completed 1 post-baseline assessment were eligible for both efficacy and safety analysis.
Daily mean scores for pain intensity (VAS, ordinal) and pain relief (ordinal) were compared by treatment using multivariate repeated measures analysis of variance. Overall scores were compared by analysis of covariance with baseline pain intensity as the covariate when possible. Length of hospital stay was compared by treatment using analysis of covariance. Opioid analgesic dose was compared by converting all doses of CR oxycodone and ST to morphine equivalents. The number of opioid analgesic administrations and number of administrations of all analgesics in phases I and II were also compared.
All adverse events were coded (COSTART IV) using preferred terms, and statistical significance was defined as p < 0.05 for a 2-tailed hypothesis.
Results
Ninety-three patients were enrolled in phase I; 70 (75.3%) completed it. There were 27 men and 43 women (mean [and standard deviation (SD)]) age 67.0 [9.4] yr). Thirty-two patients underwent total hip arthroplasty and 38 underwent total knee arthroplasty. Twenty-three patients dropped out of the study: 11 were “voluntary withdrawals,” 5 because of inadequate pain control, 1 for intercurrent illness unrelated to study drugs, 1 for protocol violation, 1 because of noncompliance, 1 for an adverse event and 3 for other reasons. One hundred and one patients were enrolled in phase II; 46 men and 55 women (mean [and SD] age 66.2 [9.5] yr). Fifty-one patients underwent total hip arthroplasty and 50 total knee arthroplasty. Twenty-nine patients did not fully complete diaries 1 and 2: 6 because of noncompliance, 4 were voluntary withdrawals, 4 because of protocol violation, 3 for intercurrent illness, 1 for an adverse event, 5 were lost to follow-up and 6 for other reasons.
Mean (and SD) baseline pain, recorded on postoperative day 2, was 63.3 (17.2) mm in phase I and 42.0 (29.9) mm in phase II. Baseline pain in phase II was recorded concomitant with cessation of PCA or epidural analgesia rather than waiting for pain levels to rise, and has therefore not been compared to phase I, in which pain was allowed to develop before administration of the first dose. Subsequent to the measurement of baseline pain, patients in phase I reported mean (and SD) VAS pain scores of 43.4 (23.8) mm on day 2, a significant reduction from baseline VAS pain scores (p < 0.001), with a steady decrease in pain over the 3-week period (31.0, 24.7, 18.6 mm at weeks 1, 2 and 3, respectively; p < 0.001). Pain intensity VAS scores reported in phase I were not significantly different from those in phase II (37.3, 32.3, 21.2, 15.6 mm at day 2 and weeks 1, 2 and 3, respectively; p = 0.080, p = 0.638, p = 0.252 and p = 0.262). Patients in phase I also showed a significant decrease in ordinal pain scores from baseline to weeks 1, 2 and 3 (p < 0.001). Ordinal pain levels for weeks 1, 2 and 3 reported in phase II were not significantly different from those in phase I (p = 0.060, p = 0.684 and p = 0.926, respectively).
Assessments at discharge showed that the mean (and SD) length of hospital stay was 5.5 (1.5) days in phase I compared with 6.4 (1.3) days in phase II (p < 0.001). Mean (and SD) pain intensity at discharge was significantly higher in phase II (20.2 [17.9] v. 27.7 [SD 21.5] mm; p = 0.021). Mean (and SD) patient satisfaction for phase I and phase II groups was 2.7 (0.5) and 2.6 (0.7) respectively (p = 0.177) measured on a scale from 0 to 3. Of patients on CR oxycodone and ST, 74% and 67% respectively were “very satisfied” with their pain relief.
The average number of hospital administrations of opioid analgesics was significantly greater for patients in phase II than for those in phase I during week 1 (p < 0.001). When the administrations of other analgesics, such as acetaminophen, were included, the mean (and SD) daily number of administrations of all analgesics for postoperative days 2–6 was significantly higher for patients in phase II than those in phase I (2.1 [0.5] v. 3.5 [1.0] respectively; p < 0.05; Fig. 1).
