Abstract
Background
The optimal route of nutrition in severe pancreatitis is controversial. Parenteral nutrition (PN) is preferred, but enteral nutrition (EN) promises to attenuate inflammation and prevent sepsis. We hypothesized that EN was at least equivalent to PN in reducing inflammation, providing effective nutrition and being cost-effective.
Methods
We conducted a randomized controlled trial comparing PN to EN in pancreatitis in an academic, multi-institutional, tertiary care health system. We screened 728 consecutive patients. Twenty-eight patients with a Ranson's score greater than 2 who did not tolerate clear fluids 4 days after admission were randomized: 18 to PN and 10 to EN. Both groups were provided daily 105 kJ (25 kcal)/kg and 1.5 g/kg of protein, respectively, until they could tolerate a regular diet.
Results
C-reactive protein in EN patients was reduced by 50% 5 days faster than PN patients (Wilcoxon test, p = 0.09). Both groups received a similar number of kilojoules and achieved near normal prealbumin and 24-hour urinary nitrogen values. Neither regimen caused a change in cholecystokinin levels. Overall mortality was 4.9% (3 patients in the PN group). In 5 patients (4 PN, 1 EN) there were infected pancreatic collections. Nine EN patients dislodged the nasojejunal tube. EN had an average cost of $1375 per patient compared with $2608 for PN (p = 0.08). After sensitivity analysis, EN cost $957 compared with $2608 for PN (p = 0.03).
Conclusions
EN or PN is safe and provides adequate nutrition in severe pancreatitis. EN shows a trend toward faster attenuation of inflammation, with fewer septic complications and is the dominant therapy in terms of cost-effectiveness. This study favours EN for nutritional support in severe pancreatitis.
Abstract
Contexte
La voie optimale de nutrition dans les cas de pancréatite sévère soulève la controverse. On préfère la nutrition parentérale (NP), mais la nutrition entérale (NE) promet d'atténuer l'inflammation et de prévenir la septicémie. Nous avons posé comme hypothèse que la NE était au moins équivalente à la NP sur les plans de la réduction de l'inflammation, de l'apport d'une nutrition efficace et de l'efficacité des coûts.
Méthodes
Nous avons procédé à un essai contrôlé et randomisé pour comparer la NP à la NE dans des cas de pancréatite dans un réseau universitaire à établissements multiples de soins de santé tertiaires. Nous avons filtré 728 patients consécutifs. Vingt-huit patients qui ont obtenu un score de Ranson de plus de 2 et qui ne toléraient pas les liquides clairs quatre jours après l'admission ont été affectés par randomisation : 18 à la NP et 10 à la NE. Les deux groupes ont reçu tous les jours 105 kJ (25 kcal)/kg et 1,5 g/kg de protéines respectivement, jusqu'à ce qu'ils puissent tolérer une alimentation régulière.
Résultats
La protéine C-réactive chez les patients nourris par NE a diminué de 50 % cinq jours plus rapidement que chez les patients nourris par NP (test de Wilcoxon, p = 0,09). Les deux groupes ont reçu un nombre semblable de kilojoules et ont obtenu des valeurs quasi normales pour la préalbumine et l'azote uréique à 24 heures. Aucun des deux régimes n'a modifié les concentrations de cholécystokinine. Le taux global de mortalité s'est établi à 4,9 % (trois patients du groupe NP). Chez cinq patients (4 NP, 1 NE), on a prélevé des échantillons pancréatiques infectés. Neuf patients nourris par NE ont délogé le tube nasojéjunal. La NE a coûté en moyenne 1375 $ par personne comparativement à 2608 $ pour la NP (p = 0,08). Après analyse de sensibilité, les coûts s'élevaient à 957 $ pour la NE comparativement à 2608 $ pour la NP (p = 0,03).
Conclusions
La NE ou la NP sont sans danger et fournissent une nutrition adéquate en cas de pancréatite sévère. La NE a tendance à atténuer plus rapidement l'inflammation, à réduire le nombre de complications septicémiques et constitue la thérapie dominante par sa rentabilité. Cette étude favorise la NE pour le soutien nutritionnel dans des cas de pancréatite sévère.
The use of nutrition to support patients with acute pancreatitis has evolved over time. Traditional approaches prescribed fasting, under the presumption that resting the pancreas avoided stimulation and production of pancreatic digestive enzymes that would further promote the disease process. Parenteral nutrition (PN) became the standard nutritional support after Feller and associates1 reported a reduction in mortality and complications for patients supported with PN. Currently, enteral nutrition (EN) is being used more frequently in acute pancreatitis2,3,4,5 after recent studies of trauma and burn management showed that EN has fewer complications, offers the potential for immune modulation and disease attenuation, reduces the incidence of sepsis and is less expensive.6,7
It is generally agreed that mild, acute pancreatitis has little impact on the patient's nutritional status. Accordingly, most patients with mild pancreatitis are kept fasting until they can resume oral intake. Patients with severe, acute pancreatitis, however, suffer increased resting energy requirements and reductions in protein mass. Prolonged fasting in these patients exacerbates these metabolic changes and may influence the outcome. Therefore, nutritional support may improve outcome and reduce complications in severe pancreatitis.
The optimal route of administering nutrition to support patients with severe, acute pancreatitis is controversial. PN provides a simple and consistent flow of kilojoules and maintains lean body mass while avoiding problems with adynamic ileus. But, it is limited by problems of catheter-related sepsis, may worsen the inflammatory process, alters gut permeability and has not improved the death rate. EN promises gut integrity with immune modulation, attenuation of the inflammatory response, fewer septic complications and less cost; but there are concerns about delivery with adynamic ileus and pancreatic stimulation.
We conducted a randomized, controlled trial and economic evaluation comparing PN to EN in severe acute pancreatitis. The primary outcome was attenuation of the inflammatory response. The secondary outcomes were the effectiveness of nutrition, the natural history and morbidity of pancreatitis, the morbidity from each nutritional modality and an economic evaluation of the nutrition technology.
Methods
Between July 15, 1999, and Dec. 15, 2001, patients with pancreatitis of all causes were identified and screened for eligibility at 3 teaching hospitals associated with the University of Alberta, serving a population of more than 1 million. Eligible patients were required to have acute pancreatitis, a Ranson's score8 (calculated by counting 1 point for each of the criteria met over the 48-hour period) of 3 or greater and inability to tolerate oral fluids after a maximum time from admission of 96 hours. Patients were excluded if they did not meet these criteria (Appendix 1), if they were younger than 18 years, unable to accept enteral nutrition via the gastrointestinal tract or already receiving nutritional support. After giving their informed consent, patients were blindly randomized to receive either PN or EN and stratified by hospital, by means of computer-generated assignment placed in sealed, opaque envelopes. The University of Alberta Health Research Ethics Board approved this protocol.
General management of pancreatitis
The attending medical staff were instructed to manage patients according to the standard of care within the region. Care of patients with severe, acute pancreatitis at the 3 teaching hospitals associated with the University of Alberta is consistent. Medical management, including intravenous fluids, analgesia, deep venous prophylaxis, institution of oral diet and other supportive care, were left to the discretion of the attending physician and house staff team. Antibiotic coverage was provided to 79% of patients in the study. As part of standard hospital protocol, all patients underwent capillary blood glucose sampling at the initiation of supplemental nutrition. Patients with blood glucose levels greater than 11 mmol/L on 2 consecutive readings were deemed to have a day of elevated blood glucose. Patients were aggressively treated with use of the Insulin Sliding Scale to maintain blood glucose levels between 6 and 10 mmol/L.
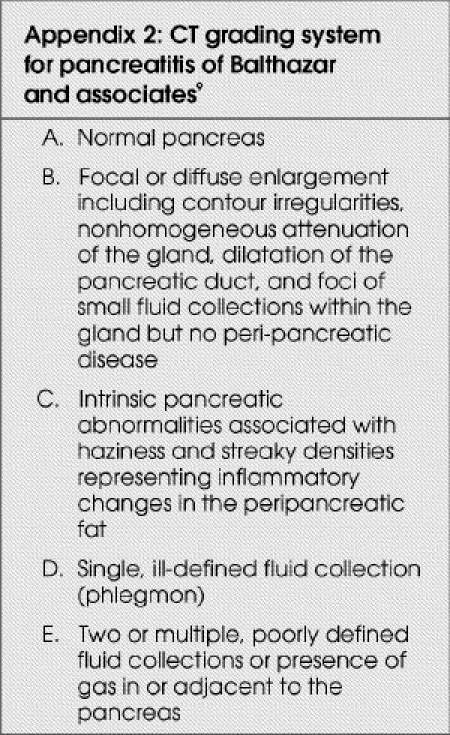
Surgical consultation and intervention were obtained when the clinical and radiologic situation dictated. All cases were assessed by CT and graded, according to the classification of Balthazar and associates,9 by a single radiologist. This grading system classified pancreatitis into 5 categories: (A) normal pancreas; (B) focal or diffuse enlargement; (C) intrinsic pancreatic abnormalities; (D) single, ill-defined fluid collection; and (E) 2 or multiple, poorly defined fluid collections or the presence of gas in or adjacent to the pancreas (Appendix 2 9). Percutaneous aspiration of acute pancreatic fluid collections is used in our centre only in exceptional circumstances.
Therapeutic intervention
Nutritional support, supplying, daily, 105 kJ (25 kcal)/kg and 1.5 g/kg of protein, based on ideal body weight, was provided within 24 hours of enrolment (Fig. 1). In the PN group, long-term vascular catheters were placed percutaneously and confirmed radiographically. PN was initially infused with a 10% dextrose solution and Intralipid (Baxter) at half of the calculated energy requirements; then increased over 2 days to achieve 100% of the target energy rate. In the EN group, nasojejunal (NJ) feeding tubes were placed via gastroscopy and confirmed radiographically. Peptamen (Nestlé), a semielemental product with low fat content, was infused at 25 mL/h and increased by 10 mL/h every 6 hours, until the target rate was achieved.