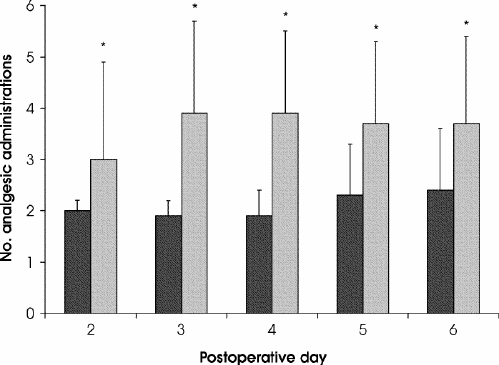
FIG. 1. Average daily number of administered analgesic doses for postoperative days 2 through 6. * p < 0.001. Black columns = controlled-release oxycodone, grey columns = standard therapy.
When the average oral morphine equivalent dose of opioids taken by day and week in each group was calculated,14 using standard opioid conversions, less opioid was administered in phase II on postoperative days 2 and 3, whereas, on days 4–6, higher amounts of opioid were administered in phase II (Fig. 2). Overall, during week 1, significantly higher amounts of opioid were administered in the hospital in phase II than in phase I (96.1 and 89.4 mg/d respectively; p < 0.001). During week 2, significantly higher amounts of opioid were taken by patients in phase II than in phase I (41.8 and 35.3 mg/d respectively; p = 0.005). During week 3, significantly higher amounts of opioid were taken in phase I than in phase II (20.7 and 5.7 mg/d respectively; p < 0.001).
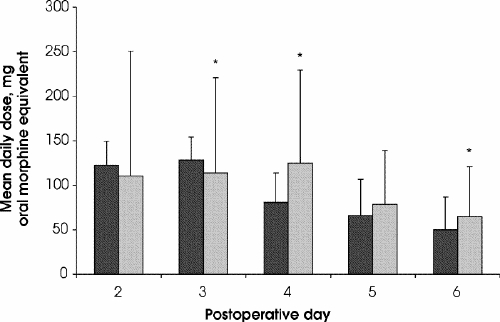
FIG. 2. Postoperative use of narcotic analgesics for postoperative days 2 through 6. * p < 0.05. Black columns = controlled-release oxycodone, grey columns = standard therapy.
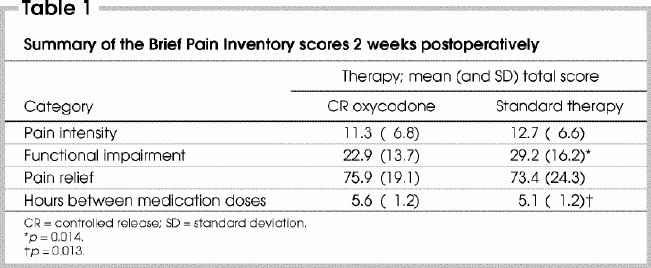
Summary of the BPI at 2 weeks postoperatively found pain equally well controlled between phases, although patients displayed less functional impairment in phase I (Table 1).
Table 1
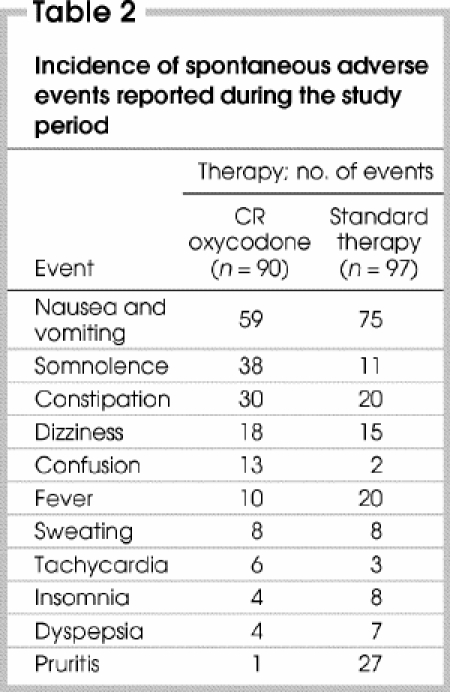
The ST group reported more nausea and vomiting, pruritis and fever but less somnolence, constipation, dizziness, confusion and tachycardia than the CR oxycodone group (Table 2). Ninety patients in phase I (97% of enrolled patients), including those who dropped out, and 97 patients in phase II (96% of enrolled patients) reported an adverse event during the study.