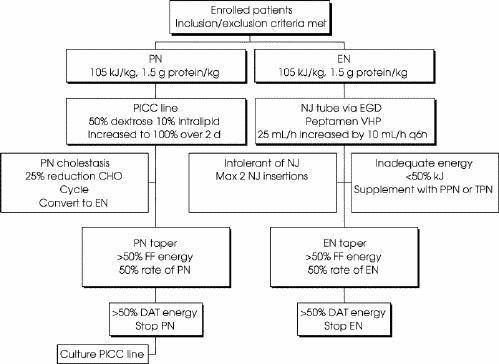
FIG. 1. Therapeutic intervention. After randomization, patients are to receive, daily, either parenteral nutrition (PN) or enteral nutrition (EN) at 105 kJ/kg and 1.5 g protein/kg. Failures are denoted by PN cholestasis, intolerance of nasojejunal tube and inability to deliver adequate energy. Nutrition is tapered as oral intake is increased. CHO = carbohyrdates, DAT = diet as tolerated, FF = full fluids, EGD = esophagogastroduodenoscopy, NJ = nasojejunal tube, PICC = percutaneous intravenous central catheter, PPN = peripheral parenteral nutrition, VHP = very high protein.
Failure to provide adequate nutrition was defined in the following 3 instances:
PN-induced cholestasis (defined as a doubling of alkaline phosphatase and bilirubin levels without other causes), if the cholestasis did not respond to a reduction in carbohydrates by 25% for 2 consecutive days and, if necessary, followed by cycled PN for 2 days;
inability to maintain placement of the NJ tube with confusion or self-removal of more than 1 NJ tube despite medical treatment of confusion, and physical and chemical restraints; and
inability after 5 days of EN to achieve 50% of maintenance energy.
Failures of PN were converted to EN. Failures of EN were either supplemented with PN or converted to PN.
As the clinical condition permitted, an oral diet was gradually instituted, starting with clear fluids and progressing to full fluids and diet as tolerated. Kilojoule counts were instituted when patients were placed on a full fluid diet. When patients were able to tolerate 50% of their maintenance energy requirements on a full fluid diet, the rate of PN or EN was halved. When the patient achieved this goal and started on diet as tolerated, the PN or EN was stopped. Patients who were on diet as tolerated and were able to maintain their energy intake for 24 hours were discharged from the study.
Measures of nutrition and inflammation
Indices of nutritional adequacy (serum albumin and prealbumin) were collected twice a week. In addition, 24-hour urinary nitrogen balance was obtained on enrolment, then every 7 days and 24 hours after a full fluid diet was consumed. Indices of inflammation (C-reactive protein and lipase) were collected twice a week. The serum cholecystokinin level was measured just before initiation and after 24 hours of nutritional support to assess the level of pancreatic stimulation related to the institution of nutrition. General laboratory tests to assess the patient's ongoing status were obtained twice a week.
Measures of cost
Direct marginal cost of nutrition was determined by calculating the cost per millilitre of nutrition for each group and capturing the volume of nutrition dispensed (used and wasted) per patient. Indirect costs for overhead in a particular department were allocated to each specific cost such as the production of PN, the placement of NJ tubes or the insertion of percutaneous indwelling catheters. Direct radiology costs per patient were captured for CT, ultrasonography and insertion of percutaneous drainage catheters, after calculating a unit cost for each investigation. For patients who had an operative procedure, costs were determined by obtaining direct patient costs, including overhead, from the cost accounting office of the regional health authority. For nonoperative complications, an average per-diem cost for general and intensive care was calculated and applied to the length of stay associated with each complication. Costs that were common to both groups such as laboratory tests, routine chest radiography for line or tube placements were not collected because they would “zero sum” in the calculation of cost-effectiveness ratios (CERs).
Formal economic evaluation was conducted in parallel to the clinical study. The intention was to calculate CERs using the formula described by Drummond:10 CER = (cost of strategy 2 – cost of strategy one)/(unit of outcome 2 – unit of outcome one).
If nutritional effectiveness was equivalent in the 2 groups, then a cost-minimization approach would be used. For this analysis, a regional health authority or hospital-based perspective was used to determine which costs were included. The costs, effects and outcomes were not discounted, considering the short duration of the study. To account for cost variability, a 1-way sensitivity analysis was completed.
Statistical analysis
The null hypothesis was that EN and PN were equivalent in reducing inflammation. We conservatively assumed a clinically important difference to be a mean of 2 days, with a standard deviation of 3 days, to achieve a 50% reduction in C-reactive protein levels. To observe a difference between the groups, the total sample size was estimated at 58 participants to achieve an α = 0.05 and a β = 0.2. Analyses were performed, using the Statistical Package for the Social Sciences (SPSS 10), on an intent-to-treat basis, using descriptive and comparative statistics, including parametric (t tests, χ2 analysis, Fisher's exact test) and non-parametric (Mann–Whitney) tests as appropriate. Time to 50% reduction in C-reactive protein and lipase levels were assessed using Wilcoxon rank sum and log rank tests. Adjusted analyses were performed using a proportional hazards model.
Results
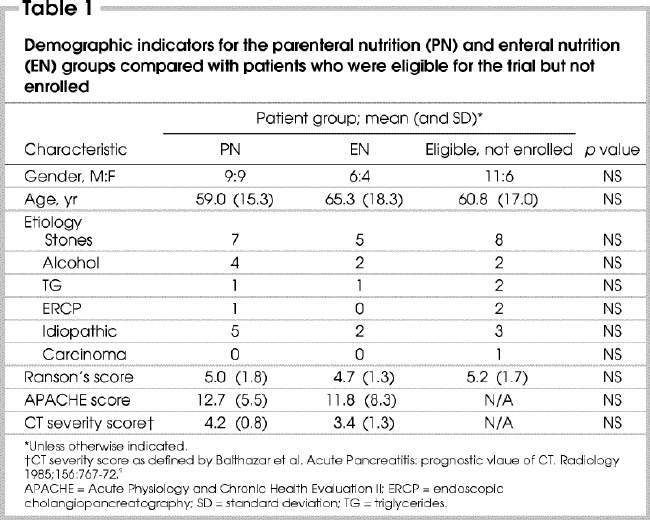
Seven hundred and twenty-eight cases of acute pancreatitis were screened for potential enrolment. One hundred and eighty-four patients had a Ranson's severity score of 3 or greater. Of these, 142 patients were excluded because they tolerated oral intake within 5 days of admission (120), died during the screening period (6), experienced intestinal perforation during endoscopic retrograde cholangiopancreatography (5), were enrolled in other studies (3) or met other exclusion criteria (8). Forty-two patients met all inclusion criteria and were eligible for consent. Eight refused to participate, and the study team did not identify 6 patients. In all, 28 patients consented to participate and completed the study. Eighteen were randomized to PN and 10 to EN (Fig. 2). The demographic distribution and baseline characteristics (Table 1 9) of the 2 groups were similar. Characteristics of enrolled patients were also similar to those who were eligible but refused to participate, were not identified or were enrolled in other studies.
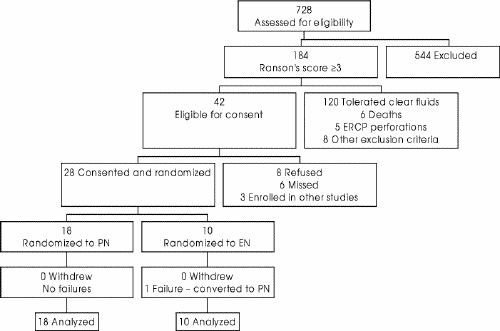
FIG. 2. Overall results: the progress of the 728 patients assessed for eligibility and screened to reach the 28 patients randomized and followed up in the study. EN = enteral nutrition, ERCP = endoscopic retrograde cholangiopancreatography, PN = parenteral nutrition.
Table 1
Reduction in inflammation
Inflammation, as measured by C-reactive protein, was reduced to 50% of enrolment levels a median of 5 days faster for EN (6 d) than PN (11 d) (Wilcoxon p = 0.09; log rank = 0.20). In contrast, lipase was reduced to 50% of enrolment levels in the EN group at 12 days compared with 5 days for the PN group (Wilcoxon p = 0.86, log rank = 0.68). In both cases, adjusting for baseline Ranson's score did not affect the results (p = 0.23 and p = 0.83) respectively.
Measures of effective nutrition
The baseline nutritional status was similar for both groups in terms of body mass index, ideal body weight, albumin, prealbumin and 24-hour urinary nitrogen levels. Patients were held fasting for a similar number of days before the initiation of nutritional support (Table 2).
Table 2
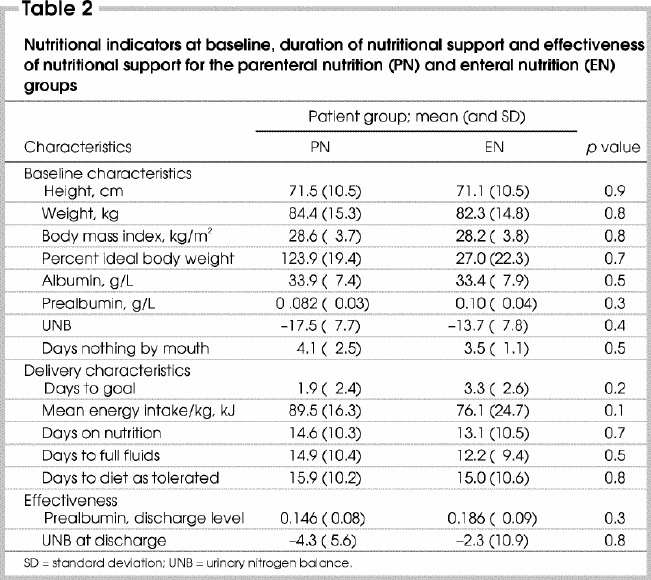
The ability to deliver and provide nutrition was the same in the 2 groups. Both groups reached the targeted energy intake at similar times and the daily average kilojoules per kilogram provided to patients were also similar. The duration of nutrition (EN or PN), as the sole source of nutrition, was similar as was the number of days to tolerate a full fluid diet and a full diet (Table 2).
The effectiveness of nutrition was assessed according to 24-hour urinary nitrogen and prealbumin levels. Both groups approached a neutral metabolic state with near-balanced urinary nitrogen levels and increasing prealbumin levels at the time of discharge from the study (Table 2).