Table 2
Discussion
The rising demand for total joint replacement is associated with increased pressures on hospital beds and, therefore, a need to safely reduce hospital stay. Inadequate postoperative pain control may result in slower patient mobilization and prolonged stay. Therefore, there is a need for therapy that provides timely and aggressive postoperative analgesia to accelerate rehabilitation and rapid, functional recovery.
Although immediate-release opioids, primarily codeine combination preparations, are frequently prescribed on an as-needed basis for pain management after joint replacement when oral therapy is tolerated, it is recommended that all patients requiring opioid analgesics for longer than 48 hours postoperatively be administered these drugs on a fixed-dose schedule.7 As in dosing with other drugs that require a steady blood level to remain effective, interruption of an around-the-clock dosage schedule, especially during the hours of sleep, may cause pain. CR oxycodone is designed to rapidly achieve and maintain effective blood concentrations of the drug over a 12-hour interval, thereby reducing the number of daily doses, facilitating uninterrupted nighttime sleep and decreasing nursing time for drug administration in comparison with immediate-release opioid preparations.
The onset of analgesia with CR oxycodone is equivalent to that of immediate-release preparations.12 The delivery system in the CR oxycodone formulation provides a biphasic absorption profile characterized by an initial prompt release, which results in onset of action within 1 hour in most patients.12
The phase I study results suggest that the fixed dose de-escalation protocol for CR oxycodone provides adequate pain control during the postoperative period for most patients. There was a significant reduction in pain intensity during the early postoperative period. Similarly, a tapering dose of CR oxycodone in patients who underwent outpatient anterior cruciate ligament surgery has been shown to improve postoperative sleep patterns and overall analgesic efficacy compared with shorter-acting opioid analgesia.8 Use of CR opioids, in combination with local anesthetic infiltration and adjuvant agents, has been suggested for the management of severe postoperative pain in patients who undergo more involved surgical procedures, more painful procedures, or in those who might otherwise be expected to have a high postoperative opioid dose requirement.15
In this indirect comparison of CR oxycodone and ST, results showed that patients receiving CR oxycodone experienced pain control similar to those receiving ST, as measured by pain intensity and relief by day or week. All patients experienced a stepwise reduction in pain intensity during the first 5 postoperative days, reaching a plateau at the end of the first week, with an approximately 55% decrease from the baseline. At the time of discharge from hospital, patients in the CR oxycodone group had significantly lower mean pain intensity scores than those in the ST group.
Patients in the CR oxycodone group had a significantly shorter hospital stay than patients in the ST group. This may have been due to more consistent control of pain, as evidenced by the lower VAS pain scores at the time of discharge.
After conversion of administered opioids to daily oral morphine equivalents, patients on ST were administered significantly higher opioid doses in hospital than those receiving CR oxycodone. Also, the number of administrations of analgesics during days 2–6 was significantly higher in the ST group than the CR oxycodone group.
In post-discharge assessments, patients in both treatment groups experienced a significant decrease in their pain intensity scores at each weekly assessment. There was a steady decline in the mean scores over the 3-week period.
Both ST and CR oxycodone therapy were well tolerated. The patients in both groups experienced typical opioid-related adverse events, although patients receiving CR oxycodone had a markedly lower level of pruritus and a greater incidence of transient confusion. However, confusion was not of sufficient clinical severity to adversely impact compliance with postoperative rehabilitation. It should be noted that patients in the CR oxycodone group had a shorter total hospital stay despite the reported incidence of postoperative confusion.
Limitations
Outcome differences between phases may have to be interpreted in light of the limitations in study design (i.e., 2 separate studies rather than a prospective comparison). Different analgesic rescue used between phases could potentially affect pain in different ways. Although specific criteria exist at the site regarding hospital discharge, none were formalized in the study protocol, thus the difference in length of stay between treatments may have to be interpreted with caution.
As the demand for total joint replacement grows, the pressure on existing hospital and rehabilitation resources necessitates a clear focus on timely recovery of function with effective control of symptoms. The results of this study show that the safety and efficacy of CR oxycodone given every 12 hours were similar to ST in the treatment of postoperative pain. However, CR oxycodone therapy was associated with a shorter length of hospital stay and lower frequency of analgesic administration.