Serum cholecystokinin was used as a measure of pancreatic stimulation. Patients receiving PN had pre-nutrition levels of 42 pmol/L and after 24 hours of nutrition of 32 pmol/L (p = 0.5). Similarly, patients receiving EN had pre-nutrition levels of 56 pmol/L and post-nutrition levels of 55 pmol/L (p = 0.2).
Mortality
Nine deaths occurred in patients with a Ranson's score of 3 or greater, for a mortality of 4.9% (9 of 184). Six deaths occurred within the screening period and were not included in the study. Within the study, there were 3 deaths. All patients were male and randomized to PN. They had an average Ranson's score of 6.3, APACHE score of 18.3 and all had CT evidence of severe pancreatitis. Independent observers not involved in the study externally reviewed the circumstances of these deaths. All deaths were attributed to specific complications of pancreatitis rather than to the mode of nutrition. Adjusted analyses were not completed given the small number of events and small sample size.
Morbidity secondary to pancreatitis
Morbidity from pancreatitis was either from organ failure or from acute pancreatic fluid collections (Table 3). In 9 PN patients acute pancreatic fluid collections were identified on CT and 4 subsequently became infected and required operative debridement. Three patients had polymicrobial infections. In 1 of these Staphylococcus aureus was grown in both the pancreatic culture and the PICC (percutaneous intravenous central catheter) culture. In the fourth patient Staphylococcus epidermis was found.
Table 3
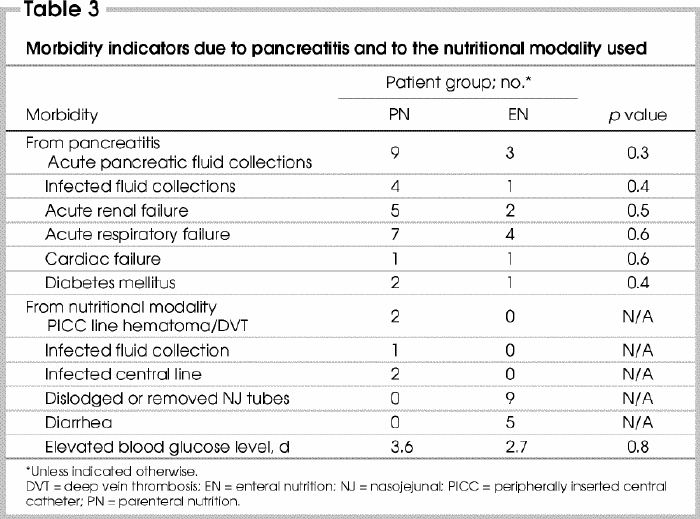
Three acute pancreatic fluid collections developed in the EN group. One patient underwent ultrasound-guided percutaneous aspiration of a pancreatic fluid collection for intermittent persisting fevers and inconclusive radiologic findings. The aspirate was sterile. Following percutaneous drainage, the patient failed to progress. He subsequently was found at laparotomy to have 2 small-bowel perforations secondary to the percutaneous drainage catheter. Intraoperative cultures were polymicrobial. When adjusted for baseline Ranson's score (p = 0.29), no differences between the groups were identified.
Morbidity secondary to nutritional practices
There were no major complications related to the insertion of PICC lines or placement of NJ tubes for nutritional support (Table 3). In the PN group, the pancreatic fluid collection that was infected with S. epidermis was presumed to originate from the PICC line. Unfortunately, we did not obtain laboratory confirmation because of a lost specimen. In the EN arm, nearly all NJ tubes were either pulled out or dislodged, thereby requiring temporary or early cessation of EN. Three patients required 2 NJ tubes, and 6 patients required temporary cessation of feeding while radiography confirmed the location of the tube. The EN failure was in a patient who had delirium tremens and removed his NJ tube. We believed that he would persist in removing the tubes, so PN was instituted.
Minor morbidity was also encountered. Transient diarrhea (>48 h–<4 d) was encountered in 5 patients after institution of EN. No patient required cessation of feeding. All patients were treated symptomatically with bulk-forming agents. Elevated blood glucose levels were encountered for an average of 2.7 days in the EN group compared with 3.6 days in the PN group.
Cost analysis
The cost of PN was greater than EN ($2608 v. $1375, p = 0.08). Other costs categories were equivalent: radiology ($852 v. $732, p = 0.5), intensive care ($21 495 v. $21 022, p = 0.9) and operative costs ($4662 v. $3039, p = 0.8) The cost of morbidity from pancreatitis was approximated by intensive care unit costs (organ failure) and operative costs to treat infected pancreatic fluid collections. The cost of morbidity from the nutritional modality was minor. Replacement or confirmation of placement of removed or dislodged NJ tubes generated additional costs of $289 per EN patient.
When we performed the 1-way sensitivity analysis, we considered 2 scenarios. First, patients were assumed to require 1 NJ tube placement. The rate of dislodgment or removal was greater than 90%; however, only 13 tubes were placed in 10 patients. Therefore, we assumed that 1 tube per patient could be achieved with minor changes in tube placement. When only 1 NJ tube is used, the average cost of PN is $2608 compared with $1086 for EN (p = 0.03; 95% confidence interval of the cost difference $163–$2880). Second, 1 patient who was an EN failure only received 3 hours of nutrition. This patient would not, under normal clinical circumstances, receive a feeding tube while in alcohol withdrawal. When it was assumed this patient received PN only, and all other NJ patients required 1 tube, the cost of EN falls to $957 (p = 0.03; 95% CI of the cost difference $230–$3073) and is significantly different from the cost of PN.
Cost-minimization analysis
In this study, the primary results show that the cost of EN is less than that of PN in all situations and that there was a trend to greater reduction in C-reactive protein with EN. EN is, therefore, less costly and produces at least an equivalent outcome in terms of reducing inflammation. Under such circumstances, there is no need to calculate a cost-effectiveness ratio because the interpretation is clear: adopt technologies that cost less and produce equivalent or better effect.11 Thus, EN becomes a dominant technology over PN with respect to economic evaluation.
Discussion
There is mounting evidence to confirm that EN is safe and effective in acute pancreatitis.4,5,12 Whereas these studies included patients with mild and severe pancreatitis, our study and the study by Kalfarentzos and associates3 included only patients with severe, acute pancreatitis as defined by Ranson's score and Imrie criteria. Both trials used NJ tubes and delivered EN to support patients with severe, acute pancreatitis with minimal morbidity from the EN.
One of the presumed difficulties with EN is the ability to meet targeted energy needs. In our study as well as others,4,5 EN led to patients receiving a lower-than-targeted energy intake. Several reasons can be cited for the lower per kilogram kilojoule count. First, as required by hospital policy, patients with EN also had their feeds held in preparation for radiologic examinations, transportation and procedures, thereby contributing to a period of reduced energy intake. Second, NJ tubes were at times dislodged or pulled out inadvertently by patients. In these instances, feeds were held until placement could be reconfirmed by plain-film radiography to be distal to the ligament of Treitz. Third, our protocol for both types of nutrition required incremental feeding to reach energy targets. Interestingly, patients receiving PN did not receive 100% of targeted energy needs either because PN formulations were adjusted for hypertriglyceridemia and renal failure, often leading to administration of fewer kilojoules.
Despite the nuances of enteral feeding and smaller per kilogram kilojoule intakes, the measures of nutritional effectiveness (urinary nitrogen balance and prealbumin level) were comparable to those receiving PN. Whether this is related to attenuation of the inflammatory response or a function of maintenance of the gut mucosa is difficult to determine. Normalization of prealbumin levels may also represent hepatic reprioritization of prealbumin synthesis as the pancreatitis resolves.13 What these findings suggest is that concerns about adynamic ileus are less of an issue than anticipated with enteral feeding. Delivery beyond the ligament of Treitz appears to bypass an apparent gastric ileus that is more consistent with acute pancreatitis.
Physicians have also expressed concern over using EN in severe pancreatitis for fear of stimulating the pancreas and worsening the clinical condition. It appears that neither PN nor jejunal EN stimulated the pancreas. However, EN may lead to slower reduction in lipase levels than PN, suggesting that EN may stimulate the pancreas. This, however, did not have a clinical impact and may reflect the sensitivity of lipase to detect subclinical pancreatic injury. When studies involving patients with chronic pancreatitis used serum cholecystokinin levels as a marker of pancreatic stimulation, oral intake of Peptamen caused a rise in serum cholecystokinin levels within 24 hours of intake. Similar rises in cholecystokinin were not found with jejunal administration of the same feed.14,15 In the present study, serum cholecystokinin levels in both groups remained relatively stable before and after 24 hours of nutritional supplementation, confirming that jejunal feeding does not stimulate the pancreas.
One of the major differences between our study and others3,4,5 is the time at which nutrition was initiated. The protocol was designed with 2 issues in mind: to simulate clinical practice in our centre and to enrol a group of patients with severe, acute pancreatitis. As a result, we enrolled patients with severe pancreatitis who truly required nutritional support and we identified patients with severe pancreatitis who did not require nutritional support. Sixty-five percent of patients with criteria for severe pancreatitis tolerated clear fluids within the screening period, leaving less than 35% of those initially defined as severe cases requiring nutritional support. This suggests that nearly two-thirds of patients with criteria for severe pancreatitis in other studies underwent procedures to deliver nutrition and were exposed to risks that many not have been necessary. There is no tool to predict which patients will require and benefit from nutritional support. Thus, it appears appropriate to initiate a trial of fasting, followed by clear fluids if the patient is clinically stable, as one such strategy for selecting suitable candidates.
This waiting period, however, placed us at odds with others who believe that early EN is associated with attenuation of the inflammatory cascade and reduced septic events.3,4,5 The evidence that early EN has an impact on attenuating the inflammatory response is inconsistent. Windsor and colleagues5 reported a significant reduction in C-reactive protein for EN after 7 days of nutrition, measured within 48 hours of admission and after 7 days of nutrition. The mean C-reactive protein level at 48 hours was 156 mg/L and after 7 days was 84 mg/L. C-reactive protein levels in the PN group were unchanged after 7 days of nutrition (125 mg/L v. 124 mg/L). Powell and colleagues,12 in comparing EN to fasting with limited C-reactive protein data, showed that EN had nonsignificant lower C-reactive protein levels from admission to day 4. More importantly, C-reactive protein levels for both EN and fasting groups moved in parallel, peaked at day 2 and decreased at similar rates. They concluded that EN had no impact in regard to attenuating the inflammatory response.