Abstract presented at the Canadian Pain Society Annual Conference, Winnipeg, Man., May 23–25, 2002.
Acknowlegements: Special thanks to Stacey Dumoulin, RN, and Laurie Kinnear, RN, for their work in coordinating phases I and II of the study, and to Danielle Petruccelli, MLIS, for assistance with manuscript preparation.
Supported by a research grant from Purdue Pharma, Canada.
Competing interests: Authors A.C Darke, G.A.E. Donnelly, L.W. Payne, J.L. Reiz and Z. Harsanyi are full-time employees of Purdue Pharma and P.C. Micelli is a past employee and is a paid consultant for Purdue Pharma.
Correspondence to: Dr. Justin de V. de Beer, Hamilton Arthroplasty Group, Hamilton Health Sciences Henderson Hospital, 711 Concession St., Hamilton ON L8V 1C3; fax 905 389-5617; petrucce@hhsc.ca
Accepted for publication Feb. 17, 2004.
References
- 1.Bernard AA, Zrinzo LU. Joint replacement. The final solution? Adv Exp Med Biol 1999;455:415-61. [PubMed]
- 2.Canadian Joint Replacement Registry (CJRR). New national initiative to provide better information on hip and knee surgeries. Newswire 2000:June 2.
- 3.Canadian Institute for Health Information. Number of total hip and total knee replacement procedures performed in Canada, 1994/1995 to 1999/2000 [Figure 1]. Hospital Morbidity Database, 2002 Jan 30. Available: http://secure.cihi.ca/cihiweb/en/media_30jan2002_fig1_e.html (accessed 2005 May 8).
- 4.Gold BS, Kitz DS, Lecky JH, Neuhaus JM. Unanticipated admission to the hospital following ambulatory surgery. JAMA 1989;262:3008-10. [PubMed]
- 5.Ghosh S, Sallam S. Patient satisfaction and postoperative demands on hospital and community services after day surgery. Br J Surg 1994;81:1635-8. [DOI] [PubMed]
- 6.Williams BA, Deriso BM, Figallo CM, Anders JW, Engel LB, Sproul KA, et al. Benchmarking the perioperative process. III. Effects of regional anesthesia clinical pathway techniques on process efficiency and recovery profiles in ambulatory orthopedic surgery. J Clin Anesth 1998;10:570-8. [DOI] [PubMed]
- 7.Acute Pain Management Guideline Panel. Acute pain management: operative or medical procedures and trauma. Clinical practice guideline. Rockville (MD): Agency for Health Care Policy and Research, U.S. Department of Health and Human Resources, Public Health Service; 1992. AHCPR Publication No. 92-0032.
- 8.Reuben SS, Connelly NR, Maciolek H. Postoperative analgesia with controlled- release oxycodone for outpatient anterior cruciate ligament surgery. Anesth Analg 1999;88:1286-91. [DOI] [PubMed]
- 9.Cheville A, Chen A, Oster G, McGarry L, Narcessian E. A randomized trial of controlled-release oxycodone during inpatient rehabilitation following unilateral total knee arthroplasty. J Bone Joint Surg Am 2001;83:572-6. [DOI] [PubMed]
- 10.Kaiko RF, Benziger DP, Fitzmartin RD, Burke BE, Reder RF, Goldenheim PD. Pharmacokinetic–pharmacodynamic relationships of controlled-release oxycodone. Clin Pharmacol Ther 1996;59:52-61. [DOI] [PubMed]
- 11.Patt RB. Using controlled-release oxycodone for the management of chronic cancer and noncancer pain. Am Pain Soc Bull 1996;6:1-6.
- 12.Sunshine A, Olson NZ, Colon A, Rivera J, Kaiko RF, Fitzmartin RD, et al. Analgesic efficacy of controlled-release oxycodone in postoperative pain. J Clin Pharmacol 1996;36:595-603. [DOI] [PubMed]
- 13.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129-38. [PubMed]
- 14.OxyContin® product monograph. In: Compendium of Pharmaceuticals and Specialties. 37th ed. Ottawa: Canadian Pharmacists Association; 2002. p. 1243-5.
- 15.Crews JC. Multimodal pain management strategies for office-based and ambulatory procedures. JAMA 2002;288:629-32. [DOI] [PubMed]