Using time-to-event analysis, we demonstrated a trend to faster reduction in C-reactive protein levels with EN than with PN (Wilcoxon p = 0.09). Wilson and colleagues16 showed in a series of patients with acute pancreatitis without other interventions that the natural progression of C-reactive protein is to peak 24–48 hours after injury to the pancreas and to fall consistently over 7 days. Peak levels greater than 210 mg/L on days 2–4 and greater than 120 mg/L on day 7 were associated with severe pancreatitis. It appears that regardless of nutrition and the time that supplemental nutrition is begun, the reduction in inflammation in these studies5,12 is very similar to the findings of Wilson and colleagues. It is also plausible that EN has only a minor impact on the inflammatory cascade, simply because of the complexity of the cascade.
C-reactive protein is an acute-phase reactant primarily synthesized by hepatocytes in response to acute and chronic inflammation. Regulated by cytokines such as interleukin-6, C-reactive protein is a sensitive, but nonspecific, marker of inflammation. Clinical application of C-reactive protein has been linked to bacterial and viral infections, pelvic inflammatory disease, arthritic conditions like systemic lupus erythematosus and rheumatoid arthritis, and renal transplantation.17 Recently, levels of C- reactive protein have been correlated with the presence of coronary artery disease18,19 and other cardiovascular conditions such as heart failure. C-reactive protein, as a marker of disease severity, is relatively new, but emerging evidence has confirmed its ability to correlate with disease severity particularly in cardiac disease.
Prevention of sepsis and sepsis-related complications was a secondary outcome in our study as well as in other studies. Pancreatic abscess requiring pancreatic debridement and necrosectomy represents significant morbidity and cost. In all 3 studies, PN was associated with more operative events and infective events than EN. Windsor and colleagues5 reported 3 occurrences of sepsis with PN compared with none for EN as well as 2 pancreatic necrosectomies in the PN group compared with none for the EN group. Similarly, Kalfarentzos and associates3 listed 4 patients with infected pancreatic or peripanceatic necrosis within the PN group compared with 1 in the EN group. Our findings were similar. Although differences were not statistically significant, the trend across 3 studies is easily recognized. Furthermore, compelling evidence from other disciplines has shown that EN limits the number of septic complications compared with PN.20,21,22 Whether this is a result of maintaining gut integrity and preventing bacterial translocation has not been consistently proven in previous trials. This represents a significant opportunity for further research with EN and pancreatitis. It also provides definable and recognizable clinically based outcomes such as pancreatic abscess, necrosis and operative interventions.
Formal cost-effectiveness evaluation is lacking in the literature, although recent studies did report limited cost measurement of nutrition.3,4,5 EN has resulted in lower calculated costs in all trials, but without considering the cost per effect of treatment it is difficult to evaluate and make recommendations regarding the adoption of EN. To our knowledge, our cost-effectiveness analysis is the first one reported in terms of EN and pancreatitis. We concluded that EN is less expensive than PN and is at least equally effective. Under suggested guidelines for adoption and use of technology,11 EN warrants adoption, integration and utilization given the limited statistical power of this study.
This must also be viewed in 2 additional contexts. First, the serious adverse events related to EN were less than in the PN group. The most serious adverse events were infected pancreatic fluid collections. Even though there was a high rate of tube dislodgement, only an average of 1.3 tubes per EN patient were used. Minor morbidity such as diarrhea was transient and symptoms were easily controlled. Elevated blood glucose levels were similar in both groups, suggesting that overfeeding was unlikely to have caused these elevated levels. Patients were supported on the basis of ideal body weight and the kilojoules per kilogram were less than 105 in both groups. It is more likely that severe pancreatitis led to islet cell dysfunction and destruction, resulting in elevated glucose levels and chronic pancreatitis as we have shown in a subsequent study.23 Second, the analysis of costs was completed as an effectiveness trial not an efficacy trial, using the most conservative assumptions — that is, a worst-case scenario. Should the assumptions included in the sensitivity analysis prove to be true, this would give further support to the findings of the economic evaluation.
Our study is clearly limited by its small sample size. Our power calculation estimated a study sample size of 58 patients and we were able to enrol only 50% of our estimated sample size. This trial was terminated before achieving our sample size because of a lower-than-anticipated incidence of severe pancreatitis, difficulties with consent, problems identifying patients and movement of key study personnel. Our difficulties with enrolment were not unique to this centre. McClave and colleagues4 also encountered difficulties with study duration, patient consent and compliance. Other studies included patients with mild pancreatitis to boost enrolment. Because our study is underpowered, there is a possibility of type II error, and the results must be interpreted with this in mind.
Conclusions
In this randomized controlled trial of PN and EN in supporting patients with severe pancreatitis, we have confirmed that EN may be safely delivered to such patients without significant complications. Although there is insufficient evidence to show that EN attenuates the inflammatory process of pancreatitis, it appears that EN does not stimulate the pancreas and can provide sufficient nutritional support. The economic analysis has demonstrated that EN is the dominant choice for nutritional support in severe pancreatitis. Adoption, integration and utilization of this technology should proceed, but with knowledge of the limitations of the study. Further research into the association of EN with septic events in pancreatitis is required.
Acknowledgments
We thank Mrs. Sandra Blitz for statistical support during the study.
Appendix 1.

Appendix 2.
†Winner of the 2004 MacLean–Mueller Prize
Presented as a poster at Nutrition Week (San Diego, Calif., Feb. 23–27, 2002), the Canadian Digestive Diseases Week (Montréal, Que., Jan. 30–Feb. 5, 2002) and Digestive Diseases Week (San Francisco, Calif., May 19–22, 2002).
Competing interests: None declared.
Correspondence to: Dr. Brian E. Louie, Thoracic Oncology Program, Swedish Cancer Institute and Swedish Medical Center, 1221 Madison St., Seattle WA 98104
Accepted for publication Oct. 5, 2004.
References
- 1.Feller JH, Brown RA, Toussaint GP, Thompson AG. Changing methods in the treatment of severe pancreatitis. Am J Surg 1974;127:196-201. [DOI] [PubMed]
- 2.Simpson WG, Marsano L, Gates L. Enteral nutritional support in acute alcoholic pancreatitis. J Am Coll Nutr 1995;14:662-5. [DOI] [PubMed]
- 3.Kalfarentzos F, Kehagias J, Mead N, Kokkinis K, Gogos CA. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg 1997;84:1665-9. [PubMed]
- 4.McClave SA, Greene LM, Snider HL, Makk LJ, Cheadle WG, Owens NA, et al. Comparison of the safety of early enteral vs parenteral nutrition in mild acute pancreatitis. JPEN J Parenter Enteral Nutr 1997;21:14-20. [DOI] [PubMed]
- 5.Windsor AC, Kanwar S, Li AG, Barnes E, Guthrie JA, Spark JI, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut 1998;42:431-5. [DOI] [PMC free article] [PubMed]
- 6.Kudsk KA, Minard G, Wojtysiak SL, Croce M, Fabian T, Brown RO. Visceral protein response to enteral versus parenteral nutrition and sepsis in patients with trauma. Surgery 1994;116:516-23. [PubMed]
- 7.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg 1992;216:172-83. [DOI] [PMC free article] [PubMed]
- 8.Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974;139:69-81. [PubMed]
- 9.Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology 1985;156:767-72. [DOI] [PubMed]
- 10.Drummond MF. Methods for the economic evaluation of health care programmes. 2nd ed. New York: Oxford University Press; 1997.
- 11.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992;146:473-81. [PMC free article] [PubMed]
- 12.Powell JJ, Murchison JT, Fearon KC, Ross JA, Siriwardena AK. Randomized controlled trial of the effect of early enteral nutrition on markers of the inflammatory response in predicted severe acute pancreatitis. Br J Surg 2000;87:1375-81. [DOI] [PubMed]
- 13.Peterson VM, Moore EE, Jones TN, Rundus C, Emmett M, Moore FA, et al. Total enteral nutrition versus total parenteral nutrition after major torso injury: attenuation of hepatic protein reprioritization. Surgery 1988;104:199-207. [PubMed]
- 14.Freedman SD. Peptamen: a novel therapy in patients with chronic pancreatitis [abstract]. Gastroenterology 1997;112:A441.
- 15.Shetzline MA, Liddle RA. Neurohumoral control of the exocrine pancreas. Curr Opin Gastroenterol 1999;15:380-4. [DOI] [PubMed]
- 16.Wilson C, Heads A, Shenkin A, Imrie CW. C-reactive protein, antiproteases and complement factors as objective markers of severity in acute pancreatitis. Br J Surg 1989;76:177-81. [DOI] [PubMed]
- 17.Young B, Gleeson M, Cripps AW. C-reactive protein: a critical review. Pathology 1991;23:118-24. [DOI] [PubMed]
- 18.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350:1387-97. [DOI] [PubMed]
- 19.Haidari M, Javadi E, Sadeghi B, Hajilooi M, Ghanbili J. Evaluation of C-reactive protein, a sensitive marker of inflammation, as a risk factor for stable coronary artery disease. Clin Biochem 2001;34:309-15. [DOI] [PubMed]
- 20.Voitk A, Brown RA, Echave V, McArdle AH, Gurd FN, Thompson AG. Use of an elemental diet in the treatment of complicated pancreatitis. Am J Surg 1973;125:223-7. [DOI] [PubMed]
- 21.Kompan L, Kremzar B, Gadzijev E, Prosek M. Effects of early enteral nutrition on intestinal permeability and the development of multiple organ failure after multiple injury. Intensive Care Med 1999;25:157-61. [DOI] [PubMed]
- 22.Kudsk KA. Gut mucosal nutritional support–enteral nutrition as primary therapy after multiple system trauma. Gut 1994;35(1 Suppl):S52-4. [DOI] [PMC free article] [PubMed]
- 23.Hochman D, Louie B, Hamilton S, Bailey R. After severe acute pancreatitis: determination of quality of life and development of chronic pancreatitis screening questionnaire [abstract]. Can J Surg 2004;47(Suppl):19.


